Abstract
Background
Patients with rheumatoid arthritis (RA) have increased coronary heart disease (CHD) risk. Some RA therapies may modify this risk, but underlying mechanisms are unclear. HDL’s cholesterol efflux capacity is associated with reduced CHD risk in non-RA populations; however, inflammation may impair HDL’s function. We hypothesized that reduced inflammation from treatment with methotrexate (MTX), adalimumab (ADA) and tocilizumab (TOC) would increase net cholesterol efflux capacity (CEC) of HDL in patients with RA.
Methods
A longitudinal multi-center study (Treatment Efficacy and Toxicity in Rheumatoid Arthritis Database and Repository) provided clinical information and serum samples from 70 patients with RA before and 6 months after starting a new drug (MTX (n=23), ADA (n=22), and TOC (n=25)). Disease activity was measured by DAS28-ESR. Net CEC was measured in paired serum samples using THP-1 macrophages with fluorometric assay for cholesterol measurement.
Results
DAS28-ESR decreased with all treatments (P<0.001). Net CEC was not significantly changed after 6 months of new RA therapy (mean± SD, baseline, 36.9%±17.3% units vs. 6 months, 38.0%±16.9% units, P=0.58). However, change in net CEC was associated with change in DAS28-ESR (rho=−0.25, P=0.04). In post hoc analyses of patients with impaired baseline net CEC, TOC resulted in significant improvement in net CEC (baseline, 21.9%±14.7% units vs. 6 months, 31.1%±12.8% units, P=0.02), but this was not observed with MTX or ADA (all P>0.05).
Conclusion
HDL’s net CEC did not change significantly after 6 months of new RA therapy, except in those with impaired baseline CEC receiving TOC. Change in disease activity was associated with change in net CEC.
Ischemic heart disease is increased almost 2-fold in patients with RA (1). In most populations increased low density lipoprotein (LDL) and decreased high density lipoprotein (HDL) cholesterol concentrations are among the strongest treatable cardiovascular (CV) risk factors identified. However, LDL cholesterol concentrations tend to be lower and HDL cholesterol concentrations are not substantially altered in RA, and thus do not account for the increased CV risk (2).
Recent evidence from non-RA populations indicates that HDL function, specifically its ability to remove cholesterol from macrophages (termed cholesterol efflux capacity) may be more important in protecting against CV risk than HDL cholesterol concentration (3). Moreover, another function of HDL, its ability to prevent oxidation of LDL, is frequently impaired in patients with RA (4,5). Thus, novel risk markers such as these or other measures of HDL function may be better indices of CV risk, particularly in populations such as RA in whom inflammation can cause changes to lipoproteins that are not detected by measuring cholesterol concentrations. In a small study, RA patients with high disease activity had impaired cholesterol efflux capacity compared to those with low disease activity or remission (6), suggesting that HDL dysfunction could potentially be reversed. Little is known about how RA treatment affects cholesterol efflux capacity.
Many of the drugs used to treat RA can increase LDL cholesterol concentrations (7,8), raising the possibility of increased CV risk. However, several drugs for RA such as methotrexate (MTX) and adalimumab (ADA) reduce rather than increase CV risk (9). We hypothesized that this improved CV risk is due to beneficial effects of the drugs on HDL cholesterol efflux capacity as a result of reduced inflammation or through treatment-specific mechanisms. Thus, we defined the effect of three different commonly used RA drugs, MTX, ADA, and tocilizumab (TOC), on HDL-mediated cholesterol efflux capacity in patients with RA.
Materials and Methods
Study population
We performed a longitudinal study of 70 patients with RA, before starting MTX (N=23), ADA (N=22), or TOC (N=25) therapy and after 6 months of treatment with the respective drug. The patients were recruited as part of the Treatment Efficacy and Toxicity in Rheumatoid Arthritis Database and Repository (TETRAD) (NCT#01070121) from University of Alabama at Birmingham (coordinating center), Brigham and Women’s Hospital, Duke University, Johns Hopkins University, North Shore Medical Center, Stanford University, University of Colorado Denver, University of Nebraska, and University of Pittsburgh. Patients met the following inclusion criteria: age 19 years or older; fulfilment of the American College of Rheumatology 1987 classification criteria for RA (10); willingness and ability to provide informed consent; starting MTX, or previous or current use of MTX and starting (or switching to) etanercept, infliximab, ADA, rituximab, abatacept, golimumab, certolizimab, or TOC as part of routine care. Patients who also had systemic lupus erythematosus, juvenile arthritis, psoriatic arthritis, hepatitis C infection, or were currently pregnant or lactating were excluded. For the present study only patients starting MTX, ADA, or TOC (index drugs) were included. Of the 70 patients included in the study, 5 patients changed index drug, and 6 patients stopped the index drug or had missing data for index drug at month 6. A sensitivity analysis was performed excluding patients who changed or stopped the index drug or had missing data for index drug at month 6 (including 4 taking MTX, 5 taking ADA and 2 taking TOC).
Clinical and laboratory information
RA disease activity was determined by the DAS28-ESR score using erythrocyte sedimentation rate (ESR) (11). ESR was measured by the clinical laboratories at each center. Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNFα) were measured by multiplex ELISA (Millipore Corporation, Billerica, MA, USA).
Measurement of net cholesterol efflux capacity of HDL-enriched serum
Net cholesterol efflux capacity was measured as previously described (12,13) with minor modifications. Human monocyte THP-1 cells were plated in 12 multi-well plates (1×106 cells/ 1 ml RPMI1640 with 10% fetal bovine serum and 0.1% phorbol myristate acetate). After 72 hours, the cells were incubated with 100µg/ml acetylated LDL (Intracel, #RP-045) for 72 hours, resulting in foam cell formation. Medium was changed to RPMI containing 4% fatty acid free bovine serum albumin (Sigma, #A6003) for one hour. Patient serum (250 µl) was added to 100 µl of polyethylene glycol (PEG) solution (20% PEG 8000 in 200mM glycine) and incubated at room temperature for 15 minutes and then centrifuged at 1900g. The supernatant was removed and used as apolipoprotein B (apoB) depleted serum or HDL-enriched serum (14). Cells were then washed and incubated with medium containing 2% apoB depleted serum. Acetylated LDL exposed cells exposed to medium only were used as a comparator. After 24 hours of incubation, cells were washed twice and air dried. Cellular lipids were extracted with isopropanol and total cellular cholesterol was measured by fluorometric assay (15). Cholesterol content was corrected for total cellular protein for each well. Cholesterol efflux capacity was defined as the % change in total cellular cholesterol content (in µg/mg protein) between wells exposed to medium and apoB depleted serum (16,17). Samples were run in duplicate. The mean intra-assay coefficient of variation was 20.1%. We defined impaired baseline net cholesterol efflux capacity as efflux capacity below the mean.
Statistical analysis
The primary outcome of interest was the overall effect of RA treatment on net cholesterol efflux capacity of HDL-enriched serum; prespecified subgroup analysis was the effect of individual drug (MTX, ADA, and TOC) on net cholesterol efflux capacity.
With 70 paired samples, we had 90% power to detect a 6.5% unit change in net cholesterol efflux, and based on a sample size of 22 paired samples (ADA group, which had the smallest sample size among the individual treatment groups), we had 80% power to detect a 12.0% unit change in net cholesterol efflux. This permitted more than sufficient power to detect change in cholesterol efflux of less than one standard deviation (17.3% units in our study) for all treatments combined and for individual therapies. A change in cholesterol efflux capacity of one standard deviation was deemed clinically important since a decrease of this magnitude was previously associated with a 20% reduction CV risk (18).
Descriptive statistics were calculated as mean ± standard deviation (SD) for normally distributed variables, median with interquartile range (median [IQR: 25th, 75th]) for skewed continuous variables, and frequency and proportions for categorical variables. Wilcoxon signed rank tests were used to compare variables at baseline and after drug therapy. Analysis of covariance (ANCOVA) was used to compare the effect of each drug on cholesterol efflux capacity. The dependent variable was efflux at 6 months and independent variables were efflux at baseline and index drug.
Statistical analyses were performed using IBM SPSS Statistics version 22, and PS Power and Sample Size Calculations version 3.0. Two-sided P values less than or equal to 0.05 were considered statistically significant.
RESULTS
Clinical characteristics
Patients were mostly Caucasian (80%), female (84%), and rheumatoid factor positive (64%) with a mean age of 53 ± 12 years at study entry (Table 1). Disease activity by DAS28-ESR score was moderate to high at entry (mean ± SD: 4.7 units ± 1.3) (Table 1). Fifty one percent of all patients were taking prednisone; 82% of those starting ADA and 48% of those starting TOC were taking concomitant MTX (Table 1).
Table 1.
Baseline patient characteristics
| All Patients (N=70) |
Methotrexate (N=23) |
Adalimumab (N=22) |
Tocilizumab (N=25) |
|
|---|---|---|---|---|
| Age, year | 53 ± 12 | 52 ± 12 | 56 ± 11 | 53 ± 13 |
| Race, Caucasian | 56 (80) | 17 (74) | 17 (77) | 22 (88) |
| Sex,female | 59 (84) | 18 (78) | 19 (86) | 22 (88) |
| RF, positive | 45 (64) | 15 (65) | 12 (55) | 18 (72) |
| CCP, positive | 44 (63) | 17 (74) | 11 (50) | 16 (64) |
| DAS28-ESR, units | 4.7 ± 1.3 | 4.6 ±1.2 | 4.8 ±1.4 | 4.8 ±1.4 |
| Disease duration, year | 9 ± 11 | 2.0 ± 5.7 | 7.7 ± 7.5 | 16.6 ± 13.7 |
| Methotrexate use | 44 (63) | - | 18 (82) | 12 (48) |
| Leflunomide use | 6 (9) | 2 (9) | 3 (14) | 1 (4) |
| Sulfasalazine use | 1 (1) | 1 (4) | - | - |
| Azathioprine use | 2 (3) | - | 1 (5) | 1 (4) |
| Hydroxychloroquine use | 12 (17) | 6 (26) | 5 (23) | 1 (4) |
| Prednisone use | 36 (81) | 12 (86) | 11 (58) | 13 (54) |
| NSAID use | 30 (63) | 11 (73) | 7 (54) | 12 (60) |
Data are presented at mean ± standard deviation for continuous data, and number (percent) for categorical data. RF= rheumatoid factor, anti-CCP= anti-cyclic citrullinated peptide antibody, DAS28-ESR= disease activity score based on erythrocyte sedimentation rate. Prednisone use available in N=57 total. NSAID use available in N=48 total.
Change in disease activity with DMARD or Biologic therapy
DAS28-ESR scores improved significantly after 6 months of DMARD or biologic therapy (mean change: −1.4 ± 1.4 units, P<0.001); the change in disease activity was similar across initiators of individual drugs (MTX, ADA and TOC) with the mean change ranging from −1.3 to −1.5 units (Table 2). All drugs resulted in a significant reduction in ESR, and tender joint count (all P <0.05) (Table 2).
Table 2.
Disease activity and markers of inflammation before and after DMARD or biologic
| Baseline | 6 Months | Absolute Change | P value* | |
|---|---|---|---|---|
| DAS28-ESR, units | ||||
| All drugs (N=70) | 4.7 ± 1.3 | 3.4 ± 1.4 | −1.4 ± 1.4 | <0.001 |
| Methotrexate (N=23) | 4.6 ± 1.2 | 3.3 ± 1.1 | −1.3 ± 1.2 | <0.001 |
| Adalimumab (N=22) | 4.8 ± 1.4 | 3.6 ± 1.3 | −1.3 ±1.3 | 0.001 |
| Tocilizumab (N=25) | 4.8 ± 1.4 | 3.3 ± 1.8 | −1.5 ± 1.6 | <0.001 |
| ESR, mm/hr | ||||
| All drugs (N=70) | 20 [10, 38] | 8.5 [3, 23] | −8 [−17, 0] | <0.001 |
| Methotrexate (N=23) | 28 [10, 42] | 19 [11, 40] | −4 [−16, 0] | 0.01 |
| Adalimumab (N=22) | 18 [11, 30] | 7 [4, 24] | −4 [−14, 1] | 0.03 |
| Tocilizumab (N=25) | 19 [11, 40] | 7 [2, 15] | −11 [−31, −2] | <0.001 |
| Tender joint count, # | ||||
| All drugs (N=70) | 7 [3, 14] | 3 [1, 9] | −3 [−6, 0] | <0.001 |
| Methotrexate (N=23) | 5 [3, 11] | 1 [0, 3] | −2 [−6, 0] | 0.03 |
| Adalimumab (N=22) | 12 [3, 16] | 6 [1, 10] | −3 [−8, 0] | 0.003 |
| Tocilizumab (N=25) | 7 [5, 15] | 6 [1, 11] | −3 [−7, 1] | 0.006 |
| Swollen joint count, # | ||||
| All drugs (N=70) | 7 [3, 10] | 2 [1, 5] | −3 [−7, 0] | <0.001 |
| Methotrexate (N=23) | 6 [3, 8] | 1 [0, 3] | −3 [−7, −2] | 0.001 |
| Adalimumab (N=22) | 9 [4, 13] | 3 [1, 5] | −5 [−7, 0] | 0.006 |
| Tocilizumab (N=25) | 6 [2, 10] | 3 [1, 9] | −1 [−5, 1] | 0.11 |
| IL-6, pg/ml | ||||
| All drugs (N=70) | 3.9 [0.0, 17.3] | 3.1 [0.0, 26.0] | 0.0 [−6.3, 10.8] | 0.47 |
| Methotrexate (N=23) | 4.8 [0.0, 22.4] | 1.6 [0.0, 6.7] | −0.3 [−7.0, 0.9] | 0.20 |
| Adalimumab (N=22) | 4.0 [0.0, 23.1] | 0.0 [0.0, 4.8] | 0.0 [−13.5, 0.0] | 0.03 |
| Tocilizumab (N=25) | 3.7 [0.0, 15.2] | 27.5 [1.9, 61.7] | 17.8 [0.0, 43.2] | 0.004 |
| TNF-α, pg/ml | ||||
| All drugs (N=70) | 11.5 [7.4, 17.1] | 10.2 [7.5, 14.7] | −0.7 [−3.7–1.6] | 0.10 |
| Methotrexate (N=23) | 10.4 [7.3–15.3] | 11.5 [7.8, 14.7] | −0.1 [−3.7, 1.7] | 0.66 |
| Adalimumab (N=22) | 11.1 [8.5–17.3] | 9.2 [7.6, 12.4] | −1.6 [−8.7, 0.3] | 0.02 |
| Tocilizumab (N=25) | 12.5 [6.9–18.6] | 13.8 [6.8, 17.7] | 0.3 [−2.1, 2.3] | 0.85 |
Data are presented as mean ± standard deviation for normally distributed data, and median [interquartile range] for skewed data.
Using Wilcoxon signed ranks test. DAS28-ESR = disease activity score based on erythrocyte sedimentation rate, ESR = erythrocyte sedimentation rate, IL-6 = interleukin 6, TNF-α = tumor necrosis factor alpha.
Net cholesterol efflux capacity of HDL-enriched serum before and after therapy
Baseline net cholesterol efflux capacity was 36.9 ± 17.3% in the entire group (Table 3) and there was no statistically significant change in net cholesterol efflux after 6 months of therapy (mean change: 1.1 ± 16.6%, P =0.58) (Table 3). Similarly, individual drugs did not have a statistically significant effect on net cholesterol efflux capacity: MTX (−3.3 ± 20.2%, P=0.38), ADA (2.3 ± 14.6%, P=0.44), or TOC (3.9 ± 14.2%, P=0.23) (Table 3). When those 5 patients who changed index drug and those 6 patients who stopped the study drug prior to or had missing data for index drug were excluded from analysis, there was no statistically significant change in net cholesterol efflux overall (P=0.90) or with individual drugs (MTX P=0.17; ADA P=0.69; TOC P=0.35). Additionally, we found that change in the use of prednisone (P=0.68) or non-steroidal anti-inflammatories (P=0.59) did not significantly change efflux capacity and that starting or stopping these drugs did not significantly change net cholesterol efflux capacity (Supplementary Table).
Table 3.
Net cholesterol efflux capacity before and after DMARD or biologic therapy
| Baseline | 6 Months | Absolute Change | P value* | |
|---|---|---|---|---|
| All patients | ||||
| All drugs (N=70) | 36.9% ± 17.3 | 38.0% ± 16.9 | 1.1% ± 16.6 | 0.58 |
| Methotrexate (N=23) | 41.2% ±13.9 | 38.0% ±17.5 | −3.3% ± 20.2 | 0.38 |
| Adalimumab (N=22) | 36.1% ±16.8 | 38.5% ± 18.2 | 2.3% ± 14.6 | 0.44 |
| Tocilizumab (N=25) | 33.7% ± 20.1 | 37.6% ± 15.8 | 3.9% ± 14.2 | 0.23 |
| Patients with impaired baseline net cholesterol efflux | ||||
| All drugs (N=37) | 24.1% ±11.3 | 31.0% ±12.4 | 6.8% ±15.7 | 0.02 |
| Methotrexate (N=10) | 28.8% ± 9.5 | 34.0% ± 12.6 | 5.1% ± 19.7 | 0.80 |
| Adalimumab (N=12) | 22.9% ± 6.6 | 28.0% ± 14.5 | 5.1% ± 14.4 | 0.27 |
| Tocilizumab (N=15) | 21.9% ± 14.7 | 31.3% ± 12.8 | 9.4% ± 14.4 | 0.02 |
Using Wilcoxon signed ranks test.Data are presented at mean ± standard deviation.
Net cholesterol efflux capacity of HDL-enriched serum among patients with impaired baseline efflux capacity
In an exploratory analysis we examined the impact of RA therapy on net cholesterol efflux capacity among those who had impaired baseline efflux capacity, assuming that those with impaired net cholesterol efflux capacity at baseline would be more likely to improve with treatment, but those with normal or high efflux capacity would likely not likely improve further. We defined impaired baseline net cholesterol efflux capacity as efflux capacity below the mean. Among those with impaired baseline net cholesterol efflux capacity, there was a significant increase in net cholesterol efflux capacity (mean change ± SD: 6.8% ± 15.7% units, P=0.02) (Table 3). Among individual drugs, TOC significantly improved net cholesterol efflux capacity (mean change ± SD: 9.4% ± 14.4% units, P=0.02), but neither MTX (P=0.80) nor ADA (P=0.27) changed net cholesterol efflux capacity significantly (Table 3). There was no significant change in the net cholesterol efflux capacity among those patients with high cholesterol efflux capacity at baseline (P=0.06) or among individual drugs (MTX P=0.17, ADA P=0.80, TOC P=0.20). Similarly, after excluding 5 patients who changed index drug and 6 patients who stopped the study drug prior to or had missing data for index drug, the results were similar: among those with impaired baseline net cholesterol efflux there was a significant increase in net cholesterol efflux overall (P=0.045), and with use of TOC (P=0.04), but not with MTX (P=0.69) or TOC (P=0.51).
Relationship between change in disease activity and change in net cholesterol efflux capacity
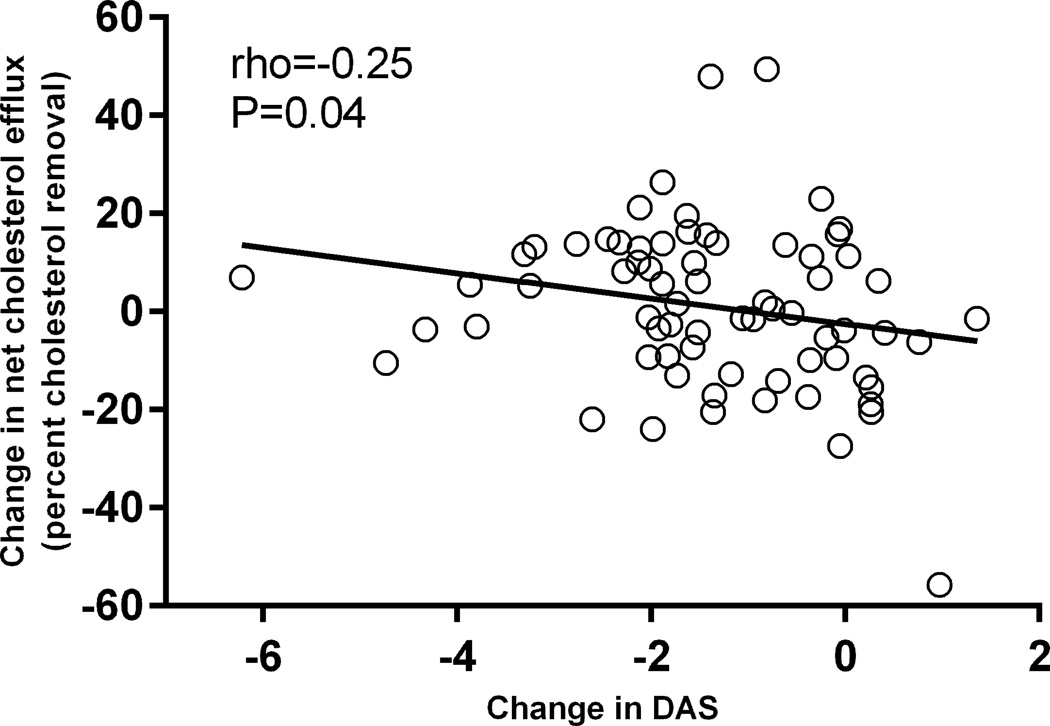
There was no significant association between baseline DAS28-ESR and baseline net cholesterol efflux capacity (rho=−0.14, P=0.24). The reduction in DAS28-ESR score after 6 months of a new DMARD or biologic therapy was correlated with an increase in cholesterol efflux capacity (rho=−0.25, P=0.04) (Figure 1).
Figure.
Relationship between absolute change in DAS28-ESR score and absolute change in net cholesterol efflux after 6 months of new drug therapy. The change in disease activity after 6 months of DMARD or biologic therapy based on the DAS28-ESR score was significantly associated with the change in net cholesterol efflux capacity of HDL-enriched serum (rho=−0.25, P=0.04).
DISCUSSION
The major findings of this study are that after 6 months of treatment with a DMARD or biologic therapy the decrease in disease activity assessed by DAS28-ESR score was correlated with increased net cholesterol efflux capacity of HDL-enriched serum. While on average the net cholesterol efflux capacity did not improve significantly after treatment of RA with MTX, ADA or TOC, there was a significant improvement in net cholesterol efflux capacity among those patients with impaired baseline net cholesterol efflux capacity, particularly those receiving TOC.
A few cross-sectional studies have examined the relationship between inflammation or disease activity and cholesterol efflux capacity in RA. In a study comparing 18 RA patients with high disease activity (DAS28-ESR > 5.1) and 7 RA patients with low disease activity or remission (DAS28-ESR < 2.6), those with high disease activity had significantly lower cholesterol efflux capacity by HDL-enriched serum, and efflux capacity was inversely associated with ESR, but not other inflammatory markers (6). In another study including 30 patients with RA, cholesterol efflux capacity of HDL-enriched serum by only one transporter, ABCG1, was inversely associated with DAS28-ESR score, but not with ESR or CRP. (19). We previously showed that among 134 patients with RA with relatively well controlled disease, there was no significant association between net cholesterol efflux capacity and disease activity or markers of inflammation (20, 21).
A few prospective studies have also examined the effect of reduction of inflammation or RA treatment on efflux capacity, a design that permits more robust examination of the relationship between inflammation and HDL function. A prospective observational cohort study of 90 patients with RA who had a reduction in CRP concentration of 10 mg/L or greater over one year found that after a substantial median reduction of CRP by 23.5mg/l and DAS28-ESR by 1.6 units, there was a small but statistically significant increase in cholesterol efflux capacity(22). There was a modest correlation between change in cholesterol efflux capacity and change in CRP (P=0.02) but not disease activity. Another study tested the effect of treatment with MTX (N=34) or ADA plus MTX (N=22) on whole serum-mediated cholesterol efflux capacity by different pathways (23). After 6 months of MTX use, SR-B1 and ABCG1-mediated cholesterol efflux capacity was increased; numerical values were not provided, but graphically the change appeared small, and contrary to what would be expected, when MTX was combined with ADA, there was no significant effect on cholesterol efflux capacity by any pathway (23).
We explored the possibility that the lack of an effect or small effect of RA therapy on HDL-mediated cholesterol efflux may be due to some patients having high baseline cholesterol efflux capacity and thus less likely to show improvement. In patients with low baseline efflux capacity we found that TOC, but not MTX or ADA, significantly increased cholesterol efflux capacity. This post hoc finding should be interpreted with caution. By splitting patients into these groups (below and above mean net cholesterol efflux capacity), we risk the possibility of our findings being due to regression to the mean even though the change in patients with high efflux capacity at baseline was not significant. Furthermore, TOC could increase HDL-mediated efflux by increasing HDL concentration rather than altering function. Though, because of the significantly decreased plasma reactive oxygen metabolites among users of TOC compared to DMARDs and anti-TNF agents (24), TOC could affect cholesterol efflux capacity though a reduction in oxidative stress, which likely affects HDL function (25,26). Future studies are needed that specifically evaluate the impact of TOC on oxidative stress and its resultant impact on HDL function and potentially CV risk reduction.
The impact of cholesterol efflux capacity on cardiovascular events in RA is currently unknown, but more is known in non-RA populations. In a prospective nested case control study from the EPIC-Norfolk study including 1745 patients with incident coronary heart disease and 1749 control subjects, for every standard deviation increase in cholesterol efflux capacity by HDL-enriched serum, the risk of incident coronary heart disease was reduced 20% independent of traditional CV risk factors (18). Similarly, in the Dallas Heart Study which included 2924 patients initially without CV disease who were followed for a median of 9.4 years, there was a 67% reduction in CV events in the lowest quartile of cholesterol efflux capacity compared to the highest quartile (27). However, not all studies have found this inverse relationship between cholesterol efflux capacity and CV disease. For example, among 1150 patients without acute coronary syndrome who underwent elective diagnostic coronary angiography, increased cholesterol efflux capacity, despite being associated with decreased prevalent coronary artery disease, was associated paradoxically with increased risk of non-fatal MI or stroke and major adverse CV events over 3 years of follow-up (28). The relationship between net cholesterol efflux capacity and CV events and clinically relevant differences in efflux capacity in RA are therefore of interest.
Considering the findings of others and those of the current study, the evidence suggests that the overall effects of DMARD or biologic treatment on HDL-mediated cholesterol efflux are likely to be small and occur in patients with high disease activity; this is likely why we previously found no significant relationship between disease activity and net cholesterol efflux capacity in a cohort of relatively well controlled patients with RA and now show a modest correlation between change in DAS28 and CEC in a cohort of patients with higher disease activity starting new therapy for RA. Other functions of HDL, such as anti-oxidant (4,5) and anti-inflammatory effects may be more strongly influenced by medications and inflammation, and may have an important role in CV disease in RA. Further studies in this area would be helpful.
Limitations
Our study has some limitations. The relationship between cholesterol efflux capacity and increased risk of CV events in patients with RA is not known. Thus, although we found overall that the reduction in disease activity was correlated with increased cholesterol efflux capacity, and efflux capacity increased among patients with impaired baseline efflux capacity after TOC, the clinical relevance of the changes are not known. Additional studies to define the relationship between HDL-mediated efflux, and other function of HDL and CV events in patients with RA will be of interest.
Supplementary Material
Acknowledgments
We thank the following TETRAD investigators: Clifton O. Bingham III - Johns Hopkins University; Stacey S. Cofield – University of Alabama at Birmingham; Richard Furie and Peter K. Gregersen - The Feinstein Institute for Medical Research and North Shore-LIJ Health System; Mark C. Genovese and William H. Robinson – Stanford University; Marc C. Levesque and Larry W. Moreland - University of Pittsburgh; Peter A. Nigrovic and Nancy A. Shadick - Brigham and Women's Hospital/Harvard University; James R. O'Dell and Geoffrey M. Thiele - University of Nebraska; E. William St Clair - Duke University;, Christopher C. Striebich - University of Colorado, Denver.
Funding: Arthritis Foundation Clinical to Research Transition Award, Rheumatology Research Foundation Disease Targeted Research Investigator Award, NIH grants: KL2 TR00046, K23 AR068443, P60 AR056116, P01 HL116263, RC2 AR058964, and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
TETRAD Investigators
Clifton O. Bingham III - Johns Hopkins University; Stacey S. Cofield – University of Alabama at Birmingham; Richard Furie and Peter K. Gregersen - The Feinstein Institute for Medical Research and North Shore-LIJ Health System; Mark C. Genovese and William H. Robinson – Stanford University; Marc C. Levesque and Larry W. Moreland - University of Pittsburgh; Peter A. Nigrovic and Nancy A. Shadick - Brigham and Women's Hospital/Harvard University; James R. O'Dell and Geoffrey M. Thiele - University of Nebraska; E. William St Clair - Duke University; , Christopher C. Striebich - University of Colorado, Denver.
Footnotes
Disclosures: None
References
- 1.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 2.Chung CP, Oeser A, Raggi P, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 3.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. New Engl JMed. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon M, Grossman J, FitzGerald J, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 5.Charles-Schoeman C, Watanabe J, Lee YY, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009;60:2870–2879. doi: 10.1002/art.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157–1162. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro-Millan I, Charles-Schoeman C, Yang S, et al. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum. 2013;65:1430–1438. doi: 10.1002/art.37916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strang AC, Bisoendial RJ, Kootte RS, et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis. 2013;229:174–181. doi: 10.1016/j.atherosclerosis.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg JD, Furer V, Farkouh ME. Cardiovascular safety of biologic therapies for the treatment of RA. Nat Rev Rheumatol. 2012;8:13–21. doi: 10.1038/nrrheum.2011.168. [DOI] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 88(31):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis and Rheumatism. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto S, Yancey PG, Ikizler TA, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Card. 2012;60:2372–2379. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanotti I, Favari E, Bernini F. Cellular cholesterol efflux pathways: impact on intracellular lipid trafficking and methodological considerations. Curr Pharm Biotechnol. 2012;13:292–302. doi: 10.2174/138920112799095383. [DOI] [PubMed] [Google Scholar]
- 14.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinet P, Wang Z, Hazen SL, Smith JD. A simple and sensitive enzymatic method for cholesterol quantification in macrophages and foam cells. J Lipid Res. 2010;51:3364–3369. doi: 10.1194/jlr.D007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou H, Shiu SW, Wong Y, Tan KC. Impaired serum capacity to induce cholesterol efflux is associated with endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res. 2009;6:238–243. doi: 10.1177/1479164109344934. [DOI] [PubMed] [Google Scholar]
- 17.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 18.Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronda N, Favari E, Borghi MO, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73:609–615. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 20.Ormseth MJ,Yancey PJ, Yamamoto S, Oeser AM, Gebretsadik T, Shintani A, Linton MF, Fazio S, Davies SS, Roberts LJ, Vickers KC, Raggi P, Kon V, Stein CM. Cholesterol efflux capacity of HDL and coronary atherosclerosis in rheumatoid arthritis. [abstract] Arthritis Rheumatol. 2014;66:S634. doi: 10.1016/j.ijcme.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ormseth MJ, Stein CM. High-density lipoprotein function in rheumatoid arthritis. Curr Opin Lipidol. 2016;27:67–75. doi: 10.1097/MOL.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao KP, Playford MP, Frits M, et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155–1164. doi: 10.1002/art.39039. [DOI] [PubMed] [Google Scholar]
- 24.Hirao M, Yamasaki N, Oze H, et al. Serum level of oxidative stress marker is dramatically low in patients with rheumatoid arthritis treated with tocilizumab. Rheumatol Int. 2012;32:4041–4045. doi: 10.1007/s00296-011-2135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rho YH, Chung CP, Oeser A, et al. Interaction between oxidative stress and high-density lipoprotein cholesterol is associated with severity of coronary artery calcification in rheumatoid arthritis. Arthritis Care Res. 2010;62:1473–1480. doi: 10.1002/acr.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivekanandan-Giri A, Slocum JL, Byun J, et al. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis. 2013;72:1725–1731. doi: 10.1136/annrheumdis-2012-202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. New Engl JMed. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XM, Tang WH, Mosior MK, et al. Paradoxical Association of Enhanced Cholesterol Efflux With Increased Incident Cardiovascular Risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.