Abstract
Despite an extensive body of reported information about peripheral and central mechanisms involved in the pathophysiology of IBS symptoms, no comprehensive disease model has emerged that would guide the development of novel, effective therapies. In this Review, we will first describe novel insights into some key components of brain–gut interactions, starting with the emerging findings of distinct functional and structural brain signatures of IBS. We will then point out emerging correlations between these brain networks and genomic, gastrointestinal, immune and gut-microbiome-related parameters. We will incorporate this new information, as well as the reported extensive literature on various peripheral mechanisms, into a systems-based disease model of IBS, and discuss the implications of such a model for improved understanding of the disorder, and for the development of more-effective treatment approaches in the future.
Introduction
IBS is the most common functional gastrointestinal disorder, occurring in up to 15% of the population worldwide.1 Even though the syndrome is defined by chronically recurring abdominal pain and discomfort associated with altered bowel habits in the absence of organic disease, increased trait anxiety, as well as comorbidity with psychiatric and other chronic pain syndromes are common.2 Despie an extensive body of reported information about peripheral3–5 and central6–9 mechanisms involved in the pathophysiology of IBS symptoms, and the development of animal models with high face and construct validity (that is, the models have many features similar to and is based on a similar pathophysiological concept as the human disease),10 no comprehensive disease model has emerged that would guide the development of novel and effective therapies. This aspect is surprising in view of the comprehensive data as well as clinical experience demonstrating the strong relationship between psychosocial factors and IBS symptoms,11 and in view of breakthroughs in the identification of several IBS-related biological abnormalities at several levels: the gut epithelium;12 immune system;4,5 neuroendocrine mechanisms;13 brain structure and function;7 stress response;14 affective,8,15 cognitive6,16–18 and pain modulation19,20 abnormalities; gene polymorphisms;3 and the gut microbiome.21,22 Despite these insights, a comprehensive understanding of how these various factors interact, and particularly to what degree they are involved, in the generation of symptoms in IBS in general or in IBS subsets (as opposed to representing epiphenomena) has not emerged. The long history of IBS research23 is full of examples of reported abnormalities (including excessive mucus production, alterations in sigmoid colon motility, slow wave abnormalities in smooth muscle, gut inflammation and others), which were reported in small sample cohorts and often not confirmed in validation sample cohorts. Typically, the majority of reported mean differences are small when compared with healthy individuals, often do not take into account sex-related differences, might only be present in subsets of patients and correlations with clinical symptoms are weak. Thus, only a very small number of findings have translated into highly effective therapies.24 These therapies include the serotonin (5-HT) 5-HT3 receptor antagonist, alosetron, the 5-HT4 agonist, tegaserod, the guanylate cyclase agonist, linaclotide, and the chloride channel blocker lubiprostone (for chronic idiopathic constipation).25 Even though effective, the first two agents were restricted or withdrawn due to serious adverse effects, whereas the latter two are primarily targeted at treating the symptom of constipation.25
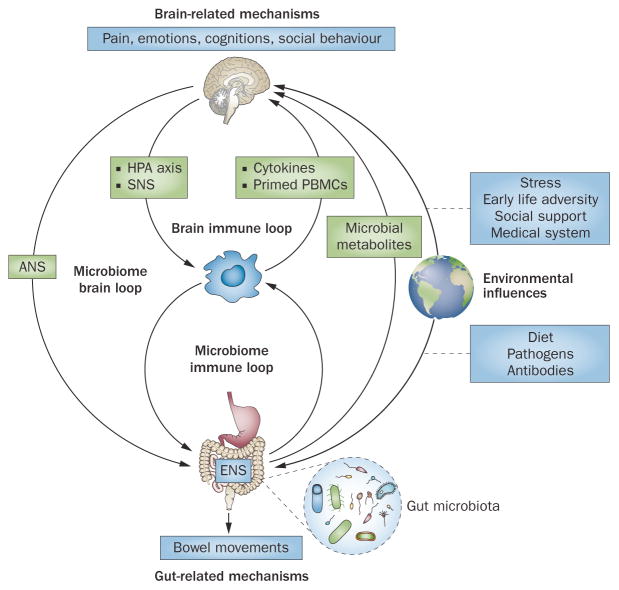
The brain and gut show reciprocal interactions in health and disease. For example, altered brain outputs via the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenal (HPA) axis have been shown to influence intestinal motility and secretion,26 intestinal epithelial permeability,3,12,27 immune function28 and gut microbial composition.21 In addition to these well-established influences of the brain on peripheral target cells, peripheral environmental gut-directed factors—in particular, dietary factors29 and intestinal pathogens30—might have an equally important role on these same processes. Regardless of primary cause for the observed peripheral findings, several of them (in particular immune and microbiota-related signalling) can feed back to the brain, setting up circular regulatory loops (Figure 1).
Figure 1.
Brain–gut axis. Schematic of the brain–gut axis, including inputs from the gut microbiota, the ENS, the immune system and the external environment. The model includes both peripheral and central components, which are in bidirectional interactions. Bottom-up influences are shown on the right side, top-down influences on the left side of the graph. Abbreviations: ENS, enteric nervous system; HPA, hypothalamic–pituitary–adrenal; PBMC, peripheral blood mononuclear cell; SNS, sympathetic nervous system. Modified with permission from Nature Publishing Group © Irwin, M.R. & Cole, S.W. Nat. Rev. Immunol. 11, 625–632 (2011).103
Major barriers exist for progress towards a comprehensive understanding of IBS pathophysiology, which incorporates such circular regulatory loops. Despite a wealth of information supporting all mechanisms listed already, controversy regarding the primary role of the brain versus peripheral factors has persisted in the field. The majority of research and drug development have focused on single, usually peripheral, targets in preclinical models (such as ion channels or specific receptors on individual neurons). Although such studies have provided major insights into mechanisms of visceral pain,10 their relevance for IBS symptom generation in humans has not firmly been established. Integration of multiple clinical, psychosocial and biological (genetic, immune, neurobiological) findings into a comprehensive disease model has yet to be achieved. For example, few studies aimed at psychosocial aspects of IBS have taken neurobiological concepts into account. The focus has been on descriptive, symptom-based rather than biology-based disease definitions. For example, different biological mechanisms might be associated with similar clinical presentations. The comprehensive identification of distinct biology-based subgroups of patients (including those based on sex), with different underlying pathophysiological components, who are differentially responsive to specific therapies has also not been achieved. A good example illustrating this point is a report on subsets of patients with IBS based on gut microbial signatures.31
Key points.
Physiological and molecular alterations have been identified in the brain–gut axis of human and rodent models of IBS, yet a comprehensive disease model to guide effective drug development has not emerged
Studies have identified distinct brain signatures in patients with IBS, which provide plausible neurobiological substrates of many previously reported behavioural and psychosocial observations
Emerging evidence demonstrates correlations of these brain signatures with alterations in genetics, immune system and gut microbiota in IBS, even though the causality of these interactions remains unknown
A systems-biology-based model is proposed to integrate the growing number of central, peripheral and behavioural IBS-related alterations, and to identify targets for more effective therapies
In this Review, we will first describe novel insights into some key components of brain–gut–microbiome axis, starting with evolving concepts about alterations in defined structural and functional brain networks. We will discuss emerging evidence on how these brain network alterations are correlated with the immune system and the gut microbiota. We will then incorporate this information, as well as the reported extensive literature on various peripheral mechanisms, into a systems-based disease model of IBS. Rather than simply synthesizing the current knowledge about the disease, we will use this model to discuss the implications for better understanding of the disorder and for the development of more-effective treatment approaches in the future.
The nervous system component
Several clinical observations support a major role of the brain in IBS symptoms: the brain is ultimately responsible for generating the subjective experience of abdominal pain, discomfort and anxiety; stressful life events in early life have a major role in vulnerability to develop IBS, and psychosocial stressors in adulthood play crucial parts in the first onset, and perceived severity of symptoms;32 centrally targeted pharmacological treatments and cognitive behavioural strategies are some of the most-effective treatment strategies.33 The role of the enteric nervous system (ENS) in the regulation and coordination of motor and secretory functions of the gastrointestinal tract, in sensory function and its interactions with the brain have been extensively reviewed.34,35 Consistent with the theme of this Review, the complexity of the interactions between multiple gut-based cell types (intrinsic and extrinsic sensory neurons, enteric glia, immune cells and innervated enteroendocrine cells) has been referred to as the ‘gut connectome’.36 The interactions between the ENS and central nervous system (CNS), two key components of the brain–gut axis, are mediated by the sympathetic and parasympathetic branches of the ANS,6 and by multiple sensory and endocrine pathways.34 Although it is difficult to characterize alterations of the ENS or of ANS-mediated brain–gut connections in living humans directly, functional, structural and metabolic brain imaging approaches have become a highly productive avenue to study brain–gut–microbiota interactions in health and disease.8,9,37,38
Although it has long been assumed that specific brain functions such as pain processing, emotion or cognition are attributable to the isolated operations of single brain regions, these processes are now viewed as resulting from the dynamic interactions of distributed brain areas operating in large-scale networks (Figure 2). These networks and their properties have been assessed by using neuroanatomical and neurophysiological studies in animals,10 as well as different brain imaging techniques and analyses in humans.39–46 In humans, several types of networks have been reported: functional brain networks based on evoked responses37 or intrinsic connectivity of the brain during rest;39–41,44,45 structural networks based on grey matter parameters47 and white matter properties; and anatomical networks based on white matter connectivity.48 Both evoked and resting state studies performed in patients with IBS have demonstrated abnormalities in regions and task-related networks linked to emotional arousal,49–53 central autonomic control,6,54–56 central executive control,51,57,58 sensorimotor processing6,59–61 and salience detection.57,62 IBS-related alterations in these networks have provided plausible neurobiological substrates for several information processing abnormalities reported in patients with IBS, such as biased threat appraisal and expectancy of outcomes (for example, salience network), autonomic hyperarousal (emotional arousal and central autonomic networks), and symptom-focused attention (central executive network).63
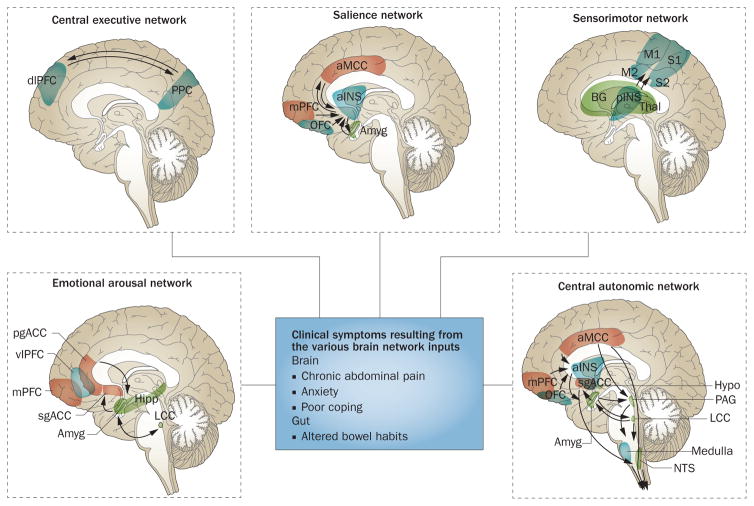
Figure 2.
Brain networks contributing to IBS symptoms. Depicted are task-related brain networks that have been described in the literature and for which structural and functional alterations have been reported in patients wiht IBS. The box in the centre describes the clinical symptoms related to the network inputs. Outputs most relevant for IBS pathophysiology occur in the form of descending pain modulation and autonomic nervous system activity. Abbreviations: Amyg, amygdala; aINS, anterior insula; aMCC, anterior midcingulate cortex; BG, basal ganglia; dlPFC, dorsolateral prefrontal cortex; Hipp, hippocampus; Hypo, hypothalamus; LCC, locus coeruleus complex; M1, primary motor cortex; M2, supplementary motor cortex; mPFC, medial prefrontal cortex; NTS, solitary nucleus; OFC, orbitofrontal cortex; PAG, periaqueductal grey; pgACC, pregenual anterior cingulate cortex; pINS, posteria insula; PPC, posterior parietal cortex; sgACC, subgenual anterior cingulate cortex; Thal, thalamus; vlPFC, ventrolateral prefrontal cortex.
In the next section, we will discuss IBS-related changes that have been identified in these task-related networks. The individual networks are depicted in Figure 2, and their basic properties are described in Table 1.
Table 1.
Brain networks with relevance to IBS
| Network | Core regions and inputs | Key features |
|---|---|---|
| Default mode network | Medial prefrontal cortex, posterior cingulate or retrosplenial cortex, inferior parietal cortex, lateral temporal cortex and hippocampal formation179,180 | Comprised of brain regions whose activity is greater during rest than goal-directed task performance181 Associated with self-related processing, including monitoring internal thoughts and future planning179,182,183 |
| Emotional arousal network | Amygdala, its positive connections with the locus coeruleus complex and inhibitory feedback projections to the amygdala from prefrontal and anterior cingulate subregions, and from the hippocampus | Activated by perceived or real perturbation of the organism’s homeostasis Generates rapid feedback inhibition of amygdala,184–186 thereby limiting the magnitude and duration of network activity and related activity in the central autonomic network |
| Central autonomic network | Control centres in the pontine-medulla (including PAG and hypothalamus), the central nucleus of the amygdala, and several cortical regions (including the anterior INS, ACC, prefrontal and motor regions)187,188 | Regions related to SNS control overlap with the executive-processing and salience-processing networks (including the ventral anterior INS), whereas regions related to parasympathetic control are more associated with the default mode network154 Provides central control and modulation of the ANS Involved in regulating respiratory, cardiovascular, endocrine and digestive system activity during cognitive, affective and motor tasks and sensation |
| Sensorimotor network | Core cortical regions are primary somatosensory cortex (S1, post central gyrus), primary motor cortex (M1; precentral gyrus), secondary somatosensory cortex (S2) and supplemental motor area189 Close connections exist between the posterior INS (primary interoceptive cortex) and S1 Sensorimotor network connectivity to the thalamus, which relays peripheral sensory information to the cortex,190 is established by 2 years of age191 |
Receives sensory input from the periphery and is important for awareness of body sensations and generation of appropriate motor responses Primary and secondary motor cortex, through their projections to the central autonomic network, might have a modulatory role in the sympathetic control of visceral function192 |
| Central executive network | Lateral prefrontal cortices and posterior parietal cortex193 | Activated during tasks involving executive functions such as attention, working memory, planning and response selection Often coactivated with regions of the salience network,39 as the brain attempts to focus its limited processing capacity to only salient information via attention, working memory, planning and response selection193 |
| Salience network | Dorsal ACC and anterior INS Core regions have strong connections to medial prefrontal and temporal regions, and subcortical regions including the amygdala, PAG and basal ganglia194–196 Dorsal portions of the anterior INS receives prefrontal input, whilst ventral portions are closely linked with the amygdala and emotional arousal system |
Anterior INS can be considered the main hub in the brain, switching from default mode network to activity-related networks, and coordinating and adjusting bodily and behavioural responses to environmental changes Responds to subjective salience of any interoceptive and exteroceptive stimulus reaching the brain, or to the expectation of such stimuli, and coordinates appropriate attentional, behavioural, affective and visceral responses to such stimuli39,197 Responds with the most appropriate responses to biologically and cognitively relevant stimuli based on maintaining homeostasis193,194 regardless of whether the subject is awake, engaged in a particular task, or asleep (achieved through close salience network connections with the other networks) |
Abbreviations: ACC, anterior cingulate cortex; ANS, autonomic nervous system; INS, insula; PAG, periaqueductal grey; SNS, sympathetic nervous system.
Emotional arousal network
This network acts as an important link between stimulus appraisal (salience network) and ANS output (generated in the central autonomic network) to peripheral targets (gastrointestinal tract, gut microbes, immune system), thereby having an important role in determining the magnitude and duration of autonomic modulation of various gut functions. A reduction in the inhibitory feedback loop within the arousal network has been demonstrated in patients with IBS compared with healthy individuals,51,64,65 and in healthy individuals as controls after decreasing central serotonin levels by acute tryptophan depletion.66 Several studies published during the past decade support an increased responsiveness of emotional arousal circuits in relation to both expected and to delivered visceral stimuli, particularly in women.67–76 A meta-analyses of functional MRI studies published between 2000 and 2010 demonstrated that during controlled rectal distension, patients with IBS show more consistent activation in regions associated with emotional arousal than healthy individuals.53 Emotional arousal circuit reactivity is associated with 5-HT-related gene polymorphisms.77 IBS-related functional alterations are accompanied by structural brain alterations in key regions of this network.78
Central autonomic network
An extensive body of knowledge derived from rodent studies26,79–82 supports an upregulation of brain circuits in response to chronic stress, in particular the input from the locus coeruleus complex to the amygdala and the hypothalamus. Information from human brain imaging studies has been more limited due to the technical difficulties in studying key regions of this network, such as the hypothalamus, locus coeruleus complex and subnuclei of the amygdala, primarily due to limited spatial resolution. However, pharmacological brain imaging studies have implicated alterations in the corticotropin-releasing factor (also known as corticoliberin)–corticotropin-releasing receptor 164,83 and norepinephrine–α adrenergic receptor signalling system in this network.84
Sensorimotor network
As with other chronic pain disorders,85–88 evidence indicates that IBS might be associated with alterations in brain networks concerned with the central processing and modulation of viscerosensory and somatosensory information. For example, compared with healthy individuals, patients with IBS showed increased low frequency power of spontaneous brain oscillations (suggesting increased neural activity) in regions belonging to the sensorimotor network.59 These functional changes seem to be accompanied by structural changes in white and grey matter. For example, patients with IBS had widespread microstructural white matter abnormalities in sensory processing and/or modulation regions.89 Female patients with IBS have cortical thickness increases in sensorimotor areas that correlated with clinical measures of symptom severity compared with healthy individuals.90 Such grey matter increases in patients with IBS were also seen in the posterior insula (INS), the primary viscerosensory cortex, and these changes were correlated with IBS symptom duration.91 Volumetric grey matter analyses in a large sample of female patients with IBS revealed increases in the primary somatosensory cortex.78 Furthermore, examining structural networks in IBS indicated that two key regions of the network (cingulate gyrus and thalamus) were found to be network hubs, indicating that these regions are more critical for information flow in IBS compared with health.78 When viewed together, current evidence supports the hypothesis that patients with chronically recurring visceral pain and/or discomfort have functional as well as neuroplastic and microstructural alterations within the brain, particularly in regions associated with the processing, integration and modulation of sensory information. The mechanism(s) underlying these alterations include chronically increased viscerosensory information flow from the gut, or from dorsal horn neurons sensitized by descending pain facilitation (see Figure 3).
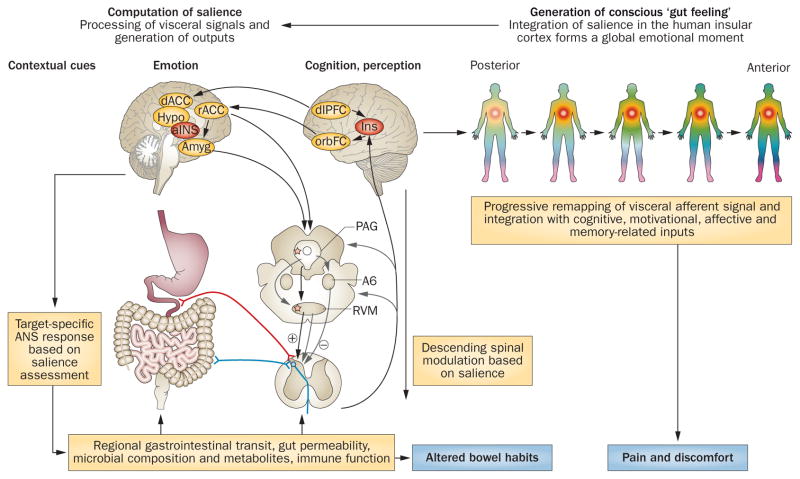
Figure 3.
Cross-sectional integrated brain–gut model of IBS pathophysiology. Proposed model for involvement of brain–gut axis in the generation of cardinal IBS symptoms (chronic abdominal pain associated with altered bowel habits). Under normal circumstances, visceral and external signals are evaluated by the salience network, which generates brain outputs in terms of targeted ANS responses (regulating gastrointestinal and immune function) and descending pain modulatory activity (regulating pain sensitivity at the dorsal horn level). Target organ alterations (either peripherally or ANS stimulated) are signalled back to the brain via neural, endocrine or immune-related channels. These signals are processed within subregions of the INS, and depending on their subjective salience, are consciously perceived (associated with activation of anterior INS) as normal gut sensations, discomfort or pain. IBS symptoms can arise from several primary peripheral or central mechanisms, but once brain–gut interactions are altered, causality is difficult to determine. Abbreviations: Amyg, amygdala; ANS, autonomic nervous system; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; Hypo, hypothalamus; INS, insula; orbFC, orbitofrontal cortex; PAG, periaqueductal grey; rACC, rostral anterior cingulate cortex; RVM, rostral ventromedial medulla.
Central executive network
Evidence indicates that patients with IBS might have functional impairments in cognitive processes associated with the central executive network.16,17 Preliminary evidence based on administration of the Attentional Network Test92 suggests that patients with IBS have greater behavioural efficiency during the alerting and orienting function of attention than healthy individuals. In comparison with healthy individuals, this greater efficiency was associated with greater activation of anterior midcingulate and insular cortices, confirming the previously reported close interactions between the central executive network and the salience network.39 Converging evidence also suggests that increased attention to gastrointestinal symptoms and contexts have an important role in the increased perceptual sensitivity to visceral stimuli characteristic of IBS.26 Patients with IBS show deficient activation of inhibitory cortical regions involved in downregulation of pain and emotion as well as attention during expectation and experience of aversive gastrointestinal stimuli.53 Selective recall of negative and gastrointestinal sensation words, as well as selective attention to threat-related stimuli, has been demonstrated in patients with IBS.93–96 Furthermore, a reduction in the effective connectivity of the central executive network circuitry (including parietal, dorsal lateral prefrontal cortex) during repeated exposure to the anticipation and experience of a threatening gastrointestinal stimulus (repeated exposure to balloon inflations) was associated with a reduction in IBS hypersensitivity.97 Data from a sample of Japanese patients with IBS, compared with healthy controls, indicated that alterations in error feedback mechanisms were associated with decreased dorsolateral prefrontal cortex activity.16 A strong negative correlation between the cortical thickness and grey matter density of the dorsolateral prefrontal cortex and pain catastrophizing has been reported.98,99 Evidence from the University of California, Los Angeles research group indicates prepulse inhibition, a process by which an organism can filter the flow of information from its internal and external environments, is altered in IBS compared with health.60 Together, these data suggest that patients with IBS have specific abnormalities in attentional processes that have a role in the increased perception of visceral stimuli and in IBS symptom severity.
Salience network
Studies performed during the past decade on brain responses to delivered and expected rectal distension have consistently reported increased engagement of the core regions of the salience network, the anterior INS and anterior midcingulate cortex in patients with IBS,7,37 which initially but incorrectly were referred to as the “pain matrix”. In addition, a close relationship between increased affect, central emotional arousal processes and enhanced visceral stimulus perception has been reported in patients with IBS.65,100,101 Three recent reports published between 2013 and 2015 in female patients with IBS have identified disease-related alterations in anterior INS activity and connectivity in the resting state58,59 and during an ambiguous abdominal pain threat,102 confirming a key role of salience network alterations in IBS. Reported alterations in the response and connectivity within the salience network are consistent with the prediction error characteristic of patients with IBS about the likelihood and severity of future gastrointestinal symptoms (catastrophizing).15
Integration of brain networks
An extensive body of literature supports the model of the central role of aberrant salience computation underlying key clinical IBS symptoms (depicted in Figure 3). Well-established biological and behavioural consequences exist with such a biased appraisal: the engagement of altered ANS outputs to targets in the gastrointestinal tract (including ENS activity, gut permeability, gastrointestinal motility and secretion,3 gut microbial composition and metabolites),21,22 and to extraintestinal targets (including the immune system);103 shifting of the balance of endogenous pain modulation systems towards increased descending pain facilitation;20 increased engagement of the central executive network resulting in selective attention to gastrointestinal symptoms; and development of prediction errors about likelihood and severity of symptoms (so-called catastrophizing).104,105 The model also provides a plausible biological basis for the effectiveness of different behavioural interventions such as cognitive behavioural therapy (normalizing salience, executive control and emotional arousal networks), self-relaxation techniques (normalizing emotional arousal and central autonomic networks) and mindfulness-based stress reduction (normalizing salience and executive control networks).63
Genetics and epigenetics
As with many other chronic diseases that involve the brain, IBS probably has a strong developmental component, which starts with the interactions of genetic and epigenetic factors early in life, including the prenatal period. In addition to the reported associations of gene variations with various peripheral mechanisms,106 imaging genetics studies performed in large samples of well-phenotyped individuals with and without IBS have identified interactions between early environmental factors,107 candidate gene polymorphisms and brain networks related to emotional arousal and/or central autonomic control, salience and somatosensory integration. The reported genes were related to the regulation of the HPA axis: corticotropin-releasing hormone receptor 1 (CRHR1, single nucleotide polymorphisms [SNPs] rs7209436, rs110402 and rs242924); glucocorticoid receptor gene (NR3C1, SNPs rs2963155 and rs33389);108,109 female sex hormones (progesterone receptor or PGR, SNPs rs1042838 and rs10895068);109 5-HT signalling system (HTR3A c.–42C>T SNP rs1062613),77 inflammation-related genes (IL1B, SNP rs16944);108 and catecholaminergic signalling (ADRA1D, SNP rs1556832; ADRA2B, SNP rs1042717; COMT, SNP rs174697).110 As discussed in the next section, these interactions are markedly influenced by epigenetic factors111,112 (such as a history of early adverse life events [EALs]) and by the sex of the study participant (Figure 4).
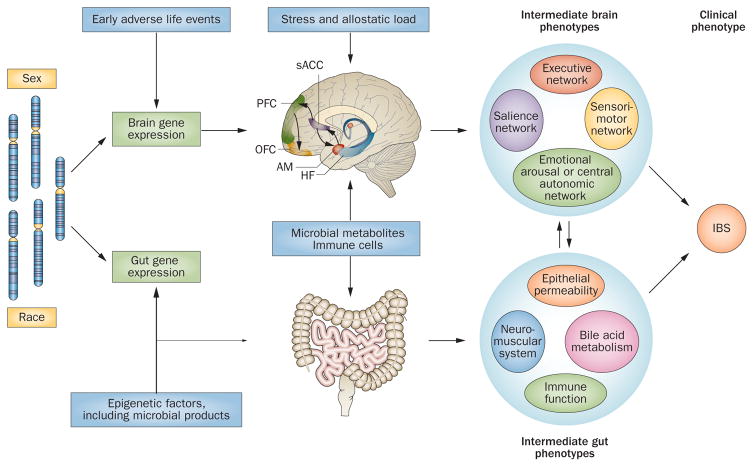
Figure 4.
Longitudinal brain–gut model of IBS pathophysiology. The interaction between genetic and epigenetic influences result in central and peripheral gene expression profiles that underlie the shaping of brain-based and gut-based intermediate phenotypes. Epigenetic factors provide the input from environmental influences on the development of intermediate phenotypes. Brain and gut intermediate phenotypes interact bidirectionally to shape the clinical phenotype of IBS. Abbreviations: AM, amygdala; HF, hippocampal formation; OFC, orbitofrontal cortex; PFC, prefrontal cortex; sACC, subgenual anterior cingulate cortex.
As with other polygenic disorders, it has become clear that no single gene variation is sufficient to explain the full clinical phenotype in IBS. However, interactions between multiple genes, early life experiences and sex probably make a small contribution to the overall variance of the peripheral and central endophenotypes.107 Validation studies in larger samples are required to confirm such contributions.
The external environment component
Various influences originating in the external environment (Figure 1) can markedly affect the development, chronicity and severity of IBS. Although some of these influences are mediated by the brain (psychosocial stress, social support, societal responses to symptoms) others are mediated by the gastrointestinal tract, including the gut microbiota, diet, gastrointestinal infections and medications.
Brain-mediated influences
Clinical literature on the role of psychosocial factors in the development, symptom persistence and symptom flares in IBS is extensive.18 In addition to the well-documented role of stressful life events in adults in preceding or exacerbating IBS symptoms,32 a history of EALs is associated with an increased vulnerability to IBS,113 disorders of mood and affect,114,115 as well as to a wide range of other chronic diseases.116 In contrast to the earlier emphasis on the role of a sexual abuse history, it is now clear that a variety of factors that disturb the quality of the interactions between the primary care giver and the child during the first 18 years of life (including serious illness of the mother, marital discord, divorce, verbal and emotional abuse) can have equally detrimental effect on adult disease vulnerability.116
Evidence from brain imaging studies have identified structural and functional brain alterations associated with self-reports of EALs. Structural alterations in regions of the emotional arousal circuitry78 and in regions associated with modulating somatosensory and viscerosensory processes90 were correlated with such reports. Alterations in the activity in the brainstem and amygdala in response to noradrenergic stimulation has been associated with increasing levels of EALs, consistent with an upregulation of central autonomic circuits in IBS.117 Self-reports of EALs were also found to be correlated with brain activity in networks involved in determining the salience of somatic, visceral or environmental stimuli in IBS.57 These results suggest that the experience of adversity early in life can lead to altered resting state activity in the salience and in the central executive network of adults with IBS, possibly leading to permanent alterations in salience computation of viscerosensory signals by the brain. The observed alterations in the functional connectivity of the emotional arousal51 and salience networks57 found in IBS might be driven by altered central noradrenergic modulation.50,64 Similar changes have also been observed in animals exposed to early life stressors,118 and have been linked to increased sympathetic nervous system responses.119 EALs are also associated with altered signalling within the HPA axis.120,121 A study published in 2009 demonstrated that self-report of EALs was associated with exaggerated HPA axis responses to an aversive visceral stimulus, an effect that was more pronounced in male participants.113
Considerable preclinical and clinical evidence supports the concept that gene expression can be influenced by EALs through epigenetic mechanisms, including DNA methylation, and that these effects can persist throughout adult life.115,122,123 As depicted in Figure 4, interactions of EALs, sex and vulnerability gene polymorphisms might increase the risk of developing IBS by shaping the connectivity of relevant brain networks (see previous section). Even though the prevalence, clinical importance and underlying molecular mechanisms of EALs have been studied in great detail, there are other factors through which the external environment can influence brain function. These include, but are not limited to, the beneficial role of a strong social support system, which can mitigate the negative effects of EALs, and societal responses to patients’ symptom reporting, which can drive a vicious cycle of symptom amplification.124
Gastrointestinal-tract-mediated influences
Factors arising from the external environment have been implicated in the pathophysiology of IBS and in the modulation of IBS symptoms. These factors have been reviewed elsewhere and so will not be discussed in detail here: dietary factors;125–128 pathogenic microorganisms;129,130 and antibiotic treatment.131,132 It remains to be determined if patients with IBS have abnormal mucosal responses to any of these factors, if symptoms reported in relation to these factors are mediated by alterations in the gut microbiota (see section on gut microbiota), or if it is simply the sensitivity of visceral perception that determines if somebody develops symptoms or can tolerate the same factors without any symptoms.
The immune system component
IBS is not an inflammatory disease, but a growing body of research suggests that dysregulation in immune function might nevertheless contribute to its aetiology or symptoms.4,5,133 Mixed data exist on whether plasma or intestinal mucosal cytokine levels are associated with IBS.5 However, other research has linked IBS to an increased reactivity of blood monocytes and increased numbers of mucosal mast cells.134–139 Some studies also indicate that peripheral blood mononuclear cells (PBMCs) from patients with IBS show abnormal release of proinflammatory cytokines such as IL-6, IL-1b and TNF.5
These observations in IBS are consistent with the broader role of the nervous system in regulating immune cell development and gene expression via neuro-endocrine signals from the brain (for example, cortisol from the HPA) and activation of sympathetic nerve fibres in the one marrow and other lymphoid organs (for example, via the sympathetic neuroeffector molecule norepinephrine).103,140–142 These regulatory interactions enable the CNS to integrate information regarding the general internal state of the body with information regarding real and perceived environmental threats (as detected by the salience network) and historical or developmental influences (for example, a history of EALs143). As one example of these regulatory effects, studies have found that stressful life circumstances are associated with the activation of a so-called conserved transcriptional response to adversity (CTRA) in PBMCs that is characterized by increased expression of proinflammatory genes and decreased expression of genes involved in innate antiviral responses (for example, type I interferons) and IgG antibody production.103,140,144,145 CTRA gene expression can also be experimentally invoked by social stress in animal models145,146 and is mediated in part by sympathetic nervous system (SNS)-induced increases in bone marrow haematopoietic production of immature and immunologically primed monocytes (CD16− in humans, Ly-6chi in mice).146 These primed monocytes can also be reciprocally recruited into the CNS by exposure of mice to social threat and aggression.147 The integration of these observations suggests a new hypothesis regarding the immune system’s role in the pathogenesis of IBS: high levels of SNS activity during early developmental periods (stemming from either genetic or environmental triggers such as EALs) might lead to increased production of immature primed monocytes that both traffic into the gut to alter local function and ENS plasticity, and traffic into the brain to affect CNS plasticity (including structures involved in salience processing and autonomic regulation). The result can be viewed as a physiological Hebbian association between gut biology and brain function, resulting in a self-perpetuating feedback system in which a sensitized gut generates ongoing adverse sensory experiences (symptom flares), to which a neurally sensitized brain responds with both greater aversion and increased sympathetic outflow, resulting in upregulated monocyte production that further promotes neural alterations in both the gut and the brain.
Several areas of empirical evidence are consistent with this systems-level brain–immune–gut hypothesis, including: brain regulation of primed monocyte production via the SNS;148 stress-induced migration of primed monocytes to the brain;147 inflammation-induced neuroplastic changes in the brain149 (for example, altering affective behaviour147); reinstatement of previous stress effect on brain and behaviour by subsequent exposure to mild stress weeks after the initial sensitization;148 and the observation that patients with IBS (and animal models of EAL) show increased stress responsiveness (for example, SNS activity150) and increased responsiveness of brain circuits related to salience detection, emotional arousal and autonomic response.151,152
Additional evidence was examined from a pilot study in which the PBMC gene expression profiles from 20 patients with IBS (12 female) and 20 healthy individuals (nine female).153 Analyses identified 280 gene transcripts showing >10% differential expression across groups (134 genes upregulated in PBMC from IBS, and 146 downregulated). Promoter-based bioinformatics analysis implicated several transcription control pathways in structuring the observed transcriptome differences, including increased activity of CREB transcription factors (which mediate β-adrenergic signalling from the SNS), growth control pathways (for example, the MAPK-responsive transcription factor ELK1), oxidative stress response pathways (NRF2), and pathways involved in growth factor and cytokine signalling (STAT). However, PBMCs derived from patients with IBS did not differ from those from healthy individuals in the activity of proinflammatory transcription factors such as NFκB or AP-1. Transcript origin analyses154 indicated that IBS upregulated genes derived predominately from monocytes and dendritic cells. Additional transcriptome representation analyses suggest that these effects stemmed at least in part from upregulation of immature (CD16–) monocytes within the PBMC pool of patients with IBS. These results are all consistent with a pattern of increased myeloid lineage cell development in patients with IBS, which might stem from tonically increased SNS signalling to bone marrow myelopoietic processes.146
Consistent with the hypothesis that CNS processes might mediate relationships between IBS and peripheral myelopoiesis, differential signalling by the myeloid lineage transcription factor MZF-1 was positively associated with the morphometry of brain regions of the emotional arousal network155 (previously reported to be altered in IBS78,90,156). These associations included bilateral amygdala, hippocampal and anterior INS volumes, and bilateral anterior INS cortical thickness (all P <0.05). Moreover, these associations were reasonably specific in that other brain regions showed either no association, or negative associations (for example, bilateral cerebellar volumes).
In conjunction with the published data reviewed here, these preliminary results suggest that key regions of the emotional arousal and/or central autonomic networks, which differ structurally between IBS and healthy individuals are also related to differential gene regulation in the peripheral immune system. We hypothesize that these chronic influences of the brain on peripheral monocytes (‘top down’) might have a role in the reported PBMC abnormalities in IBS, whereas primed monocytes migrating to the brain might have a role in the (‘bottom up’) generation of visceral hypersensitivity, anxiety and neuroplasticity during recurrent stressors.
The gut microbiota component
Evidence from rodent studies supporting bidirectional interactions between the gut microbiota and the nervous system (both CNS and ENS) has been summarized in numerous review articles.157–160 Even though various signalling mechanisms underlying such interactions have been proposed, detailed mechanistic studies regarding the relative contributions of neural, hormonal, metabolite or immune-mediated factors are required to draw definitive conclusions. Evidence from human studies using different endpoints (symptoms, brain imaging studies) confirming the rodent findings, or identifying a definitive pattern of dysbiosis in patients with IBS are limited.158
Conflicting evidence exists regarding alterations to the organization and function of the gut microbiota in patients with chronic abdominal pain and in adult and paediatric patients with IBS.21,22 Several studies examining faecal samples from patients with IBS reported decreased proportions of the genera Bifidobacterium and Lactobacillus, and increased ratios of Firmicutes:Bacteroidetes at the phylum level, even though a causal role of these microbial changes in clinical symptoms has not been established. On the other hand, one might speculate that some of these changes might be related to alterations in regional gut transit and secretion secondary to altered ANS output. A study in a cohort of well-phenotyped patients with IBS highlights the complexities of brain–gut–microbiota alterations in IBS.31 IBS subgroups were identified based on hierarchical clustering of operational taxonomic unit information from 16S ribosomal RNA analyses.31 Two IBS clusters were clearly separated from each other, from a ‘normal-like’ IBS sample and from the healthy control sample. Although the normal-like IBS sample showed normal diversity and normal Firmicutes:Bacteroidetes ratio (as in, similar to the pattern in the healthy control sample), another cluster showed diminished, and a third cluster showed increased diversity. Both of these clusters showed increased Firmicutes:Bacteroidetes ratios. Only the normal-like IBS group showed a markedly elevated rate of depression symptoms (40%). In addition, prolonged colonic transit times, common in patients with depression correlated with the prevalence of 17 taxa.31 In a randomized, placebo-controlled study of healthy men and women, psychological distress and anxiety improved after taking a Lactobacillus-containing and Bifidobacterium-containing probiotic compared to those taking a matched control product, although another study using a different Lactobacillius probiotic failed to confirm these findings.161,162
Findings from brain imaging studies in healthy individuals and patients with IBS has provided some evidence supporting reported rodent gut microbiota–brain interactions. One study has shown that chronic ingestion of a probiotic consortium (Bifididobacterium animalis ssp. lactis, Lactococcus lactis spp. lactis, Lactobacillus delbrueckii spp. bulgaricus and Streptococcus thermophilus) for 4 weeks altered functional brain responses to an emotional face recognition task in healthy women.52 Compared with two control groups (one group received a nonfermented milk product and the other no treatment), the women who had ingested the probiotic had a reduced response to the task across a wide network of brain regions that included sensory and emotional regions. Although no treatment-related changes in self-report of symptoms of anxiety or depression were seen, the findings suggest a basic change in responsiveness to negative emotional stimuli in the environment. No organizational changes in the gut microbiota were observed in this and a previous nonimaging study using the same intervention.163 The effects of the probiotic intervention on brain responsiveness were probably mediated by a change in microbial-derived metabolites; this hypothesis will have to be confirmed in future studies. Preliminary evidence investigating correlations between gut microbial metabolites and brain structure in healthy individuals and patients with IBS demonstrated statistically significant correlations between several metabolites and structural aspects of several brain regions.153
An integrated model
Hypothesis
On the evidence reviewed here, the following model is proposed that lends itself to experimental evaluation. The hypersensitive brain, and presumably ENS, shows increased responses to a variety of viscerosensory and exterosensory stimuli, which by themselves might not be consciously perceived in healthy individuals or in patients with inflammatory bowel disorders in whom intact descending inhibitory bulbospinal influences reduce dorsal horn excitability.20,164 This hyper-responsiveness might be a primary genetic or epigenetic alteration in certain brain networks (autonomic, emotional arousal or salience), or might be secondary to chronic experience of increased sensory input from the gut. Such increased viscerosensory signalling could originate from any of the elements of the gut connectome. Altered brain networks generate altered signals, which are transmitted to the periphery through the ANS, HPA axis and descending modulation to the dorsal horn. The chronically increased ANS output, results in an extensive remodelling of various peripheral cells in the immune system, ENS, gut epithelium (permeability), and in the composition and function of the gut microbiota, all contributing to sensitization of visceral afferent pathways, and increased viscerosensory feedback to the brain, reinforcing the circular regulatory loops. Memory formations at the level of the immune system, the nervous system and the gut microbiota early in life are likely to contribute to the chronicity of symptoms.
Systems biology view
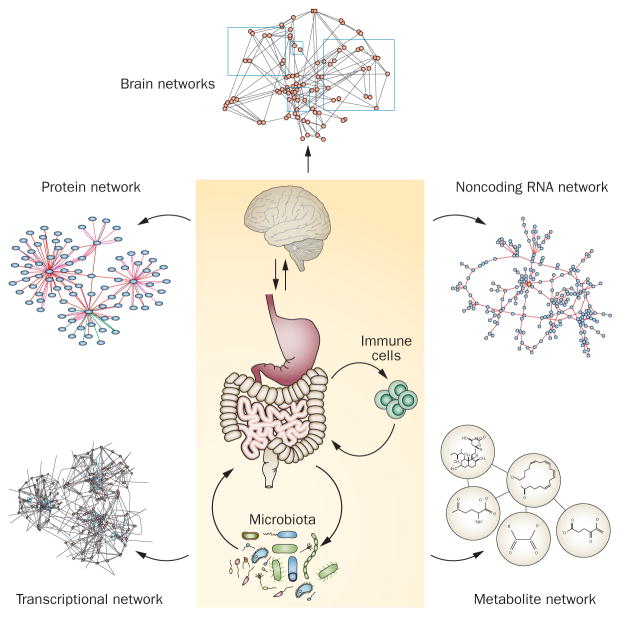
The evidence reviewed above strongly suggests that IBS is a systems disease (Figure 5), involving not only complex individual systems—nervous, immune, digestive, microbiota and the environment—but also their complex, nonlinear, reciprocal interactions. Thus, we propose a systems biological view of IBS pathophysiology, an approach taken in other areas of biology and disease165,166 involving a large number of functionally diverse, interacting components, each of which contributes only a small fraction to the variance of the symptoms. These components interact highly selectively at multiple scales and typically in a nonlinear fashion to produce coherent behaviours and outcomes.167 Applied on a macroscopic level, we propose this model to characterize the interactions of the nervous system, immune system, the gut environment (motility and secretion), the gut microbiota and the external environment (Figures 1 and 5). The nonlinearity of the model is reflected by the complexity of these macroscopic components and their macroscopic interactions, which form circular regulatory loops. Applied to the microscopic level, the model proposes the interactions between highly diverse cells types making up the various macroscopic components (Figure 5).
Figure 5.
Systems biological model of IBS. Schematic illustrating a systems biological view of components involved in the development of IBS at the cellular and molecular level. Systems-based interactions between central and peripheral components of the brain–gut axis can be studied at the level of the genome, epigenome, transcriptome, proteome, metabolome and brain connectome.
Given the complexity of the expanded gut–microbiota–brain–environment axis, and with the rapid advance of analytical tools, in particular of ‘-omics’ technologies—in the case of IBS studies, primarily genomics, transcriptomics, proteomics, metabolomics, brain connectomics and their integration into, for instance, gene and protein networks—we believe that systems biological approaches are essential to fully understand symptom generation and to identify novel treatment approaches in the future.
The multiorgan, systemic view of IBS leads to the prediction that disease models focusing on individual cellular components at the macroscopic level (brain, immune system, gut, microbiota, environment) will have limited validity. Furthermore, this view predicts that therapies targeted at single organs (for example, brain, immune system, gut), single mechanisms (for example, secretion, motility, gut microbiota, pain, diet), or single molecular targets (such as ion channels or receptors) are not likely to be successful in treating the entire syndrome, as evidenced by the limited success in drug development for IBS to date, reflected in rather small effect sizes on overall changes in IBS symptoms. This problem is compounded by the fact that different subsets of patients can be characterized by different patterns of interactions between the components of the overall system, resulting in differential responses to a particular therapy.
Indeed, each isolated mechanism proposed over the past 30 years only explains a small fraction of the variance of the clinical phenotype26 and no isolated therapies have had better effectiveness than ~10% over placebo.168 In addition, the systems view predicts that a single agent targeting multiple components of the system (tricyclic antidepressants, 5-HT3 antagonists), or combined therapies, aimed at simultaneously targeting multiple organs and mechanisms (combination of laxatives, antidiarrhoeal agents, probiotics and centrally acting drugs) are likely to be more effective in the clinic, than individual treatments by themselves. Although no controlled studies have been conducted yet and are needed, in our opinion, this suggested integrated approach is in part supported by current clinical practices and the consensus of most physicians.
Although still in their infancy, integrated multiomic approaches could be essential in the future to better understand the disease spectrum for IBS at the system level and the underlying mechanisms. Ideally, one would like to combine high-throughput microscopy and brain imaging to obtain brain connectome variables, and sequencing and mass spectrometry methods to obtain complete measurements of genomic, epigenomic, transcriptomic, proteomic and metabolomic variables simultaneously in multiple organs and tissues (in particular in gut and brain), including the microbiota. Data derived from these –omics approaches can be integratively analysed in combination with other sources of information ranging from public databases to the literature to identify, for instance, targetable hubs. This integrated –omics approach faces major obstacles stemming from the obvious difficulties of performing some of these analyses in human patients (for example, nervous system tissue). However, important steps can be taken in this direction both using rodent models10,81,169,170 and by performing partial measurements in humans.
Some of these analyses should be performed at multiple time points along the circadian cycle. Circadian rhythms are found at the molecular level in all tissues, both centrally and peripherally, and have a fundamental role in coordinating the physiology and homeostasis of the organism. Indeed integrative system biology analyses171 have revealed that about 10% of all transcripts and metabolites oscillate in a circadian manner in any tissue,172 with little overlap across different tissues beyond the core molecular clock comprising a transcriptional–translational negative feedback loop coordinated by a dozen genes.173 Furthermore, the list of oscillating molecular species in a given tissue is altered by genetic, epigenetic and environmental perturbations and thus provides a characteristic physiological signature of a tissue and its health state.174–176 Furthermore, complex reciprocal interactions exist between the molecular rhythms found in different brain regions and in other organs. Thus, in short, it is reasonable to predict that IBS should result in perturbed lists of transcripts and metabolites that oscillate in a circadian manner both centrally in specific brain areas as well as peripherally, for instance in the gut.
Conclusions
Clearly, none of the growing list of individual abnormalities identified in patients with IBS by itself can account for the variance of the clinical phenotype. For the same reason, such individual abnormalities are unlikely to represent reliable biomarkers and are not likely to represent suitable targets for the development of highly effective treatments. Rather, as depicted schematically in Figures 1 and 5, the clinical phenotype emerges from the interactions of multiple systems in the periphery (gut connectome, microbiome, genome and epigenome) and in the brain (connectome) interacting with each other in bidirectional ways. Most consistent with a systems view is the concept that central and peripheral abnormalities form circular loops that reinforce each other. In the absence of comprehensive phenotyping studies in patients with IBS performed longitudinally with or without therapeutic interventions, and without targeted mechanistic animal studies, it remains unclear which of these reported abnormalities are primary and which are secondary. On the basis of this assessment, we suggest that in the future, high-throughput –omics measurement across both tissues and time, combined with comprehensive characterization of clinical, behavioural and brain endophenotypes, should enable more accurate differential analyses, uncover complex system-level interactions and, ultimately, help develop more efficient, multi-pronged therapies.177 Such a system biological approach might not only hold promise when applied to IBS and related functional gastrointestinal disorders, but also for inflammatory diseases of the gut, such as IBD and coeliac disease.178
Acknowledgments
Supported by funding National Institutes of Health grants P50 DK064539 (E.A.M.), R01 DK048351 (E.A.M.) and P30 DK041301 (E.R.). The authors thank Cathy Liu and Arpana Gupta for invaluable editorial assistance.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
The article was conceptualized and composed by E.A.M. Inputs to the following sections came from the following co-authors: immune system (S.W.C.); brain networks (J.S.L.); gut microbiota (K.T.); and systems biology (P.B.).
References
- 1.Longstreth GF, et al. In: ROME III: The Functional Gastrointestinal Disorders. Drossman DA, et al., editors. Degnon Associates; 2006. pp. 487–556. [Google Scholar]
- 2.Mayer EA, Bushnell MC. In: Functional Pain Syndromes: Presentation and Pathophysiology. Mayer EA, Bushnell MC, editors. IASP Press; 2009. pp. 531–565. [Google Scholar]
- 3.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 4.Hughes PA, et al. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066–1074. doi: 10.1038/ajg.2013.120. [DOI] [PubMed] [Google Scholar]
- 5.Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 6.Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain. 2013;154(Suppl 1):S63–S70. doi: 10.1016/j.pain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Mayer EA, Gupta A, Kilpatrick LK, Hong JY. Chronic visceral pain. Pain. 2015;156(Suppl 1):S50–S63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsenbruch S. Abdominal pain in irritable bowel syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Al Omran Y, Aziz Q. Functional brain imaging in gastroenterology: to new beginnings. Nat Rev Gastroenterol Hepatol. 2014;11:565–576. doi: 10.1038/nrgastro.2014.89. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood-Van Meerveld B, Prusator DK, Johnson AC. Animal models of visceral pain: pathophysiology, translational relevance and challenges. Am J Physiol Gastrointest Liver Physiol. 2015;308:G885–G903. doi: 10.1152/ajpgi.00463.2014. [DOI] [PubMed] [Google Scholar]
- 11.Lackner JM. In: Functional and GI Motility Disorders. Quigley EMM, Hongo M, Fukudo S, editors. Karger Medical and Scientific Publishers; 2014. pp. 104–116. [Google Scholar]
- 12.Bischoff SC, et al. Intestinal permeability —a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsenbruch S. How positive and negative expectations shape the experience of visceral pain. Handb Exp Pharmacol. 2014;225:97–119. doi: 10.1007/978-3-662-44519-8_6. [DOI] [PubMed] [Google Scholar]
- 16.Aizawa E, et al. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology. 2012;143:1188–1198. doi: 10.1053/j.gastro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy PJ, et al. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychol Med. 2014;44:1553–1566. doi: 10.1017/S0033291713002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–139. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piche M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010;148:49–58. doi: 10.1016/j.pain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut. 2011;60:1589–1599. doi: 10.1136/gutjnl-2011-300253. [DOI] [PubMed] [Google Scholar]
- 21.Mayer EA, Savidge T, Shulman RJ. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simren M, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drossman DA. In: The Growth of Gastroenterologic Knowledge in the 20th Century. Kirsner JB, editor. Lea & Febiger; 1993. pp. 419–432. [Google Scholar]
- 24.Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24:919–933. doi: 10.1111/j.1365-2036.2006.03078.x. [DOI] [PubMed] [Google Scholar]
- 25.Barboza JL, Talley NJ, Moshiree B. Current and emerging pharmacotherapeutic options for irritable bowel syndrome. Drugs. 2014;74:1849–1870. doi: 10.1007/s40265-014-0292-7. [DOI] [PubMed] [Google Scholar]
- 26.Mayer EA, et al. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keita AV, Soderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 28.Elenkov IJ, Chrousos GP. Stress system—organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13:257–267. doi: 10.1159/000104853. [DOI] [PubMed] [Google Scholar]
- 29.Hayes PA, Fraher MH, Quigley EM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol (N Y) 2014;10:164–174. [PMC free article] [PubMed] [Google Scholar]
- 30.Spiller R, Lam C. An Update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil. 2012;18:258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeffery IB, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 32.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford AC, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350–1365. doi: 10.1038/ajg.2014.148. [DOI] [PubMed] [Google Scholar]
- 34.Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 36.Bohorquez DV, Liddle RA. The gut connectome: making sense of what you eat. J Clin Invest. 2015;125:888–890. doi: 10.1172/JCI81121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer EA, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 2009;21:579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farmer AD, Aziz Q, Tack J, Van Oudenhove L. The future of neuroscientific research in functional gastrointestinal disorders: integration towards multidimensional (visceral) pain endophenotypes? J Psychosom Res. 2010;68:475–481. doi: 10.1016/j.jpsychores.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo CC, et al. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage. 2012;61:1471–1483. doi: 10.1016/j.neuroimage.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bajaj S, Adhikari BM, Dhamala M. Higher frequency network activity flow predicts lower frequency node activity in intrinsic low-frequency BOLD fluctuations. PLoS ONE. 2013;8:e64466. doi: 10.1371/journal.pone.0064466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 43.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Hutchison RM, et al. Resting-state connectivity identifies distinct functional networks in macaque cingulate cortex. Cereb Cortex. 2012;22:1294–1308. doi: 10.1093/cercor/bhr181. [DOI] [PubMed] [Google Scholar]
- 45.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sporns O. Networks of the Brain. The MIT Press; 2010. [Google Scholar]
- 47.Tijms BM, Series P, Willshaw DJ, Lawrie SM. Similarity-based extraction of individual networks from gray matter MRI scans. Cereb Cortex. 2012;22:1530–1541. doi: 10.1093/cercor/bhr221. [DOI] [PubMed] [Google Scholar]
- 48.Hagmann P, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS ONE. 2007;2:e597. doi: 10.1371/journal.pone.0000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradford K, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390. e1–e3. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickhaus B, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol. 2003;98:135–143. doi: 10.1111/j.1572-0241.2003.07156.x. [DOI] [PubMed] [Google Scholar]
- 51.Labus JS, et al. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013;154:2088–2099. doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tillisch K, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. e1–4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farmer AD, Aziz Q. Visceral pain hypersensitivity in functional gastrointestinal disorders. Br Med Bull. 2009;91:123–136. doi: 10.1093/bmb/ldp026. [DOI] [PubMed] [Google Scholar]
- 55.Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8:451–463. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Tillisch K, et al. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta A, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76:404–412. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong JY, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34:14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong JY, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33:11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kilpatrick LA, et al. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS) Biol Psychol. 2010;84:272–278. doi: 10.1016/j.biopsycho.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Oudenhove L, Vandenberghe J, Vos R, Holvoet L, Tack J. Factors associated with co-morbid irritable bowel syndrome and chronic fatigue-like symptoms in functional dyspepsia. Neurogastroenterol Motil. 2011;23:524–e202. doi: 10.1111/j.1365-2982.2010.01667.x. [DOI] [PubMed] [Google Scholar]
- 62.Naliboff BD, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 63.Naliboff BN, Lackner J, Mayer EA. In: Principles of Clinical Gastroenterology. Yamada T, et al., editors. Wiley-Blackwell Publishing; 2008. [Google Scholar]
- 64.Hubbard CS, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall GB, et al. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil. 2010;22:276–e80. doi: 10.1111/j.1365-2982.2009.01436.x. [DOI] [PubMed] [Google Scholar]
- 66.Labus JS, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011;60:1196–1203. doi: 10.1136/gut.2010.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 68.Porro CA, et al. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22:3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bornhovd K, et al. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 71.Verne GN, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 72.Seifert F, et al. Brain activity during sympathetic response in anticipation and experience of pain. Hum Brain Mapp. 2013;34:1768–1782. doi: 10.1002/hbm.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yaguez L, et al. Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology. 2005;128:1819–1829. doi: 10.1053/j.gastro.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 74.Berman SM, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stephan E, et al. Functional neuroimaging of gastric distention. J Gastrointest Surg. 2003;7:740–749. doi: 10.1016/s1091-255x(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 76.Lawal A, et al. Neurocognitive processing of esophageal central sensitization in the insula and cingulate gyrus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G787–G794. doi: 10.1152/ajpgi.00421.2007. [DOI] [PubMed] [Google Scholar]
- 77.Kilpatrick LA, et al. The HTR3A polymorphism c. –42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140:1943–1951. doi: 10.1053/j.gastro.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Labus JS, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155:137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 80.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holschneider DP, Bradesi S, Mayer EA. The role of experimental models in developing new treatments for irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2011;5:43–57. doi: 10.1586/egh.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tache Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labus JS, et al. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology. 2013;145:1253–1261. e1–3. doi: 10.1053/j.gastro.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubio A, et al. Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system—a fMRI study in healthy volunteers. Neuroimage. 2014;107C:110–22. doi: 10.1016/j.neuroimage.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 85.Kilpatrick LA, et al. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. 2014;192:947–955. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Apkarian AV, Thomas PS, Krauss BR, Szeverenyi NM. Prefrontal cortical hyperactivity in patients with sympathetically mediated chronic pain. Neurosci Lett. 2001;311:193–197. doi: 10.1016/s0304-3940(01)02122-x. [DOI] [PubMed] [Google Scholar]
- 87.Becerra L, et al. Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. Neuroimage Clin. 2014;6:347–369. doi: 10.1016/j.nicl.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DaSilva AF, et al. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS ONE. 2008;3:e3396. doi: 10.1371/journal.pone.0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ellingson BM, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154:1528–1541. doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang Z, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS ONE. 2013;8:e73932. doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piche M, et al. Thicker posterior insula is associated with disease duration in women with irritable bowel syndrome (IBS) whereas thicker orbitofrontal cortex predicts reduced pain inhibition in both IBS patients and controls. J Pain. 2013;14:1217–1226. doi: 10.1016/j.jpain.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 92.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 93.Afzal M, Potokar JP, Probert CS, Munafo MR. Selective processing of gastrointestinal symptom-related stimuli in irritable bowel syndrome. Psychosom Med. 2006;68:758–761. doi: 10.1097/01.psy.0000232270.78071.28. [DOI] [PubMed] [Google Scholar]
- 94.Gibbs-Gallagher N, et al. Selective recall of gastrointestinal-sensation words: evidence for a cognitive-behavioral contribution to irritable bowel syndrome. Am J Gastroenterol. 2001;96:1133–1138. doi: 10.1111/j.1572-0241.2001.03759.x. [DOI] [PubMed] [Google Scholar]
- 95.Phillips K, Wright BJ, Kent S. Irritable bowel syndrome and symptom severity: Evidence of negative attention bias, diminished vigour, and autonomic dysregulation. J Psychosom Res. 2014;77:13–19. doi: 10.1016/j.jpsychores.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Tkalcic M, Domijan D, Pletikosic S, Setic M, Hauser G. Attentional biases in irritable bowel syndrome patients. Clin Res Hepatol Gastroenterol. 2014;38:621–628. doi: 10.1016/j.clinre.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 97.Labus JS, et al. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage. 2009;47:952–960. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 99.Seminowicz DA, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14:1573–1584. doi: 10.1016/j.jpain.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elsenbruch S, et al. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 101.Elsenbruch S, et al. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59:489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- 102.Hong Jui-Yang, et al. IBS patients show altered brain responses during uncertain, but not certain expectation of painful stimulation of the abdominal wall [abstract] Gastroenterology. 2014;148(Suppl):S384. [Google Scholar]
- 103.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lackner JM, Gurtman MB. Pain catastrophizing and interpersonal problems: a circumplex analysis of the communal coping model. Pain. 2004;110:597–604. doi: 10.1016/j.pain.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 105.van Tilburg MA, Palsson OS, Whitehead WE. Which psychological factors exacerbate irritable bowel syndrome? Development of a comprehensive model. J Psychosom Res. 2013;74:486–492. doi: 10.1016/j.jpsychores.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Camilleri M. Genetics of human gastrointestinal sensation. Neurogastroenterol Motil. 2013;25:458–466. doi: 10.1111/nmo.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saito YA, Mitra N, Mayer EA. Genetic approaches to functional gastrointestinal disorders. Gastroenterology. 2010;138:1276–1285. doi: 10.1053/j.gastro.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gupta A, et al. Early life adversity: interactions with stress related gene polymorphisms to impact regional brain structure in females. Brain Struct Funct. doi: 10.1007/s00429-015-0996-9. http://dx.doi.org/10.1007/s00429-015-0996-9. [DOI] [PMC free article] [PubMed]
- 109.Kilpatrick L, Gupta A, Heendeniya N, Labus J, Mayer EA. Corticotropin releasing hormone receptor 1 (crh-r1) and progestoerone receptor (pgr) polymorphisms interact with early life trauma in healthy controls (hc) and patients with irritable bowel syndrome (IBS) [abstract 666] Gastroenterology. 2013;144(Suppl 1):S121. [Google Scholar]
- 110.Gupta A, et al. Catecholaminergic gene polymorphisms are associated with GI symptoms and morphological brain changes in irritable bowel syndrome. PLoS ONE. doi: 10.1371/journal.pone.0135910. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong S, Zheng G, Wiley JW. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology. 2015;148:148–157.e7. doi: 10.1053/j.gastro.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tran L, Schulkin J, Ligon CO, Greenwood-Van Meerveld B. Epigenetic modulation of chronic anxiety and pain by histone deacetylation. Mol Psychiatry. doi: 10.1038/mp.2014.122. http://dx.doi.org/10.1038/mp.2014.122. [DOI] [PubMed]
- 113.Videlock EJ, et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 115.Klengel T, Binder EB. Gene-environment interactions in major depressive disorder. Can J Psychiatry. 2013;58:76–83. doi: 10.1177/070674371305800203. [DOI] [PubMed] [Google Scholar]
- 116.Felitti VJ, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 117.Berman S, et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage. 2012;63:1854–1863. doi: 10.1016/j.neuroimage.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaloner A, Rao A, Al-Chaer ED, Greenwood-Van Meerveld B. Importance of neural mechanisms in colonic mucosal and muscular dysfunction in adult rats following neonatal colonic irritation. Int J Dev Neurosci. 2010;28:99–103. doi: 10.1016/j.ijdevneu.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Flugge G, van Kampen M, Meyer H, Fuchs E. α2A and α2C-adrenoceptor regulation in the brain: α2A changes persist after chronic stress. Eur J Neurosci. 2003;17:917–928. doi: 10.1046/j.1460-9568.2003.02510.x. [DOI] [PubMed] [Google Scholar]
- 120.Chaloner A, Greenwood-Van Meerveld B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci. 2013;7:13. doi: 10.3389/fnins.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Provencal N, et al. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J Neurosci. 2012;32:15626–15642. doi: 10.1523/JNEUROSCI.1470-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Provencal N, Binder EB. The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp Neurol. 2015;268:10–20. doi: 10.1016/j.expneurol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 124.Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med. 1999;130:910–921. doi: 10.7326/0003-4819-130-11-199906010-00016. [DOI] [PubMed] [Google Scholar]
- 125.Ahmad OF, Akbar A. Dietary treatment of irritable bowel syndrome. Br Med Bull. 2015;113:83–90. doi: 10.1093/bmb/ldu039. [DOI] [PubMed] [Google Scholar]
- 126.Fasano A, Sapone A, Zevallos V, Schuppan D. Non-celiac gluten sensitivity. Gastroenterology. 2015;148:1195–1204. doi: 10.1053/j.gastro.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 127.Lomer MC. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther. 2015;41:262–275. doi: 10.1111/apt.13041. [DOI] [PubMed] [Google Scholar]
- 128.Shepherd SJ, Halmos E, Glance S. The role of FODMAPs in irritable bowel syndrome. Curr Opin Clin Nutr Metab Care. 2014;17:605–609. doi: 10.1097/MCO.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 129.Grover M, Camilleri M, Smith K, Linden DR, Farrugia G. On the fiftieth anniversary. Postinfectious irritable bowel syndrome: mechanisms related to pathogens. Neurogastroenterol Motil. 2014;26:156–167. doi: 10.1111/nmo.12304. [DOI] [PubMed] [Google Scholar]
- 130.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 131.Maxwell PR, Rink E, Kumar D, Mendall MA. Antibiotics increase functional abdominal symptoms. Am J Gastroenterol. 2002;97:104–108. doi: 10.1111/j.1572-0241.2002.05428.x. [DOI] [PubMed] [Google Scholar]
- 132.Villarreal AA, Aberger FJ, Benrud R, Gundrum JD. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ. 2012;111:17–20. [PubMed] [Google Scholar]
- 133.Bashashati M, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26:1036–1048. doi: 10.1111/nmo.12358. [DOI] [PubMed] [Google Scholar]
- 134.Darkoh C, et al. Chemotactic chemokines are important in the pathogenesis of irritable bowel syndrome. PLoS ONE. 2014;9:e93144. doi: 10.1371/journal.pone.0093144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ohman L, et al. Increased TLR2 expression on blood monocytes in irritable bowel syndrome patients. Eur J Gastroenterol Hepatol. 2012;24:398–405. doi: 10.1097/MEG.0b013e3283503f39. [DOI] [PubMed] [Google Scholar]
- 136.Matricon J, et al. Review article: associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 137.Cremon C, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 138.Lee KJ, et al. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 139.Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: stress, intestinal hyperpermeability and inflammation. World J Gastroenterol. 2007;13:3027–3030. doi: 10.3748/wjg.v13.i22.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cole SW. Social regulation of human gene expression. Curr Dir Psychol Sci. 2009;18:132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miller GE, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szyf M. The early life environment and the epigenome. Biochim Biophys Acta. 2009;1790:878–885. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 143.Gupta A, et al. Early adverse life events: influence on resting state connectivity in patients with chronic abdominal pain. Psychosom Med. 2014;76:404–412. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cole SW. Human social genomics. PLoS Genet. 2014;10:e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cole SW, et al. Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci USA. 2012;109:20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Powell ND, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wohleb ES, et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wohleb ES, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tillisch K, et al. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berman S, et al. Differential noradrenergic (NE) modulation of brain metabolic and electrical activity in IBS patients and healthy controls. Gastroenterology. 2007;132:A726. [Google Scholar]
- 152.Labus J, et al. Sex-specific differences in a brain network functioning during anticipation of rectal discomfort in irritable bowel syndrome patients (IBS) Gastroenterology. 2006;130:A93. [Google Scholar]
- 153.Mayer EA, et al. Correlation between gene expression profiles in peripheral blood mononuclear cells and structural and functional brain networks in chronic visceral pain. Neuropsychopharmacology. 2014;39:S641. [Google Scholar]
- 154.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci USA. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seminowicz DA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:U48–U82. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 158.Mayer EA, Tillisch K. Gut brain axis and the microbiome. J Clin Invest. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour—epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 160.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 162.Messaoudi M, et al. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 163.McNulty NP, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3:106ra106. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mayer EA, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 165.Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- 166.Hornberg JJ, Bruggeman FJ, Westerhoff HV, Lankelma J. Cancer: a systems biology disease. Biosystems. 2006;83:81–90. doi: 10.1016/j.biosystems.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 167.Likic VA, McConville MJ, Lithgow T, Bacic A. Systems biology: the next frontier for bioinformatics. Adv Bioinformatics. 2010;2010:268925. doi: 10.1155/2010/268925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang L, Lembo A, Sultan S. American Gastroenterological Association institute technical review on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1149–1172.e2. doi: 10.1053/j.gastro.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 169.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 170.Tache Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173–177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- 171.Patel VR, Eckel-Mahan K, Sassone-Corsi P, Baldi P. CircadiOmics: integrating circadian genomics, transcriptomics, proteomics and metabolomics. Nat, Methods. 2012;9:772–773. doi: 10.1038/nmeth.2111. [DOI] [PubMed] [Google Scholar]
- 172.Patel VR, Eckel-Mahan K, Sassone-Corsi P, Baldi P. How pervasive are circadian oscillations? Trends Cell Biol. 2014;24:329–331. doi: 10.1016/j.tcb.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eckel-Mahan KL, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bellet MM, et al. Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci USA. 2013;110:9897–9902. doi: 10.1073/pnas.1120636110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 179.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 181.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 183.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Labus JS, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 186.Stein JL, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 187.Critchley HD, Nagai Y, Gray MA, Mathias CJ. Dissecting axes of autonomic control in humans: Insights from neuroimaging. Auton Neurosci. 2011;161:34–42. doi: 10.1016/j.autneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 188.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 189.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 190.Jones LM, Fontanini A, Sadacca BF, Miller P, Katz DB. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proc Natl Acad Sci USA. 2007;104:18772–18777. doi: 10.1073/pnas.0705546104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alcauter S, et al. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci. 2014;34:9067–9075. doi: 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Levinthal DJ, Strick PL. The motor cortex communicates with the kidney. J Neurosci. 2012;32:6726–6731. doi: 10.1523/JNEUROSCI.0406-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 194.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: the value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Greicius MD, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Laird AR, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]