Abstract
Proper development and function of the mammalian central nervous system (CNS) depend critically on the activity of parenchymal sentinels referred to as microglia. Although microglia were first described as ramified brain-resident phagocytes, research conducted over the past century has expanded considerably upon this narrow view and ascribed many functions to these dynamic CNS inhabitants. Microglia are now considered among the most versatile cells in the body, possessing the capacity to morphologically and functionally adapt to their ever-changing surroundings. Even in a resting state, the processes of microglia are highly dynamic and perpetually scan the CNS. Microglia are in fact vital participants in CNS homeostasis, and dysregulation of these sentinels can give rise to neurological disease. In this review, we discuss the exciting developments in our understanding of microglial biology, from their developmental origin to their participation in CNS homeostasis and pathophysiological states such as neuropsychiatric disorders, neurodegeneration, sterile injury responses, and infectious diseases. We also delve into the world of microglial dynamics recently uncovered using real-time imaging techniques.
Keywords: rain, innate immunity, myeloid cells, two-photon
INTRODUCTION
The central nervous system (CNS) has long been considered immune privileged owing to its lack of standard lymphatic drainage and reduced capacity to present antigens (1). Indeed, the CNS does have an immunological capacity that differs from most peripheral tissues, but it is now appreciated that, when necessary, this tissue can mount vigorous immune reactions that depend in part upon specialized innate cells, including microglia, macrophages, and dendritic cells (DCs). Interestingly, unique anatomical niches in the CNS such as the meninges, choroid plexus, and perivascular spaces are inhabited by specialized macrophages and DCs that serve as resident innate immune sentinels capable of orchestrating potent inflammatory responses (2). The brain parenchyma, on the other hand, is populated by microglia—the most common CNS-resident innate immune cell. Microglia comprise ~10–15% of all glial cells and are often referred to as the tissue-resident macrophages of the CNS. But unlike meningeal, choroid plexus, and perivascular macrophages, microglia originate from the yolk sac and populate the CNS prior to its vasculogenesis (3, 4). Resting microglia in the adult brain have a small cell body and are highly ramified—a morphology that distinguishes them from macrophages and DCs. These cells participate in CNS development, homeostasis, and nearly all CNS disturbances. As part of their homeostatic functions, microglial cell bodies remain stationary, but their processes continuously scan the surrounding extracellular space and communicate directly with neurons, astrocytes, and blood vessels. This perpetual state of motion allows them to respond swiftly to damage or infection by transforming into an activated phenotype and performing inflammatory functions. They are remarkably plastic and capable of responding to a vast array of challenges. Upon detection of specific factors generated by parenchymal injury, degeneration, or infection, microglia undergo morphological transformations and respond rapidly through induction of genetic programs designed to overcome and repair CNS insults. Transformations of microglial morphology, phenotype, and function are observed during almost all neuropathological conditions (e.g., degenerative diseases, infection, stroke, tumors, brain injury).
The roots of microglia research are firmly embedded in the pioneering work of Franz Nissl, Santiago Ramón y Cajal, and Pío del Río Hortega. Río Hortega, a student of Ramón y Cajal, named these cells and is considered the Father of Microglia (5, 6). He meticulously described the basic morphological features of microglia using a silver staining technique and a light microscope that are still accurate to this day. He observed the treelike processes of microglia and predicted their phagocytic function. In fact, his detailed histological analyses laid the foundation for our contemporary understanding of microglial biology. The field has expanded enormously since Río Hortega’s observations. In fact, microglia are now thought to represent an important nexus between neurological and immunological activity in the CNS. Fitting with their complex morphology and plasticity, microglia often morph to suit the needs of their ever-changing surroundings, and as predicted, microglia are indeed brain-resident phagocytes that can participate in phagocytic activities, but their functions extend well beyond this. Studies have shown that these specialized sentinels play a major role in maintaining the overall health of the CNS. In the sections that follow, we discuss exciting developments in our understanding of microglial biology, starting with their origin and progressing to their participation in states of health and disease, such as neuropsychiatric conditions, neurodegeneration, sterile injury, and CNS infection. For information regarding the role of microglia in CNS autoimmunity, we refer the reader to several outstanding reviews written on this topic (7, 8).
MICROGLIA DEVELOPMENT
Debate about microglial lineage and origin began shortly after their discovery by Río Hortega. Owing to phenotypic similarities to peripheral monocytes/macrophages and DCs, microglia were proposed to be of hematopoietic origin. In fact, a number of bone marrow reconstitution studies concluded that microglia were myeloid in origin and derived from the hematopoietic system (9–11). Initially, microglia were thought to derive from circulating monocytes. This supposition is supported by studies showing that irradiation-induced myeloablation facilitates engraftment of the CNS by blood-derived Ly-6ChiCCR2+ monocytes (12, 13). Furthermore, this engraftment process is not initiated by CNS degeneration alone (e.g., cuprizone-induced death of oligoden-drocytes) but requires preconditioning by direct tissue irradiation (12). These data prove that monocytes can enter the CNS parenchyma and convert to cells with morphological similarities to microglia; however, this occurs only under certain conditions, and it is debated whether these cells are truly microglia.
A caveat associated with irradiating mice is that a nonphysiological state of systemic injury is induced that can facilitate tissue repopulation that might not otherwise occur during steady-state conditions. In addition, transient compromise of blood-brain barrier function observed following whole-body irradiation could also facilitate aberrant cellular relocation (14, 15). Hence, it was argued that irradiation followed by bone marrow reconstitution might not reflect homeostatic microglia turnover. Supporting this argument, Wirenfeldt et al. (16) showed that only one-third of microglia remained viable following irradiation, and their proliferative capacity was severely impaired. Consequently, the injury and cellular depletion induced by whole-body irradiation favor reconstitution by bone marrow–derived precursors. To avoid the confounding variables associated with whole-body irradiation, microglial turnover was reevaluated using parabiotic mice (two mice with a shared circulatory system). In nonirradiated parabiotic mice, Ajami et al. (17) provided seminal data showing that microglia under steady-state conditions were maintained by self-renewal and were not in fact derived from bone marrow progenitors. Even after inducing neurodegeneration by facial motor neuron axotomy (which causes significant microgliosis), no engraftment by blood-derived cells was detected despite infiltration by peripheral macrophages. A similar observation was made in parabiotic mice with experimental autoimmune encephalitis (EAE) (18). Although monocyte-derived macrophages contribute to the development of EAE, they do not engraft the CNS following resolution of the disease. Thus, under steady-state, degenerative, and autoimmune conditions, microglia appear to self-renew.
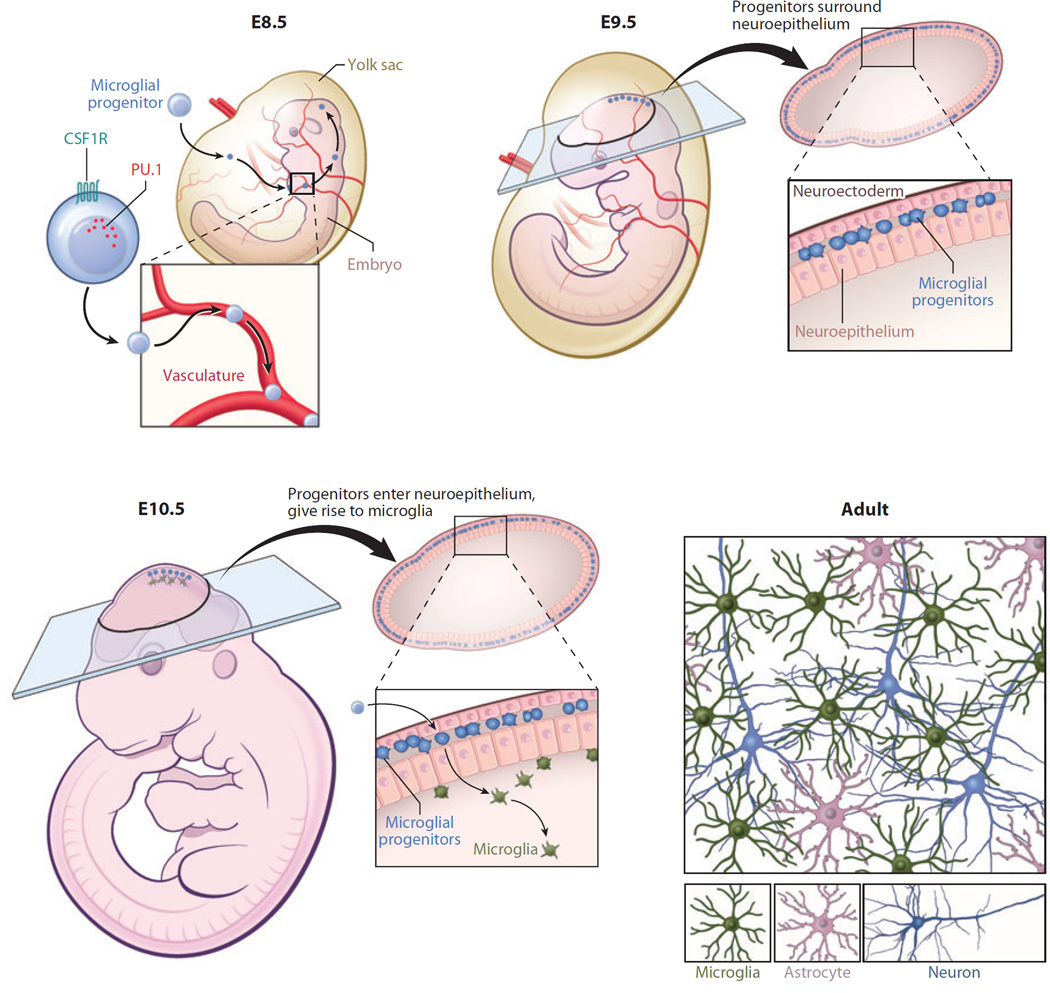
The developmental origin of microglia has also served as a subject of debate in the field. The concept of microglial derivation from the embryonic yolk sac was first suggested in the early 1990s by Cuadros et al. (19), who generated chimeras between chick embryos and quail yolk sacs to establish that primitive myeloid cells invade the brain rudiment independently of the blood supply during development. This concept was further supported by Alliot et al. (20), who showed the presence of microglia precursors in the yolk sac and later the brain rudiment of the developing embryo by embryonic day 8 (E8). Although these studies did not resolve the debate regarding microglial origin, they did provide a spatiotemporal map of where and when to find microglia during embryogenesis.
More definitive evidence was established over a decade later by Ginhoux et al. (3), who demonstrated that microglia are derived from primitive macrophages emanating from the embryonic yolk sac during development (Figure 1). In this study, mice expressing tamoxifen-inducible Cre recombinase under the Runt-related transcription factor 1 (Runx1) promoter were crossed with floxed ROSA26 reporter mice. Because Runx1 expression is restricted to the yolk sac, this elegant approach enabled Ginhoux et al. to inject tamoxifen and activate Runx1-driven Cre recombinase at different time points during embryonic development, thus permitting in vivo fate mapping of yolk-sac-derived cells. Importantly, injection of tamoxifen before E7.5 revealed that microglia progenitors come from the embryonic yolk sac. These progenitors later entered into the brain rudiment in a manner dependent on the circulatory system, as progenitors were not observed in the brain rudiment of embryos without a heartbeat (and thus no blood flow). Interestingly, injection of tamoxifen into reporter mice a day later (E8.5) failed to label yolk sac macrophages or microglia but did label circulating monocytes and leukocytes. The yolk sac derivation of microglia was confirmed by Schulz et al. (21), who established that the transcription factor Myb, which is required for hematopoiesis and the generation of hematopoietic stem cells (HSCs), was not essential for the generation of microglia. Myb−/− mice are deficient in hematopoietically derived monocytes/macrophages but have normal numbers of microglia in the CNS. Further analyses of yolk sac progenitors revealed that microglia are derived from CD45−c-kit+ erythromyeloid progenitor cells (22). These progenitors eventually convert to CD45+c-kit−CX3CR1+ microglia and invade the brain rudiment using matrix metalloproteinases (MMPs), such as MMP-8 and MMP-9. Collectively, these studies support the concept that microglia are yolk sac (not hematopoietically) derived.
Figure 1.
Microglial origin and development. Recent studies have shown that microglia are ontologically distinct from bone marrow–derived monocytes/macrophages found in peripheral tissues. Microglia are instead derived from primitive macrophages that emanate from the embryonic yolk sac during development (prior to embryonic day 8.5, E8.5) and enter the brain rudiment via the circulatory system. These progenitors surround the neuroepithelium of the developing brain around E9.5 and one day later enter the neuroepithelium and begin to colonize the CNS parenchyma. Microglia at this stage of development have an amoeboid rather than a ramified morphology. Microglia become completely ramified throughout the brain by postnatal day 28. Development and survival of microglia are critically dependent on several factors, including the transcription factor PU.1 as well as CSF1R.
During neonatal development, primitive yolk-sac-derived microglial progenitors do not bear highly ramified processes and more closely resemble macrophages. However, they eventually progress through a developmental program that includes proliferation and subsequent acquisition of ramified processes, which gives rise to the microglial architecture commonly observed in the adult CNS. Once ramified microglia are evenly spread throughout the parenchyma, several factors contribute to their homeostasis and self-renewal, and without these factors, microglia numbers would begin to wane and give rise to CNS dysfunction.
FACTORS THAT PROMOTE MICROGLIA DEVELOPMENT AND HOMEOSTASIS
During neonatal development, committed myeloid precursors differentiate into microglia, and this developmental program is controlled by various molecules that include but are not limited to transcription factors, growth factors, chemokines, MMPs, and microRNAs, among others. In the adult CNS, microglia are maintained under steady-state conditions via a balance between mitosis and apoptosis (16). Some of the key factors involved in microglial genesis and homeostasis are discussed below.
Runx1
Runx1 was initially discovered in patients with acute myeloid leukemia (AML), a heterogeneous bone marrow malignancy in which the AML1 gene on chromosome 21 is translocated and fused in frame with the ETO gene on chromosome 8 (23). Runx1 binds directly to the enhancer elements of several genes relating to hematopoietic development. For example, Runx1 binds directly to the upstream regulatory element of the PU.1 gene (a transcription factor essential for microglia development) and modulates its expression during embryonic and adult hematopoiesis (24). Interactions between Runx1 and other transcriptional elements are essential for expression of several genes that participate in lineage-specific development of multipotent progenitors. For example, expression of colony-stimulating factor receptor (CSF1R), whose function is required for myeloid cell development, is dependent on a physical interaction between Runx1 and the CCAAT enhancer-binding protein (C/EBP) (25).
Homozygous mutation of Runx1 results in embryonic lethality due to the absence of fetal liver development. In addition, embryonic stem cells lacking Runx1 do not differentiate into myeloid or erythroid progenitors of hematopoietic origin in developing embryos, although yolk sac development and erythropoiesis are maintained (26). Runx1 expression is first observed at E6.5, and around E7.5 yolk-sac-derived Runx1+ cells develop into fetal lymphoid progenitors and HSCs (27). Ginhoux et al. (3) demonstrated that yolk-sac-derived Runx1+ precursors populate the CNS and develop into microglia during gestational stages that precede brain development and vascularization. During development, nascent Runx1+ microglia have an amoeboid morphology and undergo mitosis; however, they exit their cell cycle program before downregulating Runx1. Progressive loss of Runx1 expression coincides with the morphological transformation of microglia from amoeboid to ramified, and this occurs around postnatal day 10. In the adult brain, Runx1 is reexpressed following traumatic nerve injury, suggesting an additional role for this transcription factor in the regulation of microglia activation (28).
PU.1
PU.1 is a member of the ETS (E-twenty six) family of transcription factors that are dynamically expressed in various cells, including macrophages, neutrophils, mast cells, B cells, and microglia. In addition, the PU.1 protein is constitutively expressed in both resting and activated rodent microglia as well as human microglia (29, 30). Thus, it was expected that PU.1 would play a regulatory role in the ontology of lymphoid and myeloid cells, including microglia. Indeed, the importance of PU.1 was revealed in null mice, which are severely immunocompromised and ultimately die a few days after birth unless reconstituted with wild-type bone marrow. PU.1 null mice are deficient in mature macrophages, neutrophils, B cells, and T cells but have erythrocytes and megakaryocytes (31). PU.1 is considered a master regulator for myelomonocytic differentiation during embryonic development. Olson et al. (32) demonstrated that PU.1 deficiency impedes the maturation of yolk-sac-derived myeloid progenitors, which is evidenced by a failure of these cells to express terminal myeloid differentiation markers, such as CSF1R (also known as macrophage colony-stimulating factor receptor, or M-CSFR), CD11b, and CD64. PU.1 expression is autoregulated and subjected to regulation by other hematopoietic transcription factors (e.g., C/EBPα) (33). A recent study showed that CSF1 (also referred to as M-CSF) can induce PU.1 activation in HSCs both in vitro and in vivo (34). This induces myeloid-specific gene expression in HSCs, resulting in increased myeloid cell differentiation. This finding has important implications because it shows that a cytokine such as CSF1, which is abundantly expressed during states of inflammation and stress, can direct the fate of HSCs and promote the generation of myeloid cells. Thus, the abundance of tissue-resident myeloid cells such as microglia might be influenced by developmental perturbations that modulate CSF1 levels, such as maternal microbial infections and stress.
CSF1R
CSF1R is a tyrosine kinase transmembrane receptor expressed on mononuclear myeloid cells throughout the body. Originally identified as a growth factor receptor for hematopoietic cells, CSF1R is acritical regulator of mononuclear phagocytic cell survival, differentiation, development, and chemotaxis. Mice with a frameshift mutation in CSF1 (CSF1op/op mice), a ligand for this receptor, have a profound reduction in tissue macrophages but possess relatively normal numbers of skin macrophages (Langerhans cells) and microglia (35, 36). In contrast, CSF1R−/− mice are completely deficient in microglia, which suggested the presence of another ligand for this receptor (3, 37). The discrepancy observed in CSF1- versus CSF1R–deficient mice led to the discovery of IL-34 as a complementary ligand for CSF1R (38, 39). Wang et al. (40) established that IL-34 defficiency leads to a complete loss of Langerhans cells in the epidermis and a ~20% reduction in brain microglia. In this study, the most notable reductions were observed for microglia residing in the cerebral cortex, hippocampus, and corpus callosum. Using IL-34 reporter mice, the investigators determined the source of this cytokine to be keratinocytes in the skin and neurons in the CNS. These results indicate that homeostatic CSF1R signaling in Langerhans cells and microglia is provided locally by tissue-resident cells.
IRF-8
Interferon regulatory factor 8 (IRF-8) is a transcription factor and member of the IRF family that is sometimes referred to as interferon consensus sequence-binding protein (ICSBP). In addition to their well-described inflammatory role, IRFs participate in development of the immune system by modulating transcription. IRF-8-deficient mice show enhanced proliferation and abundance of granulocytes, myelomonocytic cells, and lymphoid cells, suggesting a role for this transcription factor in modulating the proliferation and differentiation of hematopoietic progenitors (41, 42). A specific role for IRF-8 in macrophage differentiation was demonstrated by Tamura et al. (43), who used a retroviral strategy to reconstitute IRF-8 knockout myeloid progenitor cells with functional IRF-8. Retroviral transduction promoted differentiation of myeloid progenitors into phagocytic macrophages while repressing the granulocyte differentiation program. These data revealed that IRF-8 plays a key role in macrophage maturation.
Similar to macrophages, IRF-8 also has a direct impact on CNS microglia. Using reporter mice that express green fluorescent protein (GFP) under the CSF1R promoter, Minten et al. (44) revealed that IRF-8 deficiency results in increased microglial abundance in the adult brain; however, these cells also had reduced branch complexity and gross morphological changes suggestive of activation. Despite phenotypic signs of activation (e.g., increased expression of CD45, CD11b, and F4/80), no alteration in ex vivo phagocytic capacity was observed in IRF-8−/− microglia. These data suggest that IRF-8 plays an important role in maintaining microglial homeostasis in the adult brain and may also control their state of activation. More recently, Kierdorf et al. (22) linked IRF-8 to microglia development, revealing that embryonic microglia contain higher levels of IRF-8 transcripts than adult cells do. However, in contrast to previous studies (43, 44), Kierdorf et al. (22) showed decreased (not increased) parenchymal microglia in IRF-8−/− mice, which was attributed to a defect in the survival and maturation of yolk sac myeloid progenitors during embryonic development. It is conceivable that IRF-8 participates in the homeostasis and development of microglia, at least partly, by regulating apoptosis-related genes, as IRF-8-deficient myeloid cells are resistant to stimuli that induce programmed cell death (45). This supposition is consistent with the increased number of apoptotic microglial progenitors observed in IRF-8−/− mice during development (45). Although additional work is required to refine our understanding of how IRF-8 modulates microglia development and homeostasis, this transcription factor is an active participant in these processes.
miR-124
Small noncoding microRNAs regulate gene expression through posttranscriptional modification of mRNAs primarily by promoting their degradation. Translational repression is achieved by formation of an RNA-induced silencing complex (RISC) that activates an RNA cleavage pathway leading to enzymatic degradation. microRNAs are expressed from embryogenesis to adulthood and therefore play an active role in regulating gene expression (46). miR-124 is one of the most abundantly expressed microRNAs in the CNS and regulates adult neurogenesis and neuronal differentiation by altering gene expression in neurons (47–49). miR-124 is also highly expressed in resident microglia. By comparing the expression profile of miR-124 in the naive versus activated CNS, Ponomarev et al. (50) demonstrated that microglia downregulate miR-124 following activation, suggesting a role for miR-124 in maintaining microglial quiescence. This theory was corroborated by data showing that miR-124 targets the transcription factor C/EBP-α and its downstream target PU.1, greatly downregulating their protein expression. Interestingly, knockdown of miR-124 resulted in a functional switch of microglia as well as macrophages from naive to activated, demonstrating an inhibitory role for miR-124 in myeloid cell activation.
HOMEOSTATIC MICROGLIA FUNCTIONS
Microglia are indispensable for normal brain development. Given that microgliogenesis and neurogenesis occur concomitantly in the developing brain, it seemed likely that their fates would be intertwined. Indeed, studies conducted over the past decade have revealed that microglia interact with neurons during development and in the adult CNS. Disruption of these interactions can have a severe negative impact on CNS development and function. Below we discuss some vital homeostatic microglia functions that help establish and maintain the overall health of the nervous system.
Neuronal Survival
During early development, various neurotrophic factors promote survival and differentiation of specific neuronal lineages and circuit formation; during adulthood some of these very same neurotrophins promote the health and survival of neurons. Importantly, microglia contribute to the surrounding cellular milieu by releasing trophic factors that support the formation of neuronal circuits and promote their survival. For example, insulin-like growth factor-1 (IGF-1) released by surrounding microglia promotes survival of layer V cortical neurons during postnatal development (51). Immunohistochemical analyses revealed that microglia accumulate along subcerebral and callosal axon fibers early after birth (postnatal days 1 to 7), suggesting the potential for microglia to interact with and support these fibers (51). This supposition was corroborated by data showing that inhibition or depletion of microglia promoted cell death of layer V cortical neurons. In addition, this neuro-supportive role for microglia was mediated in part by fractalkine (i.e., CX3CL1-CX3CR1) signaling and IGF-1 release by microglia, as CX3CR1 deficiency or IGF-1 inhibition similarly resulted in death of layer V cortical neurons.
The mechanistic link between fractalkine and IGF-1 signaling remains unclear; nevertheless, the ability of microglia to produce IGF-1 through whatever means likely influences the fate of many cell lineages in the nervous system. For example, IGF-1 induces multipotent adult rat hippocampus-derived neural progenitor cells to differentiate into oligodendrocytes (52). Moreover, IGF-1 protects immature oligodendrocytes from glutamate-mediated apoptosis (53). Microglia secrete trophic factors other than IGF-1, such as basic fibroblast growth factor, hepatocyte growth factor, epidermal growth factor, platelet-derived growth factor, nerve growth factor, and brain-derived neurotrophic factor, which play significant roles in neuronal development, maintenance, and function throughout life (54–57). Thus, microglia are essential facilitators of neuronal health and survival in the nervous system.
Neuronal Death
About half of all immature neurons die during brain development primarily through programmed cell death. Though the reason for this extensive programmed cell death is not fully understood, it is proposed that the surplus of faulty neurons resulting from defective differentiation and migration requires elimination. In addition, neurons that fail to form proper neuronal circuits or that only perform transient functions must undergo programmed cell death. Neuronal cell death can be initiated by cell intrinsic factors or by accessory cells such as microglia that are well poised to induce programmed cell death and clean up the resultant cellular debris. An example of this was observed in the retina of the developing chick eye, where release of nerve growth factor by microglia induced apoptosis of retinal nerve cells, which is considered part of the normal developmental program (58). Similarly, neurons in the developing murine hippocampus are induced by microglia to undergo programmed cell death (59). In this anatomical location, microglia release reactive oxygen species (ROS) in a CD11b-dependent and DNAX activation protein of 12 kDa (DAP12)-dependent manner that induces neuronal death. Blockade of CD11b was shown to decrease neuronal cell death in the developing hippocampus. A similar mechanism was observed in the developing mouse cerebellum, where selective elimination of microglia reduced neuronal (Purkinje) cell death. It was further determined that release of ROS by microglia was in part responsible for inducing death of Purkinje cell neurons (60). Thus, microglia are not simply accessory cells that respond to neuronal cell death during development, but rather are active promoters of the process (at least in certain brain regions).
The amount of cell death induced during development mandates an active and efficient cleanup system to remove the resultant cellular debris. As expected, microglia participate in this cleanup through phagocytosis and, importantly, can engage phagocytic machinery without initiating an inflammatory response (60–62). The phagocytic capacity of microglia is conserved evolutionarily. Studies of the developing chick embryo spinal cord have shown that a significant number of microglia/macrophages are present at early stages of development and can be observed phagocytosing apoptotic motor neurons (63). Noninflammatory microglial phagocytosis is mediated through signaling via triggering receptor expressed on myeloid cells-2 (TREM2) (61). TREM2 signaling promotes phosphorylation of the adaptor protein DAP12, which later induces cytoskeleton reorganization and phagocytosis. Notably, proinflammatory cytokines (e.g., TNF-α, IL-1β, and NOS2) are not expressed following TREM2 signaling. In fact, overexpression of TREM2 can actually decrease the proinflammatory potential of microglia. Instead, this signaling pathway promotes CCR7 expression and microglia chemotaxis. Dying neurons in turn release factors that recruit microglia to actively participate in phagocytosis. This phagocytic activity is important not only in the developing CNS, but also for the maintenance of healthy neural networks in the adult brain. For example, release of soluble fractalkine (CX3CL1) by damaged neurons can promote microglial phagocytosis through release of milk fat globule–epidermal growth factor 8 (MFG-E8) (64). MFG-E8 is expressed by microglia and facilitates phagocytosis of apoptotic cells through activation of the integrin-associated protein CD47 signaling pathway (65). Microglia are therefore essential for removing dead cells from both the developing and the adult CNS. Without this vital function, the health of the CNS deteriorates and can give rise to neurological dysfunction.
Synaptogenesis
In addition to clearance of dead cells, microglial phagocytic activity is crucial for synaptic homeostasis (66, 67). Microglia participate in the process of neuronal pruning during development and respond to synaptic activity as well as plasticity. Proper synaptic function depends on various trophic factors and synaptogenic signals, some of which are derived from microglia. Roumier et al. (68) revealed that DAP12 signaling is essential for synaptic function and plasticity. DAP12 is a transmembrane immune receptor protein expressed by myeloid as well as lymphoid cells and is thought to play a major role in innate immune function. In the mouse brain, DAP12 expression is restricted to microglia, and its deficiency promotes a dramatic reduction in synaptic expression of tyrosine kinase receptor B (the receptor for brain-derived neurotrophic factor, commonly known as TRK-B), leading to impaired synaptic function and plasticity (68). More recently, ex vivo studies on organotypic hippocampal brain slice cultures showed that microglia can modulate synaptic activity by regulating synapse densities, glutamatergic receptors, and dendritic spine numbers (69). Microglia can also participate in experience-dependent synaptic plasticity. Ultrastructural and dynamic imaging studies of the juvenile mouse visual cortex revealed that microglia actively participate in remodeling synaptic architecture (70). Exposure to a sensory stimulus such as light influences the location and morphology of microglia in relation to synapses. In the developing visual cortex, microglia are apposed to synaptic clefts, suggesting a role in their maintenance. Interestingly, light deprivation induced microglia to generate phagocytic structures and more closely associate with what appeared to be disassembling dendritic spines. These changes were reversed following reexposure to light. These data indicate that microglia are intimately associated with synaptic structures and respond to changes in these structures elicited by environmental stimuli that modulate their activity.
Programmed axonal pruning and selective elimination of defective synapses are critical for the establishment of functional, mature neuronal circuits during brain development. Redundant and excessive neuronal processes that have the potential to hinder mature neuronal circuit formation are selectively pruned by microglia. This function is essential for normal brain development (67). Paolicelli et al. (67) followed the fate of representative pre- and postsynaptic proteins and demonstrated during synaptic maturation that these proteins localize to microglia, showing that microglia engulf synaptic structures in the uninjured brain. This synaptic pruning is mediated in part by fractalkine signaling, as mice deficient in CX3CR1 had increased dendritic spine densities and less mature synapses when assessed 2–3 weeks following birth (67).
The complement system also appears to facilitate microglial synaptic pruning. During development, the complement protein, C1q, is expressed in neurons following exposure to immature astrocytes (71). Interestingly, C1q localizes to synapses throughout the postnatal CNS and retina and can promote activation of C3. Because microglia express C3 receptors (CR3), synaptic complement activation has the potential to trigger phagocytosis and synaptic elimination. Consistent with this assertion, mice deficient in C1q or C3 showed defects in synaptic elimination, which results in the retention of excessive synaptic connections (71). Moreover, another recent study established that microglia sculpt postnatal retinogeniculate synapses in a neural activity–dependent manner using phagocytosis linked to CR3 signaling (72). Inhibition of neural activity with tetrodotoxin enhanced synaptic engulfment by microglia, whereas CR3 deficiency decreased microglia phagocytic activity and promoted a sustained defect in synaptic connectivity. Collectively, these data indicate that microglia participate in synaptic development and maintenance, which has major implications for our understanding of normal brain homeostasis, as disruption of this routine activity has the potential to cause neurological disorders.
MICROGLIAL DYSFUNCTION AND DISEASE
Obsessive-Compulsive Disorder
Given the important role of microglia in normal brain development and homeostasis, it is not surprising that certain neurological disorders are linked to microglial dysfunction. Two compelling examples that have emerged in the literature are compulsive grooming behavior, linked to the homeobox B8 (Hoxb8) gene, and Rett syndrome, which maps to a mutation in the gene encoding methyl-CpG-binding protein-2 (MECP2) (73, 74). Most mammals including humans exhibit grooming behaviors that serve diverse physiological functions. Greer & Capecchi (75) were the first to establish that Hoxb8 is necessary for normal grooming behavior in mice. The Hox genes encode a series of sequence-specific transcription factors that facilitate DNA-protein and protein-protein interactions during morphogenesis and organ development (76). Hox genes are essential for establishing correct body structures along the anteroposterior axis during embryonic development (77, 78). In addition, these genes are evolutionarily conserved from Drosophila to higher vertebrates.
To investigate the function of Hoxb8, van den Akker et al. (79) generated a Hoxb8 mutant by inserting a bacterial lacZ-coding sequence upstream of the Hoxb8 start codon. The resultant mutant mice exhibited axial skeleton abnormalities, degeneration of C2 spinal ganglion, and neurological dysfunction (79). Because the lacZ interfered with the function of genes adjacent to Hoxb8, it was argued that the phenotypic changes observed in mutant mice might not be due to Hoxb8 alone. To address this caveat, Greer & Capecchi developed a strategy to specifically delete the Hoxb8 gene. Interestingly, the resultant knockout mice showed excessive (pathological) grooming behavior that led to hair loss and self-inflicted skin lesions (75). In addition, Hoxb8−/− mice often groomed their cage mates excessively and exhibited an obsessive-compulsive behavioral pattern. It was later demonstrated by this same group that, within the CNS, Hoxb8 is expressed only by a subset of microglia (or their progenitors) (73). In the adult brain, a subpopulation of microglia appear to be derived from a Hoxb8+ cell lineage, and Hoxb8 deficiency decreases the number of steady-state microglia by ~15%. It is not clear, however, how this reduction promotes the obsessive-compulsive behavior observed in the knockout mice. To correct the aberrant behavioral pattern, two-month-old Hoxb8−/− mice were irradiated and reconstituted with wild-type bone marrow (73). This reversed symptoms in 60% of the Hoxb8−/− mice and was associated with localization of GFP+ hematopoietic cells (presumed to be microglia) to the brain parenchyma.
Given that microglia in the adult brain are not repopulated by hematopoietic precursors under steady-state conditions (17), the bone marrow reconstitution experiment performed in Hoxb8−/−mice reflects a nonphysiological state of chimerism between hematopoietically derived myeloid cells and resident microglia. Nevertheless, the reconstitution therapy corrected the aberrant behavioral pattern in Hoxb8−/− mice, signifying that bone marrow-derived myeloid cells can enter the CNS parenchyma after irradiation, replace microglia, and participate in normal homeostatic functions. This seminal study was also the first to show that a neurobehavioral disorder could map to a primary dysfunction in microglia, further supporting a crucial role for microglia in maintaining normal neural activity.
Rett Syndrome
Rett syndrome is an X-linked neurodevelopmental disorder observed primarily in females and most commonly maps to mutation of the MECP2 gene (80). MECP2 is a transcriptional repressor that binds to methylated genomic DNA via its transcriptional repressor and high-affinity methyl DNA-binding domains. The latter promotes the recruitment of histone deacetylases that repress transcription. The complex also limits the access of other transcriptional machinery to targeted promoter regions (81, 82). The temporal expression pattern of MECP2 in the CNS during the embryonic and early postnatal periods is crucial for normal neuronal development. Mutation of MECP2 promotes abnormal epigenetic regulation and consequently disrupts normal neuronal development (80).
To study Rett syndrome, various murine models have been developed in which MECP2 is mutated, knocked out, or conditionally deleted. Genetic approaches to reconstitute MECP2 in neurons have substantiated that neurological function can be restored at both early and late stages of postnatal development (83, 84). As expected, MECP2 is highly expressed in the brain, and in addition to neurons, all glial cells, including astrocytes, microglia, oligodendrocytes, and oligodendrocyte progenitor cells, express at least some MECP2. Using an in vitro coculture system, Ballas et al. (85) revealed that astrocytes containing mutant MECP2 do not support normal neuronal growth and dendritic morphology. These data show that the function of MECP2 is not necessarily cell autonomous, resulting from a defect in neurons alone. This finding was confirmed in two seminal in vivo studies in which normal MECP2 was reconstituted in astrocytes (86) and microglia (74). Lioy et al. (86) established that expression of wild-type MECP2 in MECP2 null mice restored normal neuronal morphology and improved locomotion, anxiety, respiration, and life span. Shortly thereafter, Derecki et al. (74) showed that transplantation of wild-type bone marrow into MECP2 null mice arrested disease progression. This therapeutic improvement was linked to myeloid cell chimerism in the CNS, which is induced by whole-body irradiation (17), analogous to the approach used to rescue Hoxb8−/− mice with obsessive-compulsive disorder (73). This finding demonstrates now in two distinct models that some neurological disorders can be ameliorated by bone marrow reconstitution and myeloid cell engraftment in the CNS parenchyma. Although microglia are not normally replaced by bone marrow–derived myeloid precursors, it might be possible to exploit the fact that peripheral engraftment can be induced under certain nonphysiological conditions (in this case, whole-body irradiation).
MICROGLIA AND NEURODEGENERATIVE DISEASE
Because of the close relationship between microglia and neurons, it is not surprising that microglia respond to and can sometimes exacerbate neurodegenerative conditions in which dysfunctional or defective neurons are the primary driver of disease. Neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis are all characterized by abnormal protein aggregates primarily in neurons. This in turn can trigger microglia to become reactive and eventually contribute to a chronic neuroinflammatory state in the CNS, although their initial role is often neuroprotective. How microglia respond to the two most common human neurodegenerative diseases is described in more detail below. We refer readers to other recent reviews focused on microglia and neurodegeneration for additional information on this important topic (87, 88).
Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by the excessive accumulation and sustained deposition of beta-amyloid (Aβ) as well as the formation of neurofibrillary tangles inside neurons that result from hyperphosphorylation of the tau protein. These pathological changes give rise to progressive cognitive decline in AD patients. A number of susceptibility genes for AD include amyloid precursor protein (APP), apolipoprotein E, presenilin 1 and 2, CD2-associated protein, ATP-binding cassette subfamily A member 7, membrane-spanning 4 domains subfamily A, and CD33. Microglia express several of these genes, suggesting a role for microglia in AD neuropathology. In fact, one of the risk genes associated with AD (i.e., CD33) actually impedes the ability of microglia to phagocytose Aβ (89), and it is known that human AD is associated with impaired clearance of Aβ (90). This finding suggests that the phagocytic system in the CNS is either impaired or becomes overwhelmed by the continuous deposition of (or exposure to) Aβ. Nevertheless, the precise role of microglia in the course of AD remains somewhat controversial. When evaluated in a murine model of AD, selective ablation of microglia had no impact on amyloid plaque formation or maintenance (91). Thus, it is unclear to what degree microglial phagocytosis is involved in thwarting the progression of AD. It is conceivable that microglia play an important early role in slowing the toxic effects of Aβ but are less involved later in the disease as their phagocytic function declines. In fact, a transition from phagocytosis to proinflammatory cytokine production could position microglia as late-stage exacerbators of AD.
There is now widespread support for the idea that proinflammatory cytokines produced by microglia during AD progression can promote the development of pathology and cognitive decline. For example, blockade of IL-12/IL-23 signaling (presumably on microglia) decreased pathology and cognitive decline in a murine model of AD (92). In addition, another study revealed that norepinephrine, a neurotransmitter produced in part by the locus coeruleus, suppresses microglia cytokine production induced by Aβ (93). Further, this immunosuppressive neurotransmitter promotes phagocytic uptake of Aβ by microglia. Interestingly, the locus coeruleus is damaged in the majority of AD patients, which has the potential (through reduced norepinephrine production) to decrease the phagocytic capacity of microglia and promote chronic proinflammatory cytokine production. This is consistent with data in murine models of AD showing that the phagocytic capacity of microglia declines over time (94, 95). Microglia participate in noninflammatory phagocytic activities from development to adulthood. This routine housekeeping function maintains the overall health of the brain and may slow the onset of AD; however, as phagocytic activity declines, especially during the course of a degenerative disease, the CNS becomes inflamed and can rapidly deteriorate. In a disease such as AD, inflammation and decline of microglial phagocytosis could serve as secondary contributors to pathogenesis.
Assuming that microglia do play an active role (either positive or negative) in the development of AD, it is important to understand the factors that activate and set microglia into motion. Many factors have been implicated in activating microglia and directing them toward sites of Aβ deposition. These factors include but are not limited to the receptor for advanced glycation end products (RAGE), formyl peptide receptors (FPRs), Toll-like receptors (TLRs), pattern-recognition receptors, complement receptors, scavenger receptors, fractalkine, and vascular endothelial growth factor receptor 1 (VEGFR-1). VEGFR-1 is highly expressed in human AD brain tissue and facilitates microglial migration in vitro and in vivo (96). In addition, the pattern-recognition receptors TLR2 and TLR4 (which are expressed by microglia) are involved in AD pathogenesis, as functional deficiency in either results in increased Aβ deposits and reduced phagocytic clearance (97–100). Importantly, stimulation of TLR4 signaling via systemic administration of monophosphoryl lipid A (a TLR4 agonist) reduced Aβ loads, increased microglia phagocytosis, and enhanced cognitive function in a murine model of AD (100). Thus, it might be possible to slow AD progression in humans through therapeutic manipulation of TLR signaling.
CD36 is another pattern-recognition receptor thought to contribute to AD pathogenesis. CD36 is a membrane scavenger receptor whose ligands include oxidized low-density lipoprotein, collagen, oxidized phospholipids, and long-chain fatty acids, among others. The receptor is expressed on myeloid cells such as microglia and participates in phagocytosis. Stuart et al. (101) reported that CD36 drives downstream signaling in microglia that results in cytoskeletal reorganization and locomotion in response to Aβ deposits, indicating that pattern-recognition receptors not only activate but also contribute to the movement of microglia.
On the basis of the involvement of receptors such as TLR2, TLR4, and CD36, it is becoming increasingly apparent that degenerative diseases such as AD chronically trigger the immune system through release of “danger” signals that bear some resemblance to pathogen-stimulated innate immune reactions. This is commonly referred to as sterile immunity because an immune response develops in the absence of a microbe. In line with this concept, AD studies have shown that RAGE and FPRs found on microglia can bind to Aβ, resulting in enhanced neuroinflammation and acceleration of disease (102, 103). RAGE binds to advanced glycation end products (AGE) and has numerous ligands in addition to AGE that include phosphatidylserine, high-mobility group protein B1 (HMGB1), and Aβ, among others. Transgenic expression of RAGE in microglia accelerated AD-related neuroinflammation and Aβ accumulation, leading to more rapid cognitive decline in a murine model. The pathogenic effects of RAGE overexpression were ameliorated in mice expressing a RAGE mutant defective in signaling. Another recent study demonstrated an interaction between RAGE and FPRs and suggested that FPRs play an important role in Aβ-induced microglial signal transduction (103). FPRs are G protein–coupled receptors that bind N-formyl peptides released from damaged cells and promote immune cell chemotaxis. For example, real-time imaging of a sterile injury reaction in the liver revealed that formyl peptides released from necrotic cells help guide neutrophils to sites of injury (104). Tissue injury results in the release of danger signals that promote acute inflammation (105). If Aβ promotes similar signaling (either directly or indirectly) in innate immune cells such as microglia, this would give rise to chronic CNS inflammation. Death of neurons induced by Aβ or other mechanisms could also contribute to a chronic innate immune reaction through the stimulation of damage sensors such as those described above.
The role of fractalkine signaling in murine models of AD remains controversial. A variety of different CNS myeloid sentinels (e.g., meningeal macrophages, choroid plexus macrophages, perivascular macrophages, microglia) express the fractalkine receptor CX3CR1 (2). Interactions between microglial CX3CR1 and CX3CL1 (the ligand) expressed on neurons in the naive brain are thought to help maintain microglia in a quiescent state. Thus, a fractalkine signaling deficiency is predicted to enhance microglia function and promote inflammation. Consistent with this theory, Liu et al. (106) demonstrated using the CRND8 (APP mutant) murine AD model that CX3CR1 deficiency lowers Aβ levels in the brain, fitting with an increased phagocytic capacity of microglia. In their study, no impact on neuronal cell death was observed. Reduced Aβ accumulation in the absence of CX3CR1 was observed in two additional models of AD (APP/PS1 and R1.40 mice) (107), further supporting a role for enhanced microglial phagocytosis in thwarting the progression of AD. However, using yet another murine AD model (hAPP-J20 mice), Cho et al. (108) concluded that CX3CR1 deficiency increases neuronal pathology and cognitive deficits and that this was attributable to enhanced cytokine production (e.g., IL-6 and TNF-α) rather than to an alteration in the phagocytic capacity of microglia. Finally, it was shown in 3xTg mice that express mutant APP, PS1, and tau that CX3CR1 deficiency actually decreases neuronal loss (109). These mice also had no change in Aβ accumulation when compared to littermate controls, which is in contrast to observations made in the other AD models. The discrepancy between the aforementioned studies likely reflects differences in the AD models under investigation. For example, the involvement of mutant tau in AD pathogenesis, as observed in 3xTg mice and none of the other models, may change the microglial response and role of fractalkine signaling. Understanding this variation in AD pathogenesis is important given that progression of human AD is influenced by many different genetic and environmental factors that murine models only partially reflect. It is also worth considering that CX3CR1 is not expressed solely on microglia in the CNS; therefore, deficiency might differentially modulate the functionality of other CX3CR1-GFP-expressing brain sentinels such as perivascular macrophages, meningeal macrophages, and recruited monocytes (110, 111).
Parkinson’s Disease
Parkinson’s disease (PD) is a human neurodegenerative condition characterized by neuronal accumulation of α-synuclein cytoplasmic inclusions (commonly referred to as Lewy bodies) and the loss of dopaminergic neurons in the substantia nigra pars compacta, although neuronal death is not exclusive to this brain region. Mutations in several different genes, including α-synuclein, parkin, Parkinson disease (autosomal recessive, early onset) 7, and phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase-induced putative kinase 1, are known to cause disease; however, these mutations account for only a small proportion of the cases. The most commonly studied animal models of PD involve injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or 6-hydroxydopamine, two neurotoxic agents that cause death of dopaminergic neurons in the substantia nigra, resulting in PD-like symptoms. Microglia become highly activated during the course of PD (112) and produce proinflammatory cytokines (e.g., TNF-α, IL-1β, IFN-γ), chemokines, and ROS. In addition, the brain is infiltrated by CD8+ and CD4+ T cells (113), which suggests that the adaptive immune system might also participate in disease progression. The precise contribution(s) of microglia to PD is not entirely understood, but their potential modes of participation include the chronic production of proinflammatory molecules and the modulation of adaptive immune cells.
The factors that regulate microglia during PD are complex. For example, CD200-CD200R signaling (114), fractalkine signaling (115), MMPs (116), and leucine-rich repeat kinase 2 (LRRK2) (117) were shown to modulate microglial activity in murine models of PD or following in vitro exposure of microglia to α-synuclein. MPTP studies in macaques have revealed that IFN-γ and TNF-α are elevated in the serum and CNS during the development of disease (118). Importantly, knockout studies on MPTP-treated rodents demonstrated that both of these cytokines were required for full activation of microglia (118). Both the PD susceptibility gene LRRK2 and MMPs contribute to TNF-α production by microglia (116, 117), which has the potential to create an autocrine feedback loop that results in sustained microglia activation. Inhibition of MMP-3, −8, or −9 reduces TNF-α, IL-1β, nitric oxide (NO), and ROS produced by microglia exposed to α-synuclein (116), suggesting that MMPs participate in the proinflammatory responses elicited by microglia during the course of PD. In addition, Moehle et al. (117) established that inhibition of LRRK2 decreases expression of TNF-α and inducible nitric oxide synthase (iNOS) in microglia exposed to a TLR4 agonist. Therefore, increased or aberrant expression of LRRK2 during the course of PD could foster microglia-induced inflammation. Disruption of CD200-CD200R signaling might also exacerbate microglial inflammatory responses during PD (114). Zhang et al. (114) showed that inhibition of the pathway increased inflammation, neuronal cell death, and neurological dysfunction in a rat model of PD. Because this pathway couples microglia and neurons in the naive CNS, helping maintain an uninflamed state, disruption of this pathway during the course of a neurodegenerative disease such as PD could result in chronic microglial activation.
Similar to AD, in PD microglia may not be detrimental early in the course of the disease (unless a specific genetic mutation renders them dysfunctional). Microglia participate in neuronal homeostasis and could help scavenge an overload of an aberrantly deposited protein such as α-synuclein. In support of this hypothesis, Stefanova et al. (119) showed that TLR4 deficiency impedes the ability of microglia to clear α-synuclein in a transgenic mouse model, which resulted in increased proinflammatory cytokine production, neuronal cell death, and motor dysfunction. These data suggest that the scavenging function of microglia is important in slowing the progression of neurodegenerative diseases defined by the pathologic accumulation of a particular protein. Alternatively, microglia might directly participate in neuronal cell death during the course of PD by aberrantly engulfing the neurons. A recent study in the MPTP model of PD revealed that microglia completely engulf degenerating dopaminergic neurons using a mechanism dependent on Rho-associated kinase activity (120). Whether this response is neuroprotective or neurodestructive during the course of human PD remains to be determined and depends on whether the primary defect resides in neurons or microglia.
In addition to innate immunity, compelling data implicate adaptive immune cells in PD pathogenesis. Analysis of PD brain tissue has revealed the presence of HLA-DR+ microglia in the substantia nigra pars compacta (112), and a recent genome-wide association study linked HLA-DR polymorphisms to the development of late-onset sporadic PD (121), suggesting a potential role for CD4+ T cells in the disease. Using the murine MPTP model of PD, Brochard et al. (113) demonstrated that dopaminergic cell death was markedly attenuated in mice lacking CD4+ T cells. The authors further showed that T cell-mediated neuronal damage required expression of Fas ligand (FasL). The involvement of CD4+ T cells in disease pathogenesis was corroborated and expanded upon by Harms et al. (122), who expressed full-length human α-synuclein in the substantia nigra pars compacta using an adeno-associated viral vector. This induced MHC class II (MHC-II) expression on microglia, CD4+ T cell proliferation, proinflammatory cytokine production, IgG deposition, and neuronal degeneration—a response ameliorated by MHC-II deficiency. However, a caveat of this study is that human (rather than mouse) α-synuclein was used. Because mice are not necessarily tolerant to a human protein, it is not surprising that a vigorous inflammatory response was mounted. Whether this mode of pathogenesis is operational in humans requires further study.
Interestingly, neuronal IgG deposition was observed in the damaged substantia nigra of human PD brain tissue, and the amount of IgG correlated positively with the number of HLA-DR+ microglia (123). The microglia were also shown to express FcγRI (CD64), which is the high-affinity receptor for IgG. These data suggest a potential role for humoral immunity in PD pathogenesis. Using a protracted murine model of PD, Lira et al. (124) showed that FcγR deficiency is neuroprotective, resulting in reduced microglial activation and neurological dysfunction. This neuroprotective effect was not observed in the standard, early-onset MPTP model of PD (124), suggesting that humoral immunity only becomes pathogenic when neuron cell death occurs over a long time interval. Collectively, these data imply that the adaptive immune system may participate in the pathogenesis of human PD. Given that use of nonsteroidal anti-inflammatory drugs reduces the risk of developing PD, it is worth testing other immunosuppressive drugs in PD patients (125).
MICROGLIAL RESPONSES TO INFECTION
Because of their position and dynamic nature, microglia are a critical component of the innate immune sentinel network in the CNS. They help maintain immune vigilance and must quickly respond to pathogens that enter the parenchyma, lest the CNS be overtaken by the foreign invader. Microglia are equipped with machinery that responds to a variety of pathogens, including bacteria, fungi, parasites, and viruses. Their innate pattern-recognition systems allow them to detect foreign antigens, and following activation, they can respond by producing pro- and anti-inflammatory cytokines, chemokines, complement, free radicals, and trophic factors, among others. Microglia can also proliferate and migrate to sites of inflammation. These innate programs utilized by microglia provide swift control of invading pathogens and set the stage for the arrival of adaptive immune cells such as T and B cells. Microglia are capable of presenting antigen in both MHC-I and -II complexes and thus can interact directly with pathogen-specific CD8+ and CD4+ T cells (assuming that antigen is acquired).These interactions are designed to promote pathogen clearance but can sometimes facilitate intense inflammatory reactions that result in CNS pathology. Below we describe a few select examples of how microglia respond to different classes of pathogens.
Parasitic Infections
Murine models of parasitic infection and postmortem analyses of brain tissue from parasite-infected humans have taught us a great deal about how microglia respond to pathogens such as Toxoplasma gondii, Trypanosoma brucei, and Plasmodium falciparum. For example, T. gondii is a parasitic protozoan that can infect most warm-blooded animals, including humans. Following an initial stage of replication, the parasite converts to a bradyzoite, which is a slowly dividing form of the pathogen that often clusters together and becomes tissue cysts. These cysts can be maintained for life and are found in many different tissues, including the brain. A healthy immune system can, for the most part, protect against T. gondii and localize it within tissue cysts (126). However, in immunocompromised individuals, such as those infected with HIV-1, T. gondii can emerge from tissue cysts and cause a severe disease called toxoplasmosis. During this disease, bradyzoites convert to fast-replicating tachyzoites, and when this occurs in the CNS, the end result is usually toxoplasmic encephalitis. Because T. gondii is sensitive to IFN-γ (126), microglia can cooperate with innate and adaptive immune cells in a healthy immunocompetent brain to maintain the parasite in relatively benign cysts. However, if this immune pressure is compromised, then toxoplasmic encephalitis is a potentially fatal consequence of this normally benign infection.
During toxoplasmic encephalitis, neurons, oligodendrocytes, and microglia are infected with fast-replicating tachyzoites (127, 128), and microglia may even participate in spreading the parasite throughout the brain (129), although this remains to be proven in vivo. The infection is controlled primarily through IFN-γ released by T cells as well as innate immune cells such as microglia, macrophages, and natural killer cells (130, 131). In addition, a recent study demonstrated that T. gondii cysts can be purged in a perforin-dependent manner following adoptive transfer of CD8+ T cells extracted from parasite-immune mice (132). Microglia contribute to the response against T. gondii not only by releasing IFN-γ, but also by producing T cell–recruiting chemoattractants such as CXCL9, CXCL10, and CCL5 (133, 134). In fact, Harris et al. (135) reported in an elegant two-photon imaging study that CXCL10 actually influences the search strategy of CD8+ T cells in the T. gondii–infected brain. The search strategy, commonly referred to as a Lévy walk (136), is employed to more rapidly find target cells and is characterized by periods of persistent movement interspersed with local searching. Microglia likely promote this optimal search strategy through CXCL10 release and thereby enable CD8+ T cells to more quickly find sites of T. gondii infection.
Another interesting aspect of chronic T. gondii infection is that neurodegeneration is not observed despite the presence of a persistent immune response. This suggests activation of immune-dampening mechanisms. Rozenfeld et al. (137) revealed that iNOS and NO, which have the potential to be proinflammatory, are inhibited in T. gondii–infected microglia stimulated by IFN-γ. This inhibition is dependent on transforming growth factor β1 (TGF-β1) expression, as neutralization of this cytokine restored NO production. These data indicate that infected microglia dampen inflammation in an autocrine fashion. Microglia were also reported to produce the immunosuppressive cytokine IL-10 following T. gondii infection (138). Neutralization of IL-10 increased the T cell response and decreased parasite burden during chronic infection, which indicates that this anti-inflammatory cytokine produced by microglia serves as another immune dampener (139). In addition to anti-inflammatory cytokine release, T. gondii also appears to suppress the immune system by decreasing MHC-II expression on microglia (140). Infection of rat microglia in vitro resulted in decreased expression of the class II transactivator (CTIIA) and MHC-II. Collectively, these data indicate that during chronic infection T. gondii establishes a balance in the CNS by converting to slow-growing bradyzoites that form cysts, suppressing antigen-presenting machinery, and inducing production of anti-inflammatory cytokines such as IL-10 and TGF-β. So long as this balance is maintained, the CNS is relatively tolerant of this parasite.
P. falciparum, the causative agent of cerebral malaria (CM), is another highly relevant parasite that causes CNS disease in humans. This potentially fatal disease is induced when parasitized erythrocytes adhere to the vascular endothelium of CNS microvessels. Sequestration of parasitized erythrocytes in brain vasculature is considered a hallmark of CM(141), which can give riseto severe neurological complications, including loss of consciousness, delirium, seizures, coma, and death. In human CM, microglia cluster around blood vessels in the brain parenchyma (142). Similarly, in mice infected with Plasmodium berghei ANKA (the murine model of CM), microglia showed signs of activation two to three days prior to the onset of symptoms and were observed redistributing toward retinal vessels (143). Early in the disease, microglia become globally activated and toward later stages of CM can be observed accumulating at lesion sites in dense aggregates with astrocytes in what are referred to as Durck’s granulomas (144, 145).
Adhesion of parasitized erythrocytes to CNS vasculature is mediated in part by the binding of P. falciparum erythrocyte membrane protein 1 (PfEMP1) to the endothelial protein C receptor (EPCR) (146)—a receptor involved in anticoagulation. Adhesion mediated by EPCR and other proteins such as CD36, ICAM-1, and VCAM-1 (147) likely promotes endothelial cell activation, which can elicit a compounding reaction characterized by further parasitized erythrocyte adherence and ultimately a hypoxic state once vessels become occluded. Microglia and perivascular macrophages adjacent to affected blood vessels respond by releasing proinflammatory factors that can further magnify the pathology (145), eventually resulting in vascular breakdown. Microglia produce a broad spectrum of inflammatory mediators during CM that include iNOS, cyclooxygenase-1 and −2, myeloid-related proteins (MRP8, MRP14), and TGF-β (144, 145, 148). MRP8 and MRP14 are endogenous activators of TLR4 that induce production of proinflammatory cytokines such as TNF-α and IL-1β (149), which can exacerbate the pathogenesis of CM. Microglia also synthesize angiogenic proteins such as MMP-1 and thrombospondin-1 (TSP-1) during CM (150). MMP-1 is an interstitial collagenase that breaks down extracellular matrix proteins, and TSP-1 inhibits neovascularization. In general, microglia are potent responders during CM owing to the heavy involvement of cerebral vasculature. Initially, their proinflammatory cytokine release seems focused toward clearance of the adherent parasite (a response that likely enhances vascular pathology); however, in individuals that survive CM, the CNS must repair its damaged tissue/vasculature, and it is predicted that microglia play a positive role in this process. Further studies are required to determine the relative degree to which other myeloid cell subsets such as meningeal and perivascular macrophages contribute to CM pathogenesis and recovery, as few studies differentiate between these distinct lineages during the course of disease.
Bacterial Infection
Bacteria can gain access to the CNS from adjacent focal infections during conditions such as otitis, sinusitis, and mastoiditis or from any other septic lesions that are in direct contact with the blood supply (e.g., bacterial endocarditis). Because of their ubiquitous distribution throughout the parenchyma, microglia mount a response against almost every CNS infection. In response to bacteria, microglia can proliferate (151) and carry out a variety of different functions such as pathogen detection, promotion of inflammation, phagocytosis, and neuroprotection. Bacteria such as Staphylococcus aureus can cause brain abscesses, whereas bacteria such as Streptococcus pneumoniae, Escherichia coli, Haemophilus influenzae, and Neisseria meningitidis are more commonly associated with meningitis—a disease linked to complications that include fever, fatigue, neck stiffness, seizures, coma, and sometimes death. In addition, individuals that survive bacterial abscesses or meningitis often have long-term neurological sequelae resulting from the CNS damage caused by the infection and resultant inflammatory response.
To detect bacteria, microglia constitutively express pattern-recognition receptors such as TLR2, TLR4, and TLR9, which detect bacterial lipopeptides, lipopolysaccharides, and bacterial CpG DNA, respectively (152). Bacterial components can trigger microglial responses (153–155), and stimulation of TLRs alone can induce CNS damage and neurological complications associated with bacterial meningitis (156). For example, a recent study demonstrated that intrathecal injection of a TLR2 agonist caused meningeal inflammation, increased intracranial pressure, and neuronal apoptosis—responses that were mitigated in TLR2 knockout mice (156). This study concluded that the neurotoxic effects of TLR2 agonism were mediated in part by stimulation of microglia. In addition to causing neuropathology, TLR1, TLR2, TLR4, and TLR9 agonism can enhance microglial phagocytosis and intracellular bacterial killing (157). Thus, TLR stimulation can have both positive and negative effects during bacterial infection of the CNS.
Microglia are equipped with other intracellular pattern-recognition receptors, such as nucleotide-binding oligomerization domain-2 (NOD2), which recognizes bacterial peptidoglycans. Liu et al. (158) showed that NOD2 enables microglia to generate a maximal response against intact S. pneumoniae characterized by IL-6 and TNF-α production. Upon activation, microglia secrete many proinflammatory cytokines (e.g., TNF-α, IL-6, IL-1β) (158–160) and chemoattractants that promote the recruitment of myelomonocytic cells (neurotrophils and monocytes) into the CNS (161–163). Microglia can produce iNOS as well as reactive nitrogen and oxygen species following bacterial infection (164). These antimicrobial mediators facilitate destruction of bacteria but also inhibit important CNS functions such as neurogenesis in the dentate gyrus, which can lead to cognitive impairment (164). Studies in vitro have shown that heat-inactivated group B Streptococcus can trigger TLR2-dependent NO release that causes neuronal cell death, indicating that microglial responses to bacteria are potentially neurotoxic (165).
In addition to cytokines, NO, and free radicals, microglia have many other defenses against bacteria. For example, expression of C-type lectin receptors such as CD209 (166) and complement receptors (167) facilitates bacterial uptake and destruction. Another relevant pathway was elucidated by Chauhan et al. (168), who demonstrated that microglia express the neurokinin-1 receptor (NK-1R). NK-1R binds to substance P, which is a neuropeptide that serves as a neurotransmitter as well as a potent inducer of neurogenic inflammation. Substance P can augment IL-6 and TNF-α production in N. meningitidis–infected microglia—an effect that is eliminated by NK-1R deficiency (168). In response to IL-1β and TNF-α stimulation, microglia also produce antimicrobial peptides such as cathelicidin LL-37, which is found in the cerebrospinal fluid of patients with bacterial meningitis (169). Cathelicidins are small, naturally occurring cationic antimicrobial peptides that can break down bacterial lipoprotein membranes following phagocytosis (170).
Because it is important to protect the inflamed CNS following bacterial infection, microglia are equipped with neuroprotective molecules. Several studies showed that microglia can produce activin A following bacterial infection (171, 172). Activin A is a member of the TGF-β superfamily and can modulate neuronal survival and glial cell differentiation. A recent study established that activin A released from M2 macrophages promotes oligodendrocyte differentiation during the repair process of remyelination (173). Wilms et al. (174) revealed that, following bacterial infection, microglia not only produce activin A, but also respond to it via expression of activin A receptors, suggesting the potential for autocrine feedback. Activin A induced microglial proliferation while reducing expression of NO, IL-1β, IL-6, and TNF-α (174). Thus, activin A may have both anti-inflammatory and neuroprotective properties.
Viral Infection
Among all pathogens, viruses most frequently enter the CNS, and the outcomes of these infections range from asymptomatic to fatal (175). By overcoming species barriers and adapting to new hosts, an increasing number of emerging neurotropic viruses such as West Nile virus, Chikungunya virus, lymphocytic choriomeningitis virus (LCMV), Japanese encephalitis virus (JEV), enterovirus 71, Toscana virus, Hendra virus, and Nipah virus pose a serious threat to humans and other mammalian hosts. Many neurotropic viruses induce CNS inflammatory disorders (e.g., meningitis, meningoencephalitis, encephalitis), whereas other viruses such as HIV-1, John Cunningham virus, and herpes simplex virus 1 persist (175). Similar to other types of infection, microglia are positioned as a frontline immunological defense in the CNS and must quickly sense viruses through pattern-recognition receptors to initiate a rapid inflammatory response. The outcome of the viral infection depends critically on the speed and magnitude of the inflammatory response that is set in motion by CNS sentinels such as microglia (2). In this section, we provide a few examples of how microglia mount their defense against viral invaders and set the stage for the ensuing adaptive immune response.
During an acute viral infection, microglia can produce a vast array of proinflammatory cytokines (e.g., IFN-β, IFN-γ, TNF-α, IL-1β, IL-6, IL-12), chemoattractants that direct immune traffic in the CNS, and free radicals such as NO. In fact, their response to viruses shares some similarities with the aforementioned inflammatory reactions against parasites and bacteria. Because viruses are intracellular pathogens, microglia must upregulate antigen-presenting machinery following infection and coordinate the activity of antiviral T cells. Studies have shown that costimulatory molecules (CD40, CD86) and MHC-I/II are found on the surface of microglia following viral infection (176–178). Moreover, under certain conditions, such as exposure to granulocyte-macrophage colony-stimulating factor, microglia upregulate CD11c and convert to antigen-presenting cells (APCs) that possess some DC-like functions (179). Studies in different murine viral models have concluded that activated microglia are indeed capable of presenting peptides in MHC-I and MHC-II complexes to CD8+ and CD4+ T cells, respectively (176, 178). However, the consistent conclusion is that microglia, even when activated, are less potent APCs than are peripherally derived DCs that inhabit the naive CNS and can also be recruited into the CNS following viral infection (177, 178, 180). This could be because microglia are incapable of becoming bona fide APCs (which would make sense from the perspective of minimizing inflammation in the brain parenchyma) or, alternatively, because the antigen-presenting capacity of microglia is usually evaluated ex vivo. Microglia are highly arborized cells, and removal from the CNS may impede their ability to properly present antigen because of morphological changes (e.g., the loss of processes) that occur during the tissue extraction procedure. Thus, it is important that future studies evaluate the antigen-presenting capacity of microglia in vivo. This is now possible using real-time imaging techniques such as intravital two-photon laser scanning microscopy (TPM) (2, 175, 181, 182).
A critical element of most CNS antiviral responses is the production of type I interferons (IFN-I), and microglia have the capacity to produce and respond to IFN-I following CNS viral infection (182, 183). IFN-I bind to the IFN-α/β receptor (IFNAR) and, upon doing so, can induce an antiviral state inside responding cells, including microglia (184). The purposes of this antiviral state are to impede the growth of the invading virus and to provide time for a curative adaptive immune response to develop. In the absence of IFN-I signaling, nearly every neurotropic virus tested has a replicative advantage in the CNS (184). Thus, it is incredibly important to understand the mechanics of IFN-I generation and signaling in the nervous system.
In the LCMV murine model system (185), IRF-7 and IRF-9 are heavily upregulated in microglia (among other cells) following infection, indicating a response to locally produced IFN-I (186). LCMV can induce fatal immune-mediated meningitis in mice and humans that is critically dependent on IFN-I signaling. IFNAR−/− mice infected intracerebrally with LCMV do not develop fatal meningitis but instead become asymptomatic viral carriers (187). To identify the innate immune programs that become operational in the LCMV-infected brain, we (182) conducted genomic analyses of a pure innate response to the virus (i.e., in mice that were unable to generate antiviral T cells). Unexpectedly, our studies revealed that the entire innate immune program in the LCMV-infected brain (585 differentially regulated genes) was dependent on IFN-I signaling (182). In the absence of IFN-I signaling, microglia remained quiescent despite harboring more viral antigen, and total brain gene expression was comparable to that observed in uninfected mice. These data have unveiled an Achilles’ heel in the CNS antiviral defense system; the innate antiviral response to this noncytopathic arenavirus is completely dependent on IFN-I signaling. It remains to be determined whether the CNS relies solely on IFN-I signaling to mount its response against other neurotropic viruses. We predict that cytopathic viruses stimulate non-IFN-I-dependent innate immunity through release of danger signals caused by direct cellular injury. Nevertheless, our results could explain why so many neurotropic viruses such as LCMV have acquired strategies to subvert the IFN-I pathway (188, 189).
Microglia are not only inflammatory responders to neutropic viruses; they are also directly infected by them. Many viruses such as mouse hepatitis virus (MHV), Theiler’s virus, LCMV, JEV, HIV-1, and simian immunodeficiency virus (SIV) can infect microglia, and for some viruses such as HIV-1, microglia can serve as a viral reservoir during states of persistence (190). During HIV-1 encephalopathy, microglia/macrophages become a primary target of HIV-1 and often transform into multinucleated giant cells (191), which are observed when microglia/macrophages fuse together. The HIV-1 transactivator of transcription protein induces microglial migration via release of the CCL2 and may therefore contribute to the spread of virus throughout the CNS (192).
Infection of microglia can contribute to pathological reactions in the virally infected CNS. For example, a recent study demonstrated that JEV infection of microglia stimulates TNF-α-induced glutamate synthesis and inhibition of glutamate uptake activity, which could explain the excitotoxic death of adjacent neurons (193). Microglia were also implicated in CNS demyelination following canine distemper virus and MHV infection (194, 195), although it is difficult to establish cause-and-effect relationships in these complex inflammatory reactions. Following murine leukemia virus (MuLV) infection, tissue plasminogen activator produced by microglia is thought to contribute to neurodegeneration (196). Interestingly, depletion of CNS myeloid cells with clodronate significantly reduced MuLV-induced neuropathology, suggesting a direct role for microglia in the process (196). Because clodronate depletes other myeloid cells such as perivascular and meningeal macrophages, further studies are required to prove that microglia alone are responsible for the observed pathology. Studies have also suggested that microglia are involved in disrupting dopaminergic neurotransmission following SIV infection (197), which could give rise to neurological dysfunction. But again, additional studies are required to prove this concept definitively. In general, the presence of multiple myeloid subsets in the CNS (e.g., microglia, perivascular macrophages, meningeal macrophages, choroid plexus macrophages) and the recruitment of monocyte-derived macrophages make it difficult to assign a specific function to a single subset following CNS viral infection. Furthermore, microglia and other myeloid cells are highly reactionary and thus are expected to become activated and to associate with CNS pathology. Distinguishing a neuroprotective reaction from a pathophysiologic contribution to a disease process is a major challenge in the field of microglia research.
MICROGLIAL DYNAMICS
Real-time imaging studies conducted over the past two decades have revealed that microglia are highly dynamic cells even in the naive CNS (2, 198, 199) (Figures 2 and 3; Supplemental Videos a–e; follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org). Given the energy expenditures required to maintain this state of perpetual motion, the scanning properties of microglia must be of great importance for CNS homeostasis and for mounting rapid responses against various perturbations (e.g., injury, infection, degeneration). Time-lapse video microscopy studies conducted in primary cerebral rat cultures were the first to reveal that ramified microglia are dynamic cells that continuously extend and retract their processes, suggestive of an environment-scanning behavior (200, 201). These studies also revealed that microglia possess high basal pinocytic activity. Based on these observations, microglia were hypothesized to play a housekeeping role in the normal brain by participating in fluid cleansing. Additional analysis of microglial dynamics was conducted by Brockhaus et al. (202), who revealed in acute cortical brain slices that fluorescently labeled microglia move and extend their processes toward the cut surface of the slice, which represents an injury response. Microglia were also observed phagocytosing dead cells in the injured region of brain tissue. These findings were confirmed and extended upon by Dailey and colleagues (203, 204) using hippocampal slice cultures. These studies demonstrated that in response to injured tissue (i.e., the cut surface of the brain slice) microglia transform from a ramified to an amoeboid morphology by first retracting their processes and then extending dynamic protrusions, followed by cellular locomotion (203, 204). This is a classic microglial sterile injury response. Microglia routinely migrate toward sites of sterile injury in the CNS, where they participate in numerous functions, including the cleanup of cellular debris. For example, Heppner et al. (205) used brain slice cultures to show that amoeboid microglia migrate toward and cluster around regions of neuronal degeneration induced by excitotoxic injury. Thus, microglia are major participants in reactions to CNS injury.
Figure 2.
Microglia activation following CNS injury and infection. Illustration of (a) focal and (b) diffuse microglia activation resulting from sterile brain injury and viral infection, respectively. Microglia in the naive CNS are highly ramified and continuously scan the parenchyma as part of their homeostatic program (see Figure 3; Supplemental Videos a1 and a2). (a) Upon focal brain injury that induces necrotic cell death, damaged tissue and surrounding astrocytes release extracellular ATP, which triggers activation of specific purinergic receptors expressed by microglia. For example, microglial detection of ATP via P2Y12R and P2X4R (two purinergic receptors) induces the extension of processes toward the injury epicenter and the concurrent retraction of all other processes (see Figure 3; Supplemental Video c). The extent of tissue injury likely dictates how quickly microglia convert to a phagocyte and participate in lesion cleanup. For example, detection of UDP via P2Y6R causes microglia to invest their cellular material into a single phagocytic process, which is followed by retraction of the soma into this process (see Figure 3; Supplemental Video e). The resultant phagocyte then participates in the cleanup of cellular debris. (b) Following CNS infection with a noncytopathic virus (e.g., LCMV), microglia processes become shorter and less branched but maintain a ramified structure (see Figure 3; Supplemental Video b). Microglia in this morphological state can release proinflammatory cytokines and antivirals, engage in antigen presentation, and facilitate the arrival of peripheral innate/adaptive immune cells.
Figure 3.
Morphological transformations of microglia in vivo. Representative 50 µm maximum projections of microglia (green) in the xy plane were captured through the surgically thinned skulls of CX3CR1gfp/+ mice by two-photon laser scanning microscopy. The processes of individual microglia were labeled in different colors using the filament tracer feature in Imaris. (a) Naive microglia possess multiple, highly ramified processes extending in all directions (see Supplemental Videos a1 and a2). (b) Upon diffuse activation induced by CNS viral infection with LCMV, microglial processes become shorter and less complex than naive microglia but retain a ramified structure (see Supplemental Video b). (c) Upon focal activation induced by a meningeal compression injury, microglia directionally extend multiple processes toward the focal site of injury, concurrently retracting processes in all other directions (see Supplemental Video c). (d) In response to damage at or above the glial limitans, astrocytes recruit microglial processes that line the borders between individual cells to form a continuous, honeycomb-like network composed of processes from numerous individual microglia (see Supplemental Video d). (e) In response to necrotic cell death, microglia become phagocytic and sometimes motile by first extending a single large circular extension toward the injured cell while retracting all other processes. The microglia soma is eventually pulled into the phagocytic extension along the thin connecting process (see Supplemental Video e). To view these videos, access this article on the Annual Reviews website at http://www.annualreviews.org and click on the above panels. These videos are also provided in the Supplemental Material
Because the cutting required to generate ex vivo brain slices elicits an injury response, it was important to develop new approaches to study microglia in their natural environment. The advent of TPM (181), a real-time deep-tissue imaging technique, made this possible by allowing investigators to peer into the brain and spinal cord of living animals (2, 175). Two landmark studies revealed that microglia in the naive brain [illuminated using CX3CR1-GFP+/− reporter mice (206)] are stationary, but their processes are highly dynamic, extending and retracting at a rate of ~1.5 µm/min (198, 199) (Figure 3; Supplemental Videos a1, a2). In addition, after focal laser injury, microglia projected their processes toward the damaged brain tissue using a mechanism dependent on detection of extracellular ATP by purinergic receptors (198, 199) (Figures 2 and 3; Supplemental Video c). Necrotic tissue damage triggers sterile immune reactions in part through the release of ATP and other nucleotides. Microglia express several biologically relevant purinergic receptors (e.g., P2Y6, P2Y12, and P2X4) that enable them to extend processes toward focal injury sites, migrate, and participate in phagocytosis (199, 207–211) (Figures 2 and 3; Supplemental Videos c–e). Because microglial process extension usually precedes cellular movement or phagocytosis, inhibition of any relevant purinergic receptors can negatively impact the overall dynamic response of microglia to focal injury (Figure 2).
Using a new model of mild traumatic brain injury (mTBI) referred to as meningeal compression, we (212) have observed unique morphological transformations of microglia never before seen in focal laser injury models (Figure 3; Supplemental Videos c–e). Meningeal compression injury is induced by physically depressing a thinned-skull (213) TPM viewing window downward. This injury causes physical damage in the meninges that ultimately results in breakdown of the glial limitans and cell death in the brain parenchyma, thus sharing similarities with some forms of mTBI in humans. By using TPM to study the microglial response to this injury from its inception, we (212) observed the prototypic extension of microglial processes toward sites of cell death, consistent with the observations made in focal laser injury models (198, 199) (Figure 3; Supplemental Video c). Moreover, careful examination of the glial limitans, which is often damaged during mTBI, revealed two new microglial morphologies that we refer to as the honeycomb and jellyfish reactions (Figure 3; Supplemental Videos d and e). In response to compression injury, microglia can extend honeycomb-like processes that surround individual astrocytes in the glial limitans and help fortify this important barrier structure between the meninges and brain parenchyma (Figure 3; Supplemental Video d). Upon death of astrocytes and other cells, microglia can retract all processes and reinvest this material into a single process resembling a jellyfish that participates in the phagocytic uptake of cellular debris (Figure 3; Supplemental Video e). These phagocytic microglia can remain stationary or become mobile to facilitate the cleanup process.
In contrast with sterile injury responses, CNS infection induces a completely different morphological transformation of microglia. Using TPM, we revealed that infection of the CNS with a noncytopathic virus (LCMV) causes microglia to become diffusely (rather than focally) activated (182). This results in global microglial process retraction and reduction of branch complexity, although the activated microglia do retain some shorter processes (Figures 2 and 3; Supplemental Video b). In addition, the mechanism underlying this morphological transformation was determined to be IFN-I-signaling dependent. Unexpectedly, in the absence of IFN-I signaling, microglia remained in their naive ramified state and were completely unresponsive to LCMV infection despite a 1 log increase in CNS viral titers (182). These data indicate that IFN-I rather than purinergic receptor signaling is responsible for microglial dynamics following a noncytopathic viral infection. It will be interesting in future studies to determine how microglia respond to cytopathic viruses, which have the potential to expose them simultaneously to IFN-I and cellular damage stimuli.
Dynamic imaging studies have shown that microglia can morph in several different configurations based on the environmental cues they receive (Figures 2 and 3; Supplemental Videos b–e); however, microglial dynamics are sometimes more subtle. For example, there is now evidence that microglia participate in synaptic homeostasis during development as well as in the adult CNS (66, 67, 214). During early postnatal development, microglia are in an amoeboid morphology and participate in the cleanup of unwanted synaptic circuitry (67, 215). Failure to perform this essential function can result in neurodevelopmental disorders. Between weeks 2 and 4 of postnatal development, microglia become increasingly more ramified and ultimately take on their adult morphology (215) (Figure 3; Supplemental Videos a1 and a2). In the adult brain, TPM studies have revealed that surveillant microglia make direct contact with neuronal synapses (once per hour for an average duration of 5 min), and this contact is dependent on neuronal activity (66). Interestingly, following ischemic injury, microglial contact increases in duration and is sometimes associated with the disappearance of the presynaptic bouton, suggesting that microglia participate in the cleanup of disabled or dysfunctional synaptic structures (66). Environmental stimuli can also influence microglial dynamics in relation to neuronal synapses. Tremblay et al. (70) revealed that exposing juvenile mice to light deprivation for 8–10 days increased microglial contact with dendritic spines and axon terminals. This finding was associated with a thickening of microglia processes and the appearance of phagocytic specializations. These dynamic and functional changes were reversed following a two-day reexposure to a regular light-dark cycle. Collectively, these data indicate that microglia directly monitor and respond to activity-induced changes in neuronal synapses—a function that appears essential for maintaining a healthy CNS.
Given their dynamic contribution to maintaining a healthy CNS, microglia were expected to participate dynamically in degenerative diseases. TP Manalyses in amurine model of AD (APP/PS1 mice) revealed that amyloid deposition precedes the activation and migration of microglia (216). Plaque formation was determined to be a rapidly evolving process that requires only 24h. Microglia were actively recruited to new plaques within 1–2 days but did not contribute to their removal. Thus, it was proposed that microglia may prevent the formation of new plaques and/or participate in the containment of existing plaques. A role for microglia in plaque containment is further supported by another TPM study (using the same AD model), which confirmed that microglia respond to new plaque formation (217). This study further established that plaque size fluctuates over time and that microglia associated with plaques phagocytose Aβ peptide into lysosomes and appear to restrict further plaque growth. Dynamic imaging studies have also demonstrated that fractalkine signaling in microglia may contribute to neuronal cell death in the 3xTg model of AD (109). TPM imaging revealed enhanced neuronal cell death in CX3CR1-GFP knockout mice. Real-time imaging data in murine models of AD have shown that microglia respond to plaques and may even thwart their growth via Aβ uptake. However, given the chronicity of this disease, it seems likely that the phagocytic capacity of microglia would eventually become fatigued (saturated) over time, which may result in conversion to a proinflammatory response that exacerbates AD. Thus, microglia function must always be considered in relation to disease duration and severity.
FUTURE PERSPECTIVES
The breadth of topics covered in this review encapsulates the diversity of microglia functions and showcases their ability to meet the unique demands associated with inhabiting and surveying tissues as complex as the CNS. Microglia are highly dynamic and pleomorphic, tailoring their function and morphology to optimize performance during states of health and disease. On the basis of their development, we now know that microglia are of a lineage that is distinct from bone marrow–derived monocytes/macrophages. Nevertheless, it is possible to engraft the adult CNS under certain conditions with hematopoietically derived myeloid cells and correct neuropsychiatric conditions such as Rett syndrome and obsessive compulsive disorder. These findings demonstrate that neurological disorders can be rooted in accessory cells such as microglia instead of neurons, which should change the way we think about susceptibility genes in a variety of different CNS diseases. In addition, successful engraftment of the CNS with peripherally derived myeloid cells that acquire microglia-like properties and ameliorate a complex disease phenotype opens the possibility of restorative therapies in many neurological conditions linked to aberrant microglial activity. Although it is not pragmatic to consider “conditioning” the CNS for engraftment with irradiation, other therapeutic angles could include modulation of the factors that induce microglial turnover in the adult CNS. Additional insights into microglial progenitors and turnover are required to identify alternative modes of conditioning the CNS for engraftment. Moreover, during neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s, it is important to gain additional insights into the factors that sustain the homeostatic, noninflammatory functions of microglia such as uptake of aberrant proteins and cellular debris. Numerous studies suggest that microglia can initially thwart the progression of neurodegenerative diseases but that this neuroprotective function wanes over time. Conversion of microglia to proinflammatory cytokine producers with minimal phagocytic activity has the potential to exacerbate neurodegenerative pathology and justifies use of anti-inflammatory drugs that quench their activity in the later stages of disease. When microglia become fatigued or dysfunctional, it is also worth considering the development of strategies that more rapidly promote their turnover or replacement by a less neurotoxic precursor.
During the course of acute brain injuries and infections, microglia are more likely to play a beneficial role, and thus their activities should be promoted. The purinergic-receptor-dependent sterile injury reaction that enables microglia to directionally extend processes, form honeycomb structures that fortify the glial limitans, and convert to phagocytes is highly beneficial and essential for preservation of uninjured brain tissue and initiation of the repair process. Phagocytic microglia also play an important role in the cleanup of cellular debris. Thus, future studies should focus on how best to agonize these important microglia functions. An analogous line of reasoning could be used to justify enhancement of microglia function during the course of acute CNS infections. Diffuse activation of microglia following CNS infection sets microglial programs into motion that prepare the brain to mount an antimicrobial defense and coordinate the arrival and function of peripherally derived immune cells. This early defense often holds pathogens in check and provides the CNS much needed time as it awaits the arrival of curative adaptive immune cells. It therefore seems reasonable to develop strategies that promote these microglial activities, and based on our data, modulation of IFN-I signaling is an excellent starting point. However, this same strategy might not be sensible when considering the microglial reaction to chronic infections. Similar to neurodegenerative diseases, chronic activation of microglia resulting from a persistent infection may ultimately give rise to aberrant, neurotoxic activity that needs to be quenched. Thus, the functional state of microglia must always be considered when developing immunomodulatory therapies for CNS diseases.
Beginning with microglial drawings from early pioneers, the study of microglial biology has developed into a remarkably vast and vibrant field over the past century. Each year seminal studies unveil exciting new facets of microglia development, homeostasis, and function that expand our vision of these cells and offer novel insights into how we may best harness or modulate them during the course of CNS diseases. Importantly, we now have the technologies to watch microglia operate in the living CNS, which should vertically enhance our understanding of these pleomorphic cells in the decades to come.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) intramural program. D.N. is presently supported by a NIH Intramural Competitive Fellowship. We thank Alan Hoofring and Ethan Tyler in the NIH Medical Arts Design Section for their help with the illustrations.
Footnotes
This is a work of the U.S. Government and is not subject to copyright protection in the United States.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Nayak D, Zinselmeyer BH, Corps K, McGavern DB. In vivo dynamics of innate immune sentinels in the CNS. IntraVital. 2012;1:95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013;61:121–127. doi: 10.1002/glia.22408. [DOI] [PubMed] [Google Scholar]
- 5.Del Rio-Hortega P. Microglia. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System. New York: P.B. Hoeber; 1937. pp. 481–534. [Google Scholar]
- 6.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 7.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 8.Goldmann T, Prinz M. Role of microglia in CNS autoimmunity. Clin. Dev. Immunol. 2013;2013:208093. doi: 10.1155/2013/208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eglitis MA, Mezey É. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc. Natl. Acad. Sci. USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 11.Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- 12.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 13.Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc. Natl. Acad. Sci. USA. 2012;109:18150–18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diserbo M, Agin A, Lamproglou I, Mauris J, Staali F, et al. Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study. Can. J. Physiol. Pharmacol. 2002;80:670–678. doi: 10.1139/y02-070. [DOI] [PubMed] [Google Scholar]
- 15.Li YQ, Chen P, Jain V, Reilly RM, Wong CS. Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat. Res. 2004;161:143–152. doi: 10.1667/rr3117. [DOI] [PubMed] [Google Scholar]
- 16.Wirenfeldt M, Dissing-Olesen L, Babcock AA, Nielsen M, Meldgaard M, et al. Population control of resident and immigrant microglia by mitosis and apoptosis. Am. J. Pathol. 2007;171:617–631. doi: 10.2353/ajpath.2007.061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 18.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 19.Cuadros MA, Martin C, Coltey P, Almendros A, Navascues J. First appearance, distribution, and origin of macrophages in the early development of the avian central nervous system. J. Comp. Neurol. 1993;330:113–129. doi: 10.1002/cne.903300110. [DOI] [PubMed] [Google Scholar]
- 20.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 21.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 22.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, et al. Microglia emerge from ery-thromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y, Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc. Natl. Acad. Sci. USA. 1991;88:10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang G, Zhang P, Hirai H, Elf S, Yan X, et al. PU.1 is a major downstream target of AML1 (RUNX1) in adult mouse hematopoiesis. Nat. Genet. 2008;40:51–60. doi: 10.1038/ng.2007.7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang DE, Hetherington CJ, Meyers S, Rhoades KL, Larson CJ, et al. CCAAT enhancer-binding protein (C/EBP) and AML1 (CBF alpha2) synergistically activate the macrophage colony-stimulating factor receptor promoter. Mol. Cell. Biol. 1996;16:1231–1240. doi: 10.1128/mcb.16.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 27.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 28.Zusso M, Methot L, Lo R, Greenhalgh AD, David S, Stifani S. Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1. J. Neurosci. 2012;32:11285–11298. doi: 10.1523/JNEUROSCI.6182-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton MR, Gibbons H, MacGibbon GA, Sirimanne E, Saura J, et al. PU.1 expression in microglia. J. Neuroimmunol. 2000;104:109–115. doi: 10.1016/s0165-5728(99)00262-3. [DOI] [PubMed] [Google Scholar]
- 30.Smith AM, Gibbons HM, Oldfield RL, Bergin PM, Mee EW, et al. The transcription factor PU.1 is critical for viability and function of human brain microglia. Glia. 2013;61:929–942. doi: 10.1002/glia.22486. [DOI] [PubMed] [Google Scholar]
- 31.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 32.Olson MC, Scott EW, Hack AA, Su GH, Tenen DG, et al. PU.1 is not essential for early myeloid gene expression but is required for terminal myeloid differentiation. Immunity. 1995;3:703–714. doi: 10.1016/1074-7613(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Ray-Gallet D, Zhang P, Hetherington CJ, Gonzalez DA, et al. PU.1 (Spi-1) autoregulates its expression in myeloid cells. Oncogene. 1995;11:1549–1560. [PubMed] [Google Scholar]
- 34.Mossadegh-Keller N, Sarrazin S, Kandalla PK, Espinosa L, Stanley ER, et al. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr, Ahmed-Ansari A, Sell KW, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felix R, Cecchini MG, Hofstetter W, Elford PR, Stutzer A, Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J. Bone Miner. Res. 1990;5:781–789. doi: 10.1002/jbmr.5650050716. [DOI] [PubMed] [Google Scholar]
- 37.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 38.Lin H, Lee E, Hestir K, Leo C, Huang M, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 39.Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, et al. Immunodeficiency and chronic mye-logenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 42.Scheller M, Foerster J, Heyworth CM, Waring JF, Lohler J, et al. Altered development and cytokine responses of myeloid progenitors in the absence of transcription factor, interferon consensus sequence binding protein. Blood. 1999;94:3764–3771. [PubMed] [Google Scholar]
- 43.Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. doi: 10.1016/s1074-7613(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 44.Minten C, Terry R, Deffrasnes C, King NJ, Campbell IL. IFN regulatory factor 8 is a key constitutive determinant of the morphological and molecular properties of microglia in the CNS. PLoS ONE. 2012;7:e49851. doi: 10.1371/journal.pone.0049851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabriele L, Phung J, Fukumoto J, Segal D, Wang IM, et al. Regulation of apoptosis in myeloid cells by interferon consensus sequence-binding protein. J. Exp. Med. 1999;190:411–421. doi: 10.1084/jem.190.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009;10:116–125. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp. Cell Res. 2008;314:2618–2633. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat. Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 52.Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ness JK, Wood TL. Insulin-like growth factor I, but not neurotrophin-3, sustains Akt activation and provides long-term protection of immature oligodendrocytes from glutamate-mediated apoptosis. Mol. Cell Neurosci. 2002;20:476–488. doi: 10.1006/mcne.2002.1149. [DOI] [PubMed] [Google Scholar]
- 54.Trang T, Beggs S, Salter MW. Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol. 2011;7:99–108. doi: 10.1017/S1740925X12000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S, Kurihara T. Neurotrophin secretion from cultured microglia. J. Neurosci. Res. 2001;65:322–331. doi: 10.1002/jnr.1157. [DOI] [PubMed] [Google Scholar]
- 56.Araujo DM, Cotman CW. Basic FGF in astroglial, microglial, and neuronal cultures: characterization of binding sites and modulation of release by lymphokines and trophic factors. J. Neurosci. 1992;12:1668–1678. doi: 10.1523/JNEUROSCI.12-05-01668.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamagata T, Muroya K, Mukasa T, Igarashi H, Momoi M, et al. Hepatocyte growth factor specifically expressed in microglia activated Ras in the neurons, similar to the action of neurotrophic factors. Biochem. Biophys. Res. Commun. 1995;210:231–237. doi: 10.1006/bbrc.1995.1651. [DOI] [PubMed] [Google Scholar]
- 58.Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 59.Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J. Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hristova M, Cuthill D, Zbarsky V, Acosta-Saltos A, Wallace A, et al. Activation and deactivation of periventricular white matter phagocytes during postnatal mouse development. Glia. 2010;58:11–28. doi: 10.1002/glia.20896. [DOI] [PubMed] [Google Scholar]
- 63.Calderó J, Brunet N, Ciutat D, Hereu M, Esquerda JE. Development of microglia in the chick embryo spinal cord: implications in the regulation of motoneuronal survival and death. J. Neurosci. Res. 2009;87:2447–2466. doi: 10.1002/jnr.22084. [DOI] [PubMed] [Google Scholar]
- 64.Noda M, Doi Y, Liang J, Kawanokuchi J, Sonobe Y, et al. Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J. Biol. Chem. 2011;286:2308–2319. doi: 10.1074/jbc.M110.169839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li E, Noda M, Doi Y, Parajuli B, Kawanokuchi J, et al. The neuroprotective effects of milk fat globule-EGF factor 8 against oligomeric amyloid β toxicity. J. Neuroinflamm. 2012;9:148. doi: 10.1186/1742-2094-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 68.Roumier A, Bechade C, Poncer JC, Smalla KH, Tomasello E, et al. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J. Neurosci. 2004;24:11421–11428. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS ONE. 2013;8:e56293. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 72.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen SK, Tvrdik P, Peden E, Cho S, Wu S, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- 76.Deschamps J, Meijlink F. Mammalian homeobox genes in normal development and neoplasia. Crit. Rev. Oncog. 1992;3:117–173. [PubMed] [Google Scholar]
- 77.Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen SM. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- 78.Epstein M, Pillemer G, Yelin R, Yisraeli JK, Fainsod A. Patterning of the embryo along the anterior-posterior axis: the role of the caudal genes. Development. 1997;124:3805–3814. doi: 10.1242/dev.124.19.3805. [DOI] [PubMed] [Google Scholar]
- 79.van den Akker E, Reijnen M, Korving J, Brouwer A, Meijlink F, Deschamps J. Targeted in-activation of Hoxb8 affects survival of a spinal ganglion and causes aberrant limb reflexes. Mech. Dev. 1999;89:103–114. doi: 10.1016/s0925-4773(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 80.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 81.Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- 82.Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J. Biol. Chem. 2003;278:4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- 83.Luikenhuis S, Giacometti E, Beard CF, Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl. Acad. Sci. USA. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat. Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, et al. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011;13:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 88.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 89.Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, et al. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grathwohl SA, Kälin RE, Bolmont T, Prokop S, Winkelmann G, et al. Formation and maintenance of Alzheimer’s disease β-amyloid plaques in the absence of microglia. Nat. Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vom Berg J, Prokop S, Miller KR, Obst J, Kalin RE, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 2012;18:1812–1819. doi: 10.1038/nm.2965. [DOI] [PubMed] [Google Scholar]
- 93.Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, et al. Functional impairment of microglia coincides with β-amyloid deposition in mice with Alzheimer-like pathology. PLoS ONE. 2013;8:e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryu JK, Cho T, Choi HB, Wang YT, McLarnon JG. Microglial VEGF receptor response is an integral chemotactic component in Alzheimer’s disease pathology. J. Neurosci. 2009;29:3–13. doi: 10.1523/JNEUROSCI.2888-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of Toll-like receptor signalling in Aβ uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid β1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J. Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song M, Jin J, Lim JE, Kou J, Pattanayak A, et al. TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2011;8:92. doi: 10.1186/1742-2094-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michaud JP, Halle M, Lampron A, Theriault P, Prefontaine P, et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc. Natl. Acad. Sci. USA. 2013;110:1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stuart LM, Bell SA, Stewart CR, Silver JM, Richard J, et al. CD36 signals to the actin cytoskeleton and regulates microglial migration via a p130Cas complex. J. Biol. Chem. 2007;282:27392–27401. doi: 10.1074/jbc.M702887200. [DOI] [PubMed] [Google Scholar]
- 102.Fang F, Lue LF, Yan S, Xu H, Luddy JS, et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Slowik A, Merres J, Elfgen A, Jansen S, Mohr F, et al. Involvement of formyl peptide receptors in receptor for advanced glycation end products (RAGE)-and amyloid beta 1-42-induced signal transduction in glial cells. Mol. Neurodegener. 2012;7:55. doi: 10.1186/1750-1326-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 105.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 106.Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J. Neurosci. 2010;30:17091–17101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, et al. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am. J. Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, et al. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2010;13:411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thanopoulou K, Fragkouli A, Stylianopoulou F, Georgopoulos S. Scavenger receptor class B type I (SR-BI) regulates perivascular macrophages and modifies amyloid pathology in an Alzheimer mouse model. Proc. Natl. Acad. Sci. USA. 2010;107:20816–20821. doi: 10.1073/pnas.1005888107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J. Neurosci. 2011;31:11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 113.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, et al. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J. Neuroinflamm. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shan S, Hong-Min T, Yi F, Jun-Peng G, Yue F, et al. New evidences for fractalkine/CX3CL1 involved in substantia nigral microglial activation and behavioral changes in a rat model of Parkinson’s disease. Neurobiol. Aging. 2011;32:443–458. doi: 10.1016/j.neurobiolaging.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 116.Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, et al. α-Synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J. Immunol. 2010;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 117.Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, et al. LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barcia C, Ros CM, Annese V, Gómez A, Ros-Bernal F, et al. IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2012;3:e379. doi: 10.1038/cddis.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barcia C, Ros CM, Annese V, Carrillo-de Sauvage MA, Ros-Bernal F, et al. ROCK/Cdc42-mediated microglial motility and gliapse formation lead to phagocytosis of degenerating dopaminergic neurons in vivo. Sci. Rep. 2012;2:809. doi: 10.1038/srep00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harms AS, Cao S, Rowse AL, Thome AD, Li X, et al. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci. 2013;33:9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain. 2005;128:2665–2674. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- 124.Lira A, Kulczycki J, Slack R, Anisman H, Park DS. Involvement of the Fcγ receptor in a chronic N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of dopaminergic loss. J. Biol. Chem. 2011;286:28783–28793. doi: 10.1074/jbc.M111.244830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, et al. Nonsteroidal antiinflam-matory drug use and the risk for Parkinson’s disease. Ann. Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 126.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 127.Chao CC, Hu S, Gekker G, Novick WJ, Jr, Remington JS, Peterson PK. Effects of cytokines on multiplication of Toxoplasma gondii in microglial cells. J. Immunol. 1993;150:3404–3410. [PubMed] [Google Scholar]
- 128.Luder CG, Giraldo-Velasquez M, Sendtner M, Gross U. Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp. Parasitol. 1999;93:23–32. doi: 10.1006/expr.1999.4421. [DOI] [PubMed] [Google Scholar]
- 129.Dellacasa-Lindberg I, Fuks JM, Arrighi RB, Lambert H, Wallin RP, et al. Migratory activation of primary cortical microglia upon infection with Toxoplasma gondii. Infect. Immun. 2011;79:3046–3052. doi: 10.1128/IAI.01042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sher A, Oswald IP, Hieny S, Gazzinelli RT. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J. Immunol. 1993;150:3982–3989. [PubMed] [Google Scholar]
- 131.Suzuki Y, Claflin J, Wang X, Lengi A, Kikuchi T. Microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int. J. Parasitol. 2005;35:83–90. doi: 10.1016/j.ijpara.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 132.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am. J. Pathol. 2010;176:1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Strack A, Asensio VC, Campbell IL, Schluter D, Deckert M. Chemokines are differentially expressed by astrocytes, microglia and inflammatory leukocytes in Toxoplasma encephalitis and critically regulated by interferon-γ. Acta Neuropathol. 2002;103:458–468. doi: 10.1007/s00401-001-0491-7. [DOI] [PubMed] [Google Scholar]
- 134.Norose K, Kikumura A, Luster AD, Hunter CA, Harris TH. CXCL10 is required to maintain T-cell populations and to control parasite replication during chronic ocular toxoplasmosis. Investig. Ophthalmol. Vis. Sci. 2011;52:389–398. doi: 10.1167/iovs.10-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, et al. Generalized Le´vy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Viswanathan GM, Buldyrev SV, Havlin S, da Luz MG, Raposo EP, Stanley HE. Optimizing the success of random searches. Nature. 1999;401:911–914. doi: 10.1038/44831. [DOI] [PubMed] [Google Scholar]
- 137.Rozenfeld C, Martinez R, Seabra S, Sant’anna C, Goncalves JG, et al. Toxoplasma gondii prevents neuron degeneration by interferon-γ-activated microglia in a mechanism involving inhibition of inducible nitric oxide synthase and transforming growth factor-β1 production by infected microglia. Am. J. Pathol. 2005;167:1021–1031. doi: 10.1016/s0002-9440(10)61191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schlüter D, Kaefer N, Hof H, Wiestler OD, Deckert-Schlüter M. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am. J. Pathol. 1997;150:1021–1035. [PMC free article] [PubMed] [Google Scholar]
- 139.Deckert-Schlüter M, Buck C, Weiner D, Kaefer N, Rang A, et al. Interleukin-10 downregulates the intracerebral immune response in chronic Toxoplasma encephalitis. J. Neuroimmunol. 1997;76:167–176. doi: 10.1016/s0165-5728(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 140.Luder CG, Lang C, Giraldo-Velasquez M, Algner M, Gerdes J, Gross U. Toxoplasma gondii inhibits MHC class II expression in neural antigen-presenting cells by down-regulating the class II transactivator CIITA. J. Neuroimmunol. 2003;134:12–24. doi: 10.1016/s0165-5728(02)00320-x. [DOI] [PubMed] [Google Scholar]
- 141.Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, et al. The neuropathology of fatal cerebral malaria in Malawian children. Am. J. Pathol. 2011;178:2146–2158. doi: 10.1016/j.ajpath.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Janota I, Doshi B. Cerebral malaria in the United Kingdom. J. Clin. Pathol. 1979;32:769–772. doi: 10.1136/jcp.32.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Medana IM, Hunt NH, Chan-Ling T. Early activation of microglia in the pathogenesis of fatal murine cerebral malaria. Glia. 1997;19:91–103. doi: 10.1002/(sici)1098-1136(199702)19:2<91::aid-glia1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 144.Schluesener HJ, Kremsner PG, Meyermann R. Widespread expression of MRP8 and MRP14 in human cerebral malaria by microglial cells. Acta Neuropathol. 1998;96:575–580. doi: 10.1007/s004010050938. [DOI] [PubMed] [Google Scholar]
- 145.Deininger MH, Kremsner PG, Meyermann R, Schluesener H. Macrophages/microglial cells in patients with cerebral malaria. Eur. Cytokine Netw. 2002;13:173–185. [PubMed] [Google Scholar]
- 146.Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013;498:502–505. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Exp. Rev. Mol. Med. 2009;11:e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deininger MH, Kremsner PG, Meyermann R, Schluesener HJ. Focal accumulation of cyclooxygenase-1 (COX-1) and COX-2 expressing cells in cerebral malaria. J. Neuroimmunol. 2000;106:198–205. doi: 10.1016/s0165-5728(00)00187-9. [DOI] [PubMed] [Google Scholar]
- 149.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 150.Deininger MH, Winkler S, Kremsner PG, Meyermann R, Schluesener HJ. Angiogenic proteins in brains of patients who died with cerebral malaria. J. Neuroimmunol. 2003;142:101–111. doi: 10.1016/s0165-5728(03)00250-9. [DOI] [PubMed] [Google Scholar]
- 151.Lutsik BD, Stekhnovich IV, Lutsik AD. Use of lectins in histochemical examination of microglia of the human brain in bacterial meningoencephalitis. Zh. Nevropatol. Psikhiatr. Im. S S Korsakova. 1991;91:41–44. [PubMed] [Google Scholar]
- 152.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 153.Kim YS, Tauber MG. Neurotoxicity of glia activated by gram-positive bacterial products depends on nitric oxide production. Infect. Immun. 1996;64:3148–3153. doi: 10.1128/iai.64.8.3148-3153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Prinz M, Kann O, Draheim HJ, Schumann RR, Kettenmann H, et al. Microglial activation by components of gram-positive and -negative bacteria: distinct and common routes to the induction of ion channels and cytokines. J. Neuropathol. Exp. Neurol. 1999;58:1078–1089. doi: 10.1097/00005072-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 155.Kielian T. Immunopathogenesis of brain abscess. J. Neuroinflamm. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hoffmann O, Braun JS, Becker D, Halle A, Freyer D, et al. TLR2 mediates neuroinflammation and neuronal damage. J. Immunol. 2007;178:6476–6481. doi: 10.4049/jimmunol.178.10.6476. [DOI] [PubMed] [Google Scholar]
- 157.Ribes S, Ebert S, Regen T, Agarwal A, Tauber SC, et al. Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated and encapsulated Streptococcus pneumoniae by murine microglia. Infect. Immun. 2010;78:865–871. doi: 10.1128/IAI.01110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu X, Chauhan VS, Young AB, Marriott I. NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia. 2010;58:839–847. doi: 10.1002/glia.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sharief MK, Ciardi M, Thompson EJ. Blood-brain barrier damage in patients with bacterial meningitis: association with tumor necrosis factor-α but not interleukin-1β. J. Infect. Dis. 1992;166:350–358. doi: 10.1093/infdis/166.2.350. [DOI] [PubMed] [Google Scholar]
- 160.Hanamsagar R, Torres V, Kielian T. Inflammasome activation and IL-1β/IL-18 processing are influenced by distinct pathways in microglia. J. Neurochem. 2011;119:736–748. doi: 10.1111/j.1471-4159.2011.07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Diab A, Abdalla H, Li HL, Shi FD, Zhu J, et al. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1α attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect. Immun. 1999;67:2590–2601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spanaus KS, Nadal D, Pfister HW, Seebach J, Widmer U, et al. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J. Immunol. 1997;158:1956–1964. [PubMed] [Google Scholar]
- 163.Dominguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J. Immunol. 2007;179:1842–1854. doi: 10.4049/jimmunol.179.3.1842. [DOI] [PubMed] [Google Scholar]
- 164.Hoffmann O, Mahrhofer C, Rueter N, Freyer D, Bert B, et al. Pneumococcal cell wall-induced meningitis impairs adult hippocampal neurogenesis. Infect. Immun. 2007;75:4289–4297. doi: 10.1128/IAI.01679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lehnardt S, Henneke P, Lien E, Kasper DL, Volpe JJ, et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J. Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 166.Park JY, Choi HJ, Prabagar MG, Choi WS, Kim SJ, et al. The C-type lectin CD209b is expressed on microglia and it mediates the uptake of capsular polysaccharides of Streptococcus pneumoniae. Neurosci. Lett. 2009;450:246–251. doi: 10.1016/j.neulet.2008.11.070. [DOI] [PubMed] [Google Scholar]
- 167.Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, et al. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J. Immunol. 1998;160:3543–3554. [PubMed] [Google Scholar]
- 168.Chauhan VS, Sterka DG, Jr, Gray DL, Bost KL, Marriott I. Neurogenic exacerbation of microglial and astrocyte responses to Neisseria meningitidis and Borrelia burgdorferi. J. Immunol. 2008;180:8241–8249. doi: 10.4049/jimmunol.180.12.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Wilms H, et al. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J. Neuropathol. Exp. Neurol. 2008;67:1041–1054. doi: 10.1097/NEN.0b013e31818b4801. [DOI] [PubMed] [Google Scholar]
- 170.Choi KY, Mookherjee N. Multiple immune-modulatory functions of cathelicidin host defense peptides. Front. Immunol. 2012;3:149. doi: 10.3389/fimmu.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Michel U, Gerber J, O’Connor AE, Bunkowski S, Bruck W, et al. Increased activin levels in cerebrospinal fluid of rabbits with bacterial meningitis are associated with activation of microglia. J. Neurochem. 2003;86:238–245. doi: 10.1046/j.1471-4159.2003.01834.x. [DOI] [PubMed] [Google Scholar]
- 172.Ebert S, Zeretzke M, Nau R, Michel U. Microglial cells and peritoneal macrophages release activin A upon stimulation with Toll-like receptor agonists. Neurosci. Lett. 2007;413:241–244. doi: 10.1016/j.neulet.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 173.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wilms H, Schwark T, Brandenburg LO, Sievers J, Dengler R, et al. Regulation of activin A synthesis in microglial cells: pathophysiological implications for bacterial meningitis. J. Neurosci. Res. 2010;88:16–23. doi: 10.1002/jnr.22185. [DOI] [PubMed] [Google Scholar]
- 175.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mack CL, Vanderlugt-Castaneda CL, Neville KL, Miller SD. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler’s virus model of multiple sclerosis. J. Neuroimmunol. 2003;144:68–79. doi: 10.1016/j.jneuroim.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 177.Lauterbach H, Zuniga EI, Truong P, Oldstone MB, McGavern DB. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J. Exp. Med. 2006;203:1963–1975. doi: 10.1084/jem.20060039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.D’Agostino PM, Kwak C, Vecchiarelli HA, Toth JG, Miller JM, et al. Viral-induced encephalitis initiates distinct and functional CD103+ CD11b+ brain dendritic cell populations within the olfactory bulb. Proc. Natl. Acad. Sci. USA. 2012;109:6175–6180. doi: 10.1073/pnas.1203941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, et al. Developmental plasticity of CNS microglia. Proc. Natl. Acad. Sci. USA. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 182.Nayak D, Johnson KR, Heydari S, Roth TL, Zinselmeyer BH, McGavern DB. Type I interferon programs innate myeloid dynamics and gene expression in the virally infected nervous system. PLoS Pathog. 2013;9:e1003395. doi: 10.1371/journal.ppat.1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kallfass C, Ackerman A, Lienenklaus S, Weiss S, Heimrich B, Staeheli P. Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice. J. Virol. 2012;86:11223–11230. doi: 10.1128/JVI.01093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kang SS, McGavern DB. Lymphocytic choriomeningitis infection of the central nervous system. Front. Biosci. 2008;13:4529–4543. doi: 10.2741/3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ousman SS, Wang J, Campbell IL. Differential regulation of interferon regulatory factor (IRF)-7 and IRF-9 gene expression in the central nervous system during viral infection. J. Virol. 2005;79:7514–7527. doi: 10.1128/JVI.79.12.7514-7527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 188.Martinez-Sobrido L, Zuniga EI, Rosario D, Garcia-Sastre A, de la Torre JC. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2006;80:9192–9199. doi: 10.1128/JVI.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 191.Naito M, Jogasaki M, Takahashi K, Matsumi S, Hattori T, Takatsuki K. Ultrastructural behavior of human immunodeficiency virus (HIV) in multinucleated giant cells in the brain of a Japanese hemophiliac presenting AIDS encephalopathy. Ultrastruct. Pathol. 1989;13:433–441. doi: 10.3109/01913128909048493. [DOI] [PubMed] [Google Scholar]
- 192.Eugenin EA, Dyer G, Calderon TM, Berman JW. HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1)-dependent mechanism: possible role in Neuro AIDS. Glia. 2005;49:501–510. doi: 10.1002/glia.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen CJ, Ou YC, Chang CY, Pan HC, Liao SL, et al. Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-α signaling and contributes to neuronal death. Glia. 2012;60:487–501. doi: 10.1002/glia.22282. [DOI] [PubMed] [Google Scholar]
- 194.Stein VM, Czub M, Schreiner N, Moore PF, Vandevelde M, et al. Microglial cell activation in demyelinating canine distemper lesions. J. Neuroimmunol. 2004;153:122–131. doi: 10.1016/j.jneuroim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 195.Kim TS, Perlman S. Viral expression of CCL2 is sufficient to induce demyelination in RAG1−/− mice infected with a neurotropic coronavirus. J. Virol. 2005;79:7113–7120. doi: 10.1128/JVI.79.11.7113-7120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li X, Hanson C, Cmarik JL, Ruscetti S. Neurodegeneration induced by PVC-211 murine leukemia virus is associated with increased levels of vascular endothelial growth factor and macrophage inflammatory protein 1α and is inhibited by blocking activation of microglia. J. Virol. 2009;83:4912–4922. doi: 10.1128/JVI.02343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, et al. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J. Neurochem. 2005;95:377–387. doi: 10.1111/j.1471-4159.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- 198.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 199.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 200.Thomas WE. Characterization of the dynamic nature of microglial cells. Brain Res. Bull. 1990;25:351–354. doi: 10.1016/0361-9230(90)90083-c. [DOI] [PubMed] [Google Scholar]
- 201.Booth PL, Thomas WE. Evidence for motility and pinocytosis in ramified microglia in tissue culture. Brain Res. 1991;548:163–171. doi: 10.1016/0006-8993(91)91118-k. [DOI] [PubMed] [Google Scholar]
- 202.Brockhaus J, Moller T, Kettenmann H. Phagocytozing ameboid microglial cells studied in a mouse corpus callosum slice preparation. Glia. 1996;16:81–90. doi: 10.1002/(SICI)1098-1136(199601)16:1<81::AID-GLIA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 203.Dailey ME, Waite M. Confocal imaging of microglial cell dynamics in hippocampal slice cultures. Methods. 1999;18:222–230. 177. doi: 10.1006/meth.1999.0775. [DOI] [PubMed] [Google Scholar]
- 204.Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- 205.Heppner FL, Skutella T, Hailer NP, Haas D, Nitsch R. Activated microglial cells migrate towards sites of excitotoxic neuronal injury inside organotypic hippocampal slice cultures. Eur. J. Neurosci. 1998;10:3284–3290. doi: 10.1046/j.1460-9568.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 206.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J. Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 209.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 211.Ohsawa K, Irino Y, Sanagi T, Nakamura Y, Suzuki E, et al. P2Y12 receptor-mediated integrin-β1 activation regulates microglial process extension induced by ATP. Glia. 2010;58:790–801. doi: 10.1002/glia.20963. [DOI] [PubMed] [Google Scholar]
- 212.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat. Protoc. 2010;5:201–208. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J. Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Orlowski D, Soltys Z, Janeczko K. Morphological development of microglia in the postnatal rat brain. A quantitative study. Int. J. Dev. Neurosci. 2003;21:445–450. doi: 10.1016/j.ijdevneu.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 216.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, et al. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, et al. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.McGavern DB, Truong P. Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells. J. Immunol. 2004;173:4779–4790. doi: 10.4049/jimmunol.173.8.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.