Abstract
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome. It occurs predominantly among younger females, typically in the absence of atherosclerotic coronary artery disease. Presentations vary greatly and this condition can be fatal. Given its rarity, there are no management guidelines. We present six patients with SCAD with different presentations and treatment approaches as examples in our literature review. Two patients presented with ST elevation myocardial infarction (STEMI), two with non-STEMI (NSTEMI), and two with cardiac arrest. Patients were treated according to the presentation, clinical stability, and extension and distribution of the dissection. Four patients underwent emergent percutaneous coronary intervention (PCI) and one was clinically stable and treated medically initially and underwent an elective PCI after 4 weeks when coronary angiogram showed persistent dissection. Another patient was treated medically as he was hemodynamically stable and the dissection affected a small branch. Intravascular ultrasound (IVUS) or optical coherence tomography (OCT) was used for diagnosis confirmation as well as during and after PCI to assure good stent apposition. All patients had excellent outcome. SCAD is a rare cause of acute coronary syndrome and a high index of suspicion is crucial for early diagnosis. In patients with early presentation, limited disease, and ongoing symptoms, emergent cardiac catheterization with PCI has excellent outcome. However, in stable patients, medical management and elective PCI in few weeks if the dissection persists is a more reasonable approach. IVUS and OCT are invaluable especially in ambiguous cases.
Keywords: acute coronary syndrome, coronary intervention, PCI, intravascular ultrasound, MI, non-ST elevation myocardial infarction
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome. Although essential, early diagnosis is frequently challenging and a high index of suspicion is crucial.
Case Presentation
Case 1
A 42-year-old nonpregnant female patient with no cardiac history presented to our emergency department with typical chest pain of 2 hours duration. Her electrocardiography (ECG) showed T wave inversion and ST segment depression in leads V3 to V6. Initial evaluation including the first set of cardiac enzymes was unremarkable. The patient was admitted and started on medical treatment for acute coronary syndrome. Six hours later, she developed persistent severe chest pain and hypotension with new ECG progression with T wave inversion in leads I and aVL. Troponin I and creatinine kinase MB (CKMB) were elevated. Given her hemodynamic instability, ongoing chest pain, elevated cardiac enzymes, and dynamic ECG changes, emergent coronary angiogram was performed. It was significant for dissection of the first obtuse marginal (OM1) branch of the left circumflex (LCx) artery that extended distally with severe stenosis (Fig. 1A). Optical coherence tomography (OCT; Dragonfly Duo, St. Jude Medical Inc, St. Paul, MN) showed a large false lumen compressing the true lumen of the artery (Fig. 1B). Due to ongoing chest pain, percutaneous coronary intervention (PCI) of OM1 was performed with two bare metal stents resulting in coronary blood flow restoration (Fig. 1C) and complete resolution of her chest pain and ECG changes. Distal small OM1 dissection was also present but clinically insignificant given the resolution of symptoms and ECG changes. Transthoracic echo showed normal left and right ventricular systolic function with mild hypokinesis of distal inferolateral and apical segments. The patient was discharged home on medical management. At 9-month follow-up, she was completely asymptomatic.1
Fig. 1.
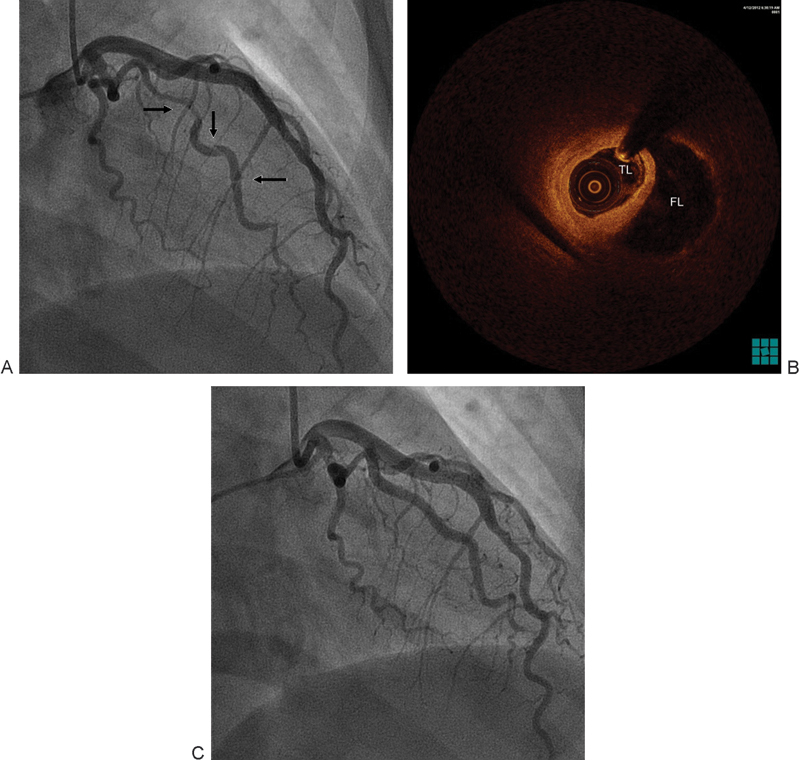
(A) Dissection (arrows) of the OM1 branch of the left. (B) OCT showed a large FL compressing the TL of the dissected artery. (C) Normal flow in OM1 after PCI. FL, false lumen; OCT, optical coherence tomography; OM1, first obtuse marginal; PCI, percutaneous coronary intervention; TL, true lumen.
Case 2
A 37-year-old female patient with a history of Sjogren syndrome presented to the emergency department with acute chest pain associated with diaphoresis and bilateral hand numbness. ECG showed ST elevation in anterolateral and inferior leads. The cardiac catheterization laboratory was activated immediately. Her troponin I was elevated at 0.191 ng/mL. Emergent coronary angiography showed large left anterior descending (LAD) artery that wraps around the apex and supplies the inferior wall with distal diffuse 99% occlusion with thrombolysis in myocardial infarction (TIMI) I flow (Fig. 2A). Intravascular ultrasound (IVUS; Eagle Eye Platinum catheter, Volcano, San Diego, CA) confirmed the presence of dissection with intramural hematoma and absence of atherosclerosis. PCI with a drug-eluting stent (DES) was performed with restoration of flow (TIMI III) (Fig. 2B) and resolution of chest pain and ST elevation. The following day, she developed intermittent pleuritic chest pain and new ST elevation in inferior, anterior, and lateral leads. A repeat coronary angiogram showed patent stent and no obstructive lesions. Transthoracic echocardiography showed a left ventricular ejection fraction (LVEF) of 40 to 45% with severe hypokinesis of apical, apical septal, apical anterior, and apical lateral segments. She was started on colchicine for post-MI pericarditis treatment with significant symptomatic improvement. A complete autoimmune inflammatory workup was negative and inflammatory markers were within normal limits; therefore, coronary vasculitis was unlikely. She was discharged on medical management. At 9-month follow-up, the patient was symptomatic.1
Fig. 2.
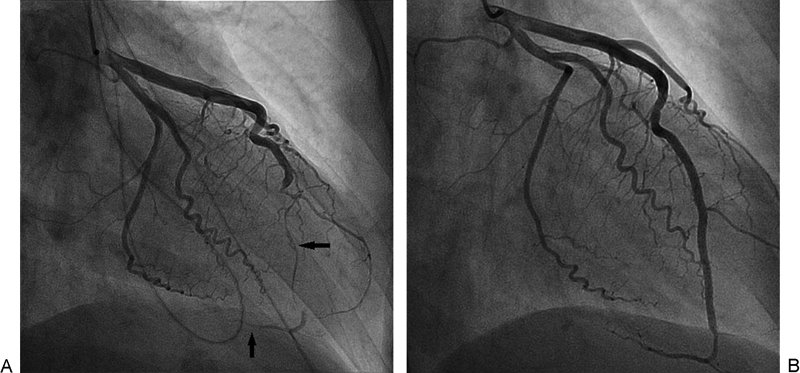
(A) Severe LAD stenosis secondary to intramural hematoma (horizontal arrow). Also seen is the large LAD that wraps around the apex and supplies the inferior wall (vertical arrow). (B) Restoration of flow in LAD post-PCI. LAD, left anterior descending; PCI, percutaneous coronary intervention.
Case 3
A 51-year-old male patient with a history of myocardial bridging of right coronary artery (RCA) had typical cardiac pain while in a cruise ship. He was given ASA, Plavix, and sublingual nitroglycerin and taken to the medical unit where he lost consciousness and coded secondary to ventricular tachycardia. He was defibrillated and received cardiopulmonary resuscitation for approximately 5 minutes, followed by return of spontaneous circulation and consciousness. Initial ECG showed ST segment elevation in leads V3 to V5. He also received low-molecular-weight heparin and was started on dopamine secondary to hypotension. Next morning, the patient arrived to our emergency department in no distress. Upon arrival, he was on dopamine infusion for hypotension. The patient was transferred to the cardiac catheterization laboratory. He underwent coronary angiography that was significant for long tubular distal LAD stenosis (Fig. 3A), with normal LCx artery and RCA. IVUS showed intramural hematoma of the mid to distal LAD compressing the true lumen, and no coronary atherosclerosis detected (Fig. 3B). Given that the patient had no ongoing symptoms and no flow limiting lesions, no PCI was performed, and aggressive medical therapy was started. Transthoracic echocardiography showed LVEF of 40 to 45% and severe apical hypokinesis (consistent with LAD lesion). The patient was discharged home on life vest with plans to repeat echocardiography and coronary angiography in 1 month to document LV function recovery and intramural hematoma healing. One month later, his repeat echocardiography showed normal LVEF with no regional wall motion abnormalities. Coronary angiography showed no improvement in the stenosis and PCI was performed with a DES (Fig. 3C). VerifyNow showed a P2Y12 reaction units of 330, so he was loaded with prasugrel. The patient had no immediate complications. He was seen by the cardiac electrophysiology service and an electrophysiology study was recommended; however, the patient preferred to be referred to an electrophysiologist near his home town (7 hours from our hospital). At 2-month follow-up, the patient was doing fine and completely asymptomatic.
Fig. 3.
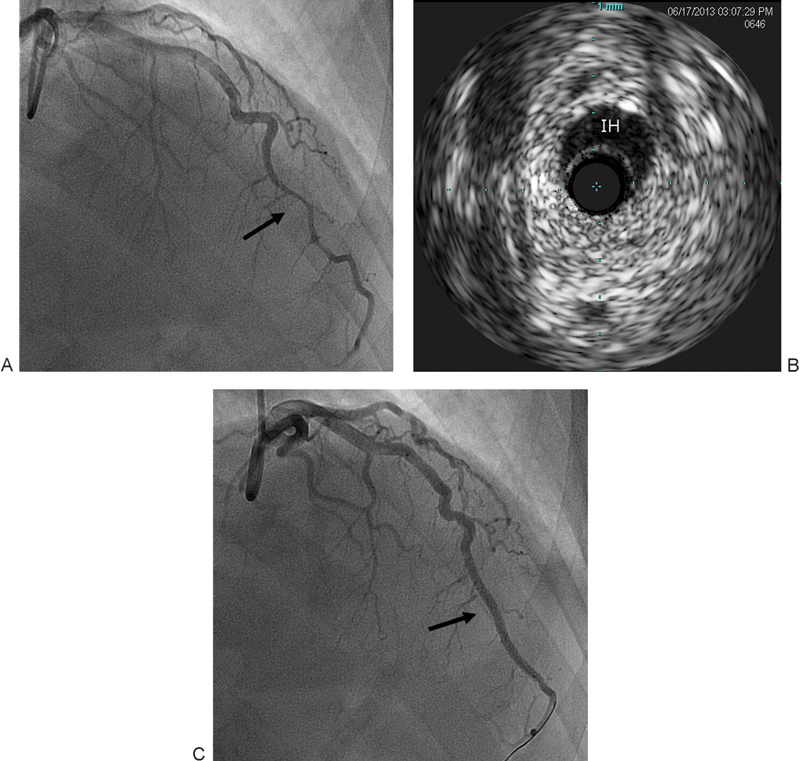
(A) Long tubular distal LAD stenosis (arrow). (B) IVUS showing IH compressing the true lumen. (C) Normal flow after PCI (arrow). IH, intramural hematoma; IVUS, intravascular ultrasound; LAD, left anterior descending; PCI, percutaneous coronary intervention.
Case 4
A 38-year-old male patient with a past medical history of cocaine abuse, chronic exertional chest pain, and cardiomyopathy was referred after he was successfully resuscitated for cardiac arrest secondary to ventricular fibrillation. Hypothermia protocol was initiated. Upon arrival, the patient was intubated and sedated. ECG showed wide QRS with right bundle branch block. His initial laboratory workup was remarkable only for leukocytosis and he was extubated the next day. Transthoracic echocardiography showed septal and apical akinesis with LVEF at 25 to 30% in addition to diastolic dysfunction. Coronary angiography showed normal coronary arteries with luminal irregularities. Cardiac electrophysiology service was consulted and the patient had biventricular implantable cardioverter–defibrillator insertion with no immediate complication. The patient was discharged on medical therapy. However, the patient continued to have mild intermittent chest pain. Few months later, his LVEF dropped to 20%. Given his persistent symptoms and recent drop in LVEF, coronary angiography was repeated and showed a diffuse mid-LAD haziness and filling defect (Fig. 4A). Fractional flow reserve (PressureWire Aeris, St. Jude Medical Inc, St. Paul, MN) was significant. OCT was done and showed complex healed dissection (Fig. 4B). Upon review of old catheterization films, this dissection was present and missed during the previous admission. PCI with a DES was performed (Fig. 4C) and postintervention OCT showed well apposed and expanded stent. The patient tolerated the procedure well and was discharged later on aggressive medical therapy. At 2-month follow-up, the patient was symptom free.
Fig. 4.
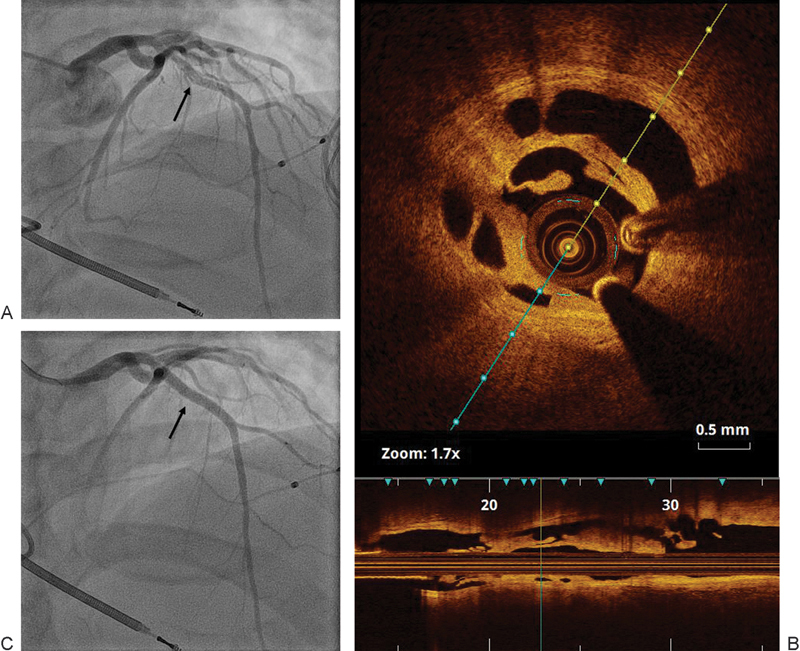
(A) Diffuse mid-LAD haziness and filling defect (arrow). (B) OCT showing complex healed dissection. (C) Post-PCI (arrow). LAD, left anterior descending; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
Case 5
A 49-year-old female patient with no cardiac history presented to our emergency department with typical chest pain of 3 to 4 hours duration. In the emergency room, her ECG showed ST elevation in the anterior leads and her troponin I and CKMB were elevated. The cardiac catheterization laboratory was activated and the patient underwent emergent coronary angiography which was significant for distal LAD 100% occlusion secondary to spontaneous dissection and a superimposed thrombus. Aspiration thrombectomy was performed followed by PCI with a DES with restoration of flow and resolution of chest pain and ST segment changes. Transthoracic echocardiography showed apical and distal segmental akinesis along with reduced LVEF at 35 to 40%. Her troponin I peak was 145 ng/mL (negative < 0.03 ng/mL). The patient was observed in the coronary care unit (CCU) and subsequently discharged home on medical therapy. At 4-month follow-up, she was completely asymptomatic and her LVEF improved to 50 to 55%. She developed atypical chest pain 2 years later and a diagnostic coronary angiography was unremarkable.
Case 6
A 32-year-old Hispanic male with no significant past medical history presented to the emergency department with typical chest pain that started few hours after strenuous exercise (heavy weight lifting). Initial ECG showed no acute changes and cardiac enzymes were negative. The second set of cardiac enzymes were elevated (troponin I 6.53 ng/dL) and ECG showed deep T wave inversions in lateral leads. Urgent coronary angiography showed a long tubular lesion of OM1 with 50 to 60% narrowing (Fig. 5A) and small branch occlusion, likely causing his non-ST elevation myocardial infarction (NSTEMI). OCT confirmed the presence of diffuse intramural hematoma from mid to distal OM1 (Fig. 5B). No visualization of the intimal flap was identified. Neither balloon angioplasty nor stenting was performed given the clinical stability and patency of OM1. The patient was admitted to the intermediate CCU, started on heparin drip (for 48 hours), Plavix, and aspirin. His transthoracic echocardiogram was unremarkable. The patient was later discharged home on medical management for NSTEMI. Table 1 summarizes the different clinical presentations and management approaches in our patients.
Fig. 5.
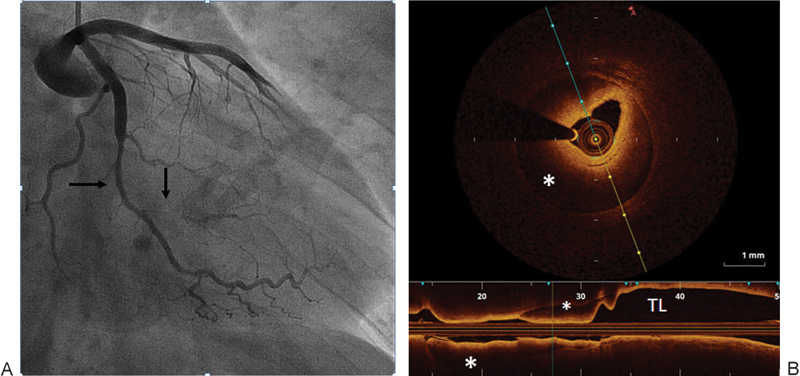
(A) Long tubular narrowing of OM1 (horizontal arrow) with small branch occlusion (vertical arrow). (B) OCT demonstrating diffuse intramural hematoma (*) compressing the TL, no intimal flap was identified. OCT, optical coherence tomography; OM1, first obtuse marginal; TL, true lumen.
Table 1. Summary of baseline characteristics, presentations, diagnostic findings, and treatment approaches in our patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age (y) | 42 | 37 | 51 | 38 | 49 | 32 |
| Sex | Female | Female | Male | Male | Female | Male |
| CAD risk factors | None | None | None | HTN, HLD | None | None |
| Smoking | Never | Never | Never | Active smoker | Never | Never |
| Drug abuse | Never | Never | Never | Cocaine | Never | Never |
| Recent pregnancy | No | No | N/A | N/A | No | N/A |
| CTD | No | Sjogren | No | No | No | No |
| Presentation | NSTEMI | STEMI | Cardiac arrest | Chronic angina | STEMI | NSTEMI |
| Coronary angiogram | Spiral dissection | Subtotal occlusion | Long stenosis | Vessel haziness | Complete occlusion | Long stenosis, side branch occlusion |
| Vessel affected | OM1 | LAD | LAD | LAD | LAD | OM1 |
| OCT/IVUS | False and true lumens, intimal flap | Intramural hematoma | Intramural hematoma | Complex dissection | N/A | Intramural hematoma |
| Treatment | Emergent PCI with BMS | Emergent PCI with DES | Early conservative, followed by elective PCI with DES | Elective PCI with DES | Emergent PCI with DES | Conservative |
Abbreviations: BMS, bare metal stents; CAD, coronary artery disease, CTD, connective tissue disease, DES, drug-eluting stent; HLD, hyperlipidemia; HTN, hypertension; IVUS, intravascular ultrasound; LAD, left anterior descending; N/A, not available; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
Discussion
Definition
SCAD is a rare cause of acute coronary syndrome. It is defined as a nontraumatic and noniatrogenic separation of the coronary arterial wall by intramural hemorrhage creating a false lumen and can happen with or without an intimal tear.2
Epidemiology
The first case was described in 19313 and its incidence among cases of acute coronary syndrome is 0.2% in angiographic studies4 5 and 0.5% after autopsy in patients who had sudden cardiac death.6 However, the true incidence is underestimated as many patients are misdiagnosed with atherosclerosis, especially in the absence of intimal tear.2
SCAD is more frequently diagnosed in the left coronary artery, with a female-to-male ratio of 2:1.4 Although the LAD artery is the most commonly affected artery in females, RCA tends to be more frequently involved in male patients.7
SCAD has been historically strongly associated with pregnancy or postpartum period. Although it remains an important risk factor, recent data challenged the frequency of the association between pregnancy and SCAD. Recent studies suggest that pregnancy accounts for a minority of SCAD cases. This is consistent with our findings as none of the patients reported in this article is pregnant or in the peripartum period.2
A subtype of SCAD is associated with connective tissue diseases such as Marfan syndrome, Ehlers–Danlos disease, several autoimmune diseases (Kawasaki, systemic lupus erythematosus, and rheumatoid arthritis), and fibromuscular dysplasia of the coronaries or other vessels.5 8 SCAD associated with atherosclerosis, intense exercise, cocaine abuse, and cyclosporine and female hormonal treatments such as oral contraceptives has been also reported.
Morphology and Pathophysiology
Coronary artery dissection is characterized by separation of the layers of the arterial wall. This results in a false lumen or an intramural hematoma between the intima and the media or the media and the adventitia, causing compression of the true lumen of the artery,9 and resulting in myocardial ischemia. There are two recognized types of SCAD according to the mechanism of injury. The first type is caused by an intimal tear resulting in medial dissection. This appears as multiple radiolucent lumens on coronary angiogram, with slow contrast clearing. The second type is probably caused by rupture of vasa vasorum, resulting in intramural hemorrhage without an intimal tear. It may appear angiographically only as luminal narrowing mimicking atherosclerotic lesions. The use of intracoronary imaging modalities is particularly important in this type.2
To be classified as spontaneous, dissection must occur in the absence of trauma, previous surgery or catheterization, or an extension of aortic dissection.
The pathogenesis of SCAD is not yet fully understood.4 The initial event that leads to dissection is not clear, and no single factor has been found to be causative.3 10 Proposed mechanisms pertain to changes in vascular wall properties that lead to weakening of the media and arterial wall connective tissue. These include changes in smooth muscle cell metabolism, the effect of proteases released from eosinophilic infiltrates, and pregnancy-related connective tissue changes.11 Peripartum patients with SCAD have higher rates of multivessel involvement supporting the hypothesis of arterial wall changes during pregnancy.12
Clinical Presentation
The clinical presentation of SCAD varies according to the degree of true lumen compression and flow impairment, number of involved arteries, extent of involvement, and rate of progression. It can range from a completely asymptomatic state to acute coronary syndrome, cardiogenic shock, cardiac arrest, and sudden cardiac death.13 Another extremely rare symptom is chronic cardiac pain as presented in one of our patients. However, in the vast majority of review articles, the diagnosis of SCAD was made mainly angiographically, which may significantly underestimate the prevalence of this disease without the help of intracoronary imaging modalities, as we will discuss later.
Clinical features that raise the suspicion of SCAD in myocardial infarction patients include young age, female sex, absence of cardiovascular risk factors, no atherosclerotic lesions in other arteries, history of fibromuscular dysplasia, peripartum state, history of connective tissue disease or systemic inflammatory conditions, and recent strenuous exercise or stress.14
Diagnosis
Coronary Angiography
Early and accurate diagnosis of SCAD is challenging but very important as it will completely change the management.15 Coronary angiography remains the primary diagnostic modality for this entity. SCAD has three distinct angiographic patterns. The first, which is the classical pattern, is characterized by the dye staining the arterial wall with multiple radiolucent lines in addition to slow dye clearance. The second pattern manifests angiographically as diffuse and smooth-walled stenosis with abrupt change in vessel diameter. The third pattern mimics atherosclerosis but the absence of atherosclerotic changes in other vessels, long lesions, linear, and hazy stenosis should raise the suspicion for SCAD.14 It is important to keep in mind that intubation of the coronary ostium and contrast injection in patients suspected to have SCAD can be potentially dangerous, as forceful maneuvers during coronary angiography may lead to propagation of the dissection.
Coronary angiography gives a two-dimensional image, sometimes unable to differentiate forms of luminal obstruction. In cases of SCAD with intimal tear, radiolucent lines separating true and false lumens can be seen. In the absence of intimal tear, SCAD appears as a smooth-walled luminal narrowing mimicking atherosclerosis and requiring the use of OCT or IVUS to confirm or exclude the diagnosis. Again, a high index of suspicion is crucial.2
Optical Coherence Tomography and Intravascular Ultrasound
As SCAD is primarily a disease of the vessel wall, coronary imaging modalities as OCT or IVUS are particularly important in confirming or ruling out the diagnosis, in addition to conventional coronary angiography that can only assess the lumen rather than the wall itself.14
The use of intracoronary imaging with IVUS or OCT provides more detailed morphological information of the lesion and dissection planes in the arterial wall.16 OCT is very useful in ruling out the disease when angiographic images mimic SCAD. OCT is an accurate modality that can provide important information identifying the entry tear, the extent and dimensions of the intramural hematoma, intraluminal thrombi, false lumen distribution, and the degree of true lumen compromise.14 15
IVUS has lower special resolution but better penetration and allows more complete vessel visualization and better appreciation of the extent of intramural hematoma.14
Cardiac Computed Tomography
Cardiac computed tomographic (CT) angiography is not routinely used for SCAD diagnosis, due to lower special resolution and inadequate visualization of distal and small coronary artery branches, which are affected by a large proportion of SCAD.14 However, if the dissection is located in a proximal segment of the coronary arteries, follow-up by CT angiography rather than coronary angiography is reasonable, to avoid the risk of further propagation of the dissection.17
Management
Medical Management
Due to its rarity, there are no randomized clinical trials or consensus in regard to SCAD management.9 Most of the literature consists of case reports and some case series. Furthermore, many reported cases have been diagnosed postmortem after sudden cardiac deaths.18
Conservative medical management is the preferred treatment strategy in stable patients given the overall good prognosis and the good probability of spontaneous healing. In a prospective study with 6-year follow-up, repeat angiography showed “disappearance” of the dissection image in 7 out of 13 patients (54%).19 Aspirin should be used for all cases of SCAD. There is little evidence about the appropriate duration of antiplatelet therapy in patients without underlying atherosclerosis.12 The role of dual antiplatelet therapy is unclear in SCAD. Some authors proposed that reducing the thrombus burden in the false lumen could theoretically reduce true lumen compression and routinely administer dual antiplatelet therapy for 1 year, then continue aspirin lifelong.2 Beta-blockers might be beneficial by reducing contractility and shear stress on the affected vessel, thus preventing expansion of the lesion.17 Nitrates and calcium channel blockers were sometimes given to prevent coronary spasm and resulting increase wall stress.12
Revascularization
If the patient is symptom free, and initial angiography showed limited disease, conservative management is a reasonable approach followed by repeat angiogram to document spontaneous healing. If the dissection is still present, angiography at that time may better define the dissection features, resulting in a safer and simpler PCI.20
PCI and stenting is the preferred strategy in situations of ongoing ischemia or infarction with limited disease and early presentation after the identification of the true and false lumen.16 21 22 Advancing the guide wire may be challenging and may lead to expansion of the lesion or perforation of the coronary artery if it was advanced through the false lumen. After confirming the site of the guide wire, stent placement must start distally in the nondissected part of the vessel, and continue proximally to prevent distal expansion of the lesion or propagation of the intramural hematoma.17 Conservative stenting approach was recommended by some authors, restricting treatment to the most proximal segment of the disease with adequate sealing of the intimal tear, and leaving distal dissections if causing no significant stenosis or located in small vessels.15 Technical difficulties of PCI in SCAD include inability to gain true lumen access, extension of dissection, and need for multiple or long stents increasing the risk of stent thrombosis and restenosis.14 Another challenge is sealing the entry point with an appropriate stent. Furthermore, intramural hematoma tends to undergo resorption with time causing stent malapposition which increases the risk of thrombosis especially after stopping the dual antiplatelet therapy.2 20 Bioabsorbable stents have theoretical advantage avoiding the long-term effects of malapposition described earlier.14 During PCI, OCT can be used to confirm the location of the guide wire in the true lumen. It can also assure adequate stent coverage, extension, and apposition.15
Cases with multivessel involvement, left main coronary artery dissection, or failed PCI may need to be treated by coronary artery bypass graft. Fibrinolysis is not recommended due to the increased risk of bleeding.10
Patients treated for SCAD should be closely monitored in an inpatient setting, preferably in the CCU. If symptoms reoccur or the patient continues to have chest pain, coronary blood flow should be evaluated by coronary angiography.17 In general, most patients who survive their initial SCAD tend to have good long-term prognosis, with 92% 10-year survival.8 After a primary SCAD event, the 10-year SCAD recurrence rate was reported to be as high as 29%. Recurrence timing is unpredictable with median time to second episode ranges from few days to more than 10 years. The recurrence can occur in previously unaffected coronary artery.8
Conclusion
SCAD is a rare cause of acute coronary syndrome, more common in female patients with no or few risk factors for coronary artery disease. It can also be seen in association with fibromuscular dysplasia, connective tissue disease, and pregnancy/postpartum period. Diagnosis is sometimes challenging and requires high index of suspicion. Intracoronary imaging modalities such as OCT and IVUS are frequently important in confirming or ruling out the diagnosis. Treatment of this condition should be individualized to patients depending on the clinical presentation, location, severity of the disease, and associated risk factors and comorbidities. In patients with early presentation, limited disease, and ongoing symptoms, emergent cardiac catheterization with PCI has excellent outcome. However, in stable patients, medical management and elective PCI in a few weeks if the dissection persists are a more reasonable approach.
Footnotes
Conflict of Interest The authors have no conflict of interest to disclose.
References
- 1.Al Emam A R, Almomani A, Gilani S A. Spontaneous coronary artery dissection and hemodynamic instability: can emergent PCI be life saving? Report of two cases and literature review. Int J Angiol. 2014;23(4):275–280. doi: 10.1055/s-0033-1349163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saw J. Spontaneous coronary artery dissection. Can J Cardiol. 2013;29(9):1027–1033. doi: 10.1016/j.cjca.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Pretty H C. Dissecting aneurysm of coronary artery in a woman aged 42. BMJ. 1931;i:667. [Google Scholar]
- 4.Verma P K, Sandhu M S, Mittal B R. et al. Large spontaneous coronary artery dissections-a study of three cases, literature review, and possible therapeutic strategies. Angiology. 2004;55(3):309–318. doi: 10.1177/000331970405500311. [DOI] [PubMed] [Google Scholar]
- 5.Azzarelli S, Fiscella D, Amico F, Giacoppo M, Argentino V, Fiscella A. Multivessel spontaneous coronary artery dissection in a postpartum woman treated with multiple drug-eluting stents. J Cardiovasc Med (Hagerstown) 2009;10(4):340–343. doi: 10.2459/JCM.0b013e3283276dee. [DOI] [PubMed] [Google Scholar]
- 6.Sherrid M V, Mieres J, Mogtader A, Menezes N, Steinberg G. Onset during exercise of spontaneous coronary artery dissection and sudden death. Occurrence in a trained athlete: case report and review of prior cases. Chest. 1995;108(1):284–287. doi: 10.1378/chest.108.1.284. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen M B, Aharonian V, Mansukhani P, Mahrer P R. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J. 1994;127(5):1382–1387. doi: 10.1016/0002-8703(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 8.Tweet M S, Hayes S N, Pitta S R. et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126(5):579–588. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 9.Auer J, Punzengruber C, Berent R. et al. Spontaneous coronary artery dissection involving the left main stem: assessment by intravascular ultrasound. Heart. 2004;90(7):e39. doi: 10.1136/hrt.2004.035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler R, Webster M WI, Davies G. et al. Spontaneous dissection of native coronary arteries. Heart. 2005;91(2):223–224. doi: 10.1136/hrt.2003.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein J, Hakimian J, Makaryus A N. Spontaneous right coronary artery dissection: causing myocardial infarction in a 36-year-old woman. Tex Heart Inst J. 2012;39(1):95–98. [PMC free article] [PubMed] [Google Scholar]
- 12.Maeder M, Ammann P, Angehrn W, Rickli H. Idiopathic spontaneous coronary artery dissection: incidence, diagnosis and treatment. Int J Cardiol. 2005;101(3):363–369. doi: 10.1016/j.ijcard.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 13.Cheung S, Mithani V, Watson R M. Healing of spontaneous coronary dissection in the context of glycoprotein IIB/IIIA inhibitor therapy: a case report. Catheter Cardiovasc Interv. 2000;51(1):95–100. doi: 10.1002/1522-726x(200009)51:1<95::aid-ccd22>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84(7):1115–1122. doi: 10.1002/ccd.25293. [DOI] [PubMed] [Google Scholar]
- 15.Alfonso F, Paulo M, Gonzalo N. et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59(12):1073–1079. doi: 10.1016/j.jacc.2011.08.082. [DOI] [PubMed] [Google Scholar]
- 16.Schmid J, Auer J. Spontaneous coronary artery dissection in a young man - case report. J Cardiothorac Surg. 2011;6:22. doi: 10.1186/1749-8090-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wain-Hobson J, Roule V, Dahdouh Z, Sabatier R, Lognoné T, Grollier G. Spontaneous coronary artery dissection: one entity with several therapeutic options. Cardiovasc Revasc Med. 2012;13(3):2030–2.03E6. doi: 10.1016/j.carrev.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Juszczyk M, Marnejon T, Hoffman D A. Spontaneous coronary artery dissection postpartum. J Invasive Cardiol. 2004;16(9):524–526. [PubMed] [Google Scholar]
- 19.Alfonso F, Paulo M, Lennie V. et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. 2012;5(10):1062–1070. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Arrivi A, Bazzucchi M, De Paolis M. et al. Spontaneous-idiopathic left anterior descending artery dissection: is watchful waiting better than immediate stenting? Case Rep Vasc Med. 2013;2013:639384. doi: 10.1155/2013/639384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missouris C G, Ring A, Ward D. A young woman with chest pain. Heart. 2000;84(6):E12. doi: 10.1136/heart.84.6.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roig S, Gómez J A, Fiol M. et al. Spontaneous coronary artery dissection causing acute coronary syndrome: an early diagnosis implies a good prognosis. Am J Emerg Med. 2003;21(7):549–551. doi: 10.1016/j.ajem.2003.08.010. [DOI] [PubMed] [Google Scholar]


