Abstract
For nearly a century neurobiologists have searched for the engram - the neural representation of a memory. Early studies showed that the engram is widely distributed both within and across brain areas and is supported by interactions among large networks of neurons. Subsequent research has identified engrams that support memory within dedicated functional systems for habit learning and emotional memory, but the engram for declarative memories has been elusive. Nevertheless, recent years have brought progress from molecular biological approaches that identify neurons and networks that are necessary and sufficient to support memory, and from recording approaches and population analyses that characterize the information coded by large neural networks. These new directions offer the promise of revealing the engrams for episodic and semantic memories.
The search for the engram
The search for the neural circuitry that supports memory - the “engram” (Semon, 1921; see Schacter et al., 1978). Previously, I have suggest that the notion of an engram as a distinct functional entity has replaced with a more general view that memories are stored via the plasticity properties of functional circuits throughout the brain (Eichenbaum & Cohen, 2001). Nevertheless, recent efforts have reached a new level of sophistication with new tools and new approaches that improve our understanding of how memories are embodied in functional circuitries, offering new insights into the neurons and information encoded by the neurons that compose engrams. These new findings suggest we can characterize an engram by identifying a neuronal network that is necessary and sufficient to support memory combined with revealing of the information coded by the network that supports a memory. Here I will review some of the history of the search for engrams and outline some of the recent successes in characterizing engrams.
The search for engrams began nearly a century ago with Karl Lashley’s pioneering efforts to map the cortical areas and pathways that support visual discrimination and maze learning in rats (see reviews in Lashley, 1929, 1950). Lashley’s systematic work was guided by the then prevalent and straightforward view that stimulus-response learning is supported by connections between sensory areas in the posterior cortical areas and motor areas in the frontal cortex. In one experiment Lashley removed a strip of cortex to separate visual and frontal cortex then trained the rats on visual discrimination. Despite the severing of sensory-to-motor pathways, these rats learned the task as rapidly as intact rats. Lashley went on to pursue a famous series of experiments in rats learning variants of a complex (Hebb-Williams) maze. Recognizing that the rats might use any sensory modality to solve the maze problems, Lashley separated functional areas by making knife cuts between many different interconnected areas, and found that none of the knife cuts had any effect on maze learning or retention. In other experiments he removed specific areas throughout the visual cortex, and found that removal of no particular area had any effect of visual discrimination learning. Lashley then went further in his studies on maze learning and showed that although the locus of damage did not matter, the amount of damage was correlated with the degree of impairment in maze performance.
Lashley reached two key complementary conclusions about the engram. First, he concluded that the memory trace was widely distributed both within cortical areas and throughout the cortex and that any of the neurons within cortical areas and any of those areas could support the engram – he called this principle “equipotentiality”. Second, based on the observation that the severity of memory loss was correlated with the number of connections or elements removed, Lashley concluded that the many involved areas acted together to support the engram – he called this principle “mass action”. After a career spent failing to identify a specific cortical area or pathway in rats essential to maze learning, Lashley who famously wrote, “I sometimes feel, in reviewing the evidence on the localization of the memory trace, that the necessary conclusion is that learning just is not possible. It is difficult to conceive of a mechanism which can satisfy the conditions set for it. Nevertheless, in spite of such evidence against it, learning does sometimes occur” (1950, pp. 477–478). However, Lashley demonstrated the distributed nature of the engram both within and among brain areas and his principles of equipotentiality and mass action described fundamental features of localization would have to be incorporated into a successful characterization of the engram.
Finding engrams
In the years since Lashley wrote his summary, we have made a lot of progress in finding engrams. One major part of this progress was the realization that there are different forms of memory, and different kinds of memory are supported by distinct brain areas and pathways (reviewed in Eichenbaum & Cohen, 2001; Squire, 2004; White et al., 2013). Thus, there have been many successes in localizing functionally distinct areas and pathways since Lashley’s work, and with regard to memory, multiple areas and pathways support different kinds of memory. Notably, these pathways highlight the key roles of subcortical areas, none of which were targeted in Lashley’s program of research, which goes a long way in explaining why Lashley had such difficulty in blocking memories with lesions confined to the cortex.
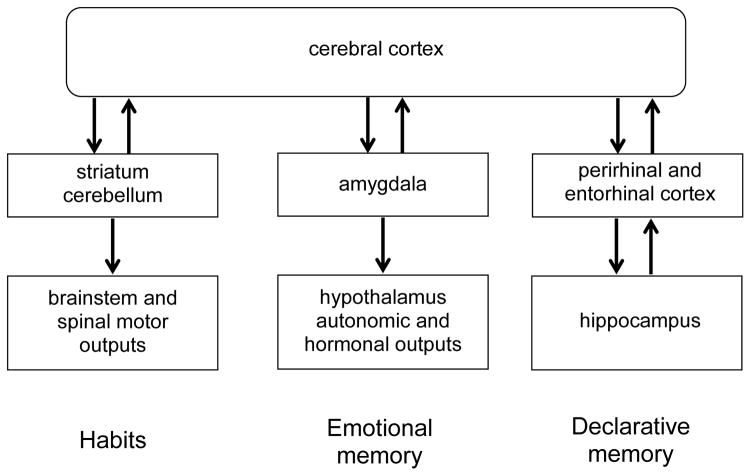
There are three main memory systems that involve different pathways of information processing related to distinct memory functions. A simple characterization of these three major systems is that they support three different types of associations: a habit learning system that supports associations between stimuli and behavioral responses, an emotional learning system that supports associations between stimuli and appetitive or aversive consequences, and a declarative memory system that learns associations between perceptually distinct events that together compose a unique experience (episodic memories) and the organization of the knowledge acquired by those experiences (semantic memory; Figure 1). I will first describe example successes in identifying engrams within the habit and emotional memory systems, then turn to the more complex nature of the declarative system.
Figure 1.
Outline of brain systems that support different forms of memory.
Habits
The habit system involves cortical and subcortical inputs to two well-studied brain areas that are critical nodal points in information processing leading to direct output effectors (Figure 1). One of these nodes is the striatum, which receives input from widespread cortical areas and is critical to associating sensory and movement information with voluntary behavioral responses via the brainstem motor system (Jog et al., 1999). Another node of this system is the cerebellum, which may be more involved in fine timing and coordination of sensory-motor associations. Here I will summarize studies that revealed an engram within the cerebellar pathway.
Studies on the cerebellar pathway examined a model of classical eye blink conditioning that includes a central set of elements by which the CS input is sent via the brainstem pontine nuclei to the interpositus nucleus as well as to the cortex of the cerebellum (reviewed in Thompson, 1976; Steinmetz, 1996; Poulos & Thompson, 2015). The US input is relayed by the trigeminal nucleus and inferior olive of the brain stem to the same cerebellar sites where the essential plasticity occurs. Outputs for the CR are then mediated by projections from the interpositus nucleus to the red nucleus, which projects to the accessory abducens motor nucleus, which also executes the UR via direct inputs from the trigeminal nucleus.
A series of clever experiments have converged in supporting this model for the engram of eyeblink conditioning. Most impressive were studies that involved dissociations between the effects of inactivation of specific components of this circuitry. Thus, inactivation of the motor nuclei that are essential for production of the CR and UR prevented the elicitation of behavior during training. However, in trials immediately following removal of the inactivation, CRs appeared in full form, showing that the neural circuit that supports UR production is not the critical site for the engram per se. A similar pattern of results was obtained with inactivation of the axons leaving the interpositus or their target in the red nucleus, showing that the final pathway for CR production is also not required to establish the memory trace (Krupa et al., 1993). By contrast, inactivation of the anterior interpositus nucleus and overlying cortex by drugs (muscimol, lidocaine) or temporary cooling did not affect reflexive blinking, yet resulted in failure of CR development during inactivation and the absence of savings in learning after removal of the inactivation. These results point to a small area of the anterior interpositus nucleus and overlying cerebellar cortex as the essential locus of plasticity, i.e., the engram.
Furthermore, complementary recording studies have shed light on the nature of the neural coding in the cerebellar cortex and interpositus nucleus that mediates the conditioning. During the course of training, neurons in both areas developed increased firing to the CS. During subsequent extinction trials, the CR gradually disappeared while interpositus cells ceased firing. By contrast the neural code remained in the activity of the cerebellar cortex long after extinction. These findings support the view that the cortical and subcortical components of the cerebellum may contain different engrams with different roles in maintaining and modulating this form of motor learning.
Emotional memory
Another major memory system involves the amygdala as a nodal stage in the association of exteroceptive sensory inputs to emotional outputs effected via the hypothalamic-pituitary axis and autonomic nervous system (Figure 1). The putative involvement of this pathway in such processing functions has led many to consider this system as specialized for “emotional memory” and that plasticity in synaptic connections specifically in the amygdala constitute an engram (Fanselow & LeDoux, 1999; Maren & Quirk, 2004). These studies have employed a fear conditioning behavioral paradigm developed by LeDoux and colleagues wherein rodents are first exposed to a novel environmental context, then in “cued-conditioning”, are presented with a tone then shock, or in “contextual conditioning”, only the shock is presented. In subsequent retention tests, the association between the tone and shock is reflected by the animal freezing during tone presentation in a novel environment – and this association is known to depend on the amygdala (Phillips & LeDoux, 1992). The association between the context and shock is reflected by freezing in the conditioning context (without the tone) and is dependent on the hippocampus as well as the amygdala.
Several studies have elucidated the physiology of the neurons in the direct thalamic and thalamo-cortical auditory pathways to the amygdala (LeDoux, 1992; Quirk et al., 1995). Cells in both the medial geniculate nuclei that project directly to the amygdala and in those in the thalamic nucleus that projects to the cortex demonstrate a variety of auditory responses. Finer auditory tuning was observed in the ventral medial geniculate than in areas that project directly to the amygdala. However, cells in the ventral nucleus responded only to auditory stimuli whereas neurons in the medial geniculate nuclei that project to the amygdala also responded to foot shock stimulation. Furthermore, some amygdala-projecting cells that responded to somatosensory stimulation but not auditory stimulation showed potentiated responses to simultaneous presentation of both stimuli. Studies that tracked the locus of plasticity showed that neuronal responses to the conditioning stimulus are enhanced by training in both the medial geniculate and lateral amygdala. However, blocking plasticity in the lateral amygdala is sufficient to prevent permanent memory formation, and the fear response is correlated with the magnitude of the evoked response to the conditioning stimulus in the lateral amygdala but not in the medial geniculate. Therefore a critical site of plasticity – the engram - is in the lateral amygdala itself.
Within the amygdala, cells in the lateral nucleus that receives thalamic input were responsive to auditory stimuli at both short (12–25 msec) and long (60–150 msec) latencies (reviewed in Maren & Quirk, 2004). Some cells had clear tuning curves, whereas others responded to a broad spectrum of sounds. Cells in the lateral amygdala could also be driven by electrical stimulation of the medial geniculate, and their responses were typically shorter than those in the basolateral amygdala. In addition, there are several lines of evidence suggesting that direct medial geniculate-lateral amygdala inputs exhibit learning related plasticity, including evidence for alterations in synaptic efficacy based on the molecular cascades. At the level of neuronal firing patterns, fear conditioning selectively enhances the short latency auditory responses of lateral amygdala neurons. Furthermore, some cells that were not responsive to tones prior to training showed post-conditioning short latency responses. Two different populations of neurons in the lateral amygdala show learning related plasticity prior to the first conditioned fear responses. Neurons in the dorsal part of the lateral amygdala exhibited the short latency responses (< 20ms) and those responses were transient and disappeared after learning. In contrast neurons in the ventral part of the lateral amygdala had longer latency responses, but these responses were maintained after learning and even when the fear response was extinguished. Thus different populations of lateral amygdala neurons signal the initiation of learning and the maintenance of a memory trace, and therefore represent distinctive engrams for fear memories.
Note that in both the systems discussed above, there is strong evidence that identifies unique and necessary roles of specific components of the cerebellum and amygdala. Also revealed in these studies is the nature of functional activity of neurons that reflect the neural representation of a memory, that is, the “memory code”. Via the combination of these studies, we know which cells embody the code and how they code for memories in specialized systems that support habits and emotional memories, thus satisfying the defining features of an engram.
But what about the brain system supports our memories for everyday facts and events, that is, declarative memory? We have known since the pioneering studies on the patient HM that the hippocampus plays a selective and critical role in declarative memory (Scoville & Milner, 1957), and many studies on human amnesia and many studies using functional imaging have confirmed an essential role of the human hippocampus and associated medial temporal cortical areas in declarative memory (Figure 1; e.g. recent reviews by Squire & Wixted, 2011; Schiller et al., 2015). In addition there has been significant progress in the development of valid animal models of declarative memory in animals, based on parallels in characteristics of declarative memory in humans also observed in animals. Thus, declarative memory in humans is commonly defined the ability to “recollect” prior experiences, and studies that employ objective measures of recollection (specifically receiver operating characteristic analysis) have revealed recollection-like characteristics of memory in rodents (Fortin et al., 2004). Also, the ability to remember the order of events in experiences as a defining feature of episodic memory has been modeled in rodents (Fortin et al., 2002), as has the ability to create semantic-like organizations of related memories (Bunsey & Eichenbaum, 1996; Dusek & Eichenbaum, 2007). Notably all of these capacities are dependent on the hippocampus in animals, thus providing a valid model for studies that seek to identify neuronal networks of the hippocampus that constitute an engram for declarative memories. However, localizing and characterizing the cells that participate and the memory code of the engram within the hippocampus has proved difficult. The remainder of this review will consider the engram for declarative memory in the hippocampus.
The modern search for the engram
In recent years, Lashley’s findings on distributed memory representations have been validated and at the same time rescued in significance by recent application of a large set of sophisticated molecular biological approaches to identifying and controlling cellular activity (Tonegawa et al., 2015; Josselyn et al., 2015). These studies have focused on the engram in hippocampus, and also that in the lateral nucleus of the amygdala, supporting different forms of fear conditioning introduced above. Using multiple molecular techniques that label cells that were activated during learning or retrieval, investigators have identified neurons that participate in the conditioning events in widespread areas, including the hippocampus, amygdala, and cortical areas, and these same areas are reactivated during the subsequent retention test (Reijmers et al., 2007; Deng et al., 2013; Taylor et al., 2013). These findings support the idea that the same neuronal networks that participate in learning also participate in retrieval of a memory. Notably, these observational studies do not inform us about whether these particular neurons are essential to the memory, in that they could play a role in non-memory processing including perception of the stimuli or in execution of the behavioral response. Showing that these cells are elements of the engram required additional manipulations.
In some of these studies, additional molecular techniques were used to inactivate or ablate the hippocampal (or amygdala) neurons that were labeled during learning, and these studies have shown that when these cells specifically are ablated or when the activity of these specific cells is subsequently blocked, the fear memory fails and the deficit is lasting (Han et al., 2009; Denny et al., 2014; Tanaka et al., 2014). The loss of memory was not due simply to loss of function in a subset of the cell population – inactivation of other cells that were not involved in learning had no effect and silencing of cells that were active during conditioning in one environment did not affect recall of fear conditioned in another environment. Furthermore, when hippocampal or amygdala cells that participated in a fear memory are ablated, other cells are recruited to support new memories during retraining (Han et al., 2009; Tanaka et al., 2014). These findings indicate that the particular neuronal networks of the hippocampus and amygdala that were activated during learning are essential to memory retrieval at a later time. And the findings also provide an exquisite replication of Lashley’s finding of equipotentiality in that distinct neural networks in these areas are each sufficient to support memory.
The molecular approaches have gone even further in providing complementary evidence about the sufficiency for memory of neural networks that were activated during learning (Liu et al., 2012; Kim et al., 2014; Yiu et al., 2014). In these experiments, during conditioning neurons in the hippocampus or amygdala were labeled as described above. But in these studies the labels were also linked to molecules that could subsequently reactivate the neurons artificially by optical stimulation or a drug. Indeed, when these cells were selectively reactivated in a neutral environment, the fearful response was expressed. In other words, these studies have shown that reactivation of the very same cells that were earlier activated during learning is sufficient to evoke the learned behavior. Notably, the level of fear expression is somewhat less following artificial reactivation than that evoked naturally by the conditioning cues. This may be due to the crudeness by which artificial activation activates all the elements of the network simultaneously instead of reproducing the natural spatiotemoral pattern evoked by natural memory cues. Nevertheless, these experiments show that even a crude artificial reactivation of a specific network involved in memory is sufficient to drive expression of this form of memory.
With justification, these studies claim to have found the engram Lashley sought (Tonegawa et al., 2015; Josselyn et al., 2015). The results show that neural networks that were active in specific areas during learning are reactivated during retrieval, and they show that activation of these specific networks is both necessary and sufficient to successful memory. Also, these studies have shown that the cells that are activated during learning are widely distributed, both within the hippocampus and amygdala, and across cortical areas and elsewhere. Thus both the widespread distribution of involvement (mass action) and equipotentiality of cells composing the memory trace as characterized by Lashley were validated.
What is the “memory code” in the hippocampus?
The above described studies provide compelling evidence that identifies the networks of neurons that encode memories, and shows the specificity of particular sets of neurons that participate in an engram. However, these studies tell us nothing about the specific information encoded by the activated cells. They tell us nothing about the features of the learning events that are encoded by particular neurons or about the temporal patterns of activity in neurons and networks that embody the information represented within the engram. They leave open the key question, what is the “memory code”?
The remainder of this review will focus on the hippocampus and its memory code. Early studies identified hippocampal neurons that became activated during the acquisition of a classically conditioned eyeblink response, such that these cells begin to fire following the CS onset and anticipating and modeling the conditioned response (Berger et al., 1976, 1983). These experiments were among the first to identify hippocampal neurons that encode a memory and subsequent studies have shown that conditioned neural responses supporting a hippocampal dependent variant of this task are robust and lasting (Hattori et al., 2015). Other early studies showed that hippocampal neurons fire associated with diverse behaviors (Ranck, 1973) and with a rat’s location in space (O’Keefe & Dostrovsky, 1971). The latter finding of hippocampal “place cells” has captured considerable excitement and has dominated subsequent research on hippocampal neuronal activity patterns, as evidenced in the awarding of the 2014 Nobel Prize for their discovery to O’Keefe and to Edvard and May Britt Moser who discovered a different type of place cells in the cortical area that provides input to the hippocampus (i.e., grid cells in the entorhinal cortex). However, at the same time, it remains to be determined that the role of hippocampal place cells extends to the full range of declarative memory supported the hippocampus.
Place cells and declarative memory
The phenomenology of place cells maps well onto the representation of spatial memories, that is, forms of learning or learning-like experiences, where spatial locations are straightforward and preeminent elements of the behavioral events that compose a declarative memory. By way of introduction to this section, it is important to consider that there are two basic forms of declarative memory, episodic memory, which involves remembering the order of events in a specific experience, and semantic memory, which involves the integration of related experiences into a network of knowledge that incorporates information that is common across multiple experiences (Eichenbaum, 2004). Importantly, while there remains controversy about whether animal models are useful for characterizing declarative memory, new findings suggest that key properties of declarative memory in humans are conserved in animals (Fortin et al., 2004; Corballis, 2013; Crystal & Smith, 2014; reviewed in Crystal, 2013; Eichenbaum et al, 2004, 2005).
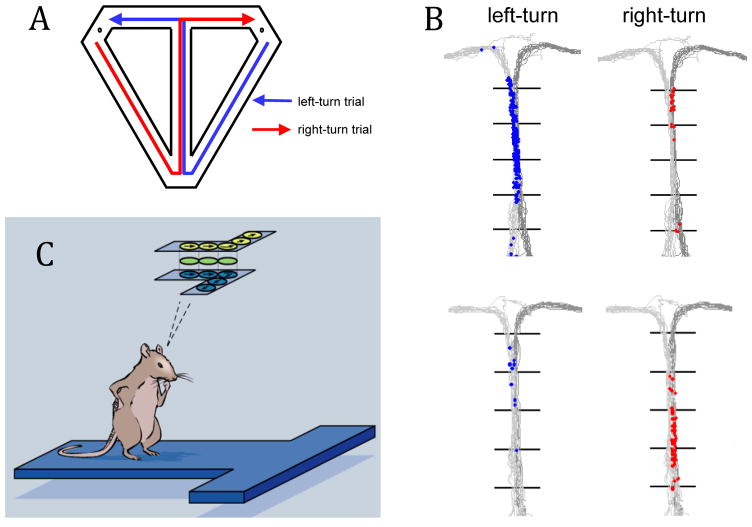
With regard to episodic memory, several studies have shown that networks of hippocampal place cells encode spatially defined memories as rats traverse or plan routes in a maze. The specificity of episode coding is revealed in task designs in which the animal traverses the same maze arm as part of different overall routes with distinct goals. Thus, for example, in T-maze alternation, on both left-turn and right-turn trials, rats traverse a maze arm that forms the “stem” of the T leading up to the choice point (Figure 1). Recordings of place cells show that, as rats accurately traverse the stem, distinct networks of hippocampal neurons fire sequentially, mapping the series of locations on the stem that correspond either to the left-turn path or the right-turn path the animal will later complete. That is, different neural networks represent the same series of locations depending on whether a left-turn or right-turn episode is ongoing, rather than on the animal’s location per se (e.g., Frank et al., 2000; Wood et al., 2000, Shapiro et al., 2006, Ainge et al., 2007). Furthermore, these episode-specific firing sequences predict the accuracy of memory performance, such that place cells exhibit path-specific firing sequences when subsequent memory choices are correct but place cells in the normal sequence fire less or not at all prior to errors (Robitsek et al 2013).
In addition, path-specific hippocampal representations associated with alternative choice paths in a maze predict acquisition of learned performance in spatial alternation (Singer et al., 2013). Furthermore, path-specific representations can be observed in place-cell sequences that anticipate the series of locations that a rat is about to traverse as it is about to choose one of two paths in a T-maze (Johnson & Redish, 2007) or as it is about to take a novel path toward a goal in an open field (Pfeiffer & Foster, 2013). These findings satisfy the criteria for characterizing the information coded in engrams of spatial episodic memories in their specificity and their association with successful spatial memory.
Furthermore, there is evidence that episode-specific firing sequences play a role in post-learning processing that may contribute to the consolidation of memories. This evidence comes from studies that record ensembles of place cells that fire in sequential locations as animals traverse a path though a maze, and find that the same ensembles subsequently also ‘replay’ the corresponding sequence of firings during subsequent ‘off-line’ periods, including sleep and quiet wakefulness when the animal is not moving through those locations (Carr et al., 2011). Thus, spatial coding observed as rats actively run through a maze is recapitulated in temporally coded firing sequences when the rat is not moving. Conversely, disruption of these replay events impairs spatial learning (Jadhav et al., 2012; Ego-Stengel & Wilson, 2010). These complementary lines of evidence support the notion that replays of specific place cell sequences serve as an engram of spatial episodic memories.
Other evidence is consistent with the notion that networks of place cells provide the representation of an entire environment as a model of semantic memory of a geographic space. In studies recording place cells in animals foraging for food in an open field, a typical observation is that the locations associated with heightened activity (the “place fields”) tile the entire environment as if to form a map of its topography (Dabaghian et al., 2014). These maps are allocentric in that the firing patterns of place cells in animals foraging throughout an environment do not depend on head or movement direction (Muller, 1996). Thus, the hippocampal spatial map bears similarity with a semantic mapping of the organization of external space that is not dependent on any particular spatial episode. Notably, across environments, the same pool of hippocampal neurons contributes to the maps of many environments, but the subset of cells involved in any particular map is independent of those involved in others (Alme et al., 2014), consistent with the notion that each map is distributed among large collective networks of cells that contain many spatial maps.
Furthermore, several experiments have revealed ensemble place cell representations that rapidly incorporate new memories into the spatial maps. For example, Dupret et al. (2010) showed that place cell representations reorganize in the same environment when goal locations are changed, suggesting accommodation of the spatial map to new memories that challenge the existing organization. Mckenzie et al., (2013) more directly examined assimilation of new memory representations into place cell ensemble representations by adding goal sites to a pre-existing set of goals in an environment. They found that many place cells represented multiple goal locations, suggesting linkage of functionally equivalent places in the spatial map. Furthermore, they found that, as new goals were added, the same neurons that previously fired at existing goals began to fire at the new locations, consistent with rapid assimilation of new goal sites in the existing spatial organization. Later, the firing patterns associated with new and old goals diverged, indicating a slow reorganization of the spatial map to both associate and distinguish competing goal locations. These properties of spatial organizations show that specific important events are incorporated into the organization of a map of geographic space.
Importantly, these geographic maps in hippocampal network activity are not simply or solely a product of the spatial cues in the environment. Many studies have shown that alterations in behavioral demands result in a “remapping” of spatial representations, that is, a very different set of place cell firing patterns within the same environment when behavioral demands are altered. Thus, for example, remapping occurs when a task is changed from foraging randomly for food to making directed movements for food (Markus et al., 1995). In another set of experiments, rats were switched between use of “response” or “place” strategies on the identical plus-maze. In the “response”-strategy variant, for example, when they began a trial on the North arm they turned left to East arm for reward, and when they began on the South arm they also turned left to enter the West arm for reward. By contrast, in the “place”-strategy variant, regardless of whether they began in the North or South arms, they were required to enter the East arm to find reward. Place cell maps were observed in both strategies but the cells participating and their firing patterns in the same maze were unrelated (Eschencko & Mizumori, 2007); similar remapping has been observed in animals switching between response and object choice strategies (Lee & Kim 2010) and between objects or positions of the same objects within an environment (Muzzio et al., 2009). Remapping has also been observed in a T-maze delayed non-matching to place task where distinct firing patterns were observed between sample trials, where the animal must encode its path, and choice trials, where the animal must remember the correct path (Griffin et al., 2007; also see Hallock and Griffin, 2013). In yet another task, remapping was observed when rats switched between start and goal arms while performing the same spatial memory task in the same maze (Bahar et al., 2011). In parallel with these studies, remapping also occurs when a neutral environment is made aversive by fear conditioning (Wang et al., 2012). Taken together, these studies show in a variety of ways that distinct memories govern the organization of the hippocampal map of the environment in which specific events must be remembered. Furthermore, the combination of findings discussed here indicates that the role of place cells is to provide a spatial framework for organizing where distinct events occurred as a major part of the characterization of the memory code in the hippocampus.
Beyond place cells – how do hippocampal networks represent declarative memories that are not organized within a spatial framework?
Experiments that demonstrate place cell sequences that mirror spatial paths and place cell organizations of environments have led Buzsaki & Moser (2013) to emphasize the parallels between place cell activity patterns and the properties of episodic and semantic memory, respectively. While the parallels in spatial memory are compelling (Eichenbaum & Cohen, 2014), other findings do not so readily connect place cells to the scope of memory supported by the hippocampus.
With regard to representing sequences of events as a fundamental property of episodic memory, the hippocampus plays a critical role in remembering the order of sequences of non-spatial events, including sequences of object and verbal stimuli in humans and monkeys (Hsieh et al., 2014; Ezzyat & Davachi, 2014; Naya & Suzuki, 2011) and sequences of odors in rats (Fortin et al., 2002), even when these events all occur in the same location. Conversely, humans and animals are impaired in sequence memory following hippocampal damage and hippocampal neurons are activated associated with encoding and retrieval of both non-spatial and spatial events.
The capacity for temporal organization of memories may be supported by temporal (not spatial) coding properties of hippocampal neurons (Eichenbaum, 2014). These temporal properties were first revealed in a study of neural ensemble activity patterns in the hippocampus that gradually changed while rats sampled sequences of odors, and this signal of continuously evolving temporal context predicted success in remembering the odor sequence (Manns et al., 2007). Since then, several studies have now identified hippocampal principal neurons that fire at a particular moments in time of a temporally structured event (Pastalkova et al., 2011; Kraus et al., 2013; Naya & Suzuki, 2011; MacDonald et al., 2013). These “time cells” compose temporal maps of specific experiences and the memories contained within, parallel to how place cells maps events in a spatial context. In these studies the location of the animal is held constant or firing patterns associated with elapsed time are distinguished from those associated with spatial and behavioral variables, and the firing patterns of these cells are dependent on the critical temporal parameters that characterize the task. Time cells have been observed in a variety of behavioral paradigms that involve bridging a temporal gap, including during delay periods in maze tasks and while bridging temporal gaps between associated non-spatial cues and in trace eyelid conditioning (reviewed in Eichenbaum, 2014). Furthermore, some of these studies have closely linked the emergence of time cell ensemble sequences to the encoding of specific memories and to subsequent memory performance, thus satisfying the criteria of importance to memory and containing information about the temporal flow of events in specific experiences.
With regard to semantic memory, in early studies aimed at identifying a role for the hippocampus in the organization of non-spatial memories, we found that the hippocampus is essential to assimilating related events into networks of memories as reflected in the ability to make inferences between events that are only indirectly related within the network. For example Bunsey & Eichenbaum (1996) showed that normal rats link overlapping paired associates (e.g., associations between A & B, and between B & C) as demonstrated by their ability to make transitive inferences about the indirectly related elements A and C, and this capacity depends on the hippocampus. Also Dusek & Eichenbaum (1997) extended these observations to a paradigm that involved a hierarchical series of stimulus elements. In this experiment normal rats could learn a series of choices (choose object A over B, choose B over C, choose C over D, and choose D over E) and could make the transitive choice B over D. Rats with hippocampal damage could learn the trained associations but could not perform the transitive inference between B and D, indicating they had not acquired the hierarchical organization. Importantly, while there were concerns about different types of representation that could support transitive inferences, recent evidence indicates that the form of organized representation that supports inference is dependent on the hippocampus (Moses et al., 2006; Lazareva et al., 2015).
In addition, Tse et al. (2007) showed that rats develop a organization of locations where different foods are buried in particular environments, and that new context-specific memories are assimilated rapidly to become hippocampal independent as they are presumably incorporated into a pre-existing organization. Consistent with these findings on rodents, several fMRI studies have shown that the hippocampus is engaged as related memories are assimilated and integrated to support novel transitive inferences in humans (Heckers et al., 2004; Preston et al., 2004; Zalesak & Heckers, 2009; Kumaran et al., 2009; Zeithmova & Preston, 2010, 2012; Milivojevic et al., 2015). Notably, these roles in organizing memories extend to a range of non-spatial tasks including learning a hierarchical organization (Piaget’s transitive inference task) and associative organizations (the associative inference task and acquired equivalence; Shohamy & Wagner, 2008; Wimmer & Shohamy, 2012; see Zeithamova et al., 2012; Milivojevic et al., 2015).
Furthermore, another recent study has shown that the hippocampus plays a role in organizing social space. In this study, Tavares et al., (2014) employed a role playing game in which human participants imagined they had moved to a new town and their goal was to find a job and place to live. To accomplish this, the participants conversed with local people in the search for a job or home through different responses in which they could comply with a character’s demand or make demands (increasing or decreasing the power of the character) and engage or not engage in personal conversation and physical interaction (increasing or decreasing affiliation with the character). The outcomes of these social interactions positioned each character relative to the subject along a vector described by axes of power and affiliation. By scanning subjects during the task, they showed that the fMRI signal in the left hippocampus correlated with the vector angle in two dimensional social space, indicating that the hippocampal network identified each character’s position in social space as an interaction of their power and affiliation relations. Thus, this study shows that the scope of semantic “space” supported by the hippocampus is indeed very broad, potentially extending to all manner of abstract spatial dimensions (Eichenbaum & Cohen, 2014; Milivojevic & Doeller, 2013). Based on these observations, I have proposed that the contribution of the hippocampus to semantic memory is the creation of a “memory space” that associates events along relevant dimensions that link memories (Eichenbaum et al., 1999; Eichenbaum and Cohen, 2014; Schiller et al. 2015).
How can we map a “memory space”?
A major challenge is how to extend the observations on spatial firing properties of hippocampal neurons to incorporate the wealth and diversity and non-spatial information we remember in a memory space. Here we get some help from many observations that, in tasks where non-spatial cues are relevant, place cells incorporate these non-spatial cues – they become only partly or not at all spatial. Thus, when animals are not moving, the engagement of the hippocampus in processing both non-spatial and spatial information is readily observed. When animals are immobilized, hippocampal neurons prominently encode non-spatial events (Berger et al., 1983; MacDonald et al., 2013; Naya & Suzuki, 2011) and when animals are still following movement to locations where salient events occur, hippocampal neurons are driven by specific events in particular places, including auditory (Moita et al., 2003; Itskov et al., 2012), object (Komorowski et al., 2009) and somatosensory (Itskov et al., 2011) stimuli. In particular, in one study rats performed a non-matching to sample task where any of several different odors could be presented in any of a large number locations on an open field, and the animals had to identify the current odor as different from that on the immediately preceding trial. In this task, hippocampal neurons encoded the same stimulus, the match or non-match meaningful feature of stimuli, or behavioral events at multiple locations, along with other cells that encoded a combination of odors and their location or meaning in the task (Wood et al., 1999). In another task where choice performance is guided by odor cues and not their spatial locations, hippocampal cellular activity was strongly bound to the odors and not to their spatial locations (Muzzio et al., 2009). In addition, other studies show that non-spatial dimensions can predominate when spatial variation is eliminated or made irrelevant to task demands. Thus, in virtual reality, spatial selectivity is markedly reduced while distance coding is prevalent (Ravassard et al., 2013). Also, in animals running in place and in head-fixed animals, hippocampal neurons show robust temporal firing patterns (reviewed in Eichenbaum, 2014). These studies show that hippocampal neurons can encode a broad domain of stimulus and behavioral events in addition to or even independent of their spatial location.
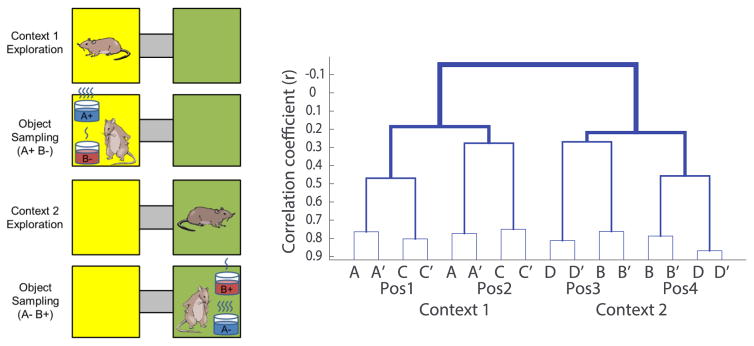
Furthermore, in tasks where animals acquire memories that are characterized by diverse features, hippocampal neurons very often integrate multiple dimensions of events. In a series of studies we have observed such “mixed selectivity” of hippocampal neurons as rats learn about objects and the locations and spatial contexts in which the objects are associated with distinct reward values. These observations suggest that hippocampal neurons encode all the information salient in the everyday way we use spatial and meaningful contexts to retrieve memories that are appropriate for that context. In our model of context-guided memory, mice (Rajji et al., 2006) and rats (Komorowski et al., 2009) learn to use the current spatial context to guide memory for object-reward associations. Animals move between two environmental contexts where they are presented with a pair of objects distinguished by olfactory, visual, and tactile cues (Figure 2 left). In Context 1, one of the objects (A+) contains a buried reward and the other (B−) does not, whereas in Context 2, the contingency is reversed (A−/B+) regardless of the positions of the objects within each context. When this initial set of objects are learned the paradigm is extended to add, on alternative trials, two additional objects (C & D) presented under the same rules, permitting us to distinguish firing patterns associated with the identity of objects from their reward assignments at each location (McKenzie et al., 2014).
Figure 2.
A. T-maze alternation task. B. Left turn (light gray) and right turn (dark gray) paths through the maze and spiking patterns for left-turn and right-turn paths. C. Cartoon summary of locations of place fields on left-turn (yellow) and right-turn (blue) paths. Some cells fired equally on both paths, suggesting a mechanism of connecting the two types of episodes (from Wood et al., 2000).
Normal learning in this task is hippocampal dependent (Komorowski et al., 2013; Rajji et al., 2006) and we have identified a large fraction of hippocampal neurons that fired during stimulus sampling associated with multiple dimensions of the stimulus (Komorowski et al., 2009; McKenzie et al., 2014). We found that many of the hippocampal neurons fire only as the rat samples a particular object when presented in a particular location within one of the contexts. Different neurons encode the object, reward association, position within a context, or context to varying degrees and in various combinations. The challenge, then, is how to find a way to reveal the nature of the organization of the memories for each combination of a particular object and its reward value in a particular position within each of the two contexts.
Note that, the mapping of all these related memories constitutes an example of semantic organization in declarative memory. But unlike the straightforward connection between place cells and mapping geographic space, there is no straightforward connection between mixed selectivity neurons and the “memory space” of a collection of related events. We could simply say that each memory is embedded within the map of the spatial contexts, but this tells us nothing about non-spatial relations among the memories, such as how are functionally equivalent objects (e.g. in the task as described above, objects A and C that have the same reward association in each location), are related within the memory organization. In other words, to fully characterize how memories are organized in the hippocampal memory space, we need a mapping not only of physical space but also of all dimensions by which memories are related. But what kind of organization can map memories by many dimensions?
Characterizing the organization of the memory code in the hippocampus
Mixed selectivity of neurons may be a common rule, especially in higher order brain areas. In the case where neurons show such cross-modal, mixed selectivity, Rigotti et al (2013) have argued that analysis of neural population activity patterns can reveal the nature and organization of multiple dimensions represented. In the their study, firing properties of cells in the prefrontal cortex were analyzed as monkeys learned the order in which objects were presented. In general, the firing patterns of individual neurons were jointly conditional on the interaction of object identity, the order of object presentation, and the nature of the memory demands, that is, they were characterized by mixed selectivity. Rigotti et al showed that a conjunctive code was highly informative on the population level despite the inability to extract specific information from the single cell responses. Furthermore, this ensemble conjunctive code greatly expanded the dimensionality of the representational space thus allowing for a greater computational complexity that correlated with task performance.
Furthermore, the approach to population coding by mixed selectivity neurons owes much to Hebb’s (1948) conceptions of cell assemblies and phase sequences. Despite early knowledge about some of the specific firing properties of cerebral neurons, Hebb’s formulation of the mechanisms of memory did not rely on identifying neurons with specific trigger features or receptive fields, such as place cells. In his view, particular events were represented by a collection of activated neurons, which he called a cell assembly, whose activity pattern was coordinated through increased connectively within the cell assembly via the so-called “Hebb rule” of neural plasticity. Thus each cell assembly, in which each individual cell could encode multiple features of an event, was viewed as representing the full concept of a particular event. Hebb went on to propose that associative learning was based on a linking of cell assemblies via overlapping neuronal elements, and that a set of overlapping cell assemblies formed what he called a phase sequence. Furthermore, Hebb proposed, networks of concept representations can be linked through shared elements of a larger set of cell assemblies. In his generic example, Hebb described three cell assemblies that were pairwise associated by overlapping elements, such that the phase sequences could support an indirect association – an inference – between concepts in two cell assemblies that had no overlapping elements. This example neatly parallels the paradigm of associative inference described above as an example of hippocampal function in the development of a memory space (Bunsey & Eichenbaum, 1996; Preston et al., 2004).
How does one reveal the structure of the neural representation – the memory code – for a organization based on Hebb’s principles of cell assemblies and phase sequences? Our approach, an example of Representational Similarity Analysis (RSA; Kriegeskorte et al., 2008), provides a metric to measure the degree of overlap between cell assemblies that represent specific events, by assessing the similarity of the ensemble firing patterns for those events. Our interpretation of these similarity measures is that two events that evoke highly similar ensemble firing patterns have high overlap and are therefore close in representational space – a very tight phase sequence - and events that evoke less correlated ensemble activity are farther apart – perhaps reflecting indirectly linked cell assemblies. We measure the similarities ensemble firing patterns among all pairwise comparisons between events and then apply a dendrogram analysis to iteratively cluster event representations to reveal the organization of the memory space, as will be described next.
Applying this approach to the context-guided memory task introduced above, our RSA begins by calculating firing rates for each hippocampal neuron recorded during the period of object sampling prior to the behavioral response on each trial for hundreds of trials in a recording session. To obtain the ensemble representation of each trial, the firing rates of all cells are combined in a list, called a population firing rate vector, that characterizes the ensemble firing pattern associated with each event. Within the task described above, animals acquire 16 distinct memories, one for each combination an object (A, B, C, or D) with a specific reward assignment in either of 2 positions within each of 2 contexts. Our RSA is a simple and highly straightforward set of computations that measure the similarity of population vectors for each event using a Pearson correlation, then we compare correlation coefficients to measure the representational distances between different types of events. Initially, we construct a population vector composed of the z-normalized firing rates of all simultaneously recorded neurons for each object-sampling event. Then we cross correlate all pairs of population vectors, using the correlation coefficient (r) as a measure of representational distance between events. These r-values are averaged in specific ways to determine whether the average r-value for a task dimension (e.g. object A+ vs object A+ in the same position and context) is different from chance. Then we use the decrease in average-r when a specific variable differs (object A+ vs C+ in the same position and context) to measure the representational distance between events associated with that dimension (in this case, object identity).
Finally, to graphically illustrate the organization of event representations, we employ a clustering algorithm to iteratively group distinct events by the strengths of their similarities. In recordings from hippocampal cells, RSA revealed a systematic hierarchical organization of ensemble representations of distinct events – the engram of the memory space (Mckenzie et al., 2014). Figure 2 right illustrates the relationships between representations of each of the different events (x-axis) as related (y-axis) by context, position, reward association, and object identity (right). At the top of this memory space, events that occur in different contexts are widely separated (r ~ 0.2) in representational space, indicated by anti-correlation between events that occur in different contexts. Within each context-based network, events are uncorrelated (r ~ 0) across positions within a context, i.e., events across positions are coded independently. Next, within each position representation, events with different reward associations (valences) are linked (r ~ 0.1– 0.25), then different objects with the same valence are more closely linked (r ~ 0.3–0.5). Finally, not shown is that identical events within a position are hardly separated (r ~ 0.8–0.9; pattern completion).
Notably, the RSA reveals an emergent network representation of the organization of memories that animals acquire in the task that could not be observed from single neuron firing patterns. Furthermore, these observations strongly support the notion that the hippocampus develops an organized representation of related memories that reflects both spatial and non-spatial features of events, and the organization that goes beyond explanation by current principles of spatial representation in studies of remapping (Colgin et al., 2008). First, the sub-networks are not statistically “independent”, as predicted by processes of global remapping and pattern separation (Alme et al., 2014), but rather are anti-correlated, suggesting active competition rather than independence. Second, memories for functionally equivalent events (objects with the same reward association in the same places) are neither independent nor generalized (highly overlapping) but rather show an intermediate level of similarity consistent with linkage within a schema structure that associates events first by reward valence then by object identity. By contrast, identical events do show strong pattern completion as high levels of representational similarity. These observations show that the hippocampus does more than distinguish or generalize memories - it organizes related memories into a memory space that constitutes a semantic engram.
Conclusions
Despite his disappointment, Lashley paved the way for the current understanding of the engram as a distributed representation of multipotent neurons and neuronal circuits, each of which performs information processing that contributes to memory in as yet only partially understood ways. Some engrams may be built from relatively straightforward circuits with dedicated functions, such as the timing of motor responses in the cerebellum and perhaps attaching emotional expressions to otherwise arbitrary events. However, brain areas and pathways that are employed to solve more generalized problems in declarative memory organize memory representations at the population level in ways that Lashley and Hebb presciently envisioned.
The population coding approach, combined with earlier described molecular biological approaches, provides the beginnings of a full understanding of the long sought engram, particularly that for declarative memory. The molecular biological approach has the major strength that it can identify all of the neuronal elements throughout the brain that participate in the engram for any particular memory or a set of related memories. And this approach can exquisitely manipulate cell assemblies in each brain area to demonstrate they satisfy necessary and sufficient roles in expressing memories. Population analyses on many neuron recordings have the complementary advantage of identifying the content of information encoded in the network of engram cells – the memory code. Furthermore, characterization of population activity that reflects the information encoded within cell assemblies and phase sequences, reveals the emergent properties of the full memory space. This approach, combined with methods for testing the necessity for these representations, provides an exciting new direction for revealing the long sought engram.
Figure 3.
Left. Context guided memory task. Right: Dendrogram illustrating the hierarchical organization of memories in the hippocampal memory space. X-axis indicates the 8 distinct rewarded events. Lines indicate mean correlation coefficients (r) between events and clusters of events. A & A′, etc., refer to odd and even numbered identical events. Pos = positions within each context (from Mackenzie et al., 2014).
Acknowledgments
This work was supported by NIMH grants MH094263, MH051570, MH052090, and MH095297.
References
- Ainge JA, Tamosiunaite M, Woergoetter F, Dudchencko PA. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. Journal of Neuroscience. 2007;27:9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alme CB, Miao C, Jezek K, Treves A, Moser EI, Moser MB. Place cells in the hippocampus: eleven maps for eleven rooms. Proceedings of the National Academy of Sciences U S A. 2014;111:18428–18435. doi: 10.1073/pnas.1421056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar AS, Shirvalkar PR, Shapiro ML. Memory-guided learning: CA1 and CA3 neuronal ensembles differentially encode the commonalities and differences between situations. Journal of Neuroscience. 2011;31:12270–12281. doi: 10.1523/JNEUROSCI.1671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger TW, Alger BE, Thompson RF. Neuronal substrates of classical conditioning in the hippocampus. Science. 1976;192:483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- Berger TW, Rinaldi PC, Weisz DJ, Thompson RF. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. Journal of Neurophsiology. 1983;50:1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: A potential substracte for memory consolidation and retrieval. Nature Neuroscience. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends in Neuroscience. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Mental time travel: a case for evolutionary continuity. Trends in Cognitive Sciences. 2013;17:5–6. doi: 10.1016/j.tics.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Remembering the past and planning for the future in rats. Behavioural Processes. 2013;93:39–49. doi: 10.1016/j.beproc.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Smith AE. Binding of episodic memories in the rat. Current Biology. 2014;24:2957–2961. doi: 10.1016/j.cub.2014.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabaghian Y, Brandt VL, Frank LM. Reconceiving the hippocampal map as a topological template. Elife. 2014 Aug;20:3, e03476. doi: 10.7554/eLife.03476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Mayford M, Gage FH. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. Elife. 2013 Mar;20:2, e00312. doi: 10.7554/eLife.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nature Neuroscience. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchencko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford University Press; 2001. [Google Scholar]
- Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin NJ, Ergorul C, Wright S, Agster P. Episodic recollection in animals: If it walks like a duck and quacks like a duck…. Learning and Motivation. 2005;36:190–207. [Google Scholar]
- Eichenbaum H. Time cells in the hippocampus: A new dimension for mapping memories. Nature Reviews Neuroscience. 2014;15:732–744. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views of hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Mizumori SJ. Memory influences on hippocampal and striatal neural codes: effects of a shift between task rules. Neurobiology of Learning and Memory. 2007;87:495–509. doi: 10.1016/j.nlm.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. Similarity breeds proximity: Pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014;81:1179–1189. doi: 10.1016/j.neuron.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum H. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–78. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Griffin AL, Eichenbaum H, Hasselmo ME. Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. Journal of Neuroscience. 2007;27:2416–2423. doi: 10.1523/JNEUROSCI.4083-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Griffin AL. Dynamic coding of dorsal hippocampal neurons between tasks that differ in structure and memory demand. Hippocampus. 2013;23:169–186. doi: 10.1002/hipo.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Hattori S, Chen L, Weiss C, Disterhoft JF. Robust hippocampal responsivity during retrieval of consolidated associative memory. Hippocampus. 2015;25:655–669. doi: 10.1002/hipo.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, Ranganath C. Hippocampal activity patterns carry information about temporal context. Neuron. 2014;81:1165–1178. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskov PM, Vinnik E, Diamond ME. Hippocampal representation of touch-guided behavior in rats: Persistent and independent traces of stimulus and reward location. PLoS ONE. 2011;6(1):e16462. doi: 10.1371/journal.pone.0016462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskov PM, Vinnik E, Honey C, Schnupp J, Diamond ME. Sound sensitivity of neurons in rat hippocampus during performance of a sound-guided task. Journal of Neurophysiology. 2012;107:1822–1834. doi: 10.1152/jn.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building Neural Representations of Habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. Journal of Neuroscience. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Köhler S, Frankland PW. Finding the engram. Nature Reviews Neuroscience. 2015;16:521–534. doi: 10.1038/nrn4000. [DOI] [PubMed] [Google Scholar]
- Kim J, Kwon JT, Kim HS, Josselyn SA, Han JH. Memory recall and modifications by activating neurons with elevated CREB. Nature Neuroscience. 2014;17:65–72. doi: 10.1038/nn.3592. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens. Journal of Neuroscience. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ, II, White JA, Eichenbaum H, Hasselmo ME. Hippocampal ‘time cells’: Time versus path integration. Neuron. 2013;78:1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis - connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompsom RF. Localization of a memory trace in the mammalian brain. Nature. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Lashley KS. Brain Mechanisms and Intelligence: A Quantitative Study of Injuries to the Brain. New York: Dover; 1929. 1963 edition. [Google Scholar]
- Lashley KS. In search of the engram. Symposiums of the Society of Experimental Biology. 1950;4:454–482. [Google Scholar]
- Lazareva OF, Kandray K, Acerbo MJ. Hippocampal lesion and transitive inference: Dissociation of inference based and reinforcement based strategies in pegeons. Hippocampus. 2015;25:219–226. doi: 10.1002/hipo.22366. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Current Opinion in Neurobiology. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- Lee I, Kim J. The shift from a response strategy to object-in-place strategy during learning is accompanied by a matching shift in neural firing correlates in the hippocampus. Learning and Memory. 2010;17:381–393. doi: 10.1101/lm.1829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R, Eichenbaum H. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. Journal of Neuroscience. 2013;33:14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard M, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin Y-L, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. Journal of Neuroscience. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Robinson NTM, Herrera L, Churchill JC, Eichenbaum H. Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. Journal of Neuroscience. 2013;33:10243–10256. doi: 10.1523/JNEUROSCI.0879-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, Eichenbaum H. Hippocampal representation of related and opposing memories develop within distinct, hierarchically-organized neural schemas. Neuron. 2014;83:202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic B, Vicente-Grabovetsky A, Doeller CF. Insight reconfigures hippocampal-prefrontal memories. Current Biology. 2015;25:821–830. doi: 10.1016/j.cub.2015.01.033. [DOI] [PubMed] [Google Scholar]
- Milivojevic B, Doeller CF. Mnemonic networks in the hippocampal formation: from spatial maps to temporal and conceptual codes. Journal of Experimental Psychology: General. 2013;142:1231–1241. doi: 10.1037/a0033746. [DOI] [PubMed] [Google Scholar]
- Moita MAP, Moisis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location specific location specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Moses SN, Villate C, Ryan JD. An investigation of learning strategy supporting transitive inference performance in humans compared to other species. Neuropsychologia. 2006;44:1370–1387. doi: 10.1016/j.neuropsychologia.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Muller RU. A quarter of a century of place cells. Neuron. 1996;17:813–822. doi: 10.1016/s0896-6273(00)80214-7. [DOI] [PubMed] [Google Scholar]
- Muzzio IA, Levita L, Kulkarni J, Monaco J, Kentros C, Stead M, Abbott LF, Kandel ER. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. PLoS Biology. 2009;7(6):e1000140. doi: 10.1371/journal.pbio.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–27. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. Hippocampal place cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Thompson RF. Localization and characterization of an essential associative memory trace in the mammalian brain. Brain Research. 2015;1621:252–259. doi: 10.1016/j.brainres.2014.10.068. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JD. Hippocampal Contribution to the Novel Use of Relational Information in Declarative Memory. Hippocampus. 2004;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. Journal of Neuroscience. 2006;26:908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. Part I. Behavioral correlates and firing repertoires. Experimental Neurology. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Ravassard P, Kees A, Willers B, Ho D, Aharoni D, Cushman J, Aghajan ZM, Mehta MR. Multisensory control of hippocampal spatiotemporal selectivity. Science. 2013;40:1342–1346. doi: 10.1126/science.1232655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, Fusi S. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–590. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek JR, White J, Eichenbaum H. Place cell activation predicts subsequent memory. Behavioural Brain Research. 2013;254:65–72. doi: 10.1016/j.bbr.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Eich JE, Tulving E. Richard Semon’s theory of memory. Journal of Verbal Learning and Verbal Behavior. 1978;17:721–743. [Google Scholar]
- Schiller D, Eichenbaum H, Buffalo EA, Davachi L, Foster DJ, Leutgeb S, Ranganath C. Memory and space: Towards an understanding of the cognitive map. Journal of Neuroscience. 2015;35:13904–13911. doi: 10.1523/JNEUROSCI.2618-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology Neurosurgury and Psychiatry. 1957;20:11–12. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Kennedy PJ, Ferbinteanu J. Representing episodes in the mammalian brain. Current Opinion in Neurobiology. 2006;16:701–709. doi: 10.1016/j.conb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: Hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiology of Learning and Memory. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annual Review of Neuroscience. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE. The brain substrates of classical eyeblink conditioning in rabbits. In: Bloedel JR, Ebner TJ, Wise SP, editors. The Acquisition of Motor Behavior in Vertebrates. Cambridge, MA: The MIT Press; 1996. pp. 89–114. [Google Scholar]
- Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, Wiltgen BJ. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 2014;84:347–354. doi: 10.1016/j.neuron.2014.09.037. [DOI] [PubMed] [Google Scholar]
- Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, Schiller D. A Map for Social Navigation in the Human Brain. Neuron. 2015;87:231–243. doi: 10.1016/j.neuron.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Current Biology. 2013;3:99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Thompson RF. The search for the engram. American Psychologist. 1976;31:209–227. doi: 10.1037//0003-066x.31.3.209. [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, Redondo R. Memory Engram Cells Have Come of Age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Wang ME, Wann EG, Yuan RK, Ramos Álvarez MM, Stead SM, Muzzio IA. Long-term stabilization of place cell remapping produced by a fearful experience. Journal of Neuroscience. 2012;32:15802–15814. doi: 10.1523/JNEUROSCI.0480-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, Packard MG, McDonald RJ. Dissociation of memory systems: The story unfolds. Behavioral Neuroscience. 2013;127:813–834. doi: 10.1037/a0034859. [DOI] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: How memory mechanisms in the hippocampus bias decisions. Science. 2012;338:270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Wood E, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko P, Robitsek JR, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, Woodin MA, Frankland PW, Josselyn SA. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Zalesak M, Heckers S. The role of the hippocampus in transitive inference. Psychiatric Research: Neuroimaging. 2009;172:24–30. doi: 10.1016/j.pscychresns.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR. Flexible memories: differential roles for medial temporal lobe and prefrontal cortex in cross-episode binding. Journal of Neuroscience. 2010;30:14676–14684. doi: 10.1523/JNEUROSCI.3250-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]