Abstract
Objective
Systemic lupus erythematosus is a relapsing autoimmune disease that affects multiple organ systems. T cells play an important role in the pathogenesis of lupus, however, early T cell events triggering disease flares are incompletely understood. We studied DNA methylation in naïve CD4+ T cells from lupus patients to determine if epigenetic remodeling in CD4+ T cells is an early event in lupus flares.
Methods
A total of 74 lupus patients with disease activity ranging from 0–18 as measured by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) were included in this study. Naïve CD4+ T cells were isolated from peripheral blood samples and DNA extracted for genome-wide methylation assessment. RNA was also extracted from a subset of patients to determine the relationship between epigenetic changes and transcriptional activity using RNA sequencing and microRNA arrays.
Results
We demonstrate that naïve CD4+ T cells in lupus undergo an epigenetic pro-inflammatory shift implicating effector T cell responses in lupus flare. This epigenetic landscape change occurs without expression changes of corresponding genes, and poises naïve CD4+ T cells for Th2, Th17, and Tfh immune responses, and opposes inhibitory TGF-β signaling. Bioinformatics analyses indicate that the epigenetic modulator EZH2 might be playing an important role in shifting the epigenetic landscape with increased disease activity in lupus naïve CD4+ T cells. Further, the expression of miR26a which is sensitive to glucose availability and which targets EZH2 was negatively correlated with disease activity in lupus patients.
Conclusion
An epigenetic landscape shift in naïve CD4+ T cells that favors T cell activation and non-Th1 immune responses predates transcriptional activity and correlates with lupus activity. A role for EZH2 dysregulation in triggering lupus flares warrants further investigation.
Keywords: Lupus, CD4+ T cells, epigenetics, DNA methylation, disease flare, Th2, Th17, TGF-β, Tfh, EZH2
Introduction
Systemic lupus erythematosus (SLE or lupus) represents a heterogeneous disease group characterized by the production of autoantibodies against cellular nuclear components. Lupus is a chronic relapsing disease, characterized by a disease course consisting of intermittent flares alternating with periods of relative disease quiescence in the majority of affected patients.
The etiology of lupus remains incompletely understood. A high concordance rate in monozygotic compared to dizygotic twins, familiar aggregation, and differences in disease prevalence between populations suggests a strong etiological genetic component in lupus. This has been demonstrated with a growing list of confirmed genetic susceptibility loci in lupus (1, 2). More recently, a role for inherited epigenetic changes has also been demonstrated (3).
With the exception of epigenetic accessibility of interferon-regulated genes in lupus (4), and inherited epigenetic changes that are directly influenced by genetic variants in the germline (3), DNA methylation changes in lupus T cells have been shown to be dynamic. Indeed, CD4+ T cells in lupus patients demonstrate a DNA methylation defect that is more pronounced in patients with active compared to inactive disease (5). This is explained by defective ERK signaling and reduced expression of the DNA methyltransferase enzyme DNMT1 (6). Inducing a DNA methylation defect in T cells can result in a lupus-like autoimmune disease in vivo (7, 8).
DNA methylation plays an important role in T cell differentiation and activation. Naïve CD4+ T cells selectively undergo an epigenetic shift rendering the IFNG, IL4-IL5-IL13, and IL17 loci accessible for transcription upon differentiating into Th1, Th2, and Th17 cells, respectively (9). These key cytokine gene loci are heavily methylated and hence transcriptionally silent in naïve CD4+ T cells prior to T cell activation or differentiation (9).
Because DNA methylation changes are generally dynamic, and T cells play an important role in the pathogenesis of lupus (10), we sought to determine epigenetic changes in CD4+ T cells that correlate with disease activity in lupus patients. We focused on naïve CD4+ T cells to understand the earliest T cell epigenetic changes in lupus flares that predate T cell activation. We performed RNA sequencing in the same cells to determine the relationships between epigenetic changes and transcriptional activity.
Methods
Patients: Demographics and disease activity
74 female participants previously diagnosed with lupus were included in this study. All patients fulfilled the American College of Rheumatology (ACR) classification criteria for SLE (11). The average age of patients in the study was 41 years of age, ranging from 18 to 66 years. A Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score was calculated at the clinical visit concurrently with enrollment in the study and blood draw. The SLEDAI scores of patients included in this study ranged from 0 to 18, with a mean and median SLEDAI of 3 and 2, respectively. No difference in age was present between patients with active disease (SLEDAI ≥5) and patients with less active disease (SLEDAI <5) (P= 0.21). Of the 74 patients, 49 were taking hydroxychloroquine, 26 were taking mycophenolate mofetil, 13 were taking azathioprine, and 39 were taking glucocorticoids of various doses at the time of sample collection. One patient was taking methotrexate, and no patients were receiving cyclophosphamide, cyclosporine, leflunomide, tacrolimus, rituximab, belimumab, or IVIG. Patients were recruited at the Oklahoma Medical Research Foundation, University of Michigan Health System, and Henry Ford Health System. The institutional review boards at the participating institutions approved this study. All participants signed an informed consent prior to enrollment.
Naïve CD4+ T cell isolation and purity
Naïve CD4+ T cells were isolated from whole blood by negative selection using the Naïve CD4+ T cell Isolation Kit II, human (Miltenyi Biotec, San Diego, CA, USA) according to manufacturer’s instructions. This kit uses an antibody cocktail to directly label the surface of undesired cells which are then bound to magnetic beads, allowing unlabeled naïve CD4+ T cells to be separated. The purity of the isolated populations was confirmed using surface protein staining and flow cytometry as CD3+, CD4+ and CD45RA+ using human FITC-conjugated human anti-CD3, human PE-conjugated anti-CD4, and human Pacific Blue-conjugated anti-CD45RA antibodies (BioLegend, San Diego, CA, USA). Cell population purity for all samples included in this study was >95%.
DNA and RNA extraction
Genomic DNA was extracted from isolated naïve CD4+ T cell populations using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Total RNA was also extracted simultaneously by lysing cells in TRIzol Reagent (ThermoFisher Scientific, Grand Island, NY, USA) and then purified using Direct-zol RNA MiniPrep Kit (Zymo Research, Irvine, CA, USA) according to manufacturer’s instructions.
DNA methylation assessment and analysis
500 ng of genomic DNA was bisulfite converted with the EZ DNA Methylation Kit (Zymo Research, Irvine, CA, USA) and used as input for the Infinium HumanMethylation450 BeadChip kit (Illumina, San Diego, CA, USA). Initial processing of DNA methylation data was conducted using GenomeStudio Methylation Module software (Illumina, San Diego, CA, USA) as previously described (12). All samples passed array quality control measures prior to analysis. Probe fluorescence intensity values were normalized for all samples. An average methylation value was calculated for each probe represented as a beta (β) value for all samples and used for subsequent analyses.
Bioinformatics analyses of DNA methylation data
Methylation data were pre-processed using methylumi v2.16.0 R package (13). Potential confounding factors were examined, and the race effect was removed using ComBat function from sva v.3.18.0 R package (14). Beta values for each methylation site were median-averaged for each level of SLEDAI score, and correlation was assessed using Pearson correlation coefficient. Adjusting for medication use had negligible effect on beta values with results almost identical to unadjusted data, and the correlations with SLEDAI scores were also almost identical to the unadjusted analysis. Methylation sites correlating with SLEDAI score at p-value < 0.01 were selected. Annotation of the locations of methylation sites was performed using ChIPseeker v.1.7.4 R package (15). Mapping between methylation sites and genes was performed using manufacturer-provided annotation file. Functional enrichment analysis in Gene Ontology categories and KEGG canonical pathways was performed using GOStats v.2.36.0 R package (16).
Chromosome-centric and transcription factor binding sites enrichment analyses were performed using GenomeRunner tool (17). Briefly, for chromosome-centric enrichment analysis, genomic locations (hg19) of methylation sites positively/negatively correlating with SLEDAI scores were tested for enrichment within each chromosome using Fisher’s exact test. For transcription factor binding sites enrichment analysis, genomic locations of 161 transcription factors were extracted from the wgEncodeRegTfbsClusteredV3 supertrack from the UCSC genomic database (18). Genomic locations of all methylation sites on the 450K array were used as the reference point to calculate the statistical significance of all identified genomic feature enrichments. All reported p-values are multiple testing corrected using False Discovery Rate (FDR) (19).
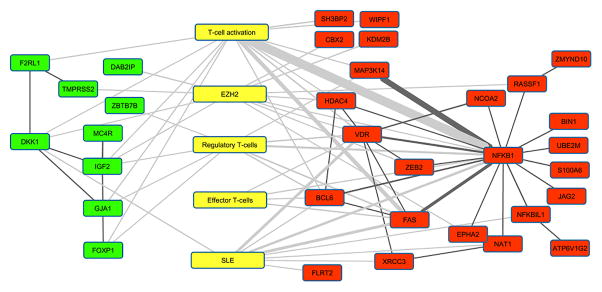
Literature analysis was conducted using IRIDESCENT (20), which uses a thesaurus of concepts (genes, diseases, phenotypes, chemicals, ontology categories, etc) to identify terms associated with these concepts within MEDLINE records. Co-mentions of terms are summed, with sentence co-mentions receiving a weight of 0.8 and abstract co-mentions receiving a weight of 0.5. Weights are based on previous studies of the probability a co-mention refers to a non-trivial relationship. The top 100 loci with the strongest correlations with SLEDAI were then analyzed, with 66 of the positively correlated loci being proximal to a gene and 69 of the negatively correlated loci being proximal to a gene. Gene lists along with associated concepts were analyzed with IRIDESCENT for their connectedness. Unconnected genes are not shown in the graph to reduce clutter.
RNA sequencing and analysis
A subset of 12 patients enrolled in the study had total RNA extracted for mRNA sequencing. The subset had an average age of 42 years and mean and median SLEDAI scores of 3 and 2.5, respectively. A sequencing library was created using the TruSeq Stranded mRNA library kit (Illumina, San Diego, CA, USA) from purified total RNA and 100bp, single-end mRNA reads were sequenced on the HiSeq 2500 sequencer (Illumina, San Diego, CA, USA). Sequenced reads were cleaned using the Trimmomatic-0.32 pre-processing workflow and then mapped to the human genome (hg19) with SHRiMP (version 2.2.3) (21, 22). Aligned reads for each gene within each sample were scaled to the number of fragments in each sample library as counts per million (CPM) for gene i of sample j using the equation . The CPM values were used for subsequent analyses. Correlation analysis with SLEDAI scores was assessed using Pearson correlation coefficient and median-averaged expression values at each SLEDAI score level.
MicroRNA microarray analysis
MicroRNA expression levels in a subset of lupus patients (n=16) was assessed using the Affymetrix miRNA 4.1 Array Strip (Affymetrix, Santa Clara, CA, USA). This array covers over 2,000 premature and 2,500 mature human miRNA sequences. Total RNA was isolated from naïve CD4+ T cells of lupus patient as described above. RNA sequences were first polyadenylated, then ligated to a biotin-labeled oligomer using the FlashTag Biotin HSR RNA Labeling Kit (Affymetrix, Santa Clara, CA, USA). Biotin-labeled sequences were hybridized to array probes, washed, and stained with streptavidin-PE. Biotin/streptavidin-PE fluorescence was measured and used for analysis in the Affymetrix Expression Console & Transcriptome Analysis Console 2.0 software (Affymetrix, Santa Clara, CA, USA). All samples passed signal intensity, polyadenylation, and ligation quality control measures. Signal intensities were background adjusted and normalized and a log2-transformed expression value for each probeset was calculated using a robust multi-array average model (23). These expression values were used for subsequent analyses. Correlation between miR-101 and miR-26a expression and SLEDAI scores was assessed using Pearson correlation coefficient and median-averaged expression values at each SLEDAI score level.
Results
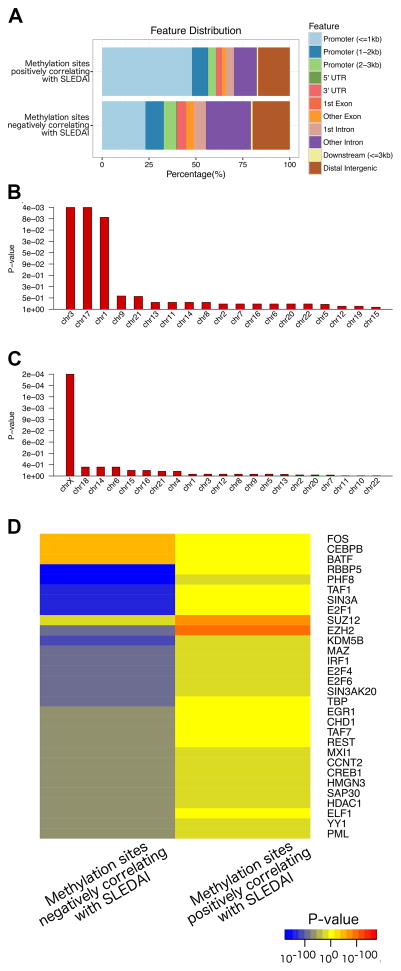
We identified 4,839 methylation sites and 1,568 methylation sites that negatively and positively correlated with disease activity in lupus, respectively, as measured by SLEDAI scores. The distribution of these methylation sites relative to transcription start sites and gene structures within the genome is depicted in Figure 1A. Compared to all methylation sites included on the 450K methylation array, methylation sites that negatively correlated with disease activity in lupus were enriched on chromosome 3, 17, and 1 (P= 3.50E-3, 2.98E-3, and 6.67E-3, respectively). Methylation sites that correlated positively with disease activity were enriched on chromosome X (P= 2.15E-4) (Figure 1B and 1C).
Figure 1.
Annotation of the locations of methylation sites that positively and negatively correlate with SLEDAI scores in lupus patients (upper and lower panels, respectively) relative to transcription start sites and gene structures (A). Enrichment by chromosome analysis of DNA methylation sites negatively correlated (B) and positively correlated (C) with disease activity as measured by SLEDAI scores in lupus patients. Y-axis shows FDR corrected enrichment p-values, X-axis shows chromosome numbers, sorted by most to least enrichments. (D) Heatmap of transcription factor binding sites (Y-axis) enriched in DNA methylation sites negatively and positively correlating with SLEDAI score (X-axis). Blue/yellow/red gradient shows significance of depletion/enrichment, respectively. Top 30 transcription factors most differentially enriched between the two sets of methylation sites are shown.
Transcription factor binding sites analysis revealed that methylation sites positively correlating with disease activity in lupus are most significantly enriched for binding sites of two repressive transcription factors, EZH2 (P = 1.06E-100) and SUZ12 (P = 3.24E-74), both are members of the polycomb repressive complex-2 (PRC2). DNA methylation sites with methylation levels negatively correlating with disease activity were enriched in binding sites for BATF, FOS, CEBPB, JUN, and STAT3 (P = 1.21E-53, 2.44E-53, 4.63E-50, 6.36E-45, and 7.10E-38, respectively). In contrast to positively correlated methylation sites, methylation sites that demonstrate reduced methylation level with disease activity are significantly depleted in EZH2 binding sites (P= 5.80E-102) (Figure 1D). This suggests that the key transcriptional regulator EZH2, which possess repressive activity, might be playing a key role in the T cell epigenetic conformational changes that predispose to disease flare in lupus. Indeed, literature mining analysis (20) independently identified an association between genes nearest the highest SLEDAI-correlated methylation sites and EZH2. The negatively correlated genes were highly associated (P <0.0001 by 2-tailed Chi-square test with Yates correction) whereas the positively correlated genes did not quite reach significance (P <0.07). This analysis also demonstrates a pro-inflammatory epigenetic landscape change in naïve CD4+ T cells as the disease becomes more active (Figure 2).
Figure 2.
Literature analysis of how concepts (yellow) are connected to genes whose methylation status is positively (green) and negatively (red) correlated with SLEDAI scores. The top 100 positively and negatively correlated methylation sites were included in this analysis. Existence of an edge indicates that the two nodes appear together in MEDLINE titles/abstracts at least 3 times, while thickness of the line correlates with frequency of the two terms being co-mentioned. Edges linking concepts and genes are shaded gray, while edges linking genes are black.
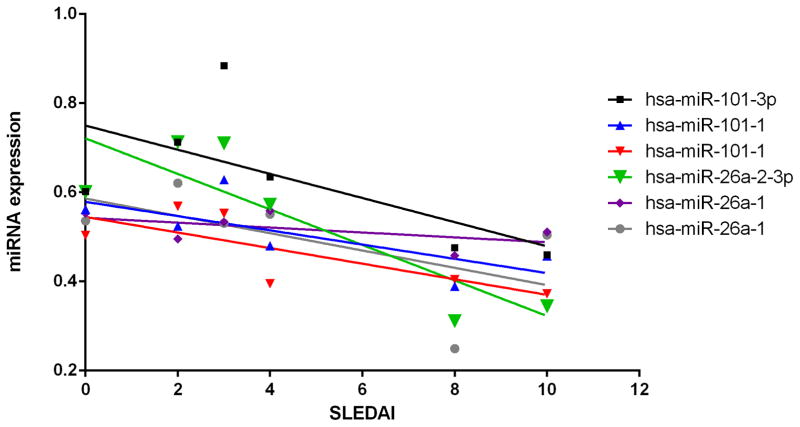
EZH2 expression in T cells has been recently shown to be inhibited by glucose restriction, via increased expression of miR-101 and mi-26a which target EZH2 (24). As CD4+ T cells in lupus patients demonstrate enhanced glycolysis (25), we hypothesized that EZH2 dysregulation upon lupus flares might be mediated by changes in miR-101 or miR-26a expression. We measured the expression of miR-101 and miR-26a microRNA species in a subset of lupus patients and show an overall negative correlation between these microRNAs and disease activity in lupus, and a significant negative correlation between miR-26a-2-3p levels in naïve CD4+ T cells and disease activity (r= −0.86, P= 0.028, Figure 3, supplementary table 1). This microRNA expression pattern is consistent with increased activity of EZH2 in naïve CD4+ T cells with lupus flares, predicted by our bioinformatics analyses.
Figure 3.
MicroRNA expression levels of miR-101 and miR-26a in naïve CD4+ T cells from lupus patients. Median values for each SLEDAI level are depicted for miR-101 and miR-26a probes with a Pearson correlation (r) with SLEDAI scores of ≥ │0.50│. The negative correlation between miR-26a-2-3p levels and SLEDAI scores was statistically significant (r=−0.86, P= 0.028). Two probe sets for each miR-101-1 and miR-26a-1 were included.
To better understand the nature of the epigenetic change in naïve CD4+ T cells that occurs upon lupus flare, we performed a functional enrichment analysis for genes that demonstrate reduced methylation (negatively correlated) and increased methylation (positively correlated) with disease activity. Genes with methylation levels that negatively correlated with SLEDAI scores in lupus were represented in several immune-related pathways including asthma, allograft rejection, autoimmune thyroid disease, and graft-versus-host disease. These pathways seem to have several cytokines in common including the Th2 cell cytokine genes IL4, IL5, and IL13, as well as several HLA class II genes (Table 1). Genes with methylation levels that positively correlated with SLEDAI scores were enriched in several pathways, most notably the inhibitory TGF-beta signaling pathway (Table 1).
Table 1.
Pathway analysis for genes with DNA methylation changes that negatively or positively correlated with disease activity in naïve CD4+ T cells in lupus
| Genes with negative correlation between
methylation status and disease activity in lupus | |||
|---|---|---|---|
| Pathway | Odds Ratio |
P value (FDR adjusted) |
Genes |
| Asthma | 8.49 | 2.67E-06 | IL13,IL4,IL5,HLA-DMB,HLA-DPA1,HLA-DRB1,HLA-DQA2,HLA-DMA,FCER1G,RNASE3,FCER1A,IL9,HLA-DQA1,HLA-DPB1,HLA-DRB5,IL3,CD40 |
| Allograft rejection | 5.81 | 1.69E-05 | IL12B,IL4,IL5,HLA-DMB,HLA-DPA1,HLA-DRB1,HLA-DQA2,HLA-A,CD86,HLA-DMA,HLA-DQA1,PRF1,HLA-DPB1,HLA-DRB5,GZMB,FAS,CD40,CD80 |
| Autoimmune thyroid disease | 4.11 | 2.94E-04 | IL4,IL5,HLA-DMB,HLA-DPA1,HLA-DRB1,HLA-DQA2,HLA-A,CD86,TPO,HLA-DMA,HLA-DQA1,PRF1,HLA-DPB1,HLA-DRB5,GZMB,FAS,CD40,CD80 |
| Graft-versus-host disease | 4.17 | 4.98E-04 | HLA-DMB,HLA-DPA1,HLA-DRB1,HLA-DQA2,HLA-A,CD86,IL6,HLA-DMA,KLRD1,HLA-DQA1,PRF1,HLA-DPB1,HLA-DRB5,GZMB,FAS,CD80 |
| Type I diabetes mellitus | 3.88 | 4.98E-04 | IL12B,HLA-DMB,HLA-DPA1,HLA-DRB1,HLA-DQA2,PTPRN2,HLA-A,CD86,HLA-DMA,CPE,HLA-DQA1,PRF1,HLA-DPB1,HLA-DRB5,GZMB,FAS,CD80 |
| Regulation of actin cytoskeleton | 1.93 | 5.47E-04 | DOCK1,VCL,TIAM2,CHRM2,FGFR2,LIMK2,FGF11,ITGB2,MYLK,CRK,ARHGEF6,FGF1,PIP5K1C,PIK3CD,ITGAD,ITGAX,FGF12,ITGB4,PDGFA,FGFR4,PIK3R1,DIAPH1, GNA12,FGF21,ITGAE,ITGB1,MYL7,MYL9,PXN,CHRM3,CYFIP1,VAV2,FGFR1,FGFR3,ITGA3,PIP4K2A,PDGFRB,MYLK2,ITGA11,BAIAP2,MYH9,NCKAP1L,ACTN4,MYH10,EGFR,GSN,ARHGEF7,PPP1CA,IQGAP2,SSH1,VAV3,PDGFD,DIAPH3,PIK3CB |
| Genes with positive correlation between
methylation status and disease activity in lupus | |||
|---|---|---|---|
| Pathway | Odds Ratio |
P value (FDR adjusted) |
Genes |
| Pathways in cancer | 2.39 | 7.62E-04 | TPM3,ARNT2,WNT10A,SMAD3,TCF7,CTNNA2,FGF12,KITLG,PIK3CD,CBL,ETS1,HDAC1,PDGFRA,BMP4,LAMA3,AXIN2,GLI3,TGFBR2,COL4A6,FGF13,BCR,FGF4,BIRC2,VEGFB,RASSF5,APC2,APC,TGFB2,MMP2,PLCG1,FGFR2,TGFBR1 |
| Neuroactive ligand-receptor interaction | 2.31 | 2.80E-03 | GALR1,MTNR1A,MC4R,GRM6,CHRM2,F2RL1,P2RX1,ADRA2A,GRM7,KISS1R,NPY1R,OPRM1,TSHR,NTSR2,GABRA5,GABRB2,HCRTR2,CHRNA3,GABRG2,CRHR1, GABBR2,GRIN1,PTGFR,PTGDR,S1PR1,MCHR1 |
| TGF-beta signaling pathway | 3.15 | 1.03E-02 | SMAD3,PPP2R1B,SMURF2,BMP4,TGFBR2,BMPR1A,ID4,ID3,PITX2,TGFB2,TGFBR1 |
| Colorectal cancer | 3.53 | 1.03E-02 | SMAD3,TCF7,PIK3CD,AXIN2,TGFBR2,APC2,APC,TGFB2,TGFBR1 |
| Glycosaminoglycan biosynthesis -chondroitin sulfate | 6.07 | 1.03E-02 | B3GAT1,CHST11,DSE,CHSY1,CHST13 |
| Phosphatidylinositol signaling system | 3.06 | 1.03E-02 | DGKZ,INPP5A,ITPR3,PIK3CD,PIP4K2A,ITPKB,DGKA,PLCB1,INPP4A,PLCG1 |
To further characterize epigenetic changes that might influence T cell differentiation in naïve CD4+ T cells upon increased disease activity, we performed a gene ontology analysis focused on “cytokine” related ontologies that are enriched in genes with methylation levels negatively and positivity correlated with disease activity (Supplementary table 2). These analyses showed significant enrichment in cytokine related ontologies in negatively correlated genes, with a pattern most consistent with an epigenetic shift towards a Th2 and possibly Th17 response, suggested by reduced methylation in key cytokine genes such as IL4, IL5, IL13, IL12B, and IL17F when disease activity is increased in lupus. Indeed, the gene ontology for Th2 cytokine production was the most enriched ontology among genes hypomethylated with disease activity (odds ratio= 17.5, P= 2.04E-02). Other inflammatory T cell cytokine genes that show reduced methylation levels with disease activity include IL9 and IL22. In contrast, genes with methylation levels that increase with disease activity include inhibitory cytokine genes including TGF-beta related genes.
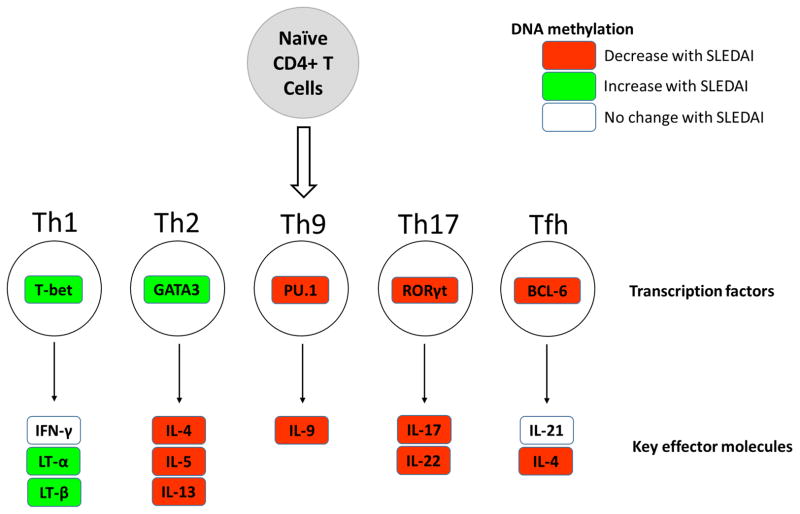
We next examined DNA methylation changes in key CD4+ T cell subsets transcription factors. Our data show reduced methylation with disease activity in the genes encoding PU.1, RORγt, and BCL-6, which promote Th9, Th17, and Tfh cell responses, respectively. Genes encoding critical Tfh cell molecules such as ICOS and CXCR5 were also hypomethylated with increased disease activity in lupus, while no methylation change with disease activity was observed in IL21. Genes encoding the Th1 and Th2 transcription factors T-bet and GATA3 showed hypermethylation with disease activity in lupus (Figure 4).
Figure 4.
Schematic representation of DNA methylation changes with disease activity in lupus naïve CD4+ T cells in key effector CD4+ T cell subsets genes.
To determine if this “epigenetic shift” towards an activated T cell phenotype precedes T cell activation and differentiation, we performed RNA sequencing in a subset of naïve CD4+ T cells included in this study. The majority of genes that showed DNA methylation changes negatively or positively correlating with disease activity in lupus demonstrated no significant change in gene expression levels with disease activity. This observation is not unexpected and is consistent with the naïve phenotype of the examined T cells, and also indicates that epigenetic changes that favor T cell activation predate gene expression changes. This is shown for the Th2 cytokine genes and the transcription factor NFkB in Figure 5. Therefore, our data suggest that a pro-inflammatory epigenomic remodeling in naïve CD4+ T cells is an early event occurring with increased lupus activity.
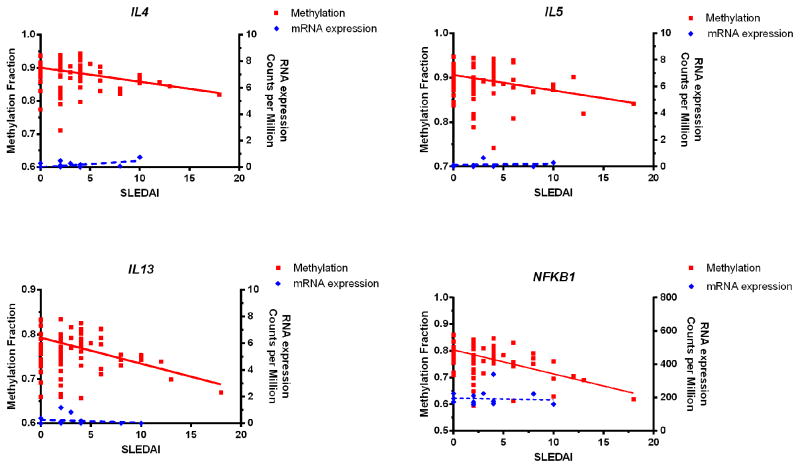
Figure 5.
Negative correlation between DNA methylation in Th2 cytokine genes (IL4, IL5, and IL13) and NFKB1 in naïve CD4+ T cells and disease activity in lupus patients (red) (r= −0.74, −0.74, −0.83, and −0.94; P= 0.0095, 0.0092, 0.0017, and 1.36E-05, respectively). Reduced methylation levels with disease flares in these genes precede active transcription as evidenced by lack of correlation between mRNA levels measured using RNAseq with disease activity (blue) (P= 0.15, 0.81, 0.63, and 0.78, respectively).
Discussion
In this study we demonstrate that an epigenetic landscape remodeling in naïve CD4+ T cells is associated with disease activity in lupus patients. With increased disease activity, as reflected by higher SLEDAI scores, naïve CD4+ T cells undergo an epigenetic shift that predisposes to an active T cell phenotype, evident at the naïve CD4+ T cells stage before T cell differentiation and activation. Indeed, by examining naïve CD4+ T cells we can predict early changes in T cell composition that accompany disease flares. Arguably, these compositional changes might be triggering or at least contributing to the initiating events resulting in a lupus flare.
The nature of the epigenetic shift detected suggests hypomethylation of genes involved in T cell activation and differentiation, and hypermethylation in inhibitory signaling pathways such as TGF-beta. Genes that demonstrate reduced methylation with increased disease activity in naïve CD4+ T cells from lupus patients include Th2 signature genes such as IL4, IL5, and IL13. Th2 cytokine gene hypomethylation is observed despite a paradoxical methylation increase in GATA3, suggesting that epigenetic poising of the Th2 cytokine gene locus with lupus flares is independent of the methylation status of GATA3. Negative correlation between DNA methylation in IL12B, IL17F, and RORC (encoding RORγt) and disease activity in lupus suggests that a Th17 response might be also an early event in lupus flares. Similarly, negative correlation between DNA methylation in the genes encoding BCL-6, ICOS, and CXCR5, and PU.1 and IL-9, suggests a role for Tfh and Th9 responses, respectively, in lupus flares. In contrast, no epigenetic changes were detected in or near the gene encoding for IFNγ, which is they key Th1 cytokine gene that typically gets demethylated upon Th1 differentiation. In addition, genes encoding other Th1 response molecules such as lymphotoxin (LT)-α, and LT-β, and the Th1 key transcription factor T-bet showed increased methylation with disease activity, and therefore are predicted to be epigenetically silenced with lupus flares. These data suggest that a non-Th1 response is the likely early T cell event occurring with increased disease activity in lupus, and underscores T cell-dependent B cell activation and plasma cell expansion in lupus flares.
The relative importance of Th2, Th17, or Tfh cell differentiation as an early flare event in lupus patients remains unclear, and is also complicated by the possibility of a transient role of these T cell responses in initiating disease relapse. Once a flare is established, it is possible that multiple other immune signaling and activating pathways become involved, which might or might not mask a predicted role for non-Th1 immune responses suggested by our data. These epigenetic changes occur without corresponding transcriptional changes indicating that a change in the epigenetic landscape in CD4+ T cells is an early event in lupus flares. The master pro-inflammatory cytokine regulator gene encoding for NFkB also is progressively demethylated with increased disease activity in lupus CD4+ T cells. In contrast, inhibitory pathways such as TGF-beta signaling are more methylated in active disease. Taken together, these data suggest that an epigenetic remodeling event shifting naïve CD4+ T cells towards activation and non-Th1 effector T cell differentiation and away from Treg differentiation could predispose to lupus flares.
To understand possible upstream events that might contribute to this epigenetic shift in naïve CD4+ T cells during disease flare, we determined if common transcription factors might be involved in regulating genes with methylation levels that positively or negatively correlated with disease activity in lupus. We found a robust enrichment for binding sites of the transcription factor EZH2 in methylation sites positively correlated with disease activity. Interestingly, methylation sites that are negatively correlated with disease activity are depleted of EZH2 binding sites. EZH2 is the catalytic member of the polycomb repressive complex-2 (PRC2). It encodes a histone methyltransferase, which methylates lysine 27 in histone H3, resulting in transcriptional repression and it can also recruit DNA methyltransferases including DNMT1, DNMT3A, and DNMT3B (26, 27). PRC2 and EZH2 play an important role in X-chromosome inactivation (28, 29), which might explain the relative enrichment of methylation sites that positivity correlated with disease activity in lupus on the X-chromosome. EZH2 plays an essential role in CD4+ T cell plasticity and differentiation. EZH2 has been implicated in the transcriptional silencing of Th2 cytokine genes, while EZH2 deficiency was shown to reduce Th1 differentiation by reducing the expression of T-bet and STAT4 (30, 31). However, other studies have shown that under Th1, Th2, and Th17 differentiation conditions, EZH2 deficiency enhances IFN-γ, IL-13, and IL-17 production, respectively (32). Further, EZH2 is critical in maintaining Treg function during T cell activation and differentiation, and EZH2 deficiency has been demonstrated to reduce Treg differentiation (32). Interestingly, EZH2 deficient Tregs as well as effector T cells demonstrate functional defects. Effector T cells deficient in EZH2 failed to expand in culture and to induce autoimmunity in a colitis model, and failed to protect against T. gondii infection which is known to elicit a Th1 response (32). In contrast, EZH2 deficiency enhanced Th2 responses in vivo in an asthma mouse model (33). Taken together, these data suggest a complex, sometimes contradictory, but crucial role for EZH2 in T cell responses. Indeed, recent studies suggest that EZH2+ T cells are polyfunctional expressing multiple effector cytokines, and that EZH2 expression is increased with T cell activation and enhances T cell survival and effector function by inhibiting Notch signaling repressors (24).
Recent evidence suggests that EZH2 expression is sensitive to glucose availability. Glucose restriction suppresses EZH2 expression by inducing the expression of two microRNAs that target EZH2 (miR-101 and miR-26a) (24). Consistent with our model of lupus flares, we find negative correlation between miR26a expression and disease activity in lupus patients. This suggests that reduced microRNA regulation of EZH2, possibly mediated by increased glycolysis and glucose availability, might result in transient increase in EZH2 activity leading to epigenetic reprograming in T cells favoring T activation. This model is consistent with recent reports showing enhanced glycolysis in CD4+ T from lupus patients and lupus mouse models (25). It should be noted that we did not observe a correlation between EZH2 mRNA expression levels and disease activity in naïve CD4+ T cells in lupus (data not shown). This might be explained by the sometimes transient nature of gene expression changes which in this case could have been missed as the epigenetic changes presumably resulting from EZH2 have been already established in naïve CD4+ T cells.
In summary, we provide evidence for epigenetic changes in CD4+ T cells in lupus that suggests a role for T cell activation and non-Th1 functions as early contributing immune responses in lupus flares. These epigenetic changes might be mediated by EZH2 which is an epigenetic regulator that is sensitive to glucose availability and plays an important role in T cell plasticity, activation, and differentiation. Further studies into the direct role of EZH2 in lupus progression and flares are warranted.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI097134.
Footnotes
Conflict of interest: None of the authors has any financial conflict of interest to report
References
- 1.Bentham J, Morris DL, Cunninghame Graham DS, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nature genetics. 2015;47(12):1457–64. doi: 10.1038/ng.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun C, Molineros JE, Looger LL, Zhou XJ, Kim K, Okada Y, et al. High-density genotyping of immune-related loci identifies new SLE risk variants in individuals with Asian ancestry. Nature genetics. 2016;48(3):323–30. doi: 10.1038/ng.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coit P, Ognenovski M, Gensterblum E, Maksimowicz-McKinnon K, Wren JD, Sawalha AH. Ethnicity-specific epigenetic variation in naive CD4+ T cells and the susceptibility to autoimmunity. Epigenetics & chromatin. 2015;8:49. doi: 10.1186/s13072-015-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coit P, Jeffries M, Altorok N, Dozmorov MG, Koelsch KA, Wren JD, et al. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naive CD4+ T cells from lupus patients. Journal of autoimmunity. 2013;43:78–84. doi: 10.1016/j.jaut.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffries MA, Sawalha AH. Autoimmune disease in the epigenetic era: how has epigenetics changed our understanding of disease and how can we expect the field to evolve? Expert review of clinical immunology. 2015;11(1):45–58. doi: 10.1586/1744666X.2015.994507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng C, Kaplan MJ, Yang J, Ray D, Zhang Z, McCune WJ, et al. Decreased Ras-mitogen-activated protein kinase signaling may cause DNA hypomethylation in T lymphocytes from lupus patients. Arthritis and rheumatism. 2001;44(2):397–407. doi: 10.1002/1529-0131(200102)44:2<397::AID-ANR59>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Sawalha AH, Jeffries M, Webb R, Lu Q, Gorelik G, Ray D, et al. Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients. Genes and immunity. 2008;9(4):368–78. doi: 10.1038/gene.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorelik G, Sawalha AH, Patel D, Johnson K, Richardson B. T cell PKCdelta kinase inactivation induces lupus-like autoimmunity in mice. Clinical immunology. 2015;158(2):193–203. doi: 10.1016/j.clim.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawalha AH. Epigenetics and T-cell immunity. Autoimmunity. 2008;41(4):245–52. doi: 10.1080/08916930802024145. [DOI] [PubMed] [Google Scholar]
- 10.Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. The Journal of clinical investigation. 2015;125(6):2220–7. doi: 10.1172/JCI78087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 12.Coit P, Renauer P, Jeffries MA, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, et al. Renal involvement in lupus is characterized by unique DNA methylation changes in naive CD4+ T cells. Journal of autoimmunity. 2015;61:29–35. doi: 10.1016/j.jaut.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis S, Du P, Bilke S, Triche TJ, Bootwalla M. R package version 2.16.0. 2015. methylumi: Handle Illumina methylation data. [Google Scholar]
- 14.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882–3. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu G, Wang LG, He QY. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31(14):2382–3. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- 16.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics. 2007;23(2):257–8. doi: 10.1093/bioinformatics/btl567. [DOI] [PubMed] [Google Scholar]
- 17.Dozmorov MG, Cara LR, Giles CB, Wren JD. GenomeRunner: automating genome exploration. Bioinformatics. 2012;28(3):419–20. doi: 10.1093/bioinformatics/btr666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbloom KR, Sloan CA, Malladi VS, Dreszer TR, Learned K, Kirkup VM, et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic acids research. 2013;41(Database issue):D56–63. doi: 10.1093/nar/gks1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storey J. A direct approachto false discovery rates. J R Stat B. 1995;64:479–98. [Google Scholar]
- 20.Wren JD, Bekeredjian R, Stewart JA, Shohet RV, Garner HR. Knowledge discovery by automated identification and ranking of implicit relationships. Bioinformatics. 2004;20(3):389–98. doi: 10.1093/bioinformatics/btg421. [DOI] [PubMed] [Google Scholar]
- 21.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rumble SM, Lacroute P, Dalca AV, Fiume M, Sidow A, Brudno M. SHRiMP: accurate mapping of short color-space reads. PLoS computational biology. 2009;5(5):e1000386. doi: 10.1371/journal.pcbi.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics (Oxford, England) 2003;4(2):249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 24.Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nature immunology. 2016;17(1):95–103. doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, et al. Normalization of CD4+ T cell metabolism reverses lupus. Science translational medicine. 2015;7(274):274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298(5595):1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 27.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439(7078):871–4. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 28.Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, Wang H, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300(5616):131–5. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- 29.Sarma K, Cifuentes-Rojas C, Ergun A, Del Rosario A, Jeon Y, White F, et al. ATRX directs binding of PRC2 to Xist RNA and Polycomb targets. Cell. 2014;159(4):869–83. doi: 10.1016/j.cell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyanagi M, Baguet A, Martens J, Margueron R, Jenuwein T, Bix M. EZH2 and histone 3 trimethyl lysine 27 associated with Il4 and Il13 gene silencing in Th1 cells. The Journal of biological chemistry. 2005;280(36):31470–7. doi: 10.1074/jbc.M504766200. [DOI] [PubMed] [Google Scholar]
- 31.Tong Q, He S, Xie F, Mochizuki K, Liu Y, Mochizuki I, et al. Ezh2 regulates transcriptional and posttranslational expression of T-bet and promotes Th1 cell responses mediating aplastic anemia in mice. Journal of immunology. 2014;192(11):5012–22. doi: 10.4049/jimmunol.1302943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang XP, Jiang K, Hirahara K, Vahedi G, Afzali B, Sciume G, et al. EZH2 is crucial for both differentiation of regulatory T cells and T effector cell expansion. Scientific reports. 2015;5:10643. doi: 10.1038/srep10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tumes DJ, Onodera A, Suzuki A, Shinoda K, Endo Y, Iwamura C, et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4(+) T helper type 1 and type 2 cells. Immunity. 2013;39(5):819–32. doi: 10.1016/j.immuni.2013.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.