Abstract
Polypharmacy in older adults results in multiple negative clinical consequences including increased risk of hospital readmissions. Precision medicine may provide tools to optimize complex medication regimens however its potential in older adults with polypharmacy is unknown. We carried out pharmacogenetic testing in an older adult with multiple chronic conditions and polypharmacy who was concerned about frequent readmissions despite receiving guideline-concordant care and being adherent to medication regimen. The testing identified patients’ CYP2D6 rapid metabolizer status. This may have resulted in decreased exposure to Carvedilol which was primary drug for CHF management in this patient. Additional nine drug-drug interactions were identified during personalized drug regimen review. We concluded that, though precision medicine has enormous potential in older adults with polypharmacy, the complexity of pharmacogenetic information requires innovative informatics solutions to support optimal workflows, decision support, and medication optimization and management in order to fully utilize its potential in routine clinical care.
Introduction
Multiple epidemiological studies clearly demonstrated that polypharmacy is highly prevalent in older adults1. Based on the 2005-2006 survey study, over 36% of people 75 and 85 years of age are taking at least five prescription medications2. A strong relationship between polypharmacy and negative clinical consequences has been described in previous research3. In older adults polypharmacy has been associated with increased health care costs, adverse drug reactions, drug interaction, medication non-adherence, impaired functional and cognitive status, falls, urinary incontinence, and malnutrition3. Not surprisingly, polypharmacy and potentially inappropriate medication use was shown to be a significant precipitating factor in frequent hospital admissions4.
Precision medicine provides tools allowing personalize medication regimens based on individual genetic variations and information about potential drug interactions obtained from comprehensive bioinformatics repositories. Pharmacogenomics is the study of how a person’s unique genetic makeup influences their response to drugs. It is the cornerstone in the concept of personalized medicine in which the use of drugs and drug combinations can be expected to be tailored to patient’s unique genetic profile. The availability of genomic testing has grown but its clinical application is still in the early stages. The US Food and Drug Administration (FDA) now require submission of pharmacogenomics data to be included in the labeling of drugs5. This has the expectation that this information may improve drug safety, identify optimal dosing, improve targeting to disease and reduce adverse drug reactions.
Particular attention in pharmacogenomics has been devoted to cytochrome P450 (CYP) enzymes involved in metabolism of over 60–70% of all prescribed drugs. The most important cytochrome P450 (CYP) enzymes involved in drug metabolism are CYP2D6, CYP2C19, CYP2C9, CYP2D6, CYP3A4 and CYP3A56. With these enzymes there may be many spectrums of genotypes resulting in poor metabolizer (patients with little to no functional activity) to ultra-rapid metabolizers (patients with increased metabolic activity)7. For example, the P450 cytochrome enzyme CYP2C9 along with VKORC1 is the primary enzymes for metabolism of Warfarin8. This is already being applied to use of Warfarin and Clopidogrel with 64% of cardiologists having reported using genomic testing9. A known allele variant has been shown to result in an 80% decrease in enzyme activity in the patients then would be expected to be very sensitive to the anticoagulants effect of Warfarin indicating need for use of lower dosing9. Clopidogrel is activated via CYP2C19 and variants affecting function are well known9. All known pharmacogenomics variants are readily available on the PharmGKB website10.
However, for the physicians and healthcare professionals in practice the clinical utility and integration in patient care remains uncertain and mostly unexplored. There are many issues regarding genetic testing from simple ones on how and where can testing be done to more complex issues as to which patients to test, how to interpret the results of testing and then how to apply the findings to decision making that may benefit the patient11. Can the use of genetic testing data be used in older adults to tailor drug treatments, reduce adverse drug effects, reduce polypharmacy and eventually improve disease outcome? Whether precision medicine has potential in providing effective means to ameliorate detrimental impact of polypharmacy in older adults is currently unknown. In this article we present a case of a frequently hospitalized older adult with polypharmacy that can provide instructive insight on potential of precision medicine in this rapidly growing population.
Method
An older adult with polypharmacy suffering from multiple chronic conditions and concerned about frequent hospital admissions was offered by a treating physician to undergo pharmacogenetic testing. Though the patient has been receiving a guideline-concordant therapy, the goal of the pharmacogenetic testing was to identify ways to further personalize the patient medication regimen. After obtaining written consent, a buccal swab was sent via overnight express mail to a CLIA-certified facility to detect common variants in genes which may affect individual response to medications (Figure 1).
Figure 1.
Pharmacogenetic panel to detect genetic cytochrome P450 variants with known clinical significance.
The results of the testing were provided by the testing facility via an online portal a week after submission of the buccal swab. The portal contained a detailed report with results of the genetic testing as well as interpretation of findings. The portal also provided basic education materials explaining general principles of pharmacogenetic testing.
Results
Pharmacogenetic testing was performed with consented patient with polypharmacy in attempt to optimize medical management. The patient was a 78 year old Puerto Rican man with history of multiple medical problems prominent of which is congestive heart failure and chronic obstructive lung disease. The patient has had 23 hospitalizations over the past five years. Most of his hospitalizations were due to decompensated heart failure. Several of the hospitalizations were for COPD exacerbations with wheezing as the presenting symptom. Many hospitalizations were deemed by his medical team to be due to exacerbation of both heart and lung disease. The patient had systolic heart failure with Left Ventricle size mildly increased. The left ventricular systolic function was moderate to severely decreased with a left ventricular ejection fraction of 30 to 35%. There was global hypokinesis with regional variations. The right ventricular size was mildly increased with right ventricular systolic function preserved. The patient also suffered from Chronic Obstructive Pulmonary Disease with FEV1 of 52% predicted. He was an approximate 50 pack/year smoker. In addition, he suffered from Atrial Fibrillation, Chronic Kidney Disease (Estimated Glomerular Filtration Rate of 23), Hyperlipidemia, Gout, BPH and Gastritis. The patient medication regimen is presented in Figure 2.
Figure 2.
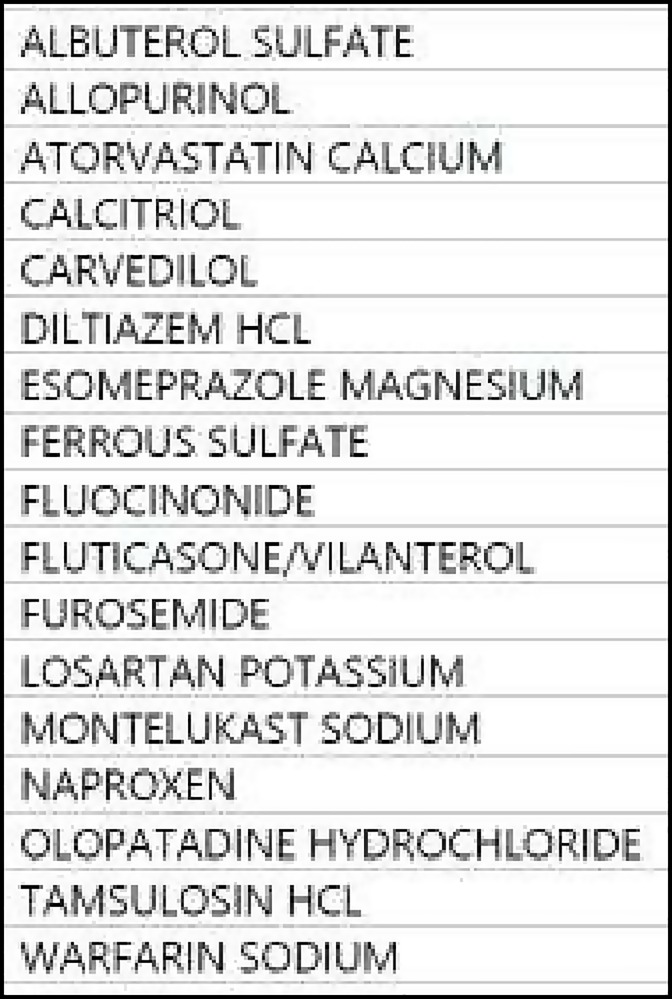
Patient medication list.
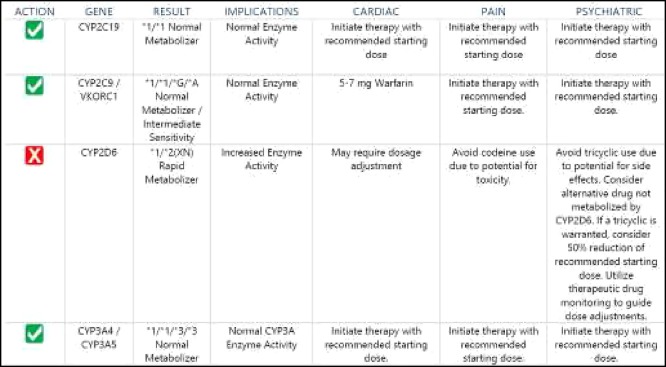
The results of pharmacogenetics testing are presented in Figure 3. The major pharmacogenetic finding was that the patient had a well described genetic polymorphism for cytochrome P450 enzyme responsible for Carvedilol metabolism - CYP2D6. Carvedilol is a major substrate of CYP2D6. Given patient’s CYPD6 Rapid Metabolizer status, patient may have decreased exposure to carvedilol. Carvedilol is main medication used to control CHF in this patient. Potentially compromised Carvedilol metabolism may affect efficacy of this drug in the patient and overall care of CHF.
Figure 3.
Genotype/phenotype results.
Additional findings included three drug-drug interactions based on cytochrome P450-mediated metabolism of moderate severity (Table 1) and seven non-CYP drug-drug interactions three of which had major severity (Table 2).
Table 1.
Drug-drug interactions based on cytochrome P450-mediated metabolism.
| Drug/Drug Interaction | Severity | Action | Mechanism |
|---|---|---|---|
| CARVEDILOL / LOSARTAN POTASSIUM | Moderate | Monitor therapy | CYP2C9 Inhibitors (Moderate) may increase the serum concentration of Carvedilol. |
| DILTIAZEM HCL / FLUTICASONE&VILANTEROL | Moderate | Monitor therapy | CYP3A4 Inhibitors (Moderate) may decrease the metabolism of CYP3A4 Substrates. |
| LOSARTAN POTASSIUM / WARFARIN SODIUM | Moderate | Monitor therapy | CYP2C9 Inhibitors (Moderate) may decrease the metabolism of CYP2C9 Substrates. |
Table 2.
Non-CYP drug-drug interactions.
| Drug/Drug Interaction | Severity | Action | Mechanism |
| ALBUTEROL SULFATE / CARVEDILOL | Major | Avoid combination | Beta-Blockers may diminish the bronchodilatory effect of Beta2-Agonists. |
| CARVEDILOL / FLUTICASONE & VILANTEROL | Major | Avoid combination | Beta-Blockers may diminish the bronchodilatory effect of Beta2-Agonists. |
| ALLOPURINOL / WARFARIN SODIUM | Moderate | Consider modification | Allopurinol may enhance the anticoagulant effect of Vitamin K Antagonists. |
| ATORVASTATIN CALCIUM / DILTIAZEM HCL (AC/DHCL) | Major | Consider modification | DHCL may increase the serum concentration of AC. AC may increase the serum concentration of DHCL. |
| CARVEDILOL / TAMSULOSIN HCL | Moderate | Consider modification | Beta-Blockers may enhance the orthostatic hypotensive effect of Alphal-Blockers |
| FUROSEMIDE / NAPROXEN | Moderate | Consider modification | Nonsteroidal Anti-Inflammatory Agents may diminish the diuretic effect of Loop Diuretics |
| NAPROXEN/ WARFARIN SODIUM | Moderate | Consider modification | NSAID (Nonselective) may enhance the anticoagulant effect of Vitamin K Antagonists |
Discussion
We performed pharmacogenomics testing on an arbitrarily selected patient with multiple medical problems that was on many medications and who was seeking answers to polypharmacy and reasons for frequent hospital admissions. The first step was to search for the appropriate laboratory that could perform the genetic testing. The first lab contacted could not be utilized because it was not licensed in the State of New York. That became the first question in seeking a lab for genetic studies. Performing the test was simple. The lab provides the educational material, consent form and buccal swab. After consent two buccal swabs are obtained, placed in the envelope provided and returned via overnight FedEx. Results were returned within 1 week via a dedicated web portal. The report included not only pharmacogenetic test results but also a list of potential drug-drug interactions with short explanations.
The patient genotype analysis is notable for “rapid metabolizer” polymorphism for the CYP2D6 enzyme. His medication Carvedilol is a primary drug in his management of congestive heart failure and a major substrate for the CYP2D6 enzyme. Rapid metabolism of the Carvedilol may be expected affect efficacy but the clinical significance is unknown. Would this polymorphism have any negative impact on the survival benefit of b-blockers in congestive heart failure? His clinical course was notable for difficulty in rate control of his atrial fibrillation requiring the use of b-blocker (which may be expected to be less effective due to “rapid metabolizer” polymorphism) and use of a calcium channel blocker Diltiazem in addition. Diltiazem is not metabolized via the CYP2D6 enzyme but is a moderate inhibitor of the CYP3A4/CYP3A5 enzymes so may be expected to impact metabolism of other CYP2D6 drugs and increase their serum levels. In this patient other affected drugs included Atorvastatin, Montelukast, Tamsulosin, Fluticasone/Vilanterol and Esomeprazole Magnesium.
The tested patient had both cardiovascular disease and other concomitant chronic conditions including chronic obstructive pulmonary disease. We recognize that cardiovascular and pulmonary airways disease often coexists as they are known to share risk factors such as age, smoking and low grade chronic inflammation12. We accept that polypharmacy is a fundamental feature in the management of cardiovascular and pulmonary airways disease. Currently, the management of cardiovascular heart disease usually requires use of b-blockers, antiplatelets agents, diuretics, statins, and ACE inhibitors or ARBs. Treatment of airways obstruction often requires use of combinations of b-agonists (short and long acting), corticosteroids, anticholinergics and Leukotriene blockers. Coexistence of both heart and airways disease is known to sometimes create therapeutic dilemmas, such as weighing the benefits of b-blockers versus the fear of exacerbating coexistent airways obstruction as well as weighing the potential increase risk of death with the use of long acting b-agonists. Even with properly prescribed medications, genetic polymorphisms of the metabolizing CYP enzymes may affect a drug’s efficacy or adverse effect potential. The use combinations of drugs may also affect the clearance via inhibition or induction of certain cytochrome metabolizing enzymes i.e. gene/drug interaction. Even in hospitalized patients there are adverse drug events that are not readily explained and raises issue if polymorphism of CYP metabolizing enzymes may be in play.
Pharmacogenomic data are currently provided in about 10% for US FDA approved medications. There is a great resource: the Pharmacogenomics Knowledge Base (PharmGKB) provides the effects of genetic variations on drug action, mechanism of drug action, dosing guidelines, information regarding available laboratories performing genetic tests and references. However use of this information may be overwhelming and its complexity may limit applicability in routine clinical care. The optimal workflows, interpretation and evidence-based follow-up for this potentially very promising approach are yet to be established. Biomedical informatics technologies have enormous potential in developing point-of-care decision support which would bring these new exciting technologies to daily clinical practice.
Majority of clinicians currently lack comprehensive skills to take full advantage of the potential of pharmacogenetic testing13. They are unsure how to carry out testing, where process samples, and how to follow-up on testing results14. Development of effective tools assisting providers in the use and interpretation of pharmacogenetic tests should be a high priority for biomedical informatics researchers. Such tools may provide support for multiple aspects pertinent to optimal use of pharmacogenetic testing. Since limited information is available on certified facilities for pharmacogenetic testing and type of services they provide, creation of a comprehensive online public resource of certified facilities which is maintained and curated by designated academic centers and industry may be warranted. To address lack of provider knowledge, introduction of online CME courses and training tools may facilitate diffusion of pharmacogenetics into routine clinical practice. Due to inherent complexity of this new methodology, introduction of evidence-based clinical pathways may promote best practices in pharmacogenetic applications15. Electronic health records have to include functionality supporting storage, exchange, and presentation of pharmacogenetic results coupled with intelligent decision support allowing account simultaneously for multiple drug-gene and drug-drug interactions and assist in medication regimen optimization16. Finally, development of interactive apps to educate patients about pharmacogenetic testing and to engage them in medication management based on genetic test results will facilitate patient-provider communication and ensure patient acceptance of pharmacogenetic testing as an opportunity to personalize their medication regimen and improve their quality of life17.
Conclusion
Precision medicine has enormous potential in optimizing medication regimen in frequently hospitalized older adults with polypharmacy. However to uncover this potential innovative biomedical informatics technologies are urgently needed to support optimal workflows, decision support, and medication optimization and management.
References
- 1.Rohrer JE, Garrison G, Oberhelman SA, Meunier MR. Epidemiology of polypharmacy among family medicine patients at hospital discharge. J Prim Care Community Health. 2013;4(2):101–5. doi: 10.1177/2150131912472905. [DOI] [PubMed] [Google Scholar]
- 2.Qato DM, Alexander GC, Conti R, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehgal V, Bajwa SJ, Sehgal R, Bajaj A, Khaira U, Kresse V. Polypharmacy and potentially inappropriate medication use as the precipitating factor in readmissions to the hospital. J Family Med Prim Care. 2013;2(2):194–9. doi: 10.4103/2249-4863.117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David SP, Johnson SG, Berger AC, Feero WG, Terry SF, Green LA, Phillips RL, Jr, Ginsburg GS. Making Personalized Health Care Even More Personalized: Insights From Activities of the IOM Genomics Roundtable. Ann Fam Med. 2015;13(4):373–80. doi: 10.1370/afm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavallari LH, Jeong H, Bress A. Role of cytochrome P450 genotype in the steps toward personalized drug therapy. Pharmgenomics Pers Med. 2011;4:123–36. doi: 10.2147/PGPM.S15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89(1):25–33. doi: 10.1016/j.mayocp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson M, Richard C, Bogdan R, Kidd R. Warfarin Dosing in a Patient with CYP2C9(*)3(*)3 and VKORC1-1639 AA Genotypes. Case Rep Genet. 2014;2014:413743. doi: 10.1155/2014/413743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman AL, Spitz J, Jacobs M, Sorrentino M, Yuen S, Danahey K, Saner D, Klein TE, Altman RB, Ratain MJ, O’Donnell PH. Evidence for Clinical Implementation of Pharmacogenomics in Cardiac Drugs. Mayo Clin Proc. 2015;90(6):716–29. doi: 10.1016/j.mayocp.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiratsuka M, Sasaki T, Mizugaki M. Genetic testing for pharmacogenetics and its clinical application in drug therapy. Clin Chim Acta. 2006;363(1-2):177–86. doi: 10.1016/j.cccn.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 11.Sim SC, Ingelman-Sundberg M. Pharmacogenomic biomarkers: new tools in current and future drug therapy. Trends Pharmacol Sci. 2011;32(2):72–81. doi: 10.1016/j.tips.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein J, Cha E, Scharf SM. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruct Pulmon Dis. 2009;4:337–49. doi: 10.2147/copd.s6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen Taber KA, Dickinson BD. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmgenomics Pers Med. 2014;7:145–62. doi: 10.2147/PGPM.S63715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clin Genet. 2012;82(4):388–94. doi: 10.1111/j.1399-0004.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169–76. doi: 10.1038/gim.2014.101. [DOI] [PubMed] [Google Scholar]
- 16.Overby CL, Erwin AL, Abul-Husn NS, Ellis SB, Scott SA, Obeng AO, Kannry JL, Hripcsak G, Bottinger EP, Gottesman O. Physician Attitudes toward Adopting Genome-Guided Prescribing through Clinical Decision Support. J Pers Med. 2014;4(1):35–49. doi: 10.3390/jpm4010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills R, Voora D, Peyser B, Haga SB. Delivering pharmacogenetic testing in a primary care setting. Pharmgenomics Pers Med. 2013;6:105–12. doi: 10.2147/PGPM.S50598. [DOI] [PMC free article] [PubMed] [Google Scholar]