Abstract
Objectives
The tooth-resin bond is the weak link of restoration, with secondary caries as a main reason for failure. Calcium phosphate-containing resins are promising for remineralization; however, calcium (Ca) and phosphate (P) ion releases last only a couple of months. The objectives of this study were to develop the first rechargeable CaP bonding agent and investigate the key factors that determine CaP ion recharge and re-release.
Methods
Nanoparticles of amorphous calcium phosphate (NACP) were synthesized. Pyromellitic glycerol dimethacrylate (PMGDM), ethoxylated bisphenol-A dimethacrylate (EBPADMA), 2-hydroxyethyl methacrylate (HEMA), and bisphenol-A glycidyl dimethacrylate (BisGMA) were used to synthesize three adhesives (denoted PE, PEH and PEHB). NACP were mixed into adhesive at 0–30% by mass. Dentin shear bond strengths were measured. Adhesive specimens were tested for Ca and P initial ion release. Then the ion-exhausted specimens were immersed in Ca and P solution to recharge the specimens, and the recharged specimens were then used to measure ion re-release for 7 days as one cycle. Then these specimens were again recharged and the re-release was measured for 7 days as the second cycle. Three recharge/re-release cycles were tested.
Results
PEHB had the highest dentin bond strength (p<0.05). Increasing NACP content from 0 to 30% did not affect dentin bond strength (p>0.1), but increased CaP release and re-release (p<0.05). PEHB-NACP had the greatest recharge/re-release, and PE-NACP had the least (p<0.05). Ion release remained high and did not decrease with increasing the number of recharge/re-release cycles (p>0.1). After the third cycle, specimens without further recharge had continuous CaP ion release for 2–3 weeks.
Significance
Rechargeable CaP bonding agents were developed for the first time to provide long-term Ca and P ions to promote remineralization and reduce caries. Incorporation of NACP into adhesive had no negative effect on dentin bond strength. Increasing NACP filler level increased the ion recharge and re-release capability. The new CaP recharge method and PMGDM-EBPAGMA-NACP composition may have wide application in adhesives, composites and cements, to combat caries and remineralize lesions.
Keywords: Dental adhesive, dentin bonding, calcium phosphate nanoparticles, ion release, ion recharge, caries inhibition
1. Introduction
Composites are popular for tooth cavity restorations due to their esthetics and direct-filling capability.1–5 Composites and adhesives have been revolutionized by developments and improvements in material compositions and placement technology.6–10 A strong and durable adhesion to dental hard tissues is a key factor in the success of the restoration.8,9,12,13 However, the oral environment poses severe challenges such as chewing forces and biofilm acids which limit the longevity of the dental restoration. Currently, the resin-tooth bonded interface represents the weak link in the restoration,13 and secondary (recurrent) caries at the margins is a chief limitation to the longevity of restorations.11–17
The mechanism of dentin bonding involves the infiltration of adhesive monomers into a demineralized dentin collagen matrix and the formation of the hybrid layer (HL).8,9,12,13 The adhesive is not only a connection between the tooth structure and the restorative composite, it also serves as a barrier to protect the demineralized collagen scaffold from the acidic and enzymatic attacks of the oral bacteria, enzymes and fluids.13,18,19 Approaches on the functionalization of adhesives have been extensively studied to improve the long-term stability of HL.20–24 Several studies incorporated antibacterial monomers into the adhesive to improve the resistance to oral bacteria and acid challenges.20,22,23 Another approach is to incorporate calcium phosphate (CaP) particles into dental resins to promote remineralization and avoid demineralization.25–28 Adhesives containing CaP particles could remineralize the remnants of tooth lesions in the cavity as well as the acid-etched dentin, and hence are promising to improve the longevity of the restorations.21,24 Recently, bonding agents containing nanoparticles of amorphous calcium phosphate (NACP) were developed.29–31 These bonding agents could release high levels of Ca and P ions to induce remineralization and combat caries.29–31 The addition of NACP did not negatively affect the dentin bond strength.29–31 Due to their small particle sizes, the NACP readily flowed with bonding agent into dentinal tubules to form resin tags.29,30 The NACP adhesive was “smart” because it could substantially increase the Ca and P ion release at a low cariogenic pH when these ions would be most needed to combat caries.31 For both total-etch and self-etch bonding systems, the bonding stability is limited by the degradation of the HL.18,32,33 The Ca and P ion release from adhesive may be highly beneficial and can serve as seed crystals to facilitate remineralization in HL and at the tooth-restoration margins.18,21 Thus the CaP adhesive may protect the exposed collagen within the bonded interface and improve the bonding stability and durability.18,34 Therefore, NACP-containing adhesive with Ca and P ion release could be meritorious in protecting the weak link of the tooth restoration.
However, a major drawback for CaP-containing dental resins is that the Ca and P ion release is short-term, lasting for only several weeks to a few months, and then the Ca and P ion release is diminished.25–27,35 For example, a previous study showed that CaP resins had ion release for one to two months.36 It would be highly desirable to develop a rechargeable CaP adhesive that can be repeatedly recharged to re-release Ca and P ions to provide a long-term capability for remineralization and inhibition of caries. Rechargeable dental materials have been reported for glass ionomer cements and resin-modified glass ionomer cements, which can be recharged with a fluoride (F) ion source to re-release F ions.37–39 A recent study showed that a NACP nanocomposite achieved tooth lesion remineralization that was 4-fold that of a commercial F-releasing composite.28 However, literature and patent searches revealed no report on rechargeable calcium phosphate dental resins.
Therefore, the objectives of this study were to develop rechargeable CaP dental adhesive for the first time, and investigate the effects of different adhesive compositions and NACP mass fractions on Ca and P ion recharge and re-release efficacy. It was hypothesized that: (1) Rechargeable CaP dental adhesive resin can be developed, and the dentin bond strength and CaP recharge and re-release efficacy will depend on the adhesive composition; (2) the incorporation of NACP into the adhesive will have no negative effect on the dentin bond strength; and (3) NACP filler level in the adhesive will significantly affect the Ca and P ion recharge and re-release capability of the adhesive.
2. Materials and Methods
2.1. NACP-containing adhesive fabrication
NACP [Ca3(PO4)2] were synthesized via a spray-drying technique as previously described.28,40 Briefly, calcium carbonate and dicalcium phosphate anhydrous were dissolved into an acetic acid solution. The concentrations of Ca and P ion concentrations were 8 mmol/L and 5.333 mmol/L, respectively, yielding a Ca/P molar ratio of 1.5. The solution was sprayed into a heated chamber to evaporate the water and volatile acid. The dried NACP powder was collected by an electrostatic precipitator. Previously studies showed that the NACP mean particle size was approximately 116 nm.28,40
Three experimental bonding agents were investigated. A pyromellitic glycerol dimethacrylate (PMGDM)-containing primer, previously reported to yield good dentin bonding properties,30 was adopted as the primer for all three groups. This primer contained PMGDM (Hampford, Stratford, CT) and 2-hydroxyethyl methacrylate (HEMA) (Esstech, Essington, PA) at a mass ratio 3.3/1, with 50% acetone solvent (all mass fractions).30
Three adhesives were formulated (Table 1). The first consisted of PMGDM and ethoxylated bisphenol A dimethacrylate (EBPADMA) (Sigma-Aldrich, St, Louis, MO) at 1:1 mass ratio, which was rendered light-curable with 1% phenylbis (2,4,6-trimethylbenzoyl) phosphine oxide (Esstech).25,27 Our preliminary study showed a high level of Ca and P ion release and recharge using the PMGDM-EBPADMA resin. PMGDM is an acidic adhesive monomer41,42 and can chelate with calcium ions from the recharging solution to achieve the recharging capability. The PMGDM-EBPADMA resin is referred to as PE.
Table 1.
Compositions (Mass %) of Experimental Adhesives of the Present Study
| Adhesive | PMGDM | EBPADMA | HEMA | Bis-GMA | BAPO |
|---|---|---|---|---|---|
| PE | 49.5 | 49.5 | - | - | 1 |
| PEH | 44.5 | 44.5 | 10 | - | 1 |
| PEHB | 44.5 | 39.5 | 10 | 5 | 1 |
PMGDM: pyromellitic glycerol dimethacrylate (Hampford, Stratford, CT). EBPADMA: ethoxylated bisphenol A dimethacrylate (Sigma-Aldrich, St, Louis, MO). Bis-GMA: bisphenol A glycidyl dimethacrylate (Esstech, Essington, PA). HEMA: 2-hydroxyethyl methacrylate (Esstech, Essington, PA). BAPO: phenylbis (2,4,6-trimethylbenzoyl) phosphine oxide (Esstech, Essington, PA).
To make the second adhesive, 10% of HEMA was added to the PE mixture to improve flowablity and hydrophilicity, following a previous study.26 This group is denoted adhesive PEH (Table 1). The third adhesive incorporated 10% HEMA and 5% bisphenol A glycidyl dimethacrylate (BisGMA) (Esstech) into the PE adhesive. Previous study showed that a small amount of BisGMA could improve the cross-linkage of monomers and the bonding properties of the adhesive.43 This adhesive is designated PEHB.
NACP fillers were mixed into each adhesive at mass fractions of 0%, 20% and 30%, following previous studies.30,31 NACP filler levels ≥ 40% were not used due to a decrease in dentin bond strength in preliminary study. Hence, nine adhesives were fabricated: (1) Adhesive PE + 0% NACP; (2) Adhesive PE + 20% NACP; (3) Adhesive PE + 30% NACP; (4) Adhesive PEH + 0% NACP; (5) Adhesive PEH + 20% NACP; (6) Adhesive PEH + 30% NACP; (7) Adhesive PEHB + 0% NACP; (8) Adhesive PEHB + 20% NACP; (9) Adhesive PEHB + 30% NACP.
For testing the dentin bond strength, two commercial bonding agents were included to provide commercial dentin bond strength values as controls. Prime & Bond NT (Dentsply, Milford, DE) served as control 1. According to the manufacturer, NT was a total-etching one-bottle bonding system and contained 30% typical methacrylates, < 10% methyl methacrylate, and 60% acetone. NT was combined with a self-cure activator (SCA) at 1:1 ratio to enable dual-cure. Scotchbond Multi-Purpose bonding system (SBMP, 3M, St. Paul, MN) served as control 2. According to the manufacturer, SBMP primer contained 35–45% HEMA, 10–20% copolymer of acrylic and itaconic acids, and 40–50% water. SBMP adhesive contained 60–70% BisGMA and 30–40% HEMA.
2.2. Dentin shear bond strength testing
Extracted human third molars were stored in 0.01% thymol solution at 4 °C. Each tooth was cut perpendicularly to the long axis of tooth to expose the mid-coronal dentin using a low speed diamond saw (Isomet, Buehler, Lake Bluff, IL) under water coolant. The dentin surface was polished with 600-grit SiC paper. Then the dentin surface was etched with 37% phosphoric acid gel for 15 seconds (s) and rinsed with water. Two coats of the primer were applied on the etched dentin with a brush-tipped applicator for 15 s. The dentin was gently blown with air for 5 s. An adhesive was then applied and light-cured for 10 s with an Optilux curing unit (VCL 401, Demeron Kerr, Danbury, CT). A stainless-steel cylindrical mold (inner diameter = 4 mm, thickness = 1.5 mm) was placed on the adhesive-treated dentin surface. A composite (TPH, Caulk/Dentsply, Milford, DE) was filled into the mold and light-cured for 60 s. The bonded specimens were stored in distilled water at 37 °C for 24 hours (h). A chisel on a Universal Testing Machine (MTS, Eden Prairie, MN) was aligned to be parallel to the composite-dentin interface.30,31 Load was applied at a cross-head of 0.5 mm/min until the bond failed. Dentin shear bond strength = 4P/(πd2), where P is the load at failure, and d is the diameter of the composite.30,31
2.3. Ca and P ion release measurement
A sodium chloride (NaCl) solution (133 mmol/L) was buffered to pH 4 with 50 mmol/L lactic acid to measure ion release, simulating a cariogenic low pH condition.31,40 For each NACP-adhesive group, three specimens of approximately 2 × 2 × 12 mm were immersed in 50 mL of solution to yield a specimen volume/solution of 2.9 mm3/mL. This was similar to a specimen volume per solution of about 3.0 mm3/mL in a previous study.35 The Ca and P ion concentrations released from the specimens were measured at 1, 3, 5, 7, 14, 21, 28, 35, and 42 days (d). At each time, aliquots of 0.5 mL were removed and replaced with fresh solution. The pH of the immersion solutions was monitored and adjusted to pH 4 with 50 mmol/L lactic acid using a combination pH electrode (Orion, Cambridge, MA).44 The aliquots were analyzed for Ca and P concentrations via a spectrophotometric method (DMS-80 UV-visible, Varian, Palo Alto, CA) using known standards and calibration curves.31,40 The release was measured as the total accumulative ion concentration versus time. This virgin ion release from the adhesive specimens was termed “initial release”, to differentiate from the subsequent recharge and re-release.
2.4. Recharge of adhesive specimens and re-release of Ca and P ions
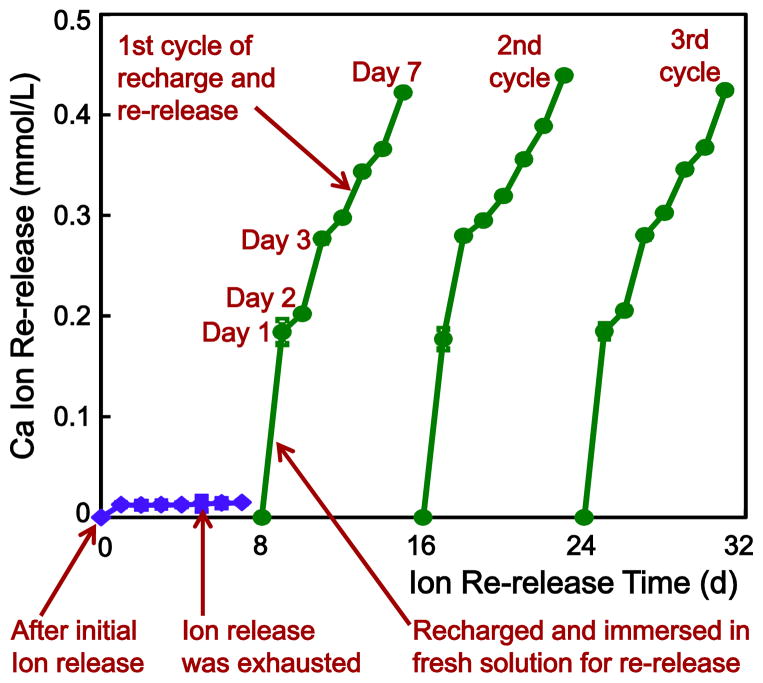
The procedures of recharge and re-release are illustrated in Fig. 1. First, specimens were immersed in the pH 4 solution for 42 d to measure the initial ion release as described in Section 2.3. After 42 d immersion, the specimens were collected and stored in 100 mL of fresh NaCl solution at pH 4 for 30 d to make sure that their ion release was exhausted. Then the specimens were removed from the immersion solution and ultrasonicated with distilled water for 30 min. These exhausted specimens were referred to as being “After initial ion release”, and constituted the starting point as indicated by the left lower arrow in Fig. 1. Then, these exhausted specimens were used for Ca and P ion measurement for 7 d, which confirmed that indeed their ion release was exhausted and there was no further release, as indicated by the second lower arrow in Fig. 1.
Fig. 1.
Illustration of the testing procedures for Ca and P ion recharge and re-release. Adhesive resin specimens were first immersed in a pH 4 solution to exhaust the ion release, as indicated by the lower left arrow. Then the specimens were immersed in a new pH 4 solution to confirm that the ion release was exhausted, as indicated by the lower middle arrow. Then the specimens were recharged in a recharge solution. The recharged specimens were tested for ion re-release for 7 d, as indicated by the right arrow at the bottom of Fig. 1. This constituted the first recharge/re-release cycle. This process was repeated for 3 cycles to test whether the recharge/re-release would decrease over time.
These exhausted exhausted specimens were then used for the recharge experiment. The calcium ion recharge solution consisted of 100 mmol/L of CaCl2 and 50 mmol/L of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer.27,45 The phosphate ion recharge solution consisted of 60 mmol/L of KHPO4 and 50 mmol/L of HEPES. The two solutions were adjusted to pH 7 using 1 mol/L of KOH.27,45 To recharge, three specimens of 2 × 2 × 12 mm were immersed into 5 mL of the Ca or P recharge solution and gently shaken on a mixing machine (Analog Vortex Mixer, Fisher, Waltham, MA) at a power level of 3 for 3 min. This immersion and shaking treatment provided movement which could also occur in the mouth-rinsing process. Then the specimens were rinsed with running distilled water for 1 min to remove any loosely attached deposits on specimen surfaces (hence only the ions recharged into the interior of the resin were measured in the subsequent re-release test). This recharge was performed at about 9:00 am, then the specimens were kept in lab air, and then they were recharged again at about 5:00 pm. Thus the specimens received two doses of recharge, simulating a mouth-rinse in the morning and in the evening. Then the specimens were used to measure the re-release.
To measure Ca and P ion re-release, the recharged specimens were immersed in 50 mL of the pH 4 solution using the method in Section 2.3, as indicated by the third arrow in the bottom of Fig. 1. The re-release was measured at 1, 2, 3, 4, 5, 6 and 7 d, as one cycle. The upper arrow in Fig. 1 indicates the measurement from 1 d to 7 d in the first cycle. To investigate whether the recharge capability of the specimens would decrease with increasing number of cycles, the recharge/re-release cycle was repeated three times. After 7 d of re-release, the specimens were recharged again, then tested for re-release as cycle 2. This was repeated for 3 cycles in the present study as illustrated in Fig. 1.
After 3 cycles of recharge/re-release, in order to investigate how long the specimens could further release Ca and P ions, the specimens after the 3rd cycle (without further recharge) were immersed in 50 mL of fresh pH 4 solution to measure ion release as described in Section 2.3. The measurements of Ca and P ion re-release from these specimens were continued for an additional 42 d. The concentrations of Ca and P ions were measured at 1, 2, 3, 4, 5, 6, 7, 14, 21, 28, 35 and 42 d as described in Section 2.3.31,40
2.5. Statistical analysis
Kolmogorov-Smirn test and Levene test were performed to confirm the normality and equal variance of data. The results of shear bond strength and Ca and P ion release were analyzed with two-way analyses of variance (ANOVA). Post hoc multiple comparisons were performed using the Tukey’s honestly significant difference test. Statistical significance was set at p < 0.05, using the SPSS 14.0 software package (SPSS, Chicago, IL, USA).
3. Results
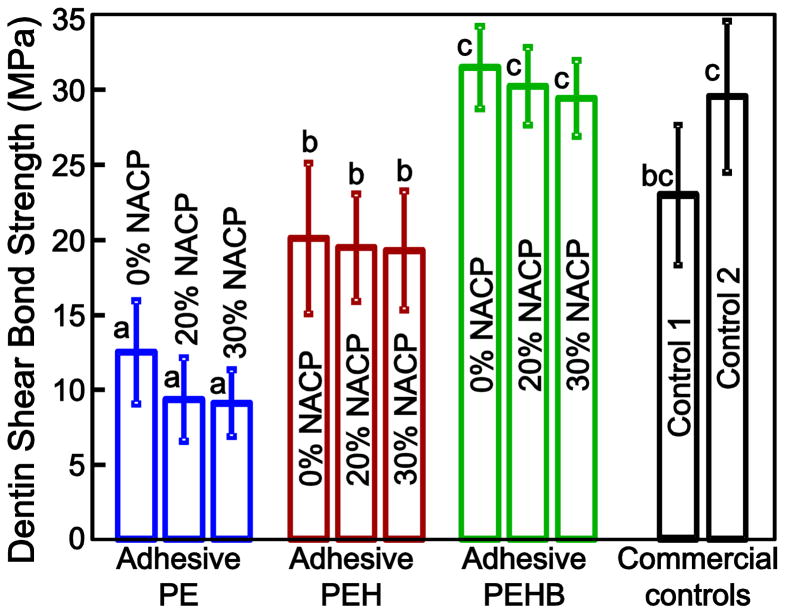
Dentin shear bond strength results are plotted in Fig. 2 (mean ± sd, n = 10). The different adhesive types had a significant effect on dentin bond strength (p < 0.05). Adhesive PEHB had the highest dentin bond strength followed by PEH, while PE had the lowest bond strength (p < 0.05). For each adhesive type, the NACP filler level of 0–30% had no significant effect on dentin bond strength (p > 0.1). Adhesive PEHB had dentin bond strength matching that of control 2 and was higher than that of control 1 (p > 0.1).
Fig. 2.
Dentin shear bond strength using extracted human teeth, tested after storage in water for 24 h (mean ± sd; n = 10). Bars with dissimilar letters indicate values that are significantly different from each other (p < 0.05).
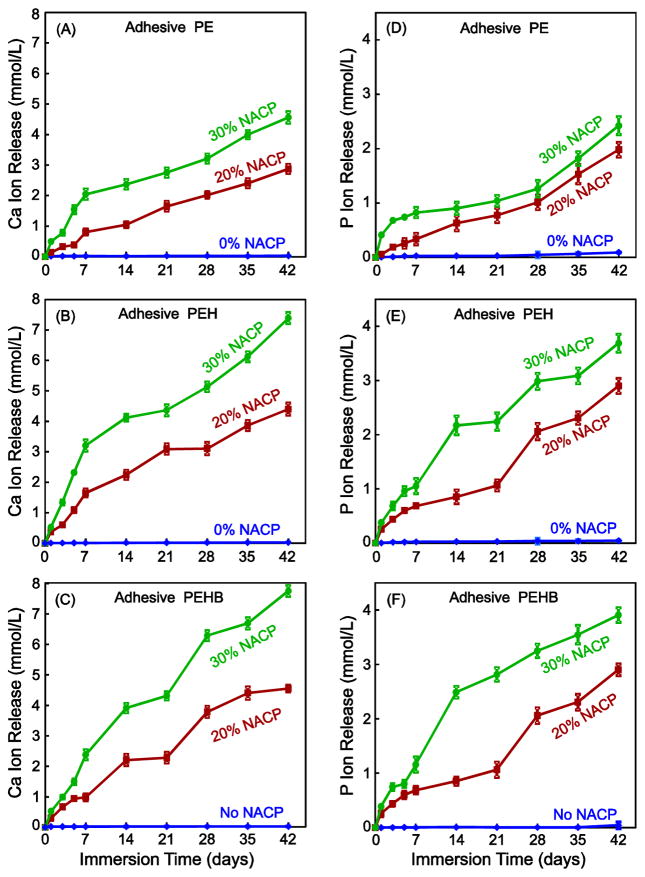
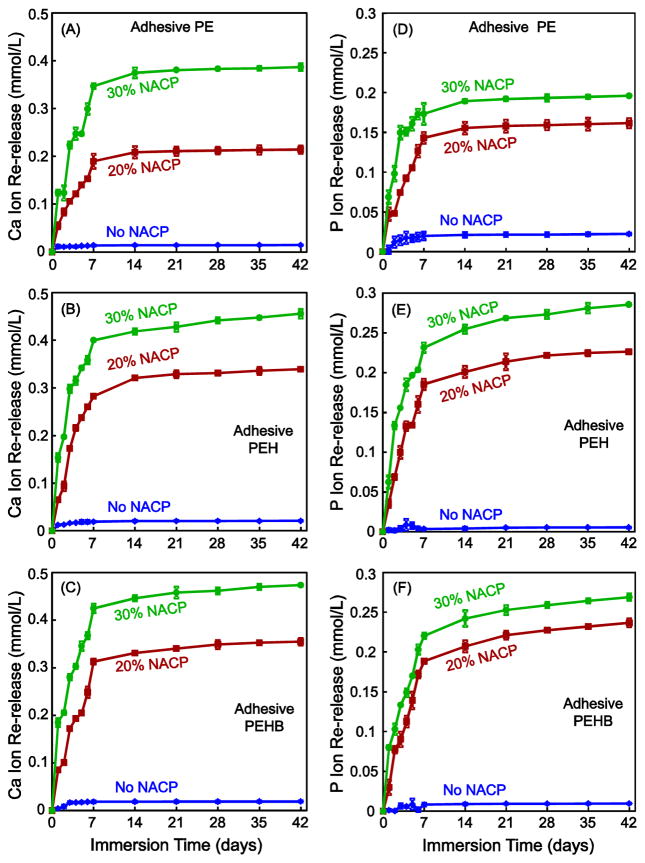
The initial Ca and P ion release from the virgin adhesive specimens are plotted in Fig. 3 (mean ± sd; n = 6). As expected, there was no Ca and P release at 0% NACP. The release significantly increased when the NACP filler level was increased from 20% to 30% (p < 0.05). PEHB had the most ion release, followed by PEH (p < 0.05). PE had the least ion release. For all NACP-containing adhesives, the ion concentrations significantly increased with time from 1 to 42 d (p < 0.05).
Fig. 3.
Initial Ca and P ion release (mean ± sd; n = 6) from the adhesive specimens. Ca and P ion release of adhesive PEH (C, D) and adhesive PEHB (E, F) were significantly higher than that of adhesive PE (A, B) with the same NACP content (p < 0.05). The released ion concentration was plotted as the total accumulative ion concentration versus time. Increasing the NACP content substantially increased the Ca and P ion release (p < 0.05)
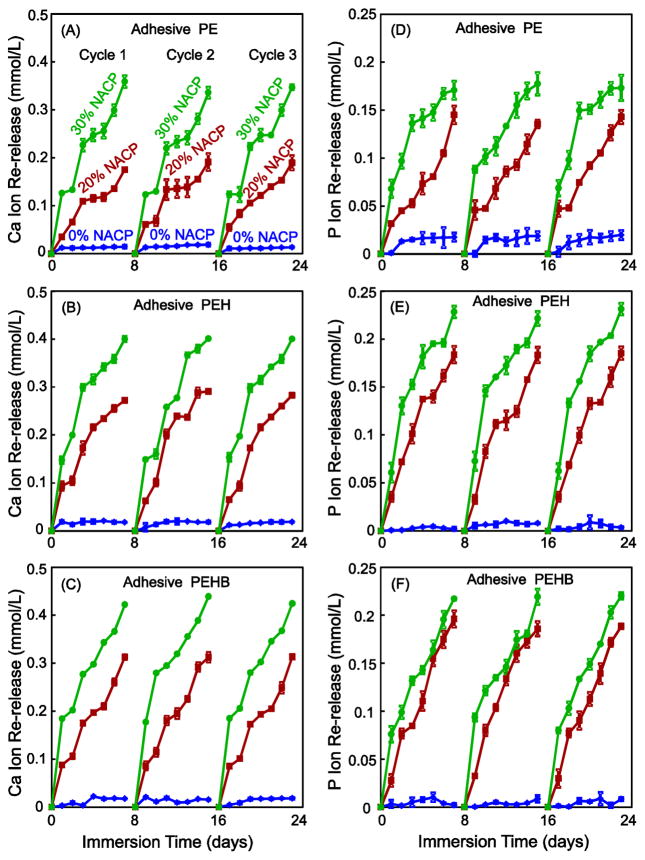
The Ca and P ion recharge and re-release results are plotted in Fig. 4 (mean ± sd, n = 3). The exhausted specimens were recharged and the ion re-release was measured for 7 d, as one cycle. Three recharge/re-release cycles were plotted in Fig. 4. Adhesives with 0% NACP showed little re-release after each recharge. For each adhesive, increasing the NACP filler level to 20% and 30% greatly increased the ion re-release (p < 0.05). For each adhesive, there was no decrease in ion release from the first recharge/re-release cycle to the third cycle. For each cycle, the ion release reached a similarly high level, demonstrating a long-term recharge/re-release capability. Comparing the three types of adhesives, PEHB had the best recharge and re-release capability, followed by PEH and then PE.
Fig. 4.
Ca and P ion re-release from adhesive resin specimens after recharge (mean ± sd; n = 3), tested for three recharge/re-release cycles. Ca and P ion re-release of adhesive PEH (C, D) and adhesive PEHB (E, F) were significantly higher than that of adhesive PE (A, B) with the same NACP content (p < 0.05). There was no decrease in the ion re-release level with increasing the recharge/re-release cycle from cycle 1 to 3 (p > 0.1).
After the third recharge/re-release cycle, the continuous Ca and P ion re-release of the specimens without further recharge was measured for 42 d (Fig. 5) (mean ± sd, n = 3). For NACP-containing adhesives, adhesive PEH and PEHB had higher Ca and P ion re-release than adhesive PE (p < 0.05). Adhesive PEH and PEHB had similar ion release levels (p > 0.1). The release was higher at higher NACP filler level (p < 0.05). The released ion concentrations significantly increased from 1 d to about 14 d and then gradually reached a plateau. These results demonstrate that after the third recharge, the specimens released Ca and P ions for 7 d in Fig. 4, and then continued to release ions for about 14 d in Fig. 5. Hence, they had significant ion release lasting for about 21 d after the recharge.
Fig. 5.
After the third recharge/re-release cycle with ion release for 7 d (after Fig. 4), the specimens without further recharge were tested for continuous Ca and P ion release for 42 d (mean ± sd; n = 3). Adhesive PEH and PEHB had greater re-release than adhesive PE (p < 0.05). The ion concentration increased for about two weeks and then plateaued. Therefore, the recharged specimens (without further recharge) could re-release ions for 7 d in Fig. 4, and then continue to release ions for two more weeks in Fig. 5.
4. Discussion
In the present study, a calcium phosphate ion rechargeable bonding agent was developed for the first time. The effects of resin composition and NACP filler level on dentin bond strength and CaP recharge and re-release were determined. Among the three adhesives tested, the adhesive PEHB with 30% NACP showed the highest initial Ca and P ion release, the greatest recharge and re-release capability, as well as the highest dentin bond strength compared to PEH and PE. Re-incorporation of minerals into the dentin HL is important because the precipitated minerals may help repair nanometer-sized voids such as nanoeakages that remain within the HL. In addition, the remineralized HL may be more resistant to degradation in the oral environment and be able to resist and neutralize biofilm acids18,19 Furthermore, according to the minimally-invasive treatment concept,4,46 more dentin tissues including some caries-infected and caries-affected dentin are recommended to be preserved in the prepared cavity, in order to save tooth structure and avoid perforating the pulp. A NACP adhesive may help remineralize the caries-infected and caries-affected lesions in the tooth cavity. Besides the existing lesions, microleakage along the restoration margins can provide pathways for new invading bacteria and lead to secondary caries along the bonded interface.47–49 Therefore, CaP resins would be highly beneficial to release Ca and P ions to combat secondary caries.40,50,51 The novel rechargeable NACP adhesive is promising to provide sustained Ca and P ion release at the margins which is the weak link, and provide long-term remineralization and caries inhibition vis repeated recharge and re-release.
PMGDM and EBPADMA (PE) are the major monomers used in all three types of adhesives in the present study. PMGDM is an acidic adhesive monomer that has been previously used in dental bonding agents and a calcium phosphate-based cement.25,30 The primer used with all three adhesives contained PMGDM, adopted from a previous study which yielded a relatively high dentin shear bond strength of about 30 MPa.30 However, the adhesive PE group showed the lowest dentin shear bond strength of only about 10 MPa. PE consisted of 49.5% of PMGDM and 49.5% of EBPADMA, and was more viscous than the other two adhesives which contained HEMA. The more viscous PE could have a low wettability on dentin surfaces, which may impair its infiltration into the demineralized dentin matrix and dentinal tubules, resulting in a lower dentin bond strength.17 The incorporation of 10% HEMA in PEH and PEHB resulted in significantly higher dentin shear bond strengths than PE. This was likely because the addition of HEMA improved the hydrophilicity and bonding ability of the adhesive.43,52 In addition, PEH and PEHB containing HEMA likely had better chemical affinity with the HEMA-containing primer than PE. Furthermore, the noticeable decrease in viscosity of the adhesive due to the addition of HEMA may facilitate the infiltration of monomers into the demineralized dentin and the formation of resin tags.17 PEHB contained 5% of BisGMA while the EBPADMA amount was reduced by 5%. To maintain a similar capability to chelate with Ca ions, the PMGDM content was the same in PEH and PEHB. BisGMA is one of the most common monomers in dental adhesives.43,53 BisGMA contains ester linkages that connect Bis-phenol-A segments to the polymerizable vinyl segments.43,53 BisGMA has bisphenol A as the core of its chemical structure, which makes it a very stiff molecule to result in a mechanically strong polymer. In addition, BisGMA has two pendant hydroxyl groups that can form strong hydrogen bonds with the hydroxyl groups on adjacent BisGMA molecules. Furthermore, the molecular weight of BisGMA is very high, which also promotes excellent mechanical properties. Indeed, adding 5% BisGMA in PEHB yielded a dentin shear bond strength of about 30 MPa, significantly higher than the nearly 20 MPa of PEH without BisGMA. However, BisGMA-rich and HEMA-rich dentin bonding agents may raise potential concerns of adhesive hydrolysis and degradation of the bonded interfaces.54,55 Therefore, the amounts of BisGMA and HEMA in the adhesive need to be carefully controlled as they may influence the bonding durability.56 The present study used only small amounts of BisGMA and HEMA (5% and 10%, respectively) to minimize any negative effect on the bonding durability. Further study needs to evaluate the effects of adhesive compositions, in particular, the PEHB, on the long-term durability of the dentin-resin bond.
All three types of adhesives showed high levels of Ca and P ion releases at relatively low NACP filler levels of 20–30% (Fig. 3), which were substantially higher than the ion releases reported for traditional CaP-filled resins.22,27,35 Traditional CaP-filled resins used CaP particle sizes of about 17 μm and 1–55 μm.25,27,35 The NACP used in the present study has a much smaller particle size of 116 nm. NACP particles had a higher surface area of 17.76 m2/g, compared to about 0.5 m2/g of traditional CaP particles in previous studies.27,35 This enabled the release of substantial Ca and P ions using relatively low NACP filler levels in the adhesive, to avoid decreasing the dentin bond strength at higher CaP filler levels. Among the three types of bonding agents, at the same NACP content, the initial Ca and P ion releases of PEHB and PEH were higher than PE. This was likely due to the incorporation of HEMA which increased the hydrophilicity and hence the ion release, consistent with a previous study.52 Previous studies showed that the Ca and P ion release was the highest at pH 4, much less at pH 5, and minimal at pH 7.31,44 Therefore, the CaP-containing resin was “smart” and could greatly increase the ion release at a cariogenic low pH during a biofilm acid challenge, when these Ca and P ions would be most needed to combat demineralization.57,58 When the cariogenic bacteria produce acids to decrease the local pH, the NACP-containing adhesive could release ions and neutralize the acid to increase the pH to a safe pH of above 5 to minimize demineralization.44
All the tested NACP-containing adhesives showed a Ca and P ions recharge and re-release capability. The recharge ability of these adhesives may be attributed to two reasons. The first is the chemical properties of the adhesive resin matrix. Our preliminary study showed that the resin matrix of BisGMA-TEGDMA at 1:1 ratio containing NACP had no recharge and re-release capability. The resin of PMGDM-EMBPDMA at 1:1 ratio containing NACP had the highest Ca and P ion recharge and re-release. The resin of BisGMA and Bis[2-(methacryloyloxy)ethyl] phosphate (Bis-MEP) containing NACP had a moderate Ca and P ion recharge and re-release capability. Thus the preliminary screening test indicated that the PMGDM-EBPAGMA resin had the best potential of Ca and P ion recharge. Therefore, the present study selected PMGDM and EMBPDMA as the major monomers in all three adhesive systems. The carboxylate groups of PMGDM can chelate with Ca ions of dentin or of the exterior environment,30,41 such as those in a recharge solution. PMGDM in the adhesive may chelate with Ca ions in the recharge solution at pH 7. After the recharge, the bond between PMGDM and Ca might break down during a cariogenic challenge such as a local pH of 4, thus inducing the re-release of the ions. Another factor in the recharge ability might be the space-occupying effect. After the initial Ca and P ion release, the sites that were previously occupied by the Ca and P ions are available for the incoming Ca and P ions from the recharge solution. Hence, for rechargeable CaP resins, the material with the higher initial Ca and P ion release should also have a higher Ca and P ion recharge and re-release. Indeed, adhesives PEHB and PEH, which had higher initial ion releases, also had higher re-release than PE. This may also explain why the adhesive with 30% NACP showed higher ion release and recharge capability than those with 20% NACP. This is consistent with previous studies on recharge of F-releasing dental materials, which showed that materials with higher initial F release also exhibited greater F recharge and re-release.37,39,59 Among the three adhesive systems developed in the present study, PEHB appeared to have the best potential for clinical applications. First, PEHB showed the highest dentin bond strength, which was not negatively affected by the incorporation of 30% NACP. Second, with the incorporation of 30% NACP, PEHB showed the highest Ca and P release and recharge capability to provide long-term remineralization.
It should be noted that the environmental pH of ion release measurement in the present study was 4, with the purpose to mimic the bacterial acidic condition. The Stephan Curve showed that the plaque pH, following a glucose mouthrinse, stayed in the cariogenic area of pH 4 or 4.5 for several minutes, and then the pH increased back to 5.5 or higher, after the bacteria had completed their metabolization of the glucose and the saliva had buffered the acid.60 Therefore, the immersion in pH 4 solution of the present study was an aggressive and accelerated test, and not an exact simulation of in vivo conditions. Most of the times the pH in vivo would be close to neutral. A previous study showed that the Ca and P ion release was minimal at pH 7, and increased dramatically at pH 4.40 Therefore, it is expected that the Ca and P ions would be largely preserved at neutral pH; then during a biofilm acid challenge, the ion release would be greatly increased, when these ions would be most needed to combat caries. Further studies are needed to investigate the ion release at neutral pH and the efficacy of combating caries under in vivo conditions via the rechargeable resins.
The recharge capability of the NACP-containing adhesives is worth noting. The resin could be recharged once in the morning and once in the evening for only a single day with a total of two doses of recharge, and then the resin could release Ca and P ions continuously for 7 days without further recharge. Even after releasing for 7 days, without further recharge, they continued to release for another two weeks before the release started to plateau (Fig. 5), at which point another recharge would be needed. Therefore, clinically it may be possible to use the Ca and P recharge solution mouth-rinse for one day to have lasting release for 2–3 weeks, which would be user friendly. Alternatively, if the patient uses the Ca and P recharge solution mouth-rinse daily, the release would likely be enhanced to provide superior remineralization and biofilm acid-neutralizing capabilities to inhibit caries. Further studies are needed to optimize the recharge method, determine the clinical efficacy, and develop a mouth-rinse for Ca and P recharge for resin restorations in vivo. The Ca and P recharge technology using PMGDM and EBPAGMA monomers and NACP is expected to have wide applicability to other dental resins, composites, adhesives, sealants, coatings, orthodontic cements, resin-modified glass ionomer cements (for both Ca and P as well as F release), to promote long-term remineralization and caries-inhibition effects.
5. Conclusion
The present study developed the first rechargeable calcium phosphate dental bonding agent to provide long-term Ca and P ions to promote remineralization and inhibit caries. Adhesive PEHB showed the highest dentin bond strength, and increasing the NACP content from 0% to 30% did not compromise the dentin bond strength. PEHB had the highest initial Ca and P ion release, followed by PEH and PE. PEHB had the greatest Ca and P ion recharge and re-release, followed by PEH and PE. After recharge, the resins had continuous release of ions for at least 2–3 weeks, before another recharge would be needed. For each adhesive, NACP at 30% yielded much better Ca and P release, recharge and re-release than 20% NACP. Among all the bonding agents tested, PEHB with 30% NACP appeared to be the best, considering its highest dentin bond strength, Ca and P ion release, and recharge and re-release. The new Ca and P recharge method and the novel PMGDM-EBPAGMA-NACP composition may be applicable to other adhesives, composites, sealants, coatings and cements, to combat secondary caries and remineralize tooth lesions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayne SC, Thompson JY, Swift EJ, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 2.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 3.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferracane JL. Resin composite - state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Lynch CD, Opdam NJ, Hickel R, Brunton PA, Gurgan S, Kakaboura A, et al. Guidance on posterior resin composites: Academy of Operative Dentistry - European Section. J Dent. 2014;42:377–83. doi: 10.1016/j.jdent.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Debnath S, Ranade R, Wunder SL, McCool J, Boberick K, Baran G. Interface effects on mechanical properties of particle-reinforced composites. Dent Mater. 2004;20:677–86. doi: 10.1016/j.dental.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. J Dent Res. 2005;84:822–826. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 8.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dental Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dental Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Lynch CD, McConnell RJ, Wilson NH. Posterior composites: the future for restoring posterior teeth? Prim Dent J. 2014;3:49–53. doi: 10.1308/205016814812143923. [DOI] [PubMed] [Google Scholar]
- 11.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent Mater. 2007;23:170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: Aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, et al. Adhesive/Dentin interface: the weak link in the composite restoration. Ann Biomed Eng. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferracane JL. Resin-based composite performance: Are there some things we can’t predict? Dent Mater. 2013;29:51–58. doi: 10.1016/j.dental.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohaty BS, Ye Q, Misra A, Sene F, Spencer P. Posterior composite restoration update: focus on factors influencing form and function. Clin Cosmet Investig Dent. 2013;15:33–42. doi: 10.2147/CCIDE.S42044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer P, Ye Q, Misra A, Goncalves SE, Laurence JS. Proteins, pathogens, and failure at the composite-tooth interface. J Dent Res. 2014;93:1243–1249. doi: 10.1177/0022034514550039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16–32. doi: 10.1016/j.dental.2013.08.201. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osorio R, Osorio E, Medina-Castillo AL, Toledano M. Polymer nanocarriers for dentin adhesion. J Dent Res. 2014;93:1258–63. doi: 10.1177/0022034514551608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–57. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 21.Tay FR, Pashley DH. Biomimetic remineralization of resinbonded acid-etched dentin. J Dent Res. 2009;88:719–724. doi: 10.1177/0022034509341826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J Dent. 2013;41:504–13. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tauböck TT, Zehnder M, Schweizer T, Stark WJ, Attin T, Mohn D. Functionalizing a dentin bonding resin to become bioactive. Dent Mater. 2014;30:868–75. doi: 10.1016/j.dental.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–66. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 26.Skrtic D, Antonucci JM, Liu DW. Ethoxylated bisphenol dimethacrylate-based amorphous calcium phosphate composites. Acta Biomater. 2006;2:85–94. doi: 10.1016/j.actbio.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langhorst SE, O’Donnell JN, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent Mater. 2009;25:884–91. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J Dent Res. 2012;91:979–84. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melo MA, Cheng L, Weir MD, Hsia RC, Rodrigues LK, Xu HH. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J Biomed Mater Res B Appl Biomater. 2013;101:620–9. doi: 10.1002/jbm.b.32864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C, Weir MD, Cheng L, Lin NJ, Lin-Gibson S, Chow LC, et al. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent Mater. 2014;30:891–901. doi: 10.1016/j.dental.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 33.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Arola DD, Gu L, Kim YK, Mai S, Liu Y, et al. Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach. Acta Biomater. 2010;6:2740–2750. doi: 10.1016/j.actbio.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regnault WF, Icenogle TB, Antonucci JM, Skrtic D. Amorphous calcium phosphate/urethane methacrylate resin composites. I. Physicochemical characterization. J Mater Sci Mater Med. 2008;19:507–15. doi: 10.1007/s10856-007-3178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu HH, Weir MD, Sun L. Nanocomposites with Ca and PO4 release: effects of reinforcement, dicalcium phosphate particle size and silanization. Dent Mater. 2007;23:1482–91. doi: 10.1016/j.dental.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003;24:2451–61. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Ling L, Wang R, Burgess JO. Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006;22:1014–23. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Ahn SJ, Lee SJ, Lee DY, Lim BS. Effects of different fluoride recharging protocols on fluoride ion release from various orthodontic adhesives. J Dent. 2011;39:196–201. doi: 10.1016/j.jdent.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venz S, Dickens B. Modified surface-active monomers for adhesive bonding to dentin. J Dent Res. 1993;72:582–586. doi: 10.1177/00220345930720030501. [DOI] [PubMed] [Google Scholar]
- 42.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dent Mater. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 44.Moreau JL, Sun L, Chow LC, Xu HH. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J Biomed Mater Res B Appl Biomater. 2011;98:80–8. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer-Lueckel H, Hopfenmuller W, von Klinggraff D, Kielbassa AM. Microradiographic study on the effects of mucin-based solutions used as saliva substitutes on demineralised bovine enamel in vitro. Arch Oral Biol. 2006;51:541–7. doi: 10.1016/j.archoralbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. J Am Dent Assoc. 2011;142:612–20. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 47.Fruits TJ, Knapp JA, Khajotia SS. Microleakage in the proximal walls of direct and indirect posterior resin slot restorations. Oper Dent. 2006;31:719–727. doi: 10.2341/05-148. [DOI] [PubMed] [Google Scholar]
- 48.Coelho-De-Souza FH, Camacho GB, Demarco FF, Powers JM. Fracture resistance and gap formation of MOD restorations: influence of restorative technique, bevel preparation and water storage. Oper Dent. 2008;33:37–43. doi: 10.2341/07-27. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Godoy F, Krämer N, Feilzer AJ, Frankenberger R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs. in vivo. Dent Mater. 2010;26:1113–8. doi: 10.1016/j.dental.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, et al. Strong nanocomposites with Ca, PO4, and F release for caries inhibition. J Dent Res. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melo MA, Weir MD, Rodrigues LK, Xu HH. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent Mater. 2013;29:231–40. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skrtic D, Antonucci JM. Dental composites based on amorphous calcium phosphate - resin composition/physicochemical properties study. J Biomater Appl. 2007;21:375–93. doi: 10.1177/0885328206064823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, et al. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20. [PubMed] [Google Scholar]
- 54.Finer Y, Santerre JP. The influence of resin chemistry on a dental composite’s biodegradation. J Biomed Mater Res A. 2004;69:233–46. doi: 10.1002/jbm.a.30000. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi M, Nakajima M, Hosaka K, Ikeda M, Foxton RM, Tagami J. Long-term evaluation of water sorption and ultimate tensile strength of HEMA-containing/-free one-step self-etch adhesives. J Dent. 2011;39:506–512. doi: 10.1016/j.jdent.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Park J, Eslick J, Ye Q, Misra A, Spencer P. The influence of chemical structure on the properties in methacrylate-based dentin adhesives. Dent Mater. 2011;27:1086–93. doi: 10.1016/j.dental.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thylstrup A, Fejerskov O. Textbook of Cariology. Copenhagen, Denmark: Munksgaard; 1986. pp. 145–146. [Google Scholar]
- 58.Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69:722–724. [PubMed] [Google Scholar]
- 59.Naoum S, Ellakwa A, Martin F, Swain M. Fluoride release, recharge and mechanical property stability of various fluoride-containing resin composites. Oper Dent. 2011;36:422–32. doi: 10.2341/10-414-L. [DOI] [PubMed] [Google Scholar]
- 60.Thylstrup A, Fejerskov O. Textbook of cariology. Copenhagen, Denmark: Munksgaard; 1986. pp. 145–146. [Google Scholar]