Abstract
Objective
Atherosclerosis is a chronic disease characterized by lipid accumulation in the arterial wall. After MI, atherosclerotic plaques are infiltrated by inflammatory myeloid cells that aggravate the disease and increase the risk of secondary myocardial ischemia. Splenic myelopoiesis provides a steady flow of myeloid cells to inflamed atherosclerotic lesions after MI. Therefore, targeting myeloid cell production in the spleen could ameliorate increased atherosclerotic plaque inflammation after MI.
Approach and Results
Here we show that MI increases splenic myelopoiesis by driving hematopoietic stem and progenitor cells into the cell cycle. In an atherosclerotic mouse model, E-selectin inhibition decreased hematopoietic stem and progenitor cell proliferation in the spleen following MI. This led to reduced extramedullary myelopoiesis and decreased myeloid cell accumulation in atherosclerotic lesions. Finally, we observed stable atherosclerotic plaque features including smaller plaque size, reduced necrotic core area and thicker fibrous cap after E-selectin inhibition.
Conclusions
Inhibiting E-selectin attenuated inflammation in atherosclerotic plaques, likely by reducing leukocyte recruitment into plaques and by mitigating hematopoietic stem and progenitor cell activation in the spleen of mice with MI.
Keywords: E-selectin, HSC, myelopoiesis, atherosclerosis
Introduction
Atherosclerosis is characterized by pathological lipid deposition in the arterial wall, which triggers an inflammatory cascade1. During progression of atherosclerosis, monocytes are recruited to lipid-rich plaques2. Recruitment is mediated by monocyte attachment to arterial endothelial cells that upregulate adhesion molecules, such as E-selectin, VCAM-1 and ICAM-13. Leukocyte homing to atherosclerotic plaques occurs in four sequential steps: 1. leukocyte tethering and rolling, mediated by selectins; 2. initial leukocyte adhesion to endothelial cells via selectins and PSGL-1; 3. integrin-mediated firm adhesion; and 4. transmigration across the endothelial layer4. Selectins mediate leukocyte tethering and rolling, which are the first steps of diapedesis. Once in the plaque, inflammatory monocytes can differentiate into macrophages and produce proteases, such as collagenase, that erode the plaque's fibrous cap. Such erosions leave plaques vulnerable to rupture that results in myocardial infarction (MI).
Recurrent ischemic injuries occur frequently and are often fatal5. In ApoE-/- mice, coronary ligation leads to extramedullary myelopoiesis that persists even 12 weeks after the ischemia6. Inflammatory myeloid cells produced in the spleen are recruited to atherosclerotic plaques via blood7. In ApoE-/- mice, MI increases plaque size, plaque monocyte numbers and monocyte phenotype, as monocytes isolated from atherosclerotic lesions after MI express higher levels of inflammatory genes6. Subsequent studies, using angiography to measure the progression of non-culprit coronary artery lesions in patients with ST elevation MI8, showed that these patients had faster advancing lesions when compared to a cohort without MI.
Since myeloid cells have high turnover rates in inflammatory tissues7, 9, they must be replenished by hematopoietic stem and progenitor cells (HSPC). MI diminishes bone marrow levels of hematopoietic stem cell (HSC) retention factors, such as CXCL12 and VCAM-1, triggering HSPC release from the bone marrow. The released HSPC seed the spleen and divide in the presence of stem cell factor to produce myeloid cells that, after being recruited to atherosclerotic plaques, render the lesions more inflamed6. This understanding of HSPC's role after MI is supported by clinical data showing higher splenic uptake of the PET tracer 18F-FDG, which is incorporated by cells with higher energy demands, such as proliferating cells. The spleens and bone marrow of patients with acute MI showed significantly higher PET tracer uptake10. Although 18F-FDG is not specific to a certain cell type, these results may indicate increased cell proliferation in the spleen in patients with acute MI. In a retrospective analysis, patients with higher splenic 18F-FDG uptake showed increased cardiovascular events following imaging11. These studies indicate that splenic activation plays a role in increased atherosclerotic lesion inflammation after MI. However, there is currently no clinically available intervention that can block this process.
HSC proliferation and differentiation are tightly regulated by niche cells, such as endothelial cells, mesenchymal stem cells, etc., in the bone marrow microenvironment12. E-selectin, a cell adhesion molecule expressed by sinusoidal bone marrow endothelial cells, regulates HSC fate by promoting progenitor proliferation13. HSC proliferation decreased in E-selectin knockout mice and after pharmacologic E-selectin inhibition. Whether similar mechanisms are active in the spleen is not well understood. We hypothesized that E-selectin inhibition dampens splenic monocyte production in hypercholesterolemic mice with experimental MI. Previously, we found that MI transiently elevated splenic progenitor proliferation after MI in wild type mice9, and more permanently in ApoE-/- mice after coronary ligation6, both resulting in increased extramedullary myelopoiesis. In the current manuscript, we describe that inhibiting E-selectin with a small molecule glycomimetic antagonist (GMI-1271) significantly decreased HSC proliferation in the spleens of mice with atherosclerosis and myocardial infarction. Reduced proliferation led to lower HSC and progenitor numbers and attenuated splenic myeloid cell output. Furthermore, after E-selectin inhibition, aortic plaque contained significantly fewer inflammatory myeloid cells. The treatment reduced atherosclerotic plaque size and necrotic core area, while plaque fibrous caps were thicker.
Materials and Methods
Please see the online-only Supplemental Data.
Results
MI triggers splenic HSPC proliferation
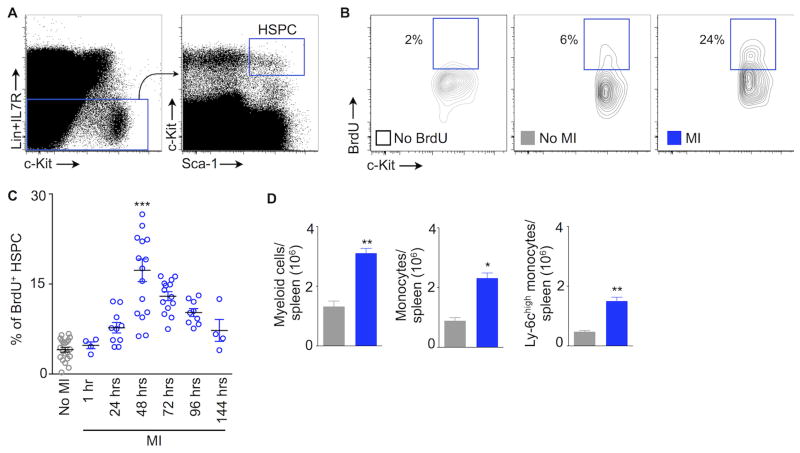
To investigate the time course of upstream splenic HSPC proliferation after MI, we ligated the left anterior descending coronary artery. At different time points after MI, we injected wild type mice with BrdU, a thymidine analogue that can be incorporated into DNA strands during the S phase of cell cycle. We found splenic HSPC proliferation progressively increased after coronary ligation. It peaked on day 3 and returned to steady-state level on day 7 (Fig. 1A-C). On day 2 after coronary ligation, HSPC proliferation was more than 4 times higher than in mice without coronary ligation (control, 4.06±0.4 BrdU+ HSPC; day 3 after coronary ligation, 17.3±1.9%). Concomitantly, splenic myeloid cell numbers increased at day 4 after myocardial ischemia (Fig. 1D), thereby indicating that proliferating HSPC differentiated into myeloid cells.
Figure 1.
MI triggers splenic HSPC proliferation. (A) Flow cytometry gating strategy for splenic hematopoietic stem and progenitor cells (HSPC). (B) Representative flow cytometric plots showing BrdU+ splenic HSPC. BrdU was injected 48 hours after MI (MI). (C) The graph depicts % of BrdU+ splenic HSPC. BrdU was injected at different time points as indicated on the x axis. Each data point indicates one mouse, *** P < 0.001. (D) Quantified expansion of splenic myeloid cells 4 days after MI. n=4-8 per group. Data are mean ± SEM, * P < 0.05, ** P < 0.01.
E-selectin inhibition decreases splenic HSC and progenitor proliferation
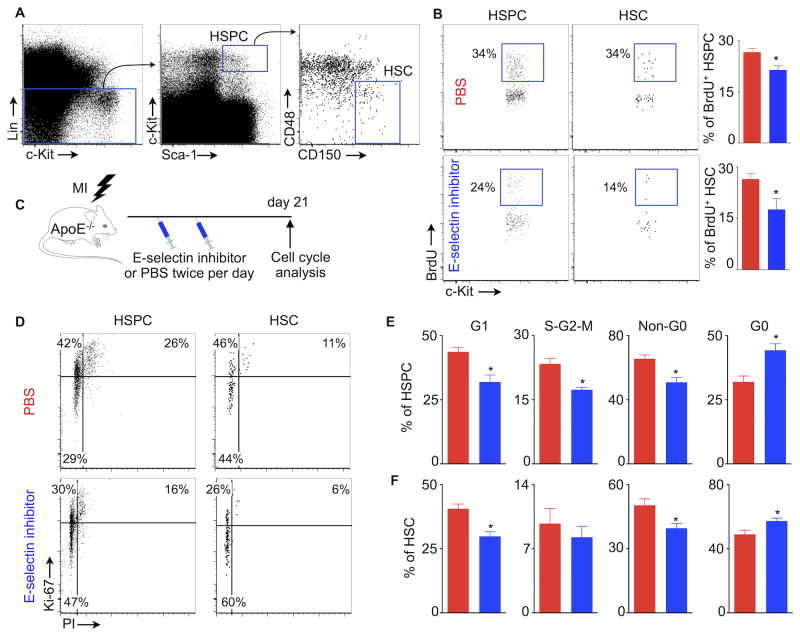
Splenic HSC and progenitor proliferation fuels extramedullary myelopoiesis7, 14. However, the regulation of splenic HSC proliferation (Fig. 2A) is not well understood. Since E-selectin expressed by bone marrow sinusoidal endothelial cells promotes HSC proliferation13, we hypothesized that inhibiting E-selectin would reduce splenic HSC proliferation. To test this hypothesis, we treated C57BL/6 mice after MI with a small molecule E-selectin inhibitor (GMI-1271). Surface plasmon resonance revealed that GMI-1271 potently inhibited E-selectin with a KD in the high nanomolar region (0.46 μM). GM-1271's IC50 for E-selectin was 1.75 μM, while it was 2.9 mM for L-selectin and > 10 mM for P-selectin. These data indicate specificity of GMI-1271 for E-selectin (Suppl. Fig. I). Treatment with GMI-1271 after coronary artery ligation resulted in significantly decreased splenic proliferation of HSC (PBS, 26.4±1.7%; GMI-1271, 17.6±3.2%) and HSPC (PBS, 26.5±1.1%; GMI-1271, 21.3±3.9%; Fig. 2B).
Figure 2.
E-selectin inhibition decreases splenic HSC and progenitor proliferation. (A) HSC gating strategy. (B) % of BrdU+ HSPC and HSC in the spleens of C57BL/6 mice. BrdU was injected 48 hours after coronary ligation. (C) Experimental design to investigate the effects of E-selectin inhibitor on HSPC and HSC proliferation in atherosclerotic mice with MI. (D) Representative flow cytometric plots depicting HSPC and HSC proliferation after MI in ApoE-/- mice treated with either PBS or E-selectin inhibitor. (E) % of splenic HSPC and HSC in different phases of the cell cycle. Data are mean ± SEM, n=5 per group, * P < 0.05.
We recently described that MI triggers splenic HSC proliferation9, leading to increased inflammatory myeloid cell production that exacerbates atherosclerosis6. To investigate the role of E-selectin in splenic HSC proliferation in atherosclerotic mice, we next performed coronary ligation in ApoE-/- mice fed a high-fat diet. Following MI, the mice received E-selectin inhibitor injections. GMI-1271 treatment did not alter blood cholesterol levels (Suppl. Fig. II). Cell cycle analysis was done on day 21 after MI (Fig. 2C). HSPC and HSC were stained for Ki-67, an antigen expressed in cycling cells. DNA marking with PI distinguished cells in S-G2-M phase (i.e. proliferating cells) from those in G1 phase (Fig. 2D). We found that E-selectin inhibition decreased the percentage of HSPC in G1, S-G2-M and Non-G0 phases, whereas the percentage of HSPC in G0 stage was increased (Fig. 2E). We detected similar effects on splenic HSC proliferation (Fig. 2F). Interestingly, GMI-1271 treatment did not change bone marrow HSC and HSPC proliferation (Suppl. Fig. III) in the same cohort of ApoE-/- mice with MI.
E-selectin inhibition lowers splenic HSC and progenitor numbers
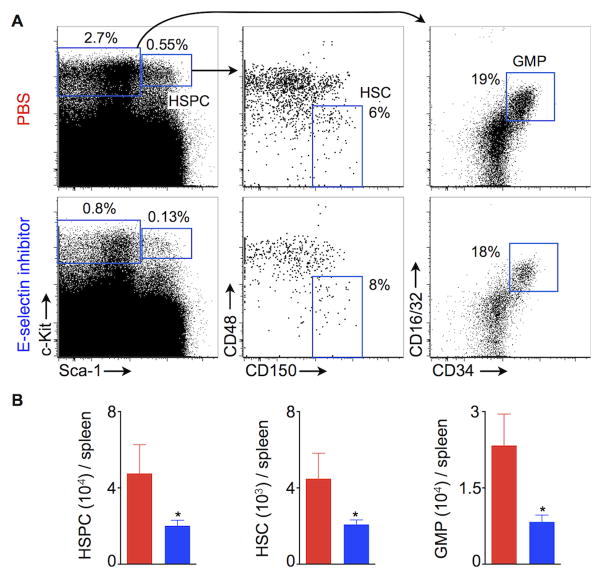
Since inhibiting E-selectin decreased HSC and HSPC proliferation, we investigated whether the treatment also decreased their numbers. For this experiment, we again used ApoE-/- mice fed a high fat diet. Fig. 3A depicts the gating strategy for HSPC (Lin- c-Kit+ Sca-1+), HSC (Lin- c-Kit+ Sca-1+ CD48- CD150+) and granulocyte monocyte progenitors (GMP) (Lin- c-Kit+ Sca-1- CD16/32+ CD34+). GMI-1271 treatment significantly reduced splenic numbers of HSPC (PBS, 47×103 ± 15× 103; GMI-1271, 20×103 ± 2×103), HSC (PBS, 4×103 ± 1×103; GMI-1271, 2×103 ± 0.2×103) and GMP (PBS, 23×103 ± 6×103; GMI-1271, 8×103 ± 1×103; Fig. 3B).
Figure 3.
E-selectin inhibition decreases splenic HSC and progenitor numbers. (A) Flow cytometric gating strategy for splenic HSC and GMP. (B) Quantification of HSPC, HSC and GMP in the spleens of ApoE-/- mice 3 weeks after coronary ligation. The mice were treated with either PBS or E-selectin inhibitor twice a day for 3 weeks. Data are mean ± SEM, n=5 per group, * P < 0.05.
E-selectin inhibition dampens myelopoiesis
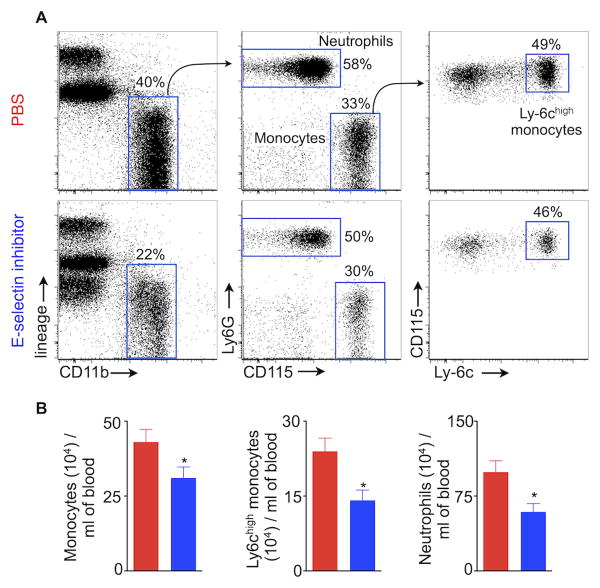
MI-induced activation of splenic HSPC contributes to a systemic rise in myeloid cell numbers6, and the spleen supplies myeloid cells in the setting of atherosclerosis7, 15. Since E-selectin inhibition significantly reduced HSC and HSPC proliferation in the spleen, we hypothesized the treatment would also reduce circulating myeloid cell numbers in mice with MI. Indeed, GMI-1271 treatment significantly reduced myeloid cell numbers in the blood (Fig. 4A, B). Fig. 4A shows the gating strategy for monocytes, the inflammatory Ly-6chigh monocyte subset and neutrophils. In Apoe-/- mice, E-selectin inhibition reduced total monocytes, Ly-6chigh monocytes and neutrophils levels by 28%, 41% and 40%, respectively, as measured 3 weeks after MI (Fig. 4B). Interestingly, ApoE-/- mice treated with GMI-1271 showed a trend towards increased lymphocyte numbers (Suppl. Fig. IV), which may indicate that E-selectin inhibition influences hemaptopoietic lineage bias, proliferation of lymphocytes or their lymphocyte migration16.
Figure 4.
E-selectin inhibition reduces blood leukocytosis in mice with atherosclerosis and MI. (A) Flow cytometric gating strategy for neutrophils, monocytes and Ly-6chigh monocytes. The flow cytometric plots depict % of myeloid cells in the blood of ApoE-/- mice treated with either PBS or E-selectin inhibitor. (B) Enumeration of neutrophils, monocytes and Ly-6chigh monocytes in ApoE-/- mice 3 weeks after MI. Data are mean ± SEM, n=5 per group, * P < 0.05.
Mitigated inflammation in atherosclerotic lesions and enhanced stable plaque phenotype after E-selectin inhibition
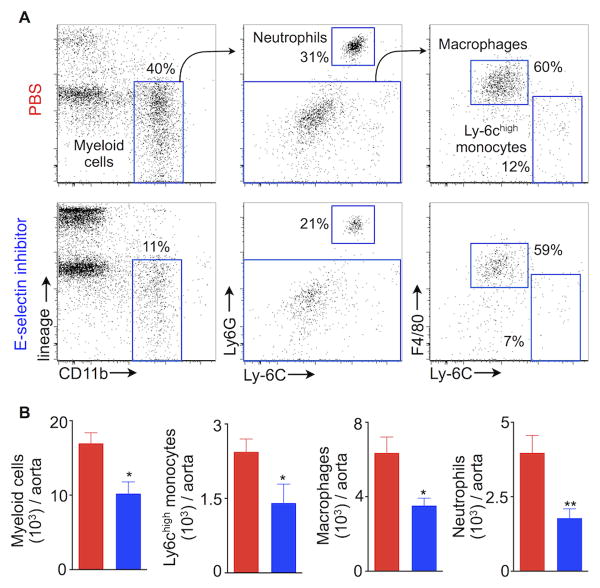
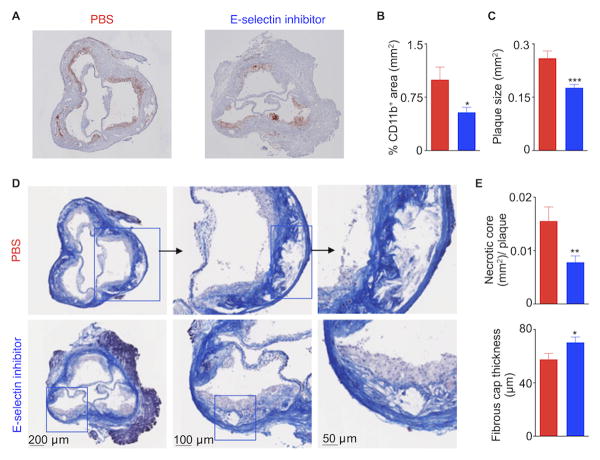
Atherosclerosis is associated with systemically increased inflammatory myeloid cells15, 17-19 that are recruited to atherosclerotic plaques, a process that partially depends on E-selectin4, 20, 21. The presence of inflammatory cells is detrimental to a stable plaque22. Since the E-selectin inhibitor effectively curbed myelopoiesis (Fig. 4), we next investigated whether GMI-1271 treatment might reduce inflammation in atherosclerotic lesions, thus promoting stable plaques. To test this hypothesis, we induced MI in ApoE-/- mice fed a high-fat diet. ApoE-/- mice were injected with either PBS or GMI-1271 for 3 weeks after coronary ligation. E-selectin inhibition significantly reduced aortic accumulation of myeloid cells, Ly-6chigh monocytes, macrophages and neutrophils (Fig. 5A, B). Diminished lesion inflammation was confirmed by histology, which revealed a smaller CD11b-stained area in sections of the aortic root (Fig. 6A, B). Furthermore, plaque size in the aortic root decreased significantly (Fig. 6C).
Figure 5.
E-selectin inhibition mitigates inflammation in atherosclerotic plaques. Aortas were excised from ApoE-/- mice fed a high-fat diet 3 weeks after coronary ligation. (A) The flow cytometric plots depict gating strategy and % of different myeloid cells in the aorta. (B) Quantification of aortic myeloid cells, Ly-6chigh monocytes, macrophages and neutrophils. Data are mean ± SEM, n=9 per group, * P < 0.05, ** P < 0.01.
Figure 6.
E-selectin inhibition improved features of stable atherosclerotic plaques. Representative images (A) and quantification (B) of CD11b+ area in aortic root sections. (C) Quantification of plaque size after E-selectin inhibitor treatment. Representative images (D) and quantification (E) of necrotic core area and fibrous cap thickness in aortic root sections. Data are mean ± SEM, n=9 per group, * P < 0.05, ** P < 0.01, *** P < 0.001.
Matrix metalloproteinases produced by inflammatory myeloid cells erode the fibrous cap, thereby allowing blood to come into contact with thrombogenic materials in the underlying necrotic core23. This may result in acute coronary thrombosis and myocardial ischemia. Accordingly, E-selectin inhibitor treatment might promote stable plaque features such as small necrotic cores and thick fibrous caps. To test this hypothesis, we performed Masson staining of aortic root sections from ApoE-/- mice 3 weeks after MI. We found that GMI-1271 treatment significantly reduced necrotic core area and increased fibrous cap thickness (Fig. 6D, E).
We also investigated the impact of E-selectin inhibition on myocardial healing after infarction by inducing MI in C57BL/6 wild type mice. Cardiac MRI was used to quantify infarct size on day 1 and measure heart failure on day 21 after MI (Suppl. Fig. VA-D). Initial infarct size was determined on day 1 after coronary artery ligation by delayed gadolinium enhancement, and was similar for both groups (Suppl. Fig. VC). That mice in the GMI-1271- and PBS-treated groups had similar ejection fraction (Suppl. Fig. IVD) indicates drug safety for the post-MI phase. Masson histology on day 21 after coronary ligation confirmed similar infarct size in both treatment groups (Suppl. Fig. VE). The treatment with GMI-1271 reduced infarct leukocytes, especially on day 7 after coronary ligation (Suppl. Fig. VI).
Discussion
The adhesion molecule E-selectin is known for its role in recruiting inflammatory leukocytes into the arterial wall. In the setting of atherosclerosis, E-selectin is expressed by activated endothelial cells and binds carbohydrate ligands on leukocytes, leading to cell adhesion to the endothelial surface. IL1 and TNF-α are known instigators of E-selectin expression by endothelial cells24. The adhesion molecule promotes a critical step in monocyte recruitment and thus contributes to plaque inflammation4. Recently, it was shown that E-selectin also promotes HSC activity in the bone marrow, and that its inhibition can reduce hematopoietic progenitor activity in this organ13. Here we describe that E-selectin also regulates splenic myelopoiesis in mice with atherosclerosis and myocardial infarction.
The spleen serves as a source of monocytes in ischemic heart disease25. Hyperlipidemia7 and myocardial injury9 lead to hematopoietic progenitor retention in this organ, which consequently supplies myeloid cells to the growing atherosclerotic plaque and to ischemic myocardium. While first clinical data indicate splenic activation in patients with acute coronary syndrome11, the regulation of splenic hematopoiesis is incompletely understood. Given previous reports on E-selectin upregulation after MI26 and its association with hyperlipidemia and cardiovascular disease in general27, we studied the effects of the E-selectin inhibitor GMI-1271, which has a good safety profile in humans28, in mice with myocardial infarction and pre-existing atherosclerosis. Previous work established that in this scenario, which mimics the occurrence of myocardial ischemia in patients with atherosclerosis, the spleen provides an overabundance of inflammatory monocytes to atherosclerotic plaques throughout the arterial system, possibly promoting re-infarction6. Our data imply that GMI-1271 efficiently dampens inflammation in mice with atherosclerosis and MI, partially through reducing splenic production of myeloid cells.
Given that E-selectin has a number of other biological roles, the observed GMI-1271 effects are likely a consequence of several mechanisms, which may include inhibition of monocyte adhesion to endothelial cells. GMI-1271 also inhibits pro-coagulatory leukocyte action29. These two mechanisms, among others that are yet unknown, may have contributed to the observed reduction in plaque size, and may be beneficial in the clinical setting.
Monocytes and macrophages are essential for efficient infarct healing. In wild type mice with reduced leukocytes, for instance due to splenectomy or macrophage depletion, rupture rates are increased and post MI remodeling leads to heart failure30, 31. In patients, this scenario is rare, as most of them have blood monocytosis and likely an overabundance of infarct macrophages. If there are too many inflammatory monocytes and macrophages residing in the infarct, resolution of inflammation is impaired, which also impedes infarct healing32. Thus, it has been postulated that there is an optimal range of monocyte supply to acute infarcts30. We therefore tested in wild type mice whether GMI-1271 influences infarct healing. E-selectin inhibition reduced myeloid cell recruitment, but did not change scar size or left ventricular remodeling. These data indicate that GMI-1271 may have a favorable safety profile in patients with acute MI. Future experiments that focus on infarct healing in a setting of systemic monocyte/macrophage overabundance, for instance in mice with pre-existing atherosclerosis32, will show whether anti-inflammatory properties of GMI-1271 support post-MI recovery of the left ventricle.
We did not observe an effect of GMI-1271 on bone marrow hematopoiesis in mice with atherosclerosis and MI. In steady state or after myelotoxic treatment, genetic deletion or E-selectin inhibition promotes HSC quiescence and survival13. We speculate that the bone marrow's state in mice with atherosclerosis and after MI may be altered and that these changes may interfere with drug distribution. An alternative explanation is that MI triggers strong and multifactorial hematopoiesis activation pathways, including via sympathetic nervous signaling6 and circulating IL-1b33, which may override effects of E-selectin inhibition.
Our study has a number of limitations. We began to investigate splenic GMI-1271 effects in the worst-case scenario, a combination of acute (MI) and chronic (atherosclerosis) inflammation. Arguably, the clinical need for decisive but specific anti-inflammatory interventions is highest in this setting. Nevertheless, it is of interest whether similar drug action is observed in ApoE-/- mice without MI. Further, given the multiple biological functions of E-selectin, our data do not provide insight to what degree GMI-1271's splenic actions contributed to the observed phenotype. Thus, future studies should decipher the precise quantitative contribution of GMI-1271 inhibition of splenic cell production versus leukocyte migration. Finally, future studies should focus on the precise E-selectin ligand(s) in the spleen, and reveal the identity of splenic cells involved in E-selectin mediated information transfer.
Supplementary Material
Highlights.
-
-
An E-selectin inhibitor dampened splenic HSC proliferation in mice with MI and atherosclerosis.
-
-
This treatment led to decreased splenic myeloid cell production and diminished supply of myeloid cells to atherosclerotic lesions.
-
-
Ultimately, E-selectin inhibition resulted in a more stable plaque phenotype.
Acknowledgments
The Flow Cytometry Core Facility (Massachusetts General Hospital, Center for Regenerative Medicine and Harvard Stem Cell Institute) provided access to an LSRII. This work was funded in part by grants from GlycoMimetics, the National Institute of Health R01-HL096576, R01-HL117829, R01-NS084863 (M.N.); K99-HL121076 (P.D.). Friedrich Felix Hoyer was funded by Deutsche Forschungsgemeinschaft (HO 5279/1-1).
John Magnani is an employee of GlycoMimetics and owns equity in the company.
Abbreviations
- MI
myocardial infarction
- HSC
hematopoietic stem cells
- HSPC
hematopoietic stem and progenitor cells
- GMP
granulocyte-macrophage progenitors
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cybulsky MI, Gimbrone MAJ. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 4.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan RC, Heckbert SR, Furberg CD, Psaty BM. Predictors of subsequent coronary events, stroke, and death among survivors of first hospitalized myocardial infarction. J Clin Epidemiol. 2002;55:654–664. doi: 10.1016/s0895-4356(02)00405-5. [DOI] [PubMed] [Google Scholar]
- 6.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins CA, Rauch P, Figueiredo J, Iwamoto Y, Gorbatov R, Etzrodt M, Weber W, Ueno T, Rooijen N, Mulligan-Kehoe M, Libby P, Nahrendorf M, Pittet M, Weissldleder R, Swirski F. Extramedullary hematopoiesis generates Ly-6chigh monocytes that infiltrate atheroscletotic lesion. Circulation. 2012;125:364–74. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Jing J, Tu S, Tian F, Xue H, Chen W, Chen J, Reiber JH, Chen Y. ST elevation acute myocardial infarction accelerates non-culprit coronary lesion atherosclerosis. Int J Cardiovasc Imaging. 2014;30:253–261. doi: 10.1007/s10554-013-0354-z. [DOI] [PubMed] [Google Scholar]
- 9.Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EJ, Kim S, Kang DO, Seo HS. The metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomographic imaging. Circ Cardiovasc Imaging. 2014;7:454–60. doi: 10.1161/CIRCIMAGING.113.001093. [DOI] [PubMed] [Google Scholar]
- 11.Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging. 2015;8:121–130. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Levesque JP. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 14.Dutta P, Hoyer FF, Grigoryeva LS, et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med. 2015;212:497–512. doi: 10.1084/jem.20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler IG, Barbier V, Radford KJ, Davis JM, Levesque JP, Smith TAG, Fogler WE, Magnani JL. Mobilization of CD8+ central memory T-cells with enhanced reconstitution potential in mice by a combination of G-CSF and GMI-1271 mediated E-selectin blockade. Blood. 2015;126:512. abstract. [Google Scholar]
- 17.Averill LE, Meagher RC, Gerrity RG. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am J Pathol. 1989;135:369–377. [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman DL, Mogelesky TC, Liptak BF, Gerrity RG. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb. 1991;11:985–994. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- 19.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nageh MF, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1997;17:1517–1520. doi: 10.1161/01.atv.17.8.1517. [DOI] [PubMed] [Google Scholar]
- 22.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 24.Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MAJ. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suefuji H, Ogawa H, Yasue H, Sakamoto T, Miyao Y, Kaikita K, Soejima H, Misumi K, Miyamoto S, Kataoka K. Increased plasma level of soluble E-selectin in acute myocardial infarction. Am Heart J. 2000;140:243–248. doi: 10.1067/mhj.2000.107544. [DOI] [PubMed] [Google Scholar]
- 27.Roldan V, Marin F, Lip GY, Blann AD. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost. 2003;90:1007–1020. doi: 10.1160/TH02-09-0083. [DOI] [PubMed] [Google Scholar]
- 28.Devata S, Sood SL, Hemmer MV, Flanner H, Kramer W, Nietubicz C, Hawley A, Angelini DE, Myers DD, Blackburn S, Froehlich J, Wakefield TW, Magnani JL, Thackray HM. First in Human Phase 1 Single Dose Escalation Studies of the E-Selectin Antagonist GMI-1271 Show a Favorable Safety, Pharmacokinetic, and Biomarker Profile. Blood. 2015;126:1004. abstract. [Google Scholar]
- 29.Myers DD, Wrobleski SK, Kelsey K, Farris D, Jose DA, Peter HK, Sood SL, Wakefield TW, Magnani JL. E-selectin inhibitor GMI-1271 works in combination with low-molecular weight heparin to decrease venous thrombosis and bleeding risk in a mouse model. Blood. 2014;124:593. abstract. [Google Scholar]
- 30.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation. 2015;132:1880–1890. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.