Abstract
Purpose
Survivors of pediatric Hodgkin Lymphoma (HL) are recognized to be at increased risk for delayed adverse health outcomes related to radiation therapy (RT). However, the necessary latency required to observe these late effects means that the estimated risks apply to out-dated treatments. We sought to compare the normal tissue dose received by children treated for HL and enrolled in the Childhood Cancer Survivor Study (CCSS) (diagnosed 1970-1986) with patients treated on recent Children's Oncology Group (COG) trials (enrolled 2002-2012).
Methods
RT treatment planning data were obtained for 50 HL survivors randomly sampled from the CCSS cohort and applied to CT planning datasets to reconstruct normal tissue dosimetry. For comparison, normal tissue dosimetry was obtained for all 191 patients with full CT-based volumetric RT planning on COG protocols AHOD0031 and AHOD0831.
Results
For early stage patients, mean female breast dose in the COG patients was on average 83.5% lower than CCSS patients, with an absolute reduction of 15.5Gy; for advanced stage patients mean breast dose decreased on average by 70% (11.6Gy average absolute dose reduction). The mean heart dose decreased on average by 22.9Gy (68.6%), and 17.6Gy (56.8%) for early and advanced stage patients, respectively. All dose comparisons for breast, heart, lung, and thyroid were significantly lower for patients on COG trials than CCSS participants. Reduction in prescribed dose was a major contributor to this dose reduction.
Conclusions
These are the first data quantifying the significant reduction in normal tissue dose based on actual rather than hypothetical treatment plans for children with HL. The findings provide some useful information when counselling families regarding the risks of contemporary RT.
It is well recognized that irradiation of normal tissues is associated with risks of late toxicity among survivors of childhood Hodgkin Lymphoma (HL). One of the largest studies to quantify these risks is the Childhood Cancer Survivors Study (CCSS), which has reported, for example, a 24.2-fold increase risk of breast cancer among female HL survivors treated with mantle field radiation therapy (RT) (1), in addition to increased risks of cardiac disease (2-4), and other second malignancies (5-8). An important consideration for inferring the risks of contemporary treatment, however, is the reduction in normal tissue volume and prescribed dose that has occurred over the last two decades, intended to minimize late-effects while maintaining high relapse-free survival. Direct application of CCSS risk estimates to contemporary treatment is very likely inaccurate for modern patients. Although prior studies have estimated the magnitude of these dose reductions with hypothetical re-planning of contemporary patients with historic mantle fields, no study has directly compared the reconstructed normal tissue dose actually delivered to historically treated patients with the analogous doses for received by patients on more contemporary trials.
Methods
We compared normal tissue radiation exposure for five-year survivors of childhood HL enrolled on the CCSS study (diagnosed 1970-1986) with patients treated on Children's Oncology Group (COG) AHOD 0081 (enrolled 2009-2012) and AHOD 0031 (enrolled 2002-2009) trials. Two-dimensional treatment data was obtained for 50 HL survivors randomly sampled from the CCSS cohort (total irradiated HL patients = 761), who received mediastinal RT. The dose reconstruction method for CCSS patients is described elsewhere (9). Briefly, treatment planning parameters outlined in the RT records were applied to a reference 3D CT dataset matched by gender, age, and thorax size, using Pinnacle, version 8 (Phillips Radiation Oncology Systems, Milpitas, CA). CCSS patients were primarily treated using parallel opposed pair to mantle fields and to the abdomen. For COG patients, normal tissue dosimetry was extracted for 191 patients with full RT planning CT datasets via the Computational Environment for Radiotherapy Research (CERR) software from the Imaging and Radiation Oncology Core Group, Rhode Island Review Center. Details of these trials are described elsewhere (10, 11). Sixty-eight consecutive patients on AHOD0031 (stage IIB-IVA disease) and 123 consecutive patients on AHOD0831 (stage IIB and IVB) had electronic RT dose plans available in CERR, and used in this study. The CCSS cases were matched based on gender, stage (I/II vs III/IV), and age (+/− 2 years ) at treatment to HL patients on the two COG trials. If a single CCSS patient had more than one COG match, then one of the matching COG patients was randomly selected and normal organ mean dose (Dmean) and volumes covered by 5Gy (V5) from the resulting two matched CCSS/COG patients were compared with paired t-tests. In supplemental analysis, if a single CCSS patient had more than one COG match, the dosimetry from all available COG matches was averaged and compared with the matching CCSS case. To evaluate the relative contribution of lower prescribed dose versus smaller treatment volume on the reduction in normal tissue dose, patients in the CCSS cohort had prescription doses re-set to match the COG prescribed dose (21Gy). Given the equal prescribed dose, the remaining reduction in normal tissue dose was then attributed to differences in irradiated volume.
Results
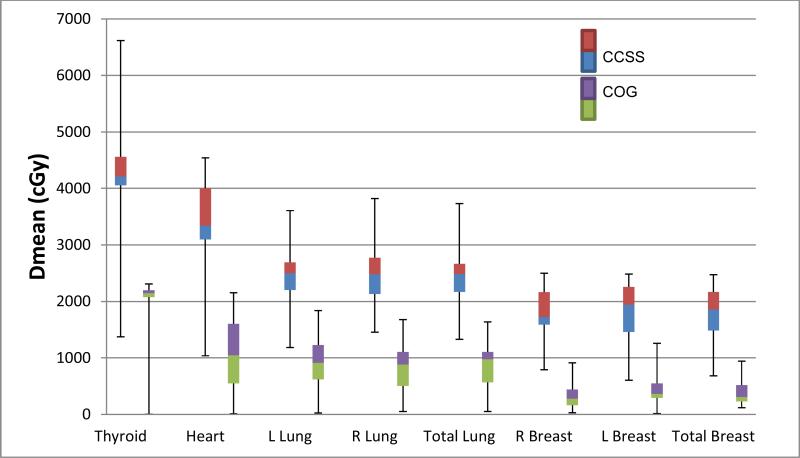
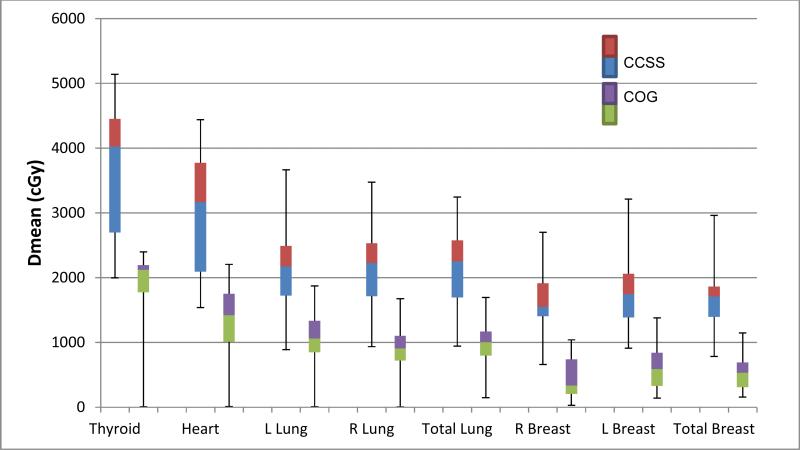
The median prescribed dose for CCSS patients was 44Gy (range 20.0-57.9Gy) while the prescribed dose for both COG trials was 21Gy. One-to-one matching produced better balance of age, gender and stage characteristics (supplemental Table 1), although differences in all dosimetric comparisons were significant regardless of matching strategy. Figures 1 and 2 compare the mean normal tissue dose in the early and advanced stage CCSS and COG studies, demonstrating a significant (P <0.001) reduction in dose in the contemporary protocols. For early stage patients, mean female breast dose in the COG patients decreased by 83.5%, corresponding to an average reduction in absolute dose of 15.5Gy. The mean heart dose was on average 22.9Gy (68.6%) lower and mean lung and thyroid doses were reduced on average by 15Gy (61%) and 20.7Gy (49%), respectively (all p-values<0.001). Similarly in late stage disease, mean breast dose decreased on average by 11.6Gy (70.0%), while mean heart dose decreased on average by 17.4Gy (55.1), mean lung dose reduced by 11.1Gy (51%), and mean thyroid dose by 19.0Gy (47.2%) (All comparisons p<0.001).
Figure 1. Mean dose to normal tissues in Stage I/II HL Childhood Cancer Survivors Study (CCSS) vs Children's Oncology Group (COG).
CCSS n=24; median prescribed dose = 44.9Gy. COG AHOD0031 and AHOD0831 n=37; median prescribed dose = 21Gy).
Figure 1. Mean dose to normal tissues in Early Stage (I/II) disease in Childhood Cancer Survivors Study (CCSS) vs Children's Oncology Group (COG). CCSS n=24; median prescribed dose=4492.5cGY. COG n=46; median prescribed dose=2100cGy.
Figure 2. Mean dose to normal tissues in Stage III/IV Childhood Cancer Survivors Study (CCSS) vs Children's Oncology Group (COG).
B) CCSS n=26; median prescribed dose = 44.0Gy. COG AHOD0031 and AHOD0831 n=133; median prescribed dose = 21Gy).
Figure 2. Mean dose to normal tissues in Late Stage (III/IV) disease in Childhood Cancer Survivors Study (CCSS) vs Children's Oncology Group (COG). CCSS n26=; median prescribed dose=4225cGy, COG n=111; median prescribed dose=2100cGy.
The volumes of normal tissue receiving low dose exposure were also significantly lower in the COG cohort. In early stage female patients, the breast V5 decreased from 61% in the CCSS cohort to 17% in the COG patients; for late stage patients breast V5 decreased from 59% to 28%. Cardiac V5 decreased from 99% in CCSS patients (both early and late stages) to 61% in early stage COG patients and 75% in late stage COG patients (all p-values < 0.001)
The relative contributions of lower prescription dose versus smaller treatment volume varied depending on tissue and stage of disease. For example, in early stage patients 40% of the reduction in mean breast dose was due to smaller irradiated volumes, while for late stage patients this proportion was 27%. Smaller treatment volumes could account for 24% of the reduction in mean heart dose and 12% of the reduction in mean lung dose among early stage patients, although only 9% and 2%, respectively of the reduction for advanced stage patients. The whole thyroid was in the treated volume for all but 12 of the COG patients, and reduction in prescribed dose accounted for essentially all of the reduction in thyroid exposure.
Discussion
These results are the first to quantify significant dose reductions in normal tissue dose associated with recent COG HL protocols compared to the historical CCSS HL patients.
These data are useful for framing discussion of the risks of modern RT in an environment in which the high incidence of late effects seen among CCSS subjects has been well described (5, 6). In many cases, children treated 1970-1986 and registered in the CCSS received radical extended-field RT, similar to that given to contemporaneously treated adults. Reports of favorable disease-free survival following 15-25Gy with combined modality therapy led to 21Gy becoming the standard dose for pediatric HL protocols in North America, and for lung and thyroid tissue, a significant component of the reduction in the normal tissue dose relates simply to this reduction in prescribed dose, while for early stage patients, the transition from extended-field to involved-field RT led to a reduction in irradiated volume that also accounted for a significant reduction in breast exposure. Further reduction in target volume is being evaluated in advanced stage patients in the ongoing COG AHOD 1331 trial, which employs involved site RT (12), and omits RT to non-bulky rapidly responding sites of involvement.
Given that the risks of radiation-induced breast and lung cancer increase with increasing normal tissue dose up to approximately 40Gy (13), one would expect that the reductions in the normal tissue dose described here will translate into lower risks of second malignancy, and there are emerging data in adults that this is the case (14). For example, based on CCSS data, Inskip et al. reported an increasing excess odds ratio of developing a breast tumor at a specific site within the breast of 0.21-0.44 per Gy (8). The observed average reduction in median breast dose of 15.5Gy and 11.6Gy in early and advanced stage patients, then, might reasonably be expected to translate into lower breast cancer risks, although the impact of simultaneous changes in chemotherapy may also influence the breast cancer risks of more modern treatment.
Similarly, dose-risk data relating to cardiac toxicity also suggest that contemporary treatment should be associated with substantially lower risks of heart disease than historic RT (4). It is also notable that the volumes of normal tissue receiving low doses (≤ 5Gy) have also reduced significantly between the CCSS and recent COG treatments.
Further work is required to better select pediatric HL patients who benefit from RT, and to further refine target volume definition. But these results should provide some reassurance that patients treated with RT on contemporary protocols are unlikely to experience the same extent of RT-related late toxicity as described for historically treated survivors.
Supplementary Material
Summary.
The Childhood Cancer Survivors Study (CCSS) is a major source of information regarding late effects among pediatric Hodgkin lymphoma (HL) survivors. In this comparison of children with HL who received radiation therapy, patients treated from 2002-2012 on Children's Oncology Group trials had normal tissue doses typically 55%-80% lower than those registered 1970-1986 on the CCSS. This finding suggests contemporary treatment should significantly reduce RT-related late toxicity compared to treatments given to patients registered in CCSS.
Acknowledgements
This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator, CCSS) and (U10 CA98543, P. Adamson, Principal Investigator, COG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
The authors of this paper have no conflicts of interest.
References
- 1.Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Novetsky Friedman D, et al. Breast cancer after chest radiation therapy for childhood cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(21):2217–23. doi: 10.1200/JCO.2013.54.4601. Epub 2014/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(14):2308–18. doi: 10.1200/JCO.2009.22.3339. Epub 2009/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(29):3673–80. doi: 10.1200/JCO.2013.49.3205. Epub 2013/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. Bmj. 2009;339:b4606. doi: 10.1136/bmj.b4606. Epub 2009/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(14):2356–62. doi: 10.1200/JCO.2008.21.1920. Epub 2009/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2010;102(14):1083–95. doi: 10.1093/jnci/djq238. Epub 2010/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the childhood cancer survivor study. Radiat Res. 2010;174(6):840–50. doi: 10.1667/RR1903.1. Epub 2010/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inskip PD, Robison LL, Stovall M, Smith SA, Hammond S, Mertens AC, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(24):3901–7. doi: 10.1200/JCO.2008.20.7738. Epub 2009/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng A, Brock KK, Sharpe MB, Moseley JL, Craig T, Hodgson DC. Individualized 3D reconstruction of normal tissue dose for patients with long-term follow-up: a step toward understanding dose risk for late toxicity. International journal of radiation oncology, biology, physics. 2012;84(4):e557–63. doi: 10.1016/j.ijrobp.2012.06.026. Epub 2012/08/30. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(32):3651–8. doi: 10.1200/JCO.2013.52.5410. Epub 2014/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combination Chemotherapy and Radiation Therapy in Treating Young Patients With Newly Diagnosed Hodgkin Lymphoma. [2015];Children's Oncology Group. 2009 [updated March 2015]; Available from: https://clinicaltrials.gov/ct2/show/study/NCT01026220.
- 12.Hodgson DC, Dieckmann K, Terezakis S, Constine L. International Lymphoma Radiation Oncology G. Implementation of contemporary radiation therapy planning concepts for pediatric Hodgkin lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Pract Radiat Oncol. 2015;5(2):85–92. doi: 10.1016/j.prro.2014.05.003. Epub 2014/11/22. [DOI] [PubMed] [Google Scholar]
- 13.Shuryak I, Hahnfeldt P, Hlatky L, Sachs RK, Brenner DJ. A new view of radiation-induced cancer: integrating short- and long-term processes. Part II: second cancer risk estimation. Radiat Environ Biophys. 2009;48(3):275–86. doi: 10.1007/s00411-009-0231-2. Epub 2009/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bruin ML, Sparidans J, Van't Veer MB, Noordijk EM, Louwman MW, Zijlstra JM, et al. Breast Cancer Risk in Female Survivors of Hodgkin's Lymphoma: Lower Risk After Smaller Radiation Volumes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(28):4239–46. doi: 10.1200/JCO.2008.19.9174. Epub 2009/08/12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.