Abstract
In recent years a substantial number of findings have been made in the area of immunometabolism, by which we mean the changes in intracellular metabolic pathways in immune cells that alter their function. Here, we provide a brief refresher course on six of the major metabolic pathways involved (specifically, glycolysis, the tricarboxylic acid (TCA) cycle, the pentose phosphate pathway, fatty acid oxidation, fatty acid synthesis and amino acid metabolism), giving specific examples of how precise changes in the metabolites of these pathways shape the immune cell response. What is emerging is a complex interplay between metabolic reprogramming and immunity, which is providing an extra dimension to our understanding of the immune system in health and disease.
Most immunologists have a distant memory of learning metabolic pathways as undergraduates. Many of these proto-immunologists saw these pathways as scientifically interesting but unlikely to impinge on their own growing research interests into the complexity of the immune response. Equally, those scientists who became immunologists later were largely unaware of how metabolic pathways might be of direct relevance to their research, unless they had a particular interest in the role of immune cells in obesity or in metabolic diseases, such as type 2 diabetes. Of course there was a minority of immunologists who were considering metabolic processes in the functioning of immune cells, with early studies from more than 30 years ago describing the requirement of certain metabolites for macrophage, neutrophil and T cell function1–4. These studies largely focused on energy production and biosynthesis, as activated macrophages or rapidly dividing T cells have huge metabolic demands. There was also a major interest in mechanistic target of rapamycin (mTOR), which is a central metabolic regulator of immunity5, and AMP kinase. mTOR is the catalytic subunit of two distinct complexes — mTOR complex 1 (mTORC1) and (mTORC2) — that can sense amino acids and growth factors and promote mRNA translation and lipid synthesis to support cell growth; beyond this, mTOR signalling regulates numerous events that are crucial for T cell and monocyte differentiation6. AMP kinase (which is activated during nutrient deprivation) promotes catabolism (for example, of fatty acids) and also inhibits mTOR activity, thereby limiting immune cell activation7.
What we have seen in the past five years or so is something of a rediscovery of metabolism by immunologists and the emergence of what is now termed the field of immunometabolism. Why did this happen? Technological advances have helped tremendously; highly sensitive metabolomic approaches allow us to define the alterations in metabolites that occur during immune cell activation and show how metabolites are directly linked to immune cell effector functions. Immunology itself as a science has advanced hugely in the past 30 years. Notable advances include the discovery of whole new immune receptor systems (most notably the pattern recognition receptors (PRRs)), the description of many cytokines and immune cell types, and a deeper understanding of the development and molecular regulation of these immune cells. Furthermore, we now have elaborate tools that facilitate the study of the immune system in a bewildering range of states, including in models of infection, autoimmunity and autoinflammation. More recently we have seen the application of newer tools (including small molecule agonists or antagonists) and approaches (such as techniques that measure the flux though metabolic pathways) to the study of the immune system, which is providing us with exciting new insights into the core of what is happening during an immune response. That core involves complex and specific metabolic changes that directly connect to those aspects of immunity and host defence so beloved by immunologists: a detailed account of the molecular regulation of events occurring in immune cells in health and disease. In this Review, we provide a refresher course of six main metabolic pathways that occur in cells and discuss their possible roles in immunity. We will focus mainly on specific examples in T cells, macrophages and dendritic cells (DCs), since most of the recent new insights have been made in these cell types. We will also provide a list of tools (shown in TABLE 1) and a glossary of key terms to encourage immunologists to bring the extra dimension of immunometabolism to their own research programmes, as we are confident this will allow them to advance their understanding of the immune processes they are interested in. We hope the readers find our account understandable, interesting and thought-provoking for their own research.
Table 1.
Small molecule agents that manipulate immunometabolism*
| Name | Target | Consequence |
|---|---|---|
| 2-deoxyglucose | Hexokinase | Inhibition of glycolysis |
| 3-bromopyruvate | Hexokinase | Inhibition of glycolysis |
| Ritonavir | Glucose transporter 1 | Inhibition of glycolysis |
| Dichloroacetate | Pyruvate dehydrogenase kinase | Inhibition of glycolysis |
| FX11 | Lactate dehydrogenase | Inhibition of glycolysis |
| 4-CIN | Monocarboxylate transporters | Inhibition of glycolysis |
| DASA and TEPP46 | Pyruvate kinase M2 | Inhibition of HIF1α |
| C75 | Fatty acid synthase | Inhibition of fatty acid synthesis |
| Etomoxir | Carnitine palmitoyl transferase I | Inhibition of fatty acid oxidation |
| AICAR | AMP kinase | Increased fatty acid oxidation |
| Metformin | AMP kinase | Increased fatty acid oxidation; inhibition of Complex I; decreased mitochondrial reactive oxygen species. |
| Cerulenin | Fatty acid synthase | Inhibition of fatty acid synthesis |
| Rotenone | Complex I | Inhibition of OXPHOS |
| BPTES | Glutaminase | Inhibition of glutaminolysis |
| Oligomycin | ATP synthase | Inhibition of OXPHOS |
| TOFA | Acetyl CoA carboxylase | Inhibition of fatty acid synthesis |
| UK5099 | Pyruvate transporter | Inhibition of the TCA cycle |
4-CIN, α-cyano-4-hydroxycinnate; AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; BPTES, bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide; DASA, diarylsulfonamide; HIF1α, hypoxia-inducible factor 1α; OXPHOS, oxidative phosphorylation; TCA, tricarboxylic acid; TOFA, tall oil fatty acid.
A number of small molecules can be used by immunologists to probe the role of metabolic reprogramming in the systems in which they are particularly interested.
An overview of metabolic pathways
Cell intrinsic and extrinsic signals regulate the activity of metabolic pathways to couple the growth and survival needs of the cell to the metabolic machinery that regulates the generation of key products to fulfil these needs. In the context of immunity, however, specific alterations in metabolic pathways couple to immune effector functions, most notably in the production of distinct sets of cytokines. FIGURE 1 illustrates how immune molecules such as interleukin-4 (IL-4), or PRRs, can promote different metabolic pathways in cells, events previously shown to be regulated by oxygen levels. Immune cells with different functions use several different metabolic pathways to generate adequate levels of energy stores to support survival and to produce numerous biosynthetic intermediates to allow for cellular growth and proliferation. These metabolic pathways, although diverse in terms of their end products, are closely linked as a consequence of shared fuel inputs, and a reliance on products from one pathway to feed into alternative pathways as key synthetic precursors. As one example of the complex interplay of metabolic pathways, the process of fatty acid synthesis — which allows for the production of cell membranes and other key lipid-based structures that are necessary for proliferation — is dependent on the availability of intermediate products from the glycolytic pathway and tricarboxylic acid (TCA) cycle metabolism. With the interconnected nature of cellular metabolic pathways in mind, here we discuss six key metabolic pathways that have an important role in the generation of key products to promote cell survival or growth. The glycolytic, TCA cycle, pentose phosphate, fatty acid oxidation, fatty acid synthesis and amino acid pathways each have a unique purpose in the cell and are regulated by cellular signalling pathways to link their activity to cellular needs (FIG. 2).
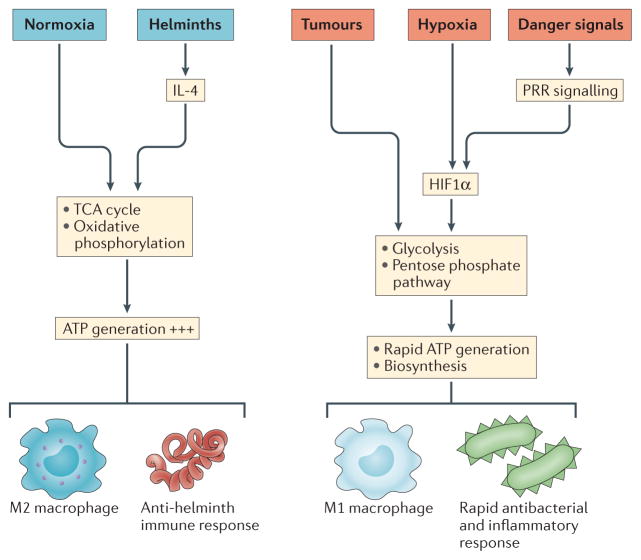
Figure 1. Metabolic reprogramming by the immune system.
Historically, oxygen levels and nutrient supply were seen as the key drivers of metabolic pathways. Normoxia supports the tricarboxylic acid (TCA) cycle and oxidative phosphorylation, whereas hypoxia leads to the activation of hypoxia-inducible factor 1α (HIF1α) and the expression of glycolytic enzymes. More recently, it has become apparent that immune stimuli can also cause metabolic reprogramming in cells. For example, stimulation of cells with interleukin-4 (IL-4) can induce oxidative phosphorylation, whereas the activation of cells through pattern recognition receptors (PRRs), such as Toll-like receptor 4 (TLR4), induces HIF1α expression to promote glycolysis. Glycolysis also predominates in tumours under normoxia in the form of aerobic glycolysis, presumably giving tumours a growth advantage in oxygen-replete tissues.
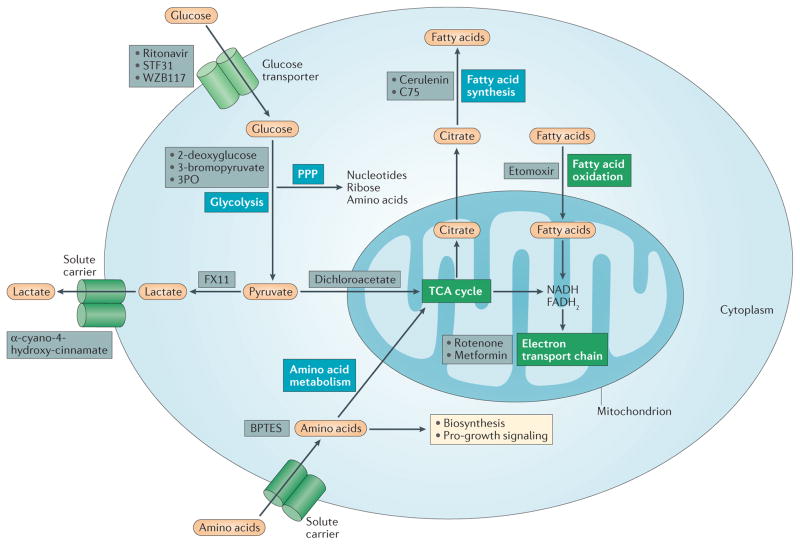
Figure 2. Six major metabolic pathways.
Glycolysis converts glucose into pyruvate, which can then either be converted into lactate, and secreted, or can enter the tricarboxylic acid (TCA) cycle in which it will generate NADH and FADH2 for the electron transport chain, leading to ATP production. Glycolysis also feeds the pentose phosphate pathway (PPP), which generates ribose for nucleotides, amino acids and NADPH. NADPH is used for fatty acid synthesis, which uses citrate withdrawn from the TCA cycle. Fatty acids can also be oxidized, generating NADH and FADH2, which again drive ATP production from the electron transport chain. Finally amino acid metabolism can feed the TCA cycle and is also important for cell growth and protein biosynthesis. In this figure, the pathways that require oxygen are indicated in green boxes, and pathways that are not oxygen dependent are indicated in blue boxes. In addition, inhibitors of metabolic pathways are indicated in grey boxes. BPTES, bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulphide.
The glycolytic metabolic pathway
The glycolytic metabolic pathway (also termed glycolysis) begins with the uptake of extracellular glucose from the environment surrounding the cell and subsequent intracellular processing of glucose in the cytosol to eventually yield pyruvate along with numerous other products. Glycolytic metabolism is a relatively inefficient pathway for the generation of cellular ATP, netting only two molecules of ATP per unit of glucose. However, glycolytic metabolism provides key benefits to cells because it also allows for the reduction of NAD+ to NADH, which is used by numerous enzymes as a cofactor, as well as enabling the diversion of intermediate products to biosynthetic growth pathways to support anabolic growth. To maintain glycolytic flux, cells often reduce pyruvate to lactate to recycle NADH and maintain NAD+ levels. Glycolytic metabolism has a key role in providing biosynthetic intermediates for the synthesis of ribose for nucleotides (glucose-6-phosphate into pentose phosphase), amino acids (3-phosphoglycerate into the serine biosynthetic pathway) and fatty acids (pyruvate into the TCA cycle for citrate). Indeed, many pro-growth signalling pathways, including the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways, promote cellular use of glycolytic metabolism. For these reasons, glycolysis is frequently observed as having a dominant and essential role in the metabolism of rapidly proliferating cells.
The TCA cycle
The TCA cycle (also known as the citric acid cycle or Krebs cycle) occurs in the matrix of the mitochondrion and is a major metabolic pathway that is thought to be used in most quiescent or non-proliferative cell settings. Although some quiescent stem cells primarily use glycolysis, the TCA cycle and oxidative phosphorylation are a highly efficient mode of ATP generation used by cells whose primary requirements are energy and longevity. The TCA cycle is a nexus for multiple nutrient inputs. Most notably, glucose-derived pyruvate or fatty acids are converted into acetyl coenzyme A (acetyl-CoA) that joins the TCA cycle by aldol condensation with oxaloacetate to form citrate. Glutamate is also a critical fuel for the TCA cycle through its direct conversion into the TCA intermediate α-ketoglutarate. Two major products of the TCA cycle are NADH and FADH2, which can transfer electrons to the electron transport chain to support oxidative phosphorylation and highly efficient ATP generation. This process provides for basal subsistence in most cell types. Similarly to what is seen in glycolysis, growth signals can promote the diversion of TCA intermediates for production of amino acids and lipids (termed cataplerosis), although this requires increased nutrient diversion to the TCA cycle (termed anaplerosis).
The pentose phosphate pathway
The pentose phosphate pathway takes place in the cytosol and serves several key purposes that support cell proliferation and survival. First, and most well-known, the pentose phosphate pathway allows the diversion of intermediates from the glycolytic pathway towards the production of nucleotide and amino acid precursors that are necessary for cell growth and proliferation. This involves the non-oxidative branch of the pentose phosphate pathway. A second key function of the pentose phosphate pathway is the generation of reducing equivalents of NADPH, which has an important role in the maintenance of a favourable cellular redox environment and is also required for fatty acid synthesis. This involves the oxidative branch of the pentose phosphate branch.
Fatty acid oxidation
The fatty acid oxidation pathway allows for the mitochondrial conversion of fatty acids into numerous products that the cell can further use to generate energy, including acetyl-CoA, NADH and FADH2. The initial step of fatty acid oxidation is the ‘activation’ of the fatty acid in the cytosol via an enzyme-mediated reaction with ATP to eventually generate a fatty acid acyl-CoA. The subsequent mechanism of fatty acid oxidation is dependent on the length of the aliphatic tail in the fatty acid. Short-chain fatty acids, which are defined as having fewer than six carbons in the aliphatic tail, are simply able to diffuse into mitochondria passively. Medium-chain and long-chain fatty acids must first be conjugated to carnitine via carnitine palmitoyl transferase I (CPT1). After this, the carnitine conjugated long-chain fatty acid is then shuttled into the mitochondria where it is converted back to a fatty acid acyl-CoA through the removal of carnitine by carnitine palmitoyl transferase II (CPT2). At this stage, β-oxidation of the fatty acid acyl-CoA commences, yielding large amounts of acetyl-CoA, NADH and FADH2 that are further used in the TCA cycle and the electron transport chain to generate ATP. CPT1A acts as a key regulatory step in fatty acid oxidation, as it is rate limiting and inhibited by the lipid synthesis intermediate malonyl-CoA, thus preventing lipid oxidation when cells are actively synthesizing lipids. Overall, fatty acid oxidation can allow the production of tremendous amounts of ATP, with the complete β-oxidation of a single palmitate molecule (a major fatty acid in mammalian cells) eventually having the potential to yield over 100 ATP molecules.
Fatty acid synthesis
The fatty acid synthesis pathway allows cells to generate lipids that are necessary for cellular growth and proliferation from precursors derived from other cell intrinsic metabolic pathways. The activity of the fatty acid synthesis pathway is closely coupled to mTOR signalling, which has been shown to promote fatty acid synthesis through regulation of many of the key enzymes responsible for de novo lipid synthesis, including SREBP (sterol regulatory element binding protein), FASN (fatty acid synthase) and ACC (acetyl CoA carboxylase), both of which are induced by SREBP. Fatty acid synthesis uses products derived from several other metabolic pathways, notably glycolysis, the TCA cycle and pentose phosphate pathway. For the synthesis of straight-chain fatty acids, TCA cycle-derived citrate may be exported from the mitochondria into the cytosol via the citrate carrier, where ATP citrate lyase converts it into acetyl-coA, along with oxaloacetate. The acetyl-CoA derived from this process may then be carboxylated by ACC to yield malonyl-CoA. Subsequently, FASN acts in an NADPH-dependent manner to elongate the nascent fatty acid chain until products such as palmitic acid are synthesized. Fatty acids with alternative chain lengths may be synthesized using palmitic acid as a substrate for elongation, while desaturation reactions may be performed to yield unsaturated fatty acids. Unlike straight-chain fatty acids, branched-chain fatty acid synthesis requires branched-chain amino acids such as valine and leucine as substrates for elongation. Furthermore, fatty acids may be condensed with glycolysis-derived glycerol to yield many possible combinations of triacylglycerols and phospholipids, which are key components of many cellular structures.
Amino acid metabolic pathways
The metabolism of various amino acids has several important roles in multiple aspects of cell biology. As can be expected with the large number of individual amino acids, there are diverse metabolic pathways that make use of amino acids as substrates. Amino acids, as a consequence of their utilization as substrates for protein synthesis, are intimately linked to important anabolic cellular signalling pathways, most notably the mTOR pathway and nucleotide synthesis. Many studies have shown a role for mTOR-containing complexes in sensing amino acid levels and adequate availability of amino acids is known to be required for mTOR-driven anabolic growth. As discussed above, aside from their well-known role as building blocks in protein synthesis, amino acids can act as precursors for the de novo synthesis of branched-chain fatty acids. Individual amino acids may play more specific roles in metabolic pathways. For instance, glutamine and aspartate are required for de novo purine and pyrimidine synthesis. Also, glutamine may be used in actively proliferating cells as an alternative input for the TCA cycle where it can be used to support ATP production or, alternatively, as a source of citrate for fatty acid synthesis. Additional amino acids, including arginine and tryptophan, are metabolized through various metabolic pathways to support cellular proliferation and anabolic growth.
Glycolysis in immunity
We now give key examples of how changes in the metabolic pathways described above directly impact on immune cell function. Firstly glycolysis has been shown to be involved in a number of immune processes (FIG. 3). Early work indicated that both activated macrophages and T cells have a voracious appetite for glucose1,2. In addition, 2-deoxyglucose was applied in various contexts and was shown to inhibit macrophage activation in vitro and to suppress inflammation in in vivo models8,9. All of these studies and indeed others pointed to glycolysis as being crucial for immune cell function. This was initially somewhat surprising as glycolysis is not the most effective way to generate ATP. As stated above, glycolysis generates 2 molecules of ATP from 1 molecule of glucose, whereas oxidative phosphorylation is much more effective, generating 36 ATP molecules from a single molecule of glucose. However, it was also realized that glycolysis could be rapidly activated via the induction of enzymes that are involved in this pathway. By contrast, boosting oxidative phosphorylation requires mitochondrial biogenesis, which is a more complex and probably slower process. As such, cells that need to make ATP swiftly will switch to glycolysis. Potentially more important than rapid ATP generation, however, is the capacity of high rates of glycolysis to provide biosynthetic intermediates to support rapid cell growth. Activating signals such as growth factors strongly promote increased glucose uptake and glycolysis, which supplies ATP, supports the TCA cycle, and donates intermediates for the PPP, glycosylation reactions and synthesis of key biomass constituents, including serine, glycine, alanine, and acetyl-CoA for lipid synthesis.
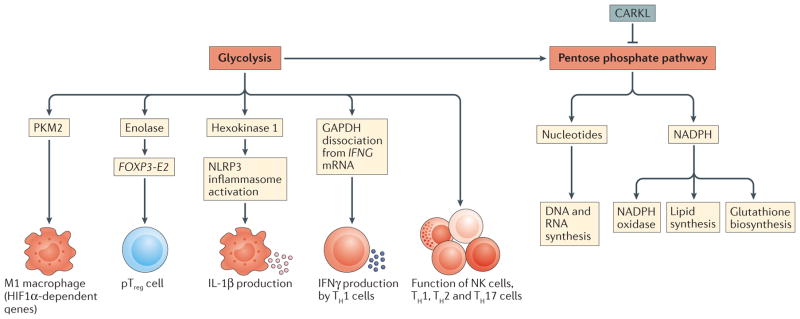
Figure 3. Glycolysis and the pentose phosphate pathway in immunity.
Glycolysis occurs in M1-like macrophages in response to hypoxia-inducible factor 1α (HIF1α) activation. HIF1α not only promotes glycolysis but also induces the expression of genes that encode inflammatory cytokines, notably interleukin-1β (IL-1β). The glycolytic enzyme hexokinase 1 has also been shown to directly interact with and activate the NLRP3 inflammasome, leading to caspase 1 activation and the processing of pro-IL-1 β. In T cells, the glycolytic enzyme glyceraldehyde 3 phosphate dehydrogenase (GAPDH) binds to mRNA encoding interferon-γ (IFNγ) and represses its translation; the switch to glycolysis that occurs in response to T cell activation leads to the dissociation of GAPDH allowing for translation of IFNγ. Glycolysis is also crucial for the functioning of natural killer (NK), T helper 1 (TH1), TH2, TH17 and peripherally induced regulatory T (pTreg) cells. The pentose phosphate pathway branches off glycolysis and generates ribose for nucleotides for DNA and RNA synthesis, but also NADPH, for NADPH oxidase or for glutathione biosynthesis, promoting an antioxidant response. In human pTreg cells, the glycolytic enzyme enolase has been shown to promote their suppressive functions by regulating the expression of FOXP3 splicing variants containing exon 2 (FOXP3-E2). CARKL, carbohydrate kinase-like protein; NLRP3, NOD-, LRR- and pyrin domain-containing 3; PKM2, pyruvate kinase isoenzyme M2.
Enhanced glycolysis occurs in lipopolysaccharide (LPS)-activated macrophages and DCs10,11, in activated natural killer (NK) cells12, in activated effector T cells13 and in activated B cells14. Effector T cell subsets all show an increase in glycolysis following activation, most notably T helper 17 (TH17) cells15, TH1 and TH2 cells13 and activated effector CD8+ T cells16. Increased mTOR pathway activity that is correlated with enhanced glycolysis seems to be associated with the initial generation of peripherally induced regulatory T (pTreg) cells, but may be detrimental to their long-term survival and lineage stability17–19. Increased glycolysis can therefore be considered a hallmark metabolic change in most immune cells undergoing rapid activation, for instance, in response to stimulation of PRRs, cytokine receptors or antigen receptors. Enhanced glycolysis enables the immune cell to generate sufficient ATP and biosynthetic intermediates to carry out its particular effector functions. For macrophages this includes phagocytosis and inflammatory cytokine production, for DCs this includes antigen presentation20 and for T cells this includes the production of effector cytokines (such as IL-17 in the case of TH17 cells15).
We have some molecular insights into the signalling pathways that trigger glycolysis during immune cell activation. For example, LPS induces activation of hypoxia-inducible factor 1α (HIF1α), a transcription factor that is crucial for the induction of several enzymes involved in glycolysis21. It also probably involves nuclear factor-κB (NF-κB) activation, as the ubiquitous isoform of phosphofructokinase-2 (uPFK2) — which is crucial for the regulation of glycolysis — is still induced by LPS in HIF1α-deficient cells10. LPS can also rapidly induce glycolysis in DCs via the activation of TANK-binding kinase 1 (TBK1) and/or inhibitor of NF-κB kinase ε (IKKε) and hexokinase 2, in a HIF1α-independent manner18.
A key mechanism for the enhanced glycolysis in LPS-activated macrophages is induction of pyruvate kinase isoenzyme M2 (PKM2)22. This form of PKM (which is an enzyme that generates pyruvate and ATP from phospho-enolpyruvate and ADP during glycolysis) is regulated to slow glycolytic flux and allow diversion of glycolytic intermediates to biosynthetic pathways. PKM2 also has a separate function outside its role in glycolysis. It translocates to the nucleus where it interacts with HIF1α and promotes the expression of HIF1α-dependent genes22,23, including those that encode the aforementioned glycolytic enzymes and also inflammatory factors, such as IL-1β. What is also of interest is the observation that a small molecule that forces PKM2 into a tetrameric state (in which it is unable to enter the nucleus and is more active in glycolysis than dimeric PKM2) reprogrammes macrophages to become more M2-like in their gene-expression profiles22. This indicates that inhibition of HIF1α (as will occur in this situation since PKM2 is no longer nuclear) will change the phenotype of the macrophage from a pro-inflammatory M1 phenotype to a pro-reparatory (or alternatively activated) M2 phenotype. A pro-inflammatory role for PKM2 in inflammatory monocytes and macrophages has also been demonstrated in human atherosclerotic coronary artery disease, further emphasizing the contribution of this glycolytic enzyme to inflammation24.
A similar role for glycolysis in immune cell reprogramming has been reported in TH17 cells, in which inhibition of glycolysis with 2-deoxyglucose converted TH17 cells into Treg cells15. In contrast, as discussed earlier, hyperactivation of mTOR pathway signalling resulting in increased glycolysis in peripheral Treg cells, but may impair their survival and lineage-commitment17–19. These studies emphasize again the link between metabolism and the phenotype of an immune cell, with glycolysis and HIF1α induction leading to the acquisition of a more inflammatory phenotype. Oxidative phosphorylation has been associated with a more anti-inflammatory cell phenotype25–27, although recent studies have shown that human Treg cells can utilize glycolysis28,29, suggesting that glycolysis may not only be associated with inflammatory cell functions. The glycolytic enzyme enolase has in fact been shown to ‘moonlight’, promoting Foxp3 splicing and the generation of pTreg cells29.
Another interesting aspect of glycolysis induction in activated immune cells is the role of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH). In TH1 cells it has been shown that GAPDH binds to mRNA that encodes interferon-γ (IFNγ)30,31, repressing its translation. Once glycolysis is triggered, however, GAPDH dissociates from IFNG mRNA, allowing it to be translated. Moreover, GAPDH can now enter the glycolytic pathway to promote further ATP production. This finding highlights another aspect of why immunometabolism is so central to immunity — the glycolytic enzyme GAPDH is controlling production of the crucial TH1-type effector cytokine IFNγ and must be recruited to the glycolytic pathway for this cytokine to be made.
In macrophages, another glycolytic enzyme, hexokinase 1, has been shown to moonlight, in this case as an NLRP3 (NOD-, LRR- and pyrin domain-containing 3) regulator32. The NLRP3 inflammasome is a crucial regulator of caspase 1, which generates mature IL-1β, as well as active IL-18, and induces a type of cell death called pyroptosis. Evidence has been presented that hexokinase interacts with NLRP3 in the outer mitochondrial membrane, enabling NLRP3 activation32. There are likely to be more examples of glycolytic enzymes having additional functions in immune cells.
As stated above, in addition to its role in ATP synthesis, glycolysis is also able to generate intermediates for biosynthesis of macromolecules. The most important example is glucose-6-phosphate, which enters the pentose phosphate pathway, which we will consider next.
The pentose phosphate pathway in immunity
As discussed above, there are two important outcomes of the pentose phosphate pathway — namely, nucleotide production (which happens during cell proliferation) and NADPH production. NADPH has multiple functions in immune cells. It is used by the NADPH oxidase to generate reactive oxygen species (ROS) during the respiratory burst, but as a counter-balance it is also used to generate glutathione and other antioxidants. During an infection, macrophages and neutrophils probably require both of these NADPH-dependent functions —rapid ROS production to clear the infectious agent followed by induction of antioxidants to prevent excessive tissue damage. DCs also use NADPH and lipid synthesis to support endoplasmic reticulum synthesis, which is necessary for DC activation and cytokine secretion20.
The pentose phosphate pathway has been shown to be elevated in LPS-activated macrophages21. A key control point is the enzyme carbohydrate kinase-like protein (CARKL; also known as SHK), which is a sedoheptulose kinase33 (FIG. 3). This enzyme limits the flux through the pentose phosphate pathway, and it has been shown to be highly expressed in M2 macrophages33. If CARKL is suppressed, macrophages become M1-like, indicating the importance of the pentose phosphate pathway for M1 macrophage function. Why nucleotide generation by the pentose phosphate pathway is elevated in M1 macrophages21 is a mystery, as these cells show low proliferative capacity. It is possible that the nucleotides are being generated for the production of different RNA populations (for example, microRNAs and the long non-coding RNAs that are important for regulating cellular function).
The TCA cycle in immunity
The TCA cycle and oxidative phosphorylation have been studied extensively in immune cells. Both are fully functional in most T cell subsets13, although, as stated above, there is a shift towards glycolysis and away from the TCA cycle in effector T cells. Furthermore, the TCA cycle is very prominent in memory CD8+ T cells34.
Of particular note, there have been observations concerning the TCA cycle in distinct macrophage subtypes (FIG. 4). In M2 macrophages, there is an intact TCA cycle that is coupled to oxidative phosphorylation25. This allows the generation of UDP-GlcNAc intermediates that are necessary for the glycosylation of M2-associated receptors, such as the mannose receptor25. However, in M1 macrophages the situation is rather different. In these cells, the TCA cycle has been shown to be broken in two places — after citrate (owing to a decrease in expression of isocitrate lyase) and after succinate21,25.
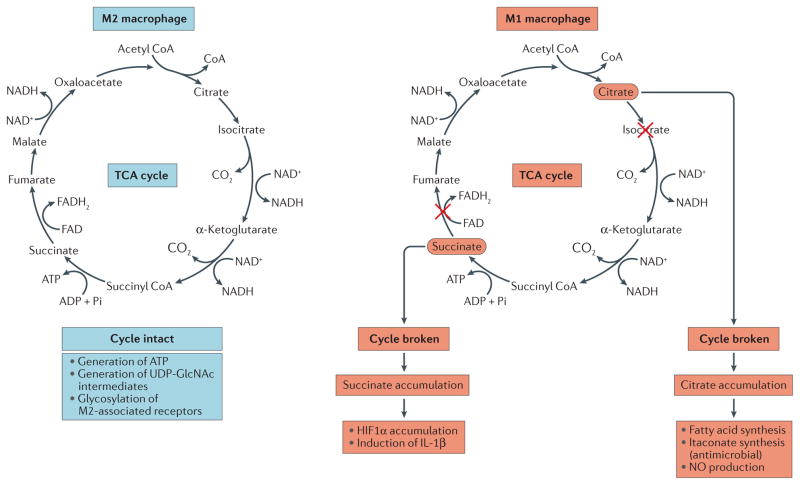
Figure 4. The TCA cycle in macrophages.
In M2-like macrophages (that is, interleukin-4 (IL-4)-activated macrophages) the tricarboxylic acid (TCA) cycle is intact and participates in oxidative phosphorylation, providing ATP for energy. In M1-like macrophages (that is, cells that have been activated by lipopolysaccharide (LPS) and interferon-γ), the TCA cycle is broken in two places — after citrate and after succinate. Citrate is used to generate fatty acids for membrane biogenesis and also for prostaglandin production. It also generates itaconic acid via the enzyme immune-responsive gene 1 (IRG1). Itaconic acid has direct antimicrobial activity against Mycobacterium tuberculosis and Salmonella sp. HIF1α, hypoxia-inducible factor 1α.
The citrate that accumulates in M1 macrophages has been shown to be exported from the mitochondria via the citrate transporter. It is then utilized for the production of fatty acids, which in turn are used for membrane biogenesis. This broken TCA cycle is also seen in activated DCs and seems to be especially important for their function, as these cells require substantial membrane production to support antigen presentation20. Excess citrate can also feed into pathways that generate nitric oxide and prostaglandins, which are key effector molecules made by macrophages35. A third metabolite generated from citrate is itaconic acid, which has been shown to have direct bactericidal effects on species such as Salmonella enterica subsp. enterica serovar Typhimurium and Mycobacterium tuberculosis36. This particular example shows how a metabolic rewiring event can generate metabolites with direct antimicrobial activity.
Succcinate that accumulates in M1 macrophages as a consequence of a broken TCA cycle has a direct impact on macrophage cytokine production21. One mechanism involved is inhibition of prolyl hydroxylases by succinate, leading to the stabilization of HIF1α and the sustained production of IL-1β21. This pathway will operate under normoxia as well as in hypoxia, and is therefore a mechanism for HIF1α activation under aerobic conditions. Taken together, these studies show that the alterations in the TCA cycle that occur in M1 macrophages lead to the mitochondrial accumulation of metabolites that can promote their immune functions. These events may also link to nitric oxide production, which has been shown to inactivate the electron transport chain in macrophages37.
Fatty acid oxidation and immune function
Fatty acid oxidation has key roles in the regulation of adaptive and innate immune responses (FIG. 5). In contrast to aerobic glycolysis that is often observed in inflammatory and rapidly proliferating immune cells, a reliance on fatty acid oxidation has been observed in many immune cells that are not inflammatory in nature and exhibit increased cellular lifespans, including M2 macrophages, Treg cells and memory T cells.
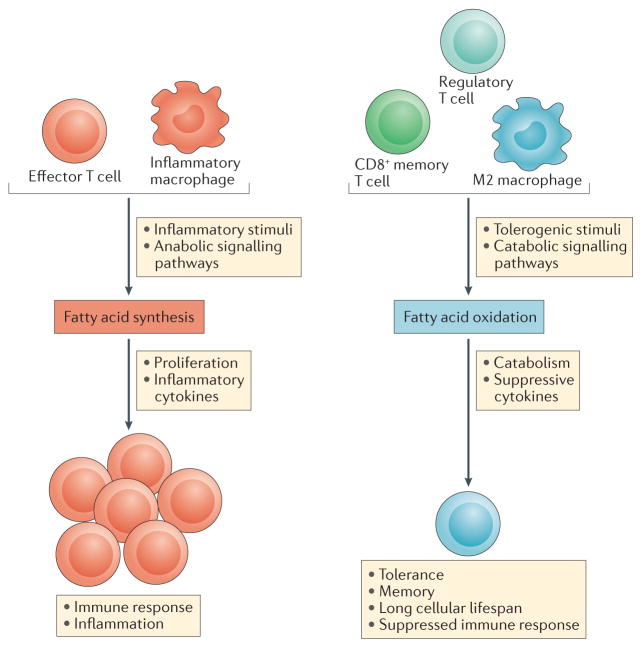
Figure 5. Fatty acid synthesis and oxidation in immunity.
Inflammatory signals drive fatty acid synthesis, which is important for immune cell proliferation and inflammatory cytokine production. By contrast, tolerogenic stimuli from the immune system drive fatty acid oxidation, which is required for the production of suppressive cytokines leading to immune tolerance and the inhibition of inflammation. Effector T cells show enhanced fatty acid synthesis and this is needed for their growth. Memory T cells show fatty acid oxidation, which limits their growth and allows them to persist.
Fatty acid oxidation and macrophage function
Fatty acid oxidation has been found to modulate the inflammatory functions of macrophages. The aberrant accumulation of fatty acids and their derivative lipoproteins in macrophages has been correlated with the generation of foam cells and the development of inflammatory pathologies, including atherosclerotic lesions38–40. Recent work showed that increased intracellular levels of unsaturated fatty acids (including oleic acid, linoleic acid and arachidonic acid), but not saturated fatty acids, stimulates the production of IL-1α in foam cells, leading to increased inflammation in vivo41. Intriguingly, enforced expression of constitutively active CPT1A (which as discussed above, transports long-chain fatty acids into the mitochondria) in macrophage cell-lines increased fatty acid oxidation, reduced lipid accumulation and reduced the production of inflammatory cytokines42. Therefore, promoting fatty acid oxidation in inflammatory macrophages may be one approach to reducing their inflammatory potential.
Fatty acid oxidation has also been found to have a key role in macrophage differentiation. Whereas M1 macrophages (induced by stimulation with IFNγ and LPS) use glycolytic metabolism, M2 macrophages (as defined by activation with IL-4) rely on a programme of fatty acid oxidation that is promoted by signal transducer and activator of transcription 6 (STAT6) and PPARγ-co-activator 1β (PGC1β) and works to inhibit inflammatory signals43,44. Added complexity has emerged, however, in a recent report showing that Cpt2 deletion did not affect M2 macrophage polarization, despite impaired fatty acid oxidation45. CPT1 may therefore have some additional role for M2 polarization other than fatty acid transport.
Fatty acid oxidation and T cell responses
Fatty acid oxidation also has an important role in regulating T cell responses. To date, fatty acid oxidation has been observed to regulate the balance between inflammatory effector T cells and suppressive Treg cells, and to promote long-lived memory T cells that are necessary for sustained immune function. In the context of regulating the balance between effector T cells and Treg cells, it has been shown that Treg cells exhibit increased fatty acid oxidation relative to TH1, TH2 and TH17 cells and that fatty acid oxidation promotes the generation of Treg cells while inhibiting effector T cell polarization13. Subsequent work showed that Treg cells have increased expression of genes involved in fatty acid oxidation, including Cpt1a, when compared to TH17 cells26. Interestingly, effector T cells have been shown to downregulate fatty acid oxidation during the activation process46. Consistent with fatty acid oxidation inhibiting effector T cell function and promoting tolerance, ligation of the inhibitory programmed death 1 (PD1) receptor on T cells resulted in increased expression of CPT1A and elevated fatty acid oxidation47.
Fatty acid oxidation also plays an important role in the generation and maintenance of long-lived memory CD8+ T cells. Memory CD8+ T cells, which slowly proliferate under steady state conditions48 but allow for a rapid innate immune response to previously encountered antigens, appear to require fatty acid oxidation to respond to antigen stimulation in a timely manner49. Stimulation of memory CD8+ T cells with IL-15 increases their expression of CPT1A and promotes fatty acid oxidation, resulting in increased cell survival50. Interestingly, although memory CD8+ T cells seem to extensively use fatty acid oxidation, they have been found to synthesize fatty acids de novo to support this process and subsequently oxidize these products in a cell intrinsic futile cycle, the reason for which is not known34. Taken together, these studies indicate key roles for fatty acid oxidation both in the generation of tolerogenic Treg cells as well as in the maintenance of long-lived memory CD8+ T cells.
Fatty acid synthesis and immune function
In contrast to fatty acid oxidation, which seems to promote the development and activity of non-inflammatory immune cells, fatty acid synthesis seems to positively regulate the generation and function of pro-inflammatory immune cells of both the innate and adaptive immune systems (FIG. 5).
Several studies indicated that inflammatory stimuli such as LPS and cytokines trigger an increase in fatty acid synthesis in macrophages51,52. Mechanistically, sterol regulatory element-binding transcription factor 1c (SREBP1c) was found to be upregulated during the differentiation of monocytes into macrophages following treatment with macrophage colony-stimulating factor (M-CSF), leading to increased expression of fatty acid synthesis-related target genes such as FASN and a functional increase in lipid synthesis. Importantly, the observed increase in fatty acid synthesis was crucial to the differentiation and inflammatory function of macrophages in this setting53. Further work on this subject indicated that mitochondrial uncoupling protein 2 (UCP2) stimulates fatty acid synthesis through regulation of FASN to promote NLRP3 inflammasome activation, which leads to a harmful inflammatory response during polymicrobial sepsis54. Taken together, this work suggests that fatty acid synthesis promotes and is required for inflammatory macrophage responses.
Interestingly, fatty acid synthesis provides a link between innate and adaptive immunity through regulation of DC function. Fatty acid synthesis was found to be upregulated during Toll-like receptor (TLR)-mediated DC activation, and this increased fatty acid synthesis was necessary for DC activation and their stimulation of CD8+ T cell responses20. Fatty acid synthesis is also key to the cell intrinsic function of T cells and B cells; synthesis of fatty acids and sterols has been shown to be necessary for cell proliferation after the activation of these cells through their antigen receptors55,56. Recent work demonstrated that T cell-specific deletion of acetyl-CoA carboxylase 1 (ACC1) — the rate-limiting enzyme in fatty acid synthesis — results in reduced blasting efficacy and lower accumulation of antigen-specific CD8+ T cells. Importantly, these defects could be overcome by supplementing ACC1-deficient T cells with exogenous fatty acids57.
As seen with fatty acid oxidation, the balance of effector and regulatory T cells is also influenced by fatty acid synthesis. Pharmacological or genetic inhibition of ACC1 in CD4+ T cell subsets showed that fatty acid synthesis is required for proper TH17 cell differentiation but not for Treg cell generation and function58. More recently, an intriguing observation was made concerning fatty acid synthesis and TH17 cells. Wang and colleagues59 showed that a protein termed CD5 antigen-like (CD5L) is expressed in so-called ‘non-pathogenic’ TH17 cells. These cells produce low levels of IL-17 but also produce the anti-inflammatory cytokine IL-10. In mice they have a homeostatic role in the gut, preventing invasion by the gut microbiota and promoting epithelial barrier function60, whereas in humans they may have a role in host defence against Staphylococcus aureus61. CD5L binds fatty acid synthase and is thought to promote the production of polyunsaturated fatty acids (PUFAs). The authors demonstrate that PUFAs are likely to modulate production of cholesterol-derived ligands for RORγt, the key transcription factor for TH17 cells, such that modified ligands for RORγt promote IL-10 production while limiting IL-23 and IL-17 (which are pro-inflammatory). In pathogenic TH17 cells, CD5L expression is low and this may favour the production of saturated fatty acids by fatty acid synthase; this in turn promotes the generation of ligands for RORγt that enhance its IL-23 and IL-17 promoting activity while limiting its ability to drive IL-10 production. What this means is that the type of fatty acid being synthesized governs the type of cytokines being made by TH17 cells — PUFAs drive production of the anti-inflammatory cytokine IL-10, while saturated fatty acids limit IL-10 production and promote the synthesis of pro-inflammatory cytokines. This study in many ways illustrates the exciting prospect of analysing immunometabolism in fine detail to provide new insights into immune cell effector function.
Overall, it seems that fatty acid oxidation and fatty acid synthesis have opposing roles in the immune system, with fatty acid oxidation being preferentially used by non-inflammatory and tolerogenic immune cells, whereas fatty acid synthesis is characteristic of inflammatory responses in the innate and adaptive immune systems. It is unclear how this distinction in fatty acid metabolism may lead to these opposing immunological functions, but the efficiency of lipid oxidation for energy generation and the necessity of lipid synthesis for biosynthesis and cell growth suggest that pro-inflammatory and regulatory immune cells show fundamental differences in their reliance on ATP generation or growth. It may be that effector immune cells upregulate nutrient uptake sufficiently so that metabolic intermediates are abundant and can be diverted to biosynthesis, whereas M2 macrophages or Treg cells are adapted to function in tissue environments where nutrients are more limiting and efficiency is crucial. It is also possible that effector cells might require fatty acid synthesis during their rapid growth to allow membrane biogenesis, while the slow growth of memory cells will occur because of fatty acid oxidation. It is also worth mentioning that there is substantial information concerning the metabolism of other lipid species in immune cell function, notably of cholesterol and sphingolipids, but space does not allow us to cover these further and interested readers are referred to recent reviews62,63.
Amino acid metabolism and immune function
In this final section, we focus on how amino acid metabolism can modulate immune cell activity. As discussed previously, the mTOR pathway has important roles in cellular metabolism, including in the sensing of amino acid levels to couple nutrient availability to cellular growth and proliferation. It is not surprising, then, that the availability and metabolism of various amino acids has been found to play a role in immune function (FIG. 6). Here, we will describe how the metabolism of three of the most well-studied amino acids — namely glutamine, arginine and tryptophan — affects the immune system.
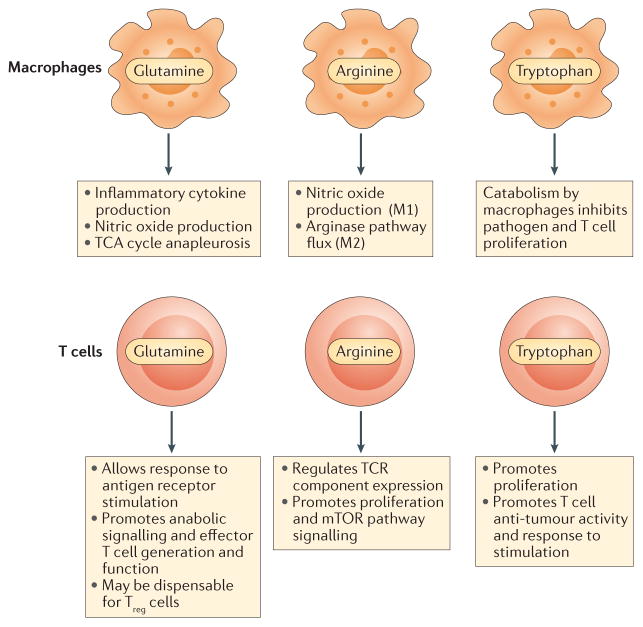
Figure 6. Amino acid metabolism in immunity.
Amino acid metabolism plays an important role in mediating functionality of the innate and adaptive immune systems. In macrophages, the amino acids glutamine and arginine are crucial for immune functions including cytokine and nitric oxide production. The fate of arginine in macrophages is a key distinction between inflammatory and tolerant cell phenotypes. Tryptophan metabolism by macrophages may suppress the activity of the adaptive immune system. In T cells, glutamine and arginine promote robust responses to T cell receptor (TCR) stimulation, including proliferation and cytokine production. Sufficient availability is necessary for proper mechanistic target of rapamycin (mTOR) pathway signalling. Tryptophan has an important role in promoting T cell proliferation, and lack of availability may mediate failure to respond to infections or tumours. TCA, tricarboxylic acid; Treg, regulatory T.
Glutamine metabolism
Glutamine catabolism regulates numerous aspects of immune cell function and has been hypothesized to play an important role in immune function in the context of serious illnesses, such as sepsis or burns64,65. Adequate supplies of glutamine have been found to be required for the induction of IL-1 by macrophages in response to LPS stimulation66. Glutamine metabolism is also important for the generation of nitric oxide through feeding into arginine synthesis67, demonstrating a role for glutamine in the cytotoxic and antimicrobial functions of macrophages. Indeed, pharmacological inhibition of glutaminase activity or glutamine withdrawal from culture medium resulted in decreased nitric oxide production by bacille Calmette-Guérin (BCG)-activated macrophages in vitro67,68. Interestingly, recent work has shown that glutamine is extensively fluxed into the TCA cycle and the hexosamine pathway and promotes M2 macrophage polarization in response to IL-4 stimulation, whereas LPS-stimulated M1 macrophages do not require glutamine for their development25.
T cell and B cell responses are also regulated by glutamine metabolism. Glutamine usage increases markedly upon both T cell and B cell activation, and both populations require glutamine to respond to antigen receptor stimulation46,69,70. In the T cell setting, heterozygous knockout of glutaminase resulted in increased ROS levels that were increased by hypoxia, indicating a role for glutamine metabolism in controlling ROS stress71. Glutamine metabolism also appears to regulate the balance between effector T cells and Treg cells, as the genetic loss of the transporter protein ASCT2 (which is responsible for the uptake of neutral amino acids such as glutamine and leucine) in T cells resulted in impaired generation and function of TH1 and TH17 cells, whereas Treg cell generation was not altered72. Illustrating the key connection between amino acid levels and mTOR pathway activity in the immune system, this same study found that ASCT2 loss or reduction of glutamine levels in culture media brought about a reduction in mTORC1 activity that coincided with the observed effector T cell defects.
Arginine metabolism
Arginine metabolism has been found to have a key role in the inflammatory function of macrophages73. Macrophages use arginine in two distinct metabolic pathways, the nitric oxide synthesis pathway and the arginase pathway. The pathway used for arginine metabolism in macrophages has profound effects on the immune function of the cell. Macrophage flux of arginine into the nitric oxide synthesis pathway is associated with an inflammatory M1 phenotype. When macrophages direct arginine into this pathway, arginine (via citrulline) is converted into nitric oxide, a process mediated by inducible nitric oxide synthase (iNOS)74. It has been known for some time that iNOS expression is itself required for inflammatory macrophage function, as macrophages derived from iNOS-deficient mice show defective killing of bacteria and tumour cells in vitro. Further pointing to an in vivo role for iNOS in mediating inflammatory activity, toxicity from LPS-induced septic shock was reduced in iNOS-deficient mice75. In contrast to the inflammatory involvement of arginine metabolism in the nitric oxide synthesis pathway, arginine flux through the arginase pathway is associated with a more tolerant immune response, often associated with wound healing76. Interestingly, arginase expression in macrophages limits the inflammatory potential of effector T cells77 and is positively correlated with the disease severity of both visceral leishmaniasis and HIV infection78. Indicating a probable immunoregulatory role for arginine metabolism beyond macrophages, arginine has been found to regulate the expression of components of the T cell receptor79 and to promote proliferation of human T cells80. Similarly to what has been observed in glutamine deficiency, mTORC1 activity in T cells is suppressed in arginine-depleted in vitro cultures81.
Tryptophan metabolism
Tryptophan is another amino acid that has been observed to play a key role in modulating immune function. Pioneering studies showed that treating animals with high doses of exogenous tryptophan resulted in the development of an autoimmune phenotype characterized by aberrant eosinophil function82,83. A great deal of research on the immunoregulatory role of tryptophan metabolism in the immune system has focused on the role of an enzyme called indoleamine-2,3-dioxygenase (IDO), which is responsible for the rate-limiting step of tryptophan catabolism. IDO expression has long been known to be stimulated by LPS exposure84 and, intriguingly, numerous studies have shown that one of many cellular responses to IFNγ is to increase the catabolic metabolism of tryptophan by driving the expression of IDO85,86. Interestingly, it has been found that tryptophan catabolism in host cells may prevent the growth of bacterial and parasitic pathogens by denying them a necessary substrate for anabolic growth87,88, suggesting that tryptophan metabolism may play an important role as a component of the immune response to pathogens. In a more direct analysis of the role of tryptophan metabolism in the immune system, it was shown that T cells require tryptophan to proliferate in vitro89, and driving IDO expression and tryptophan catabolism in antigen-presenting cells blunts T cell stimulation90. Indeed, tryptophan insufficiency can lead to an accumulation of charged tRNAs and activation of the unfolded protein response protein GCN2 (REF. 91). Taken together, these studies point to a model in which tryptophan availability is necessary for immune cell function and suggest that there may be competition for the use of tryptophan in the immune cell microenvironment. However, numerous other aspects of tryptophan metabolism, including metabolites generated from tryptophan catabolism, such as kynurenine, may play important roles in modulating immune cell function through activation of the aryl hydrocarbon receptor (AHR), which is a ligand-activated transcription factor92.
The role of tryptophan metabolism in immune function has recently become an area of intense study as it relates to the propensity of tumour cells to evade immune cell responses. In many types of tumours, IDO expression is seen in both tumour cells and in tumour-associated stromal cells93,94 and increased IDO expression levels have been correlated with a poor prognosis in some cancer types95,96. Recent work has shown that enforced expression of IDO in tumour cells impaired the antitumour immune responses of T cells, but this could be overcome by pharmacological inhibition of IDO with 1-methyltryptophan97. It is important to keep in mind, however, that as discussed in the non-cancer immune setting, there are likely to be numerous mechanisms through which tryptophan metabolism acts to modulate the immune system. Future work on amino acid metabolism in immune cells, particularly for amino acids other than those explored to date, could yield important new insights into immune cell function.
Concluding remarks
This Review is designed to inform immunologists and stimulate their interest in the field of immunometabolism and metabolic reprogramming. The substantial and growing literature on immunometabolism continues to provide us with new insights into the role of intracellular metabolites in the complex regulation of immune cells (FIG. 7). The six pathways described above can be considered in isolation, but as we discussed, they are interlinked and can be co-regulated (for example, MYC will affect glucose and glutamine metabolism, and mTOR will coordinate both glycolysis and fatty acid synthesis) adding to the complexity. We can expect further findings in this burgeoning field. BOX 1 describes key outstanding questions in the field which might serve as a ‘call to arms’ for immunologists. Recent work demonstrating profound effects of inhibiting metabolic pathways in models of systemic lupus erythematosus98 and transplantation99, without any apparent toxicity, means we can also anticipate therapeutic interventions which could provide new and badly needed approaches to treat immune and infectious diseases.
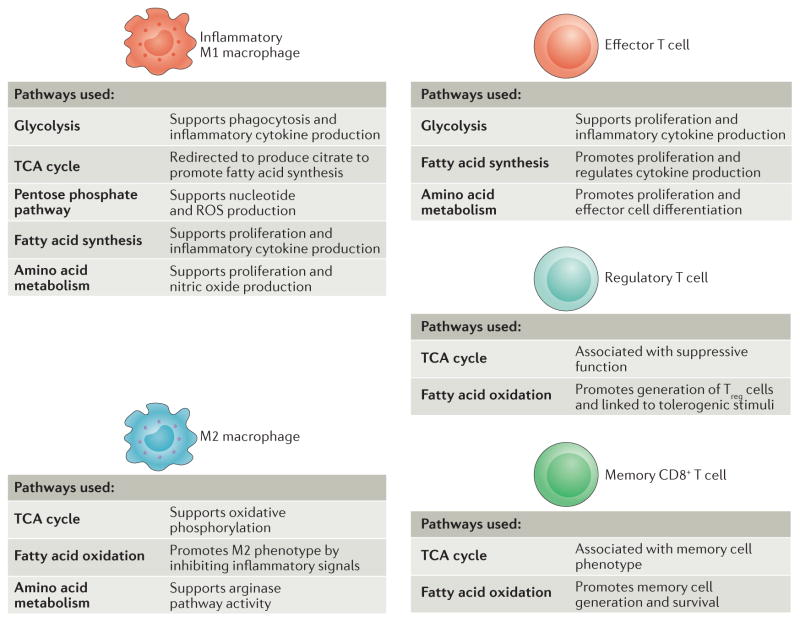
Figure 7. Metabolism of immune cell subtypes.
The various immune cell subsets exhibit a reliance on distinct metabolic pathways to promote cell survival, lineage generation and function. Inflammatory macrophages use glycolysis, the tricarboxylic acid (TCA) cycle, the pentose phosphate pathway, fatty acid synthesis and amino acid metabolism to proliferate and to support the production of inflammatory cytokines. M2 macrophages, which exhibit a more tolerant phenotype, use the TCA cycle, fatty acid oxidation and arginine flux into the arginase pathway. Rapidly proliferating effector T cells, including T helper 1 (TH1), TH17 and cytotoxic CD8+ T cells, use glycolysis, fatty acid synthesis and amino acid metabolism to promote proliferation and cytokine production. Immunosuppressive regulatory T (Treg) cells use the TCA cycle and fatty acid oxidation. Similarly, memory CD8+ T cells also require the use of the TCA cycle and fatty acid oxidation to promote increased cell lifespan. ROS, reactive oxygen species.
Box 1. Current questions in the field of immunometabolism.
In addition to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), hexokinase 1, pyruvate kinase isoenzyme M2 (PKM2) and enolase, do other metabolic enzymes have ‘moonlighting’ roles that influence immune cell function?
Can changes in cellular metabolites other than lactate and succinate also regulate non-metabolic functions in immune cells?
What is the purpose of the futile cycle from fatty acid synthesis to oxidation in dendritic cells and T cells?
What functions aside from the generation of NADPH and nucleotides might there be for the pentose phosphate pathway in immune cell activation?
Will new metabolic processes (such as shunts or metabolic regulators such as CD5 antigen-like (CD5L)) be identified that are crucial for immune cells functions?
How does immunometabolism regulate epigenetic changes during immune cell activation and innate immune memory?
Will new functions be found for other metabolites that are generated during immune cell activation?
How do pathogens (and the microbiota) manipulate immunometabolism for their own ends?
What are the similarities and differences between the immunometabolic processes seen in T cells and macrophages, and those that operate in other key immune cell populations, such as plasma cells, innate lymphoid cells, natural killer cells and granulocytes?
Will it be possible to target specific events in immunometabolism to achieve a therapeutic effect in immune and inflammatory diseases (or in the setting of transplantation) without causing toxicity?
Acknowledgments
L.A.J.O. acknowledges Science Foundation Ireland, The European Research Council and The Wellcome Trust for research funding.
Glossary
- Mechanistic target of rapamycin (mTOR)
An atypical serine/threonine kinase that is present in two distinct complexes. mTOR complex 1 (mTORC1), is composed of mTOR, Raptor, MLST8 (also known as GβL), PRAS40 and DEPTOR; it is inhibited by rapamycin
- Electron transport chain
The series of proteins in the inner mitochondrial membrane that transfer electrons in a series of redox reactions, leading to proton pumping across the membrane
- 2-deoxyglucose
A derivative of glucose that inhibit hexokinase, thereby blocking the first step in glycolysis
- Aerobic glycolysis
Glycolysis occurring when oxygen is present
- Foam cells
Fat-laden macrophages commonly seen in the plaques occurring in atherosclerosis
- Futile cycle
Two metabolic pathways running in opposite directions that seem to cancel each other out metabolically
- Metabolic enzymes
Enzymes in metabolic pathways that convert substrates into products. Major classes are dehydrogenases (which remove hydrogen from a substrate in an oxidation–reduction reaction), isomerases (which convert a molecule from one isomer to another), synthases (which link two molecules together without using ATP as an energy source), carboxylases (which add a carboxyl group to a substrate) and kinases (which add a phosphate group to a molecule)
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Newsholme P, Curi R, Gordon S, Newsholme EA. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986;239:121–125. doi: 10.1042/bj2390121. One of the key pioneering studies on macrophage metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso D, Nungester WJ. Comparative study of host resistance of guinea pigs and rats V. The effect of pneumococcal products on glycolysis and oxygen uptake by polymorphonuclear leucocytes. J Infect Dis. 1956;99:174–181. doi: 10.1093/infdis/99.2.174. [DOI] [PubMed] [Google Scholar]
- 3.Oren R, Farnham AE, Saito K, Milofsky E, Karnovsky ML. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963;17:487–501. doi: 10.1083/jcb.17.3.487. An important early study on metabolism in different types of macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuzumi M, Shinomiya H, Shimizu Y, Ohishi K, Utsumi S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect Immun. 1996;64:108–112. doi: 10.1128/iai.64.1.108-112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Zhang DT, Liu X. G mTOR signaling in T cell immunity and autoimmunity. Int Rev Immunol. 2015;34:50–66. doi: 10.3109/08830185.2014.933957. [DOI] [PubMed] [Google Scholar]
- 6.Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 8.Michl J, Ohlbaum DJ, Silverstein SC. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I Description of the inhibitory effect. J Exp Med. 1976;144:1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton JA, Vairo G, Lingelbach SR. CSF-1 stimulates glucose uptake in murine bone marrow-derived macrophages. Biochem Biophys Res Commun. 1986;138:445–454. doi: 10.1016/0006-291x(86)90301-3. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Prados JC, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 11.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. This was one of the first papers to analyse the Warburg effect in DCs activated by TLR4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly RP, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193:4477–4484. doi: 10.4049/jimmunol.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. In this paper, we see one of the first reports of different metabolic processes occurring in T cell subsets, with glycolysis and fatty acid synthesis being a feature of TH1 cells, and fatty acid oxidation being more prominent in Treg cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doughty CA, et al. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–4465. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi LZ, et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. This paper is notable for demonstrating that the phenotype of the T cell can change when glycolysis is inhibited, effectively turning from a TH17 cell into a Treg cell. This study ushered in the concept of metabolic reprogramming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubser PM, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 17.Shrestha S, et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh A, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17:277–285. doi: 10.1038/ni.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everts B, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. This is the first report to show that a TCA cycle intermediate, succinate, can serve as an activation signal in macrophages and promote IL-1β production by activating HIF1α. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palsson-McDermott EM, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo W, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirai T, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–354. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha AK, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Gerriets VA, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beier UH, et al. Essential role of mitochondrial energy metabolism in Foxp3+ T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Procaccini C, et al. The proteomic landscape of human ex vivo regulatory and conventional T cells reveals specific metabolic requirements. Immunity. 2016;44:406–421. doi: 10.1016/j.immuni.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Rosa V, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol. 2015;16:1174–1184. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem Sci. 2009;34:324–331. doi: 10.1016/j.tibs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang CH, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. An important paper that demonstrates that GAPDH ‘moonlights’ — its other role being to repress expression of IFNγ in TH1 cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon JS, et al. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Rep. 2015;12:102–115. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Haschemi A, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. The pentose phosphate pathway is shown in this study to be crucial for macrophage polarization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Sullivan D, et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infantino V, et al. The mitochondrial citrate carrier: a new player in inflammation. Biochem J. 2011;438:433–436. doi: 10.1042/BJ20111275. [DOI] [PubMed] [Google Scholar]
- 36.Michelucci A, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpenter KL, et al. Macrophages, lipid oxidation, ceroid accumulation and alpha-tocopherol depletion in human atherosclerotic lesions. Gerontology. 1995;41:53–67. doi: 10.1159/000213725. [DOI] [PubMed] [Google Scholar]
- 39.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freigang S, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 42.Malandrino MI, et al. Enhanced fatty acid oxidation in adipocytes and macrophages reduces lipid-induced triglyceride accumulation and inflammation. Am J Physiol Endocrinol Metab. 2015;308:E756–E769. doi: 10.1152/ajpendo.00362.2014. [DOI] [PubMed] [Google Scholar]
- 43.Vats D, et al. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang SC, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura M, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17:216–217. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patsoukis N, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruno L, von Boehmer H, Kirberg J. Cell division in the compartment of naive and memory T lymphocytes. Eur J Immunol. 1996;26:3179–3184. doi: 10.1002/eji.1830261251. [DOI] [PubMed] [Google Scholar]
- 49.van der Windt GJ, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci USA. 2013;110:14336–14341. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Posokhova EN, Khoshchenko OM, Chasovskikh MI, Pivovarova EN, Dushkin MI. Lipid synthesis in macrophages during inflammation in vivo: effect of agonists of peroxisome proliferator activated receptors α and γ and of retinoid X receptors. Biochem (Mosc) 2008;73:296–304. doi: 10.1134/s0006297908030097. [DOI] [PubMed] [Google Scholar]
- 52.Feingold KR, et al. Mechanisms of triglyceride accumulation in activated macrophages. J Leukoc Biol. 2012;92:829–839. doi: 10.1189/jlb.1111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ecker J, et al. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc Natl Acad Sci USA. 2010;107:7817–7822. doi: 10.1073/pnas.0912059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moon JS, et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Invest. 2015;125:665–680. doi: 10.1172/JCI78253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Chen HW, Heiniger HJ, Kandutsch AA. Relationship between sterol synthesis and DNA-synthesis in phytohemagglutinin-stimulated mouse lymphocytes. Proc Natl Acad Sci USA. 1975;72:1950–1954. doi: 10.1073/pnas.72.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dufort FJ, et al. Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for atp-citrate lyase in lipopolysaccharide-induced differentiation. J Biol Chem. 2014;289:7011–7024. doi: 10.1074/jbc.M114.551051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee J, et al. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J Immunol. 2014;192:3190–3199. doi: 10.4049/jimmunol.1302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berod L, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. In this study, fatty acid metabolism is shown to govern the fate of TH cell subtypes. [DOI] [PubMed] [Google Scholar]
- 59.Wang C, et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell. 2015;163:1413–1427. doi: 10.1016/j.cell.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guglani L, Khader SA. Th17 cytokines in mucosal immunity and inflammation. Curr Opin HIV AIDS. 2010;5:120–127. doi: 10.1097/COH.0b013e328335c2f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zielinski CE, et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 62.Fessler MB. Regulation of adaptive immunity in health and disease by cholesterol metabolism. Curr Allergy Asthma Rep. 2015;15:48. doi: 10.1007/s11882-015-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly D, Wischmeyer PE. Role of L-glutamine in critical illness: new insights. Curr Opin Clin Nutr Metab Care. 2003;6:217–222. doi: 10.1097/00075197-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Parry-Billings M, Evans J, Calder PC, Newsholme EA. Does glutamine contribute to immunosuppression after major burns? Lancet. 1990;336:523–525. doi: 10.1016/0140-6736(90)92083-t. [DOI] [PubMed] [Google Scholar]
- 66.Wallace C, Keast D. Glutamine and macrophage function. Metabolism. 1992;41:1016–1020. doi: 10.1016/0026-0495(92)90130-3. [DOI] [PubMed] [Google Scholar]
- 67.Murphy C, Newsholme P. Importance of glutamine metabolism in murine macrophages and human monocytes to L-arginine biosynthesis and rates of nitrite or urea production. Clin Sci (Lond) 1998;95:397–407. [PubMed] [Google Scholar]
- 68.Bellows CF, Jaffe BM. Glutamine is essential for nitric oxide synthesis by murine macrophages. J Surg Res. 1999;86:213–219. doi: 10.1006/jsre.1999.5713. [DOI] [PubMed] [Google Scholar]
- 69.Carr EL, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crawford J, Cohen HJ. The essential role of L-glutamine in lymphocyte differentiation in vitro. J Cell Physiol. 1985;124:275–282. doi: 10.1002/jcp.1041240216. [DOI] [PubMed] [Google Scholar]
- 71.Le A, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakaya M, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 75.MacMicking JD, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 76.Albina JE, et al. Arginine metabolism in wounds. Am J Physiol. 1988;254:E459–E467. doi: 10.1152/ajpendo.1988.254.4.E459. [DOI] [PubMed] [Google Scholar]
- 77.Pesce JT, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takele Y, et al. Arginase activity in the blood of patients with visceral leishmaniasis and HIV infection. PLoS Negl Trop Dis. 2013;7:e1977. doi: 10.1371/journal.pntd.0001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez PC, et al. Regulation of T cell receptor CD3ζ chain expression by L-arginine. J Biol Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 80.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cobbold SP, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silver RM, et al. Scleroderma, fasciitis, and eosinophilia associated with the ingestion of tryptophan. N Engl J Med. 1990;322:874–881. doi: 10.1056/NEJM199003293221302. [DOI] [PubMed] [Google Scholar]
- 83.Stahl JL, Cook EB, Pariza MA, Cook ME, Graziano FM. Effect of L-tryptophan supplementation on eosinophils and eotaxin in guinea pigs. Exp Biol Med (Maywood) 2001;226:177–184. doi: 10.1177/153537020122600304. [DOI] [PubMed] [Google Scholar]
- 84.Yoshida R, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1978;75:3998–4000. doi: 10.1073/pnas.75.8.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshida R, Imanishi J, Oku T, Kishida T, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci USA. 1981;78:129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Werner ER, et al. Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-γ. Biochem J. 1989;262:861–866. doi: 10.1042/bj2620861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfefferkorn ER. Interferon γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schroten H, et al. Potential role of human brain microvascular endothelial cells in the pathogenesis of brain abscess: inhibition of Staphylococcus aureus by activation of indoleamine 2,3-dioxygenase. Neuropediatrics. 2001;32:206–210. doi: 10.1055/s-2001-17375. [DOI] [PubMed] [Google Scholar]
- 89.Lee GK, et al. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Munn DH, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu H, et al. GCN2-dependent metabolic stress is essential for endotoxemic cytokine induction and pathology. Mol Cell Biol. 2014;34:428–438. doi: 10.1128/MCB.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bessede A, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. Tryptophan metabolism by the enzyme IDO is shown here to be crucial for antitumour immunity. [DOI] [PubMed] [Google Scholar]
- 94.Okamoto A, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 95.Munn DH, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. 2007;214:8–14. doi: 10.1159/000096906. [DOI] [PubMed] [Google Scholar]
- 97.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yin Y, et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee CF, et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep. 2015;13:760–770. doi: 10.1016/j.celrep.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]