Abstract
We investigated the effects mediated by glucocorticoid (GC) receptor (GR) blockage using RU486, a GR antagonist, and GR siRNA on the proliferative and differentiation capabilities of human bone marrow mesenchymal stromal/stem cells (MSCs), as well as on their senescence and antioxidant levels during extended in vitro culture. Treatment with either RU486 or GR siRNA for a 7 day period significantly increased the proliferation of MSCs as well as their osteogenic capabilities, as reflected by an increase in alkaline phosphatase (ALP) level after differentiation. After 4 weeks of the treatments MSCs improved or maintained their proliferation rates, while the control MSCs exhibited decreased proliferation. While all MSCs exhibited reduced osteogenic potential after 4 weeks of in vitro culture, the MSCs treated with GR inhibitors showed higher ALP levels than untreated MSCs after they were subjected to osteogenic differentiation. These treatment also significantly down-regulated the adipogenic capabilities of MSCs. Telomere lengths as well as the telomerase and superoxide dismutase activities of MSCs treated with either RU486 or GR siRNA appeared to be higher than those detected in controls. These results demonstrate that blockage of the effects mediated by the GCs normally found in fetal bovine serum may postpone senescence of these cells by up-regulating their antioxidant levels. These data suggested that blocking the effects mediated by GCs could potentially extend the lifespan of endogenous MSCs in patients who have elevated GC levels as a consequence of advancing age or estrogen depletion.
Keywords: Glucocorticoid receptor, siRNA, RU486, Mesenchymal stromal cells, Senescence
Introduction
Bone marrow mesenchymal stem/stromal cells (MSCs) are capable of differentiating into multiple cell types and replace these cells when they are lost or damaged [1–2]. Imbalances in steroid hormone levels as well as aging have been demonstrated to induce senescence of these stem cells, resulting in both decreased proliferation and osteochondrogenic differentiation and increased adipocyte differentiation [3–5]. These alterations in bone marrow MSCs can ultimately contribute to the development of osteoporosis and osteoarthritis, because these stem cells have lost their potential to contribute to bone and cartilage formation [6–8]. In addition, because of their high ex vivo proliferative capabilities and strong osteochondrogenic differentiation potential, bone marrow MSCs also have been considered a potential cell source for the tissue engineering of bone and cartilage [9–10]. Unfortunately, in vitro expansion of these cells using current culture techniques induces senescence that abruptly reduces their proliferative and osteochondrogenic capabilities and seriously limits their potential usefulness in osteochondral regeneration [11–13]. Therefore, a more thorough understanding of steroid-mediated regulation of MSC characteristics is required in order to develop protocols that might postpone their senescence. This new information is essential in order to develop an appropriate treatment to improve MSC capabilities in terms of osteochondral regeneration and develop an effective approach to prevent MSC senescence-associated osteoporosis.
Glucocorticoids (GCs) play a major role in regulating the proliferation and differentiation of MSCs via classic glucocorticoid receptors (GR), classified as the type 2 receptor for GCs [14–15]. Elevated endogenous levels of GCs potentially reduce proliferation and induce apoptosis in many cell types, including osteoblasts and osteocytes [16]. In addition, the effects mediated by GCs may accelerate cell aging and shorten telomere length by increasing oxidative stress and down-regulating the activity of telomerase and antioxidant levels [17–20]. Elevated levels of endogenous GCs also contribute to MSC senescence and the development of osteoporosis in aging patients and in those who are postmenopausal and hence estrogen depleted [12–24]. Our previously published data have shown that short-term blockage of the effects mediated by the GCs present in fetal bovine serum (FBS) using either the GR antagonist RU486, or the small interfering RNA (siRNA) for GR, significantly improve the proliferative and osteochondral differentiation capabilities of human MSCs [25–26]. However, because of the short-term nature of these previously conducted experiments, we were unable to assess the potential effectiveness of both of these treatments on the senescence and antioxidant activity of these stem cells. In order to address this major limitation, our goal in the present long-term study was to investigate the in vitro effects of both of these GR inhibitors on not only the capabilities of MSCs, including proliferation and differentiations, but also on their senescence. The data presented in this report demonstrate that blockage of the effects mediated by the GCs normally found in FBS during a long-term in vitro expansion effectively maintains the proliferative capabilities of MSCs and postpones the reduction in their osteogenic capabilities. Our new data clearly demonstrate that both of these two independent approaches for blocking GR-mediated responses increase the expression of telomerase as well as telomere length and superoxide dismutase (SOD) activity. Taken collectively, these data strongly suggest that by blocking the effects mediated by GCs we may potentially be able to develop a protocol that will improve MSC proliferation and osteochondral differentiation, as well as postpone the onset of senescence in these human stem cells.
Materials and Methods
Preparation of human MSCs
Fresh human bone marrows, donated by total six healthy male and female donors from 22 to 43 years of age, were purchased from Allcells (Emeryville, CA). Mononuclear cells were isolated using density gradient centrifugation (Histopaque-1.077; Sigma, St. Louis, MO). These cells were subsequently cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Carlsbad, CA), supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) and 1% antibiotic-antimycotic (Sigma, St. Louis, MO). Five days after plating non-adherent cells were removed after medium exchange. The adherent cells continued to proliferate and were collected when they reached 70%–80% confluence. The cells were then cultured in 100mm petri dishes (passage-1) with an initial seeding density of 105 cells/per dish. Passage-1 cells were subsequently used to test the effects of the blockage of GC-mediated responses on proliferation and differentiation of MSCs.
Treatments with RU486 and GR siRNA
In the experiments where the GR antagonist RU486 was used, a final concentration of 10−8M was used. It has been demonstrated that both cortisol (average 7μg/100 ml) and corticosterone (approximately 3.0 μg/per 100 ml) are major glucocorticoids found in bovine plasma [27]. These published data for adult bovine serum are consistent with the corticosterone levels (2.4μg/100 ml) that we have directly measured in FBS. Our previously published data demonstrated that 10−8M RU486 effectively blocks the effects mediated by these GCs normally found in FBS [25]. Untreated MSCs served as controls. GR siRNA loaded PLGA particles were prepared using a (water-in-oil)-in-water double emulsion solvent evaporation method as previously described [24]. Briefly, GR siRNA (50 μg) was added to 300 μL of a 1% aqueous solution of PVA (water1 phase). The water1 phase was emulsified into the oil phase that was prepared by dissolving 6.67 % w/v of PLGA polymer in 2 ml of dichloromethane. This primary water-in-oil emulsion was then immediately sonicated into 4 ml of aqueous phase containing 1% PVA for 30 seconds to generate the secondary emulsion. The secondary emulsion was then dropped slowly into a beaker containing 25 ml of 1% aqueous solution of PVA and stirring was continued using a magnetic stirrer until complete evaporation of DCM. The particles were collected by centrifugation at 5000g for 5 minutes, washed three times with distilled water and lyophilized overnight. For treatment with GR siRNA, 1 mg GR siRNA-loaded particles containing 0.12 μg of GR siRNA were suspended into DMEM medium and added to human MSCs cultured on a 100-mm petri dish for 24 hours. The MSCs were then washed twice with PBS to remove suspended particles. Our previous study has shown that the GR siRNA-loaded PLGA particles at this concentration effectively down-regulated GR mRNA expression in MSCs [24]. The GR mRNA level down-regulated by the siRNA treatment returned to near control levels (minus siRNA) after 5 days [24]. Thus in this study the siRNA-loaded particles at this dose were added to MSCs weekly during in vitro culture. After 1 and 4 weeks, MSCs in the different treatment groups were collected and analyzed for GR (NR3C1) level, proliferation rate, multiple differentiation potentials, telomerase expression, telomere length, and superoxide dismutase (SOD) activity.
GR level and proliferation capabilities of MSCs
After being pretreated for 1 or 4 weeks with RU486 or GR siRNA, human MSCs were collected and cultured in 96-well (1000 cells/well) plates using steroid-free medium consisting of phenol red-free DMEM supplemented with 1% charcoal-stripped FBS. MSC DNA content was measured using a DNA quantification kit (FluoReporter ® Blue Fluorometric dsDNA Quantitation Kit, Invitrogen) according to the manufacturer’s protocols. The difference in DNA content after every 48hrs was used to determine the MSC proliferation rate as described in our previous studies [25–26]. NR3C1 level after different treatments were measured using a commercially available ELISA kit (MyBioSource, San Diego, CA).
Differentiation capacities of human MSCs
In order to measure the differentiation capabilities of human MSCs, the cells were washed twice with PBS and trypsinized after 1 or 4 weeks of treatment with RU486 or GR siRNA. The cells were subsequently cultured in 6-well plates (104 cells/well) and exposed to osteogenic or adipogenic differentiation medium for up to 3 weeks. The differentiation medium for osteogenesis consisted of the basic medium supplemented with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate. The osteocalcin (OCN) expression, alkaline phosphatase (ALP) and calcium content of MSCs following osteogenic differentiation were measured in triplicate as described in our previous studies [28]. For adipogenesis, the medium was supplemented with 50 nM dexamethasone, 10 μM insulin, and 0.5 mM isobutyl-methylxanthine. We used real-time polymerase chain reaction (PCR) to quantify the expression of peroxisome proliferator-activated receptors (PPAR)-γ2 and lipoprotein lipase (LPL), which are both adipogenic differentiation markers for MSCs. The real-time PCR primers and probes for multiple differentiation markers were described in our previous study [21].
Effects of GC blockages on telomere length, telomerase expression, and SOD activity
After 4 weeks of RU486 or GR siRNA treatment, the SOD activity of MSCs was evaluated by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine using a commercial kit according to the manufacturer’s protocol (Cayman Chemical). The mRNA expression of telomerase was measured using real-time PCR. The Syber Green Real-time PCR primers and probe were: forward 5′-GGA GAA CAA GCT GTT TGC GG-3′ and reverse 5′-AGG TTT TCG CGT GGG TGA G-3′ (ABI, CA). The primers of GAPDH were purchased from Applied Biosystems. We also measured the average telomere length from total genomic DNA using a real-time PCR method [29]. This assay measures an average telomere length ratio (ATLR) by quantifying telomeric DNA with specially designed primer sequences and dividing that amount by the quantity of a single-copy gene. Briefly, genomic DNA was extracted using the Qiagen DNA kit. The forward and reverse primers for telomere were GGC TTT TAT GGG TAT GGG TAT GGG TAT GGG TAT GGG TT and TCC CGA CTT ACC CTT ACC CTT ACC CTT ACC CTT ACC CT, respectively. The 36B4 (encodes acidic ribosomal phosphoprotein PO) forward and reverse primers were TCG TCT TTA AAC CCT GCG TG and TGT CTG CTC CCA CAA TGA AAC, respectively. An individual sample of rat DNA was serially diluted over a 12-fold range of telomere concentrations to generate standard curves. Input amounts were calculated for each sample by using the same protocol. The relative input amount of the telomere PCR was divided by the relative input amount of the 36B4 PCR of the same sample. Real-time PCR was repeated a minimum of three times for every sample, and the ratio of telomere: 36B4 was calculated. The average of these ratios was reported as the average telomere length ratio (ATLR).
Statistics
All quantitative data were expressed as means ± standard deviations and descriptive statistics were conducted using SAS for Windows (v9.3, SAS Institute Inc., Cary, NC). Nonparametric Wilcoxon signed-rank test was used to detect the significant differences in proliferation rates, differentiations, and premature senescence of MSCs receiving different treatments. A p-value of less than 0.05 was used as a criterion for statistical significance, and 0.05<p<0.10 was used as a criterion for marginal significance.
Results
GR variations and MSC proliferative capabilities following in vitro treatment with RU486 or GR siRNA
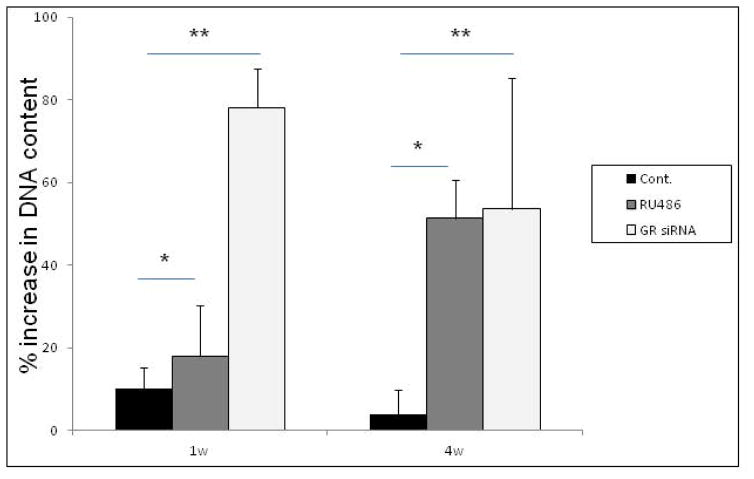
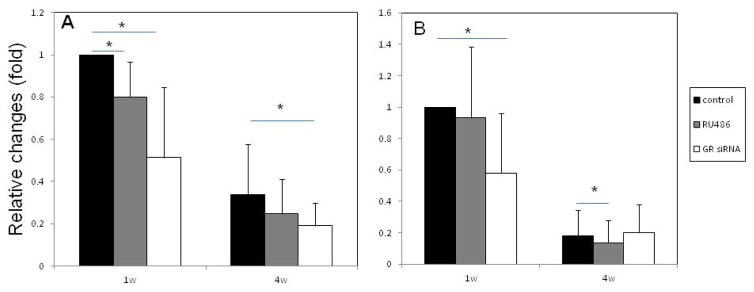
Figure 1 shows the GR level and proliferative capabilities of human bone marrow MSCs after being pretreated with RU486 or GR siRNA. After 1 week treatment with either GR siRNA or RU486, MSCs showed significantly higher proliferation rates than that of control MSCs. While the proliferation rate of control cells appeared to decrease after 4 weeks of in vitro culture, the MSCs treated with either RU486 or GR siRNA maintained high proliferation rates. The proliferation rates of MSCs 4 weeks after being treated to block endogenous GCs were significantly higher than controls (Fig. 1a). Our previous studies have demonstrated that the treatment with GR siRNA-loaded PLGA particles knocked down the expression of GR mRNA in MSCs and the GR mRNA level returned to near control levels after 5 to 7 days [26]. No significant difference of GR mRNA expression was observed in the MSCs treated with RU486 or GR siRNA compared to controls after 4 weeks when MSCs started to differentiate (Fig. 1b). The protein levels of NR3C1 in MSCs treated with GR siRNA were down-regulated at Day 7. However, the GR levels were returned to near control levels at Day 14 (Fig. 1c).
Figure 1.
The GR characterization and proliferation capabilities of human MSCs after different treatment. a: The percentage difference in DNA content after every 48hrs of MSCs after 1 and 4 weeks of pretreatment with GR siRNA or RU486 in vitro. n=6, Means ± SD; *:p<0.05, **:p<0.01. b: GR mRNA expression in MSCs treated with RU486 or GR siRNA after 4 weeks. c: The protein levels of NR3C1 in MSCs treated with GR siRNA or RU486 at different time points. All samples were measured in triplicate.
Differentiation capabilities of human MSCs following in vitro treatment with RU486 or GR siRNA
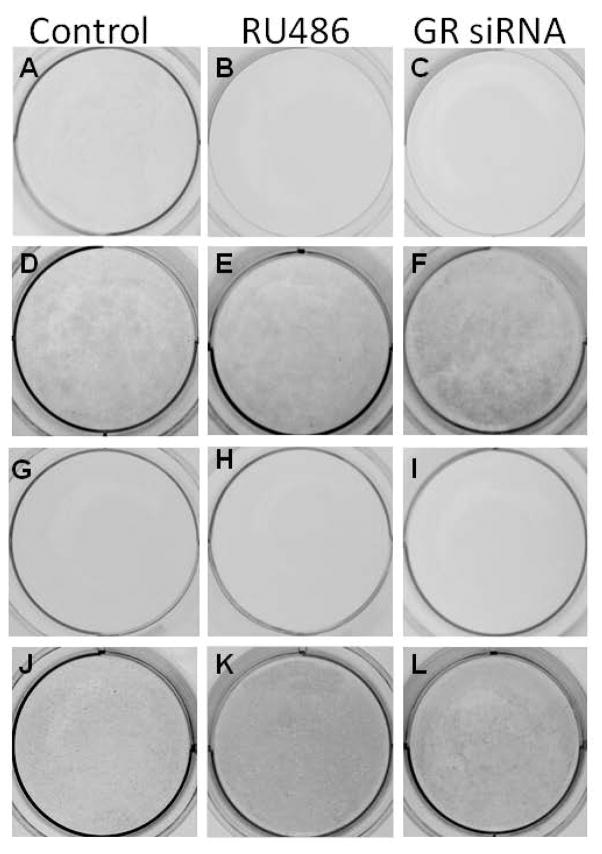
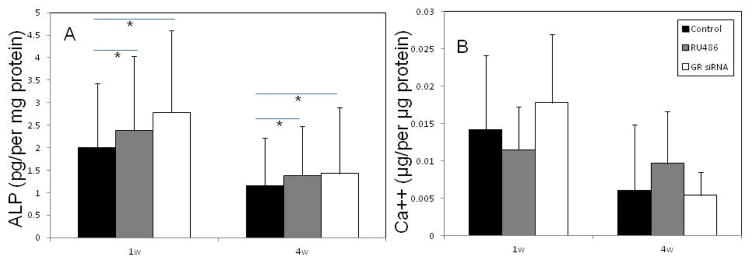
For MSCs treated with RU486 or GR siRNA for 1 week, after the MSCs were exposed to osteogenic differentiation medium for 3 weeks, mineral deposition, as indicated by blackness after von-Kossa staining, were detected in control MSCs (Fig. 2d) as well as MSCs pretreated with RU486 (Fig. 2e) or GR siRNA (Fig. 2f). Staining in MSCs that had been pretreated with GR siRNA (Fig. 2f) appeared darker than that detected in controls (Fig. 2d). For MSCs pretreated with RU486 or GR siRNA for 4 weeks, while the mineral deposition became slightly less intense in most MSCs (treated and untreated) after osteogenic differentiation for 3 weeks, MSCs that had been pretreated with RU486 appeared to stain darker than controls (Fig. 2j–l). Quantitatively, after MSCs were exposed to osteogenic differentiation medium for 2 weeks ALP levels in the MSCs that had been pretreated with GR siRNA or RU 486 for 1 week were significantly higher than the level detected in controls. The ALP levels decreased in all MSCs after 4 weeks in vitro culture with different treatments. However, the ALP level in MSCs pretreated for 4 weeks with GR siRNA or RU486 appeared to be higher than the level detected in controls (Fig. 3a). Similarly, the gene expression of OCN in the MSCs that had been pretreated with GR siRNA or RU 486 for 1 week were slight higher than the level detected in controls. The OCN levels decreased in control MSCs after 4 weeks in vitro culture. However, the OCN level in MSCs pretreated for 4 weeks with GR siRNA or RU486 appeared to be higher than the level detected in controls (Fig. 3b). The calcium content in 5 out of 6 plates of human MSCs pretreated with GR siRNA for 1 week was also higher than that of controls after these cells were exposed to osteogenic differentiation medium. The calcium content in 4 out of 6 plates of MSCs pretreated with RU486 for 4 weeks appeared higher than that of controls (Fig. 3c), although there was no statistical difference between these two groups due to a limited sample size.
Figure 2.
Images of von-Kossa stained MSCs receiving different treatments after in vitro osteogenic differentiation. MSCs pretreated with controls (a, d, g, and j), RU486 (b, e, h, and k), and GR siRNA (c, f, i and l) for 1 (a–f) and 4 (g–l) weeks after exposure to undifferentiated (a–c, g–i) and osteogenic differentiation medium (d–f, j–l), respectively.
Figure 3.
Osteogenic capabilities of MSCs that received different treatments. a: ALP activity in MSCs with different pretreatments for 1 and 4 weeks after they were osteogenic differentiated in vitro for 2 weeks. b: calcium contents in MSCs from different pretreatments for 1 and 4 weeks after they were osteogenic differentiated in vitro for 3 weeks. N=6; Mean ± SD; *:p<0.05. c: the mRNA expression of OCN in MSCs pretreated with GR siRNA or RU 486 for 1 and 4 weeks after they were osteogenic differentiated for 2 weeks. All samples were measured in triplicate.
After exposure to adipogenic differentiation medium, lipid accumulations, as detected by Oil-red-O staining, were observed in the MSCs pretreated with all groups for 1week (Fig. 4a–c). No detectable decrease in lipid accumulation appeared in MSCs receiving different pretreatments for 4 weeks (Fig. 4d–f). However, the expression of mRNA for adipogenic markers after differentiation, including PPAR (Fig. 5a) and LPL (Fig. 5b), were lower in MSCs pretreated with RU486 or GR siRNA for 1 week. All MSCs after 4 weeks of in vitro culture exhibited decreased expression of both adipogenic markers and the treatment with GR siRNA or RU486 for 4 weeks further decreased PPAR or LPL expression, respectively (Fig. 5a and b).
Figure 4.
Images of Oil-red-O stained MSCs from different treatment groups after in vitro adipogenic differentiation for 2 weeks. MSCs of controls (a and d) and pretreated with RU486 (b, and e), and GR siRNA (c and f) for 1 (a–c) and 4 (d–f) weeks after their exposure to adipogenic differentiation medium, respectively. Bar=50μm.
Figure 5.
Quantitative measurements of adipogenic capabilities of MSCs that received different pre-treatments. a and b: the mRNA expressions for PPAR-γ (a) and LPL (b) in MSCs that received different pretreatments in vitro for 1 and 4 weeks after they were exposed to adipogenic differentiation medium for 2 weeks. N=6; Mean ± SD. *p<0.05
Telomerase expression, telomere length and SOD activity in MSCs treated with RU486 or GR siRNA
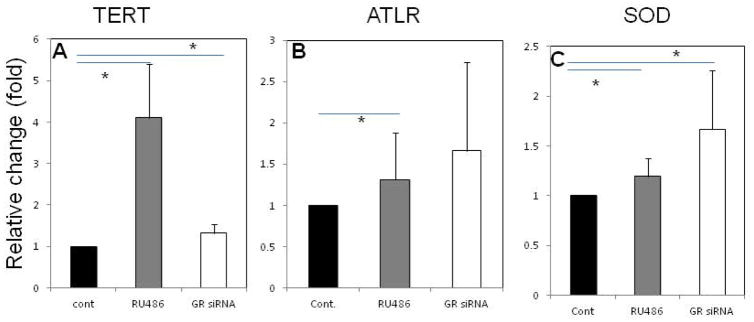
Figure 6 shows the quantitation of telomerase expression, telomere length, and SOD activity in MSCs treated with GR siRNA or RU486 for 4 weeks. Treatment with RU486 or GR siRNA significantly up-regulated the expression of telomerase mRNA (Fig. 6a). The telomere lengths of MSCs treated with RU486 were significantly longer than those in controls (Fig. 6b). The MSCs treated with GR siRNA also had relatively longer telomeres than those in controls, but the differences are marginally significant (p=0.0625). In addition, total SOD activity, which is a key antioxidant, was 30–40% higher in MSCs that had been treated with RU486 or GR siRNA than in controls and these differences were statistically significant (Fig. 6c).
Figure 6.
The senescence characteristics of MSCs 4 weeks after received different treatments in vitro. a: telomerase; b: average telomere length; c: SOD activity. N=6; Mean ± SD; *:p<0.05
Discussion
Our previous studies revealed that GCs may inhibit MSC proliferation and down-regulate the expression of fibroblast growth factor (FGF)-2, which is a stemness gene that potentially up-regulates antioxidant levels and prevents the senescence of MSCs [25]. The GC responses in MSCs are mediated via type 2 intracellular GR, and hence blockage of the effects mediated by GCs contained in culture medium using GR siRNA or RU486 has been shown to improve the capability for MSC proliferation and increase the expression of osteogenic differentiation markers [25–26]. The present study further demonstrates that both of these distinct treatments up-regulate the expression of telomerase mRNA, increase antioxidant levels, and increase MSC telomere length during in vitro expansion. These anti-senescence responses may explain the mechanism underlying the improvement mediated by both of these treatments on the proliferative and osteogenic capabilities of MSCs during in vitro culture. The progression of MSC senescence in vitro has been demonstrated to resemble that which occurs in vivo during human aging [30]. The results presented here indicate that the blockage of the effects mediated by endogenous GCs may be further developed as a protocol to effectively prevent or delay the senescence of MSCs and extend their lifespan in human patients.
Because of their high proliferative capabilities, MSCs expanded in vitro has been used for potential osteochondral regeneration. However, recent studies demonstrate that when cultured in vitro, these cells exhibit the characteristics of senescence, as evidenced by their reduced proliferation as well as their loss of osteogenic differentiation [10–13]. In the present study, we also observed the reduction in MSC proliferative capabilities as well as the osteogenic differentiation capabilities of these cells as reflected by ALP activity, OCN expression, and calcium content after 4 weeks of in vitro expansion. Although the exact mechanism(s) underlying the senescence of MSCs in vitro is not clear, increased oxidative stress levels and reduced activity of antioxidants has been observed after in vitro culture [31–33]. Enhanced oxidative stress has been demonstrated to induce senescence of these cells both in vitro as well as in vivo [20, 33]. Supplementation with exogenous antioxidants has also been shown to increase the potential for MSC proliferation and osteogenic differentiation [31]. GCs have been demonstrated to increase oxidative stress and reduce levels of antioxidants [31]. In this study we observed for the first time that there is an increase in SOD activity following in vitro treatment of MSCs with either GR siRNA or RU486. In addition, the expression of telomerase mRNA and telomere length, both of which are markers for cell aging, were increased following treatment of MSCs with GR siRNA or RU486. These data strongly suggest that blockage of the effects mediated by the GCs found in the FBS contained in complete culture medium may improve the antioxidant capabilities in MSCs and prevent or postpone the onset of senescence in these cells. We further observed that MSCs treated with GR siRNA or RU486 exhibit increased proliferation rates when compared to controls. The ALP levels detected in MSCs undergoing osteogenic differentiation were also found to be significantly increased compared to controls. Since bone-specific ALP levels of differentiated MSCs in vitro correlate with their in vivo bone regenerative capabilities [34], the enhancement of ALP in treated MSCs suggests that these cells may have higher bone regeneration potentials. These enhancements in the proliferation and osteogenic potential of MSCs treated with GR siRNA or RU486 further support the hypothesis that the blockage of GC-mediated responses can potentially prevent the senescence of MSCs.
The senescence of MSCs, which may be enhanced by elevated GC levels during aging and in postmenopausal patients, has been demonstrated to lead to increased adipogenic differentiation of these cells. These responses could potentially contribute to the development of osteoporosis and obesity in these patients. Although in the current study telomere length and telomerase expression levels were shown to be increased in MSCs that pretreated with RU486 or GR siRNA, we did not observe any significant difference in lipid accumulation. Furthermore, in the present study we observed that expression of mRNA for adipogenic differentiation markers, including PPAR gamma and LPL, were decreased in all MSCs after 4 weeks of in vitro culture. Pretreatment with GR siRNA or RU486 also down-regulated the expressions of PPARγ and RU486. Although the underlying mechanism(s) are not clear, one possibility is that the extent of MSC senescence after 4 weeks with different treatments may not have been sufficient to cause an increase in adipogenic differentiation similar to that occurs during aging and in postmenopausal patients. In addition, a previous study demonstrated that MSCs may maintain adipogenic differentiation after extended culture in vitro, although they may lose proliferative properties and osteochondral differentiation capabilities after a long-term in vitro culture [11]. These data suggest that in vitro senescence has less of an effect on the adipogenic differentiation of MSCs, and/or, that adipogenic differentiation of MSCs in vitro may have different underlying mechanism(s) compared to the mechanisms underlying this process when it occurs in patients with advanced age or after the menopause.
Steroid hormones have been demonstrated to regulate oxidative stress and the levels of antioxidants in many tissue and cell types. Physiologically, estrogens function as antioxidants and promote MSC proliferation and osteogenic differentiation [35–36]. In contrast, GCs inhibit MSC proliferation, accelerate senescence and increase oxidative stress [14, 19]. GCs have been demonstrated to play a critical role in bone metabolism by actively regulating proliferation, differentiation, and senescence of bone marrow MSCs [15–17]. Thus patients with advancing age and those who are postmenopausal, who are characterized as having decreased estrogen levels and characteristics mediated by elevated endogenous GC levels, have increased oxidative stress and reduced antioxidant activity [20–24]. These factors appear to induce the senescence of MSCs that eventually serves to trigger steroid- or age-related osteoporosis that is characterized as having decreased bone regeneration. Therefore, by effectively regulating, or blocking, these GC-mediated responses we may be able to reduce oxidative stress and increase the levels of antioxidants, and by so doing potentially prevent or delay MSC senescence and rejuvenate their physiological capabilities. This approach accomplished via a delivery system that specifically targets bone marrow MSCs could be potentially beneficial in patients with GC-induced osteoporosis because it could potentially improve the regenerative capabilities of MSCs by preventing or delaying the onset of their senescence.
Acknowledgments
This research was partially supported by the Start-up funding from the Dows Institute for Dental Research, College of Dentistry, the University of Iowa.
References
- 1.Dennis JE, Caplan AI. Porous ceramic vehicles for rat-marrow-derived (Rattus norvegicus) osteogenic cell delivery: effects of pre-treatment with fibronectin or laminin. J Oral Implantol. 1993;19:106–115. [PubMed] [Google Scholar]
- 2.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414–423. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex steroids and stem cell function. Mol Med. 2008;14:493–501. doi: 10.2119/2008-00004.Ray. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–22. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 6.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;28:9, 60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson IE, van Veen SC, Sengupta S, Kestle SR, Mauck RL. Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent. Clin Orthop Relat Res. 2011;469:2744–53. doi: 10.1007/s11999-011-1869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205:194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 12.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen TL. Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone. 2004;35:83–95. doi: 10.1016/j.bone.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Dennis JE, Caplan AI. Differentiation potential of conditionally immortalized mesenchymal progenitor cells from adult marrow of a H-2Kb-tsA58 transgenic mouse. J Cell Physiol. 1996;167:523–538. doi: 10.1002/(SICI)1097-4652(199606)167:3<523::AID-JCP16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim CH, Cheng SL, Kim GS. Effects of dexamethasone on proliferation, activity, and cytokine secretion of normal human bone marrow stromal cells: possible mechanisms of glucocorticoid-induced bone loss. J Endocrinol. 1999;162:371–379. doi: 10.1677/joe.0.1620371. [DOI] [PubMed] [Google Scholar]
- 17.Ichiyoshi H, Kiyozuka Y, Kishimoto Y, Fukuhara S, Tsubura A. Massive telomere loss and telomerase RNA expression in dexamethasone-induced apoptosis in mouse thymocytes. Exp Mol Pathol. 2003;75:178–186. doi: 10.1016/s0014-4800(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 18.Wen J, Wang L, Zhang M, Xie D, Sun L. Repression of telomerase activity during in vitro differentiation of osteosarcoma cells. Cancer Invest. 2002;20:38–45. doi: 10.1081/cnv-120000364. [DOI] [PubMed] [Google Scholar]
- 19.Costantini D, Marasco V, Møller AP. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011;181:447–456. doi: 10.1007/s00360-011-0566-2. [DOI] [PubMed] [Google Scholar]
- 20.Brandl A, Meyer M, Bechmann V, Nerlich M, Angele P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res. 2011;317:1541–7. doi: 10.1016/j.yexcr.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Kizaki T, Ookawara T, Iwabuchi K, Onoé K, Day NK, Good RA, Maruyama N, Haga S, Matsuura N, Ohira Y, Ohno H. Age-associated increase of basal corticosterone levels decreases ED2high, NF-kappaBhigh activated macrophages. J Leukoc Biol. 2000;68:21–30. [PubMed] [Google Scholar]
- 22.Nakamura J, Yakata M. Age- and sex-related differences in urinary cortisol level. Clin Chim Acta. 1984;14:77–80. doi: 10.1016/0009-8981(84)90314-0. [DOI] [PubMed] [Google Scholar]
- 23.Nicolson N, Storms C, Ponds R, Sulon J. Salivary cortisol levels and stress reactivity in human aging. J Gerontol A Biol Sci Med Sci. 1997;52:68–75. doi: 10.1093/gerona/52a.2.m68. [DOI] [PubMed] [Google Scholar]
- 24.Woods NF, Mitchell ES, Smith-Dijulio K. Cortisol levels during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2009;16:708–718. doi: 10.1097/gme.0b013e318198d6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, Wei N, Stanford C, Schmidt T, Hong L. In vitro effects of RU486 on proliferation and differentiation capabilities of human bone marrow mesenchymal stromal cells. Steroids. 2012;77:132–137. doi: 10.1016/j.steroids.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong L, Wei N, Joshi V, Yu Y, Kim N, Krishnamachari Y, Zhang Q, Salem A. Effects of glucocorticoid receptor siRNA delivered by poly lactic-co-glycolic acid microparticles on proliferation and differentiation capabilities of human mesenchymal stromal cells. Tissue Eng Part A. 2012;18:775–784. doi: 10.1089/ten.tea.2011.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganjam K, Venkataseshu3, Estergreen VL., Jr Cortisol and Corticosterone in Bovine Plasma and the Effect of Adrenocorticotropin. Journal of Dairy Science. 1970;53:480–483. doi: 10.3168/jds.S0022-0302(70)86234-8. [DOI] [PubMed] [Google Scholar]
- 28.Hong L, Colpan A, Peptan IA. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng. 2006;12:2747–2753. doi: 10.1089/ten.2006.12.2747. [DOI] [PubMed] [Google Scholar]
- 29.Gil ME, Coetzer TL. Real-time quantitative PCR of telomere length. Mol Biotechnol. 2004;27:169–172. doi: 10.1385/MB:27:2:169. [DOI] [PubMed] [Google Scholar]
- 30.Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebert R, Ulmer M, Zeck S, Meissner-Weigl J, Schneider D, Stopper H, Schupp N, Kassem M, Jakob F. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. 2006;24:1226–1235. doi: 10.1634/stemcells.2005-0117. [DOI] [PubMed] [Google Scholar]
- 32.Ho JH, Chen YF, Ma WH, Tseng TC, Chen MH, Lee OK. Cell contact accelerates replicative senescence of human mesenchymal stem cells independent of telomere shortening and p53 activation: roles of Ras and oxidative stress. Cell Transplant. 2011;20:1209–1220. doi: 10.3727/096368910X546562. [DOI] [PubMed] [Google Scholar]
- 33.Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kühnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27:1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 34.Mendes SC, Tibbe JM, Veenhof M, Both S, Oner FC, van Blitterswijk CA, de Bruijn JD. Relation between in vitro and in vivo osteogenic potential of cultured human bone marrow stromal cells. J Mater Sci Mater Med. 2004;15:1123–8. doi: 10.1023/B:JMSM.0000046394.53153.21. [DOI] [PubMed] [Google Scholar]
- 35.White RE, Gerrity R, Barman SA, Han G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids. 2010;75:788–93. doi: 10.1016/j.steroids.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baltgalvis Kristen A, Greising Sarah M, Warren Gordon L, Lowe Dawn A. Estrogen Regulates Estrogen Receptors and Antioxidant Gene Expression in Mouse Skeletal Muscle. PLoS One. 2010;5:e10164. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Numakawa T, Matsumoto T, Numakawa Y, Richards M, Yamawaki S, Kunugi H. Protective Action of Neurotrophic Factors and Estrogen against Oxidative Stress-Mediated Neurodegeneration. J Toxicol. 2011:405194. doi: 10.1155/2011/405194. [DOI] [PMC free article] [PubMed] [Google Scholar]