Abstract
Purpose
The use of electronic cigarettes (e-cigarettes) has risen dramatically in recent years. However, there is currently no published data on use of e-cigarettes among cardiac patients. The current study reports on the prevalence, reasons for use, and perceived risks of e-cigarettes among post-Acute Coronary Syndrome (ACS) patients. The relationship between e-cigarette use and post-ACS tobacco smoking cessation is also explored.
Methods
Participants were drawn from a randomized trial of smoking cessation treatments following hospitalization for ACS. The current study focuses on 49 participants that completed e-cigarette questions at 24 weeks post-ACS.
Results
51.0% of participants reported ever use of an e-cigarette and 26.5% reported using an e-cigarette at some time during the 24 weeks post-ACS. Ever use and post-ACS use were both significantly associated with lower rates of abstinence from tobacco cigarettes. Participants perceived e-cigarettes as less harmful to cardiac health than tobacco use and Chantix, and similarly harmful as nicotine replacement therapy. Participant perceived likelihood of experiencing a heart attack in the next year was 34.6% if they were to regularly use e-cigarettes only, significantly lower than perceived risk of recurrence if they were to regularly smoke only tobacco cigarettes (56.2%) and significantly higher than perceived risk of recurrence if they were to use no nicotine (15.2%).
Conclusions
A significant minority of patients are using e-cigarettes post-ACS. Providers should be prepared to discuss potential discrepancies between patient beliefs about the safety of e-cigarettes and the current state of the science.
Keywords: Electronic cigarettes, acute coronary syndrome, smoking
Electronic cigarettes (e-cigarettes) are battery-powered devices that deliver an inhaled aerosol that typically contains nicotine, flavorants, and propylene glycol and/or vegetable glycerin. They do not contain tobacco, require combustion, or produce smoke or carbon monoxide. While the prevalence of smoking traditional tobacco cigarettes has decreased over the past decade, use of e-cigarettes has risen. Between 2010 and 2013 both ever use (rose from 3.3% to 8.5%) and current use (rose from 1.0% to 2.6%) of e-cigarettes doubled among US adults1. Current and former tobacco cigarette use are the strongest predictors of e-cigarette use1, 2. 37% of current tobacco cigarette smokers have ever used an e-cigarette and 9.4% are current e-cigarette users1, 3.
Current smokers perceive electronic cigarettes to be significantly less harmful and less likely to cause health problems compared to tobacco cigarettes, including the perception that e-cigarettes are less likely to cause heart disease4. It may be the case that e-cigarettes present significantly lower cardiac risk compared to combustible cigarette use, but only preliminary data is available on this topic5.
Some data exists on the prevalence of e-cigarette use in medical patients who smoke with estimates of current use ranging from 18–27%6–9. To our knowledge, no data exists regarding 1) prevalence, reasons for use, or perceived risks of e-cigarettes or 2) the relationship between e-cigarette use and cessation of tobacco cigarettes specifically among patients with cardiovascular disease. The current study provides data on these issues in a sample of post-Acute Coronary Syndrome (ACS) patients participating in a smoking cessation trial.
Methods
Participants
Participants in the current study were drawn from participants in a randomized trial of smoking cessation treatments following ACS (NCT01964898). For the parent trial, we recruited patients that: 1) had an ACS diagnosis documented in their medical record (diagnosis of unstable angina, ST elevation myocardial infarction, or non-ST elevation myocardial infarction), 2) smoked ≥ 3 tobacco cigarettes per day, 3) were between the ages of 18–75, 4) were fluent in English, 5) had regular access to a telephone, 6) lived within a one hour drive of their admitting hospital, and 7) were willing to “strongly consider” an attempt to quit smoking at discharge. Patients were excluded if they: 1) evidenced limited mental competency, 2) presented with psychosis, serious mental illness, or suicidality, 3) were not expected to live through the study period or 4) were regularly attending counseling for depression or smoking cessation and planned to continue after hospital discharge. Patients who smoked ≥ 3 cigarettes per day who also used other tobacco products (e.g., cigars, pipe) or e-cigarettes were allowed to participate.
All participants received smoking cessation counseling during their inpatient stay for ACS consistent with the most recent national guidelines10. Subsequently, participants were randomly assigned to receive an integrated smoking cessation and mood management counseling program or mailed smoking cessation educational materials over 12 weeks after hospital discharge. Both conditions were offered an 8-week supply of nicotine patches at hospital discharge. Neither treatment specifically encouraged or discouraged e-cigarette use. If participants asked about safety of the e-cigarettes or about using them as a way to quit smoking, study staff responded that there was not enough evidence on the safety or efficacy of e-cigarettes for our team to recommend their use. This approach was consistent with the state of e-cigarette research when this protocol was designed in 2011 and is also consistent with the current literature11, 12.
Participants were assessed by staff members while in the hospital following ACS and at 12 and 24 weeks post-discharge. The current study focuses on e-cigarette questions asked at the 24-week post-discharge assessment, thus we only report on trial participants that completed this assessment. All procedures were approved by The Miriam Hospital institutional review board.
Measures
At baseline participants self-reported demographics and smoking variables (including the Fagerström Test for Cigarette Dependence13 and their confidence in ability to quit smoking rated 0–10), and medical history was pulled from clinical charts. At the 24-week assessment, patients self-reported if they had attended any cardiac rehabilitation or smoked tobacco cigarettes since hospital discharge. Seven-day point prevalence abstinence from tobacco cigarettes was established by exhaled carbon monoxide (eCO<10ppm) at the 24-week assessment.
All e-cigarette questions were asked at the 24-week assessment. Participants were asked if they had “ever used an e-cigarette or vapor device, even one or two times.” We included “vapor devices” to ensure that patients included use of so called “second” or “third generation” e-cigarette products which are known by a variety of terms (e.g., “hookah pens,” “tank systems,” “mods,”) and often look more like large fountain pens than a cigarette. Those who endorsed ever use reported date of first use, if they had used since discharge from the hospital, and why they tried e-cigarettes for the first time (responses were coded by the assessor into one of 15 categories; e.g., “to cut down on other tobacco,” “curiosity,” or “to save money”).
Those who reported ever use also reported on the relative perceived harmfulness of e-cigarettes. Specifically, participants were asked to rate e-cigarettes, the nicotine patch, the nicotine gum, the nicotine lozenge, smokeless tobacco, Chantix, and Zyban/Wellbutrin on harmfulness to cardiac health on a scale from 0–10, where 0 = “not at all harmful,” and 10 = “extremely harmful.” If participants had never heard of a product or did not know anything about it, they did not rate harmfulness of that product. In addition, participants were asked to rate from 0–100% “How likely are you to have a heart attack in the next year” if you were to 1) “regularly smoke only tobacco cigarettes?,” 2) “regularly use e-cigarettes or vapor devices only?,” or 3) “quit all nicotine products?”
Results
49 participants completed the questionnaire regarding e-cigarette use at 24 weeks post-ACS. The demographics, smoking history, and medical history of these participants are presented in Table 1.
Table 1.
Participant Characteristics By E-Cigarette Use
| Total (n = 49) | Ever-Used E-Cigarette (n = 25) | Never Used E-Cigarette (n = 24) | E-Cigarette Use Post-ACS (n = 13) | No E-Cigarette Use Post-ACS (n = 36) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 56.5 ± 10.6 | 56.0 ± 11.1 | 57.1 ± 10.3 | 53.9 ± 9.4 | 57.4 ± 11.0 |
| Married or living with committed partner | 59.2% | 56.0% | 62.5% | 53.8% | 61.1% |
| Female | 30.6% | 40% | 20.8% | 46.2% | 25.0% |
| Race | |||||
| Non-Hispanic Caucasian | 89.8% | 88.0% | 91.7% | 92.3% | 88.9% |
| African American | 6.1% | 4.0% | 8.3% | 0.0% | 8.3% |
| Hispanic Caucasian | 2.0% | 4.0% | 0.0% | 0.0% | 2.8% |
| Multiracial | 2.0% | 4.0% | 0.0% | 7.7% | 0.0% |
| Employed full or part-time | 57.1% | 52.0% | 62.5% | 61.5% | 55.6% |
| Median yearly household income, $ | 38,000 [54,675] | 44,000 [42,250] | 27,000 [51,400] | 63,000 [62,500] | 36,000 [49,050] |
| Some college education | 51.0% | 64.0% | 37.5% | 69.2% | 44.4% |
| Medical History | |||||
| Prior ACS event | 32.7% | 36.0% | 29.2% | 30.8% | 33.3% |
| Type of ACS index event | |||||
| STEMI | 53.1% | 60.0% | 45.8% | 61.5% | 50.0% |
| NSTEMI | 36.7% | 32.0% | 41.7% | 30.8% | 38.9% |
| Unstable angina | 10.2% | 8.0% | 12.5% | 7.7% | 11.1% |
| Baseline Smoking Variables | |||||
| Tobacco, cigarettes/day | 17.0 ± 9.2 | 15.9 ± 8.6 | 18.2 ± 9.9 | 14.9 ± 6.7 | 17.8 ± 9.9 |
| Total years smoking tobacco cigarettes regularly | 38.9 ± 11.6 | 38.5 ± 11.9 | 39.3 ± 11.4 | 36.1 ± 12.2 | 39.9 ± 11.4 |
| FTCD | 4.8 ± 2.2 | 5.1 ± 2.1 | 4.6 ± 2.2 | 4.6 ± 2.2 | 4.9 ± 2.2 |
| Median number of 24-hour quit attempts in lifetimea,b | 3.0 [4.0] | 5.0 [8.0] | 2.0 [2.0] | 5.0 [14.0] | 2.0 [3.75] |
| Confidence in ability to quit (rated 1–10)a,b | 7.3 ± 2.6 | 6.6 ± 2.8 | 8.1 ± 2.2 | 6.1 ± 3.1 | 7.8 ± 2.3 |
| Treatment Process and Outcome | |||||
| Randomized to mood management condition | 49.0% | 48.0% | 50.0% | 46.2% | 50.0% |
| Accepted free nicotine patches offered by studyb | 83.7% | 80.0% | 87.5% | 61.5% | 91.7% |
| Attended cardiac rehabilitation post-ACS | 36.7% | 32.0% | 41.7% | 30.8% | 38.9% |
| eCO verified 7-day point prevalence abstinence from tobacco at 24 weeks post-ACSa,b | 49.0% | 28.0% | 70.8% | 23.1% | 58.3% |
| Continuous abstinence from tobacco at 24 weeks post-ACSa,b | 36.7% | 16.0% | 58.3% | 7.7% | 47.2% |
Abbreviations: ACS, acute coronary syndrome; STEMI, ST segment elevation myocardial infarction; NSTEMI, non-ST segment elevation myocardial infarction; FTCD, Fagerström Test for Cigarette Dependence; eCO, exhaled carbon monoxide. Data presented as either mean SD, median [IQR] or %.
Ever-users differ from never-users; P < .05.
Post-ACS users differ from post-ACS non-users; P < .05.
51.0% (n=25) of participants reported ever use of an e-cigarette. 28.6% (n=14) reported first use before ACS, and 22.4% (n=11) reported first use post-ACS. 26.5% (n=13) reported using an e-cigarette at some time during the 24 weeks post-ACS. The most common reasons for trying an e-cigarette were “used to quit other tobacco” (60.0%; n=15), “used to cut down on other tobacco” (16.0%; (n=4)), and “curiosity” (28.0%; n=7). No other reason was endorsed by ≥ 10% of the sample (data not presented).
Of those who reported post-ACS use (n=13), 76.9% (n=10) reported lapsing to tobacco cigarettes prior to using an e-cigarette, 15.4% (n=2) used an e-cigarette prior to lapsing to tobacco cigarettes, and 7.7% (n=1) never lapsed to tobacco cigarettes following ACS. 12.2% (n=6) of all participants reported using an e-cigarette in the 30 days prior to the 24-week assessment.
Participants that had ever used an e-cigarette were significantly less likely to be abstinent from tobacco cigarettes at 24 weeks (28.0% vs. 70.8%; X2 (1, N = 49) = 8.99, p = .003) and to have maintained continuous abstinence post-ACS (16.0% vs. 58.3%; X2 (1, N = 49) = 9.44, p = .002) than those who had never used an e-cigarette. At baseline, participants that had ever used an e-cigarette also reported significantly more lifetime quit attempts (U=172.0, p=.01) and significantly lower confidence in their ability to quit (t(47)=2.2, p=.04) than those who had never used an e-cigarette.
Those that used e-cigarettes during the 24 weeks post-ACS were significantly less likely to be abstinent from tobacco cigarettes at 24 weeks (23.1% vs. 58.3%; X2 (1, N=49)=4.75, p = .03), to have maintained continuous abstinence (7.7% vs. 47.2%; Fisher exact test p = .02), and to accept free nicotine patches provided by the research study (61.5% vs. 91.7%; Fisher Exact test p = .02) than those who did not. At baseline, participants that used e-cigarettes during the 24 weeks post-ACS also reported significantly more lifetime quit attempts (U = 143.5; p = .04) and significantly lower confidence in their ability to quit (t(47)=2.1, p=.04) than those who did not.
Figure 1 shows participant perceived harm to cardiac health by product among ever users. Participants rated e-cigarettes as significantly less harmful to their cardiac health than regular tobacco cigarettes (paired t(24) = 10.52, p <.001), smokeless tobacco (paired t(22) = 7.89, p <.001), and Chantix (paired t(21) = 2.85, p = .01) and similarly harmful as the nicotine patch, gum, and lozenge. Participants rated Zyban/Wellbutrin as somewhat more harmful than e-cigarettes, but this difference was not significant.
Figure 1.
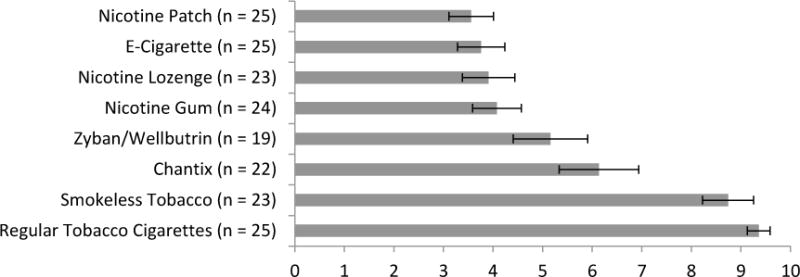
Mean Perceived Harmfulness to Cardiac Health (Rated 0–10)
Participant perceived likelihood of experiencing a heart attack in the next year was 56.2% (SD = 28.3%) if they were to regularly smoke only tobacco cigarettes, 34.6% (SD = 27.3%) if they were to regularly use e-cigarettes or vapor devices only, and 15.2% (SD = 13.4%) if they were to quit all nicotine products. Participants perceived that using e-cigarettes would significantly increase the odds of a heart attack relative to no nicotine use (paired t (24)= 4.59, p <.001), but significantly decrease the odds of a heart attack relative to tobacco cigarette use (paired t (24)= 3.87, p = .001).
Discussion
Little is known regarding e-cigarette use among cardiovascular disease patients. This study is the first to address this gap in the literature. More than half of the sample reported ever using an e-cigarette and just over 12% endorsed current use. Most interestingly, 26.5% of our sample reported using an e-cigarette at some time during the 24 weeks post-ACS and 22.4% reported using an e-cigarette for the first time post-ACS.
Although mixed, the current extant literature favors e-cigarettes helping smokers quit tobacco14. Thus, it was interesting that both ever and post-ACS e-cigarette use was associated with lower rates of point prevalence and continuous abstinence from tobacco cigarettes. We cannot conclude from this data that e-cigarette use caused relapse, particularly because the vast majority of post-ACS e-cigarette users relapsed to tobacco cigarettes prior to using an e-cigarette. Further, both ever and post-ACS e-cigarette users reported lower confidence in their ability to quit smoking and a greater number of previous failed quit attempts at baseline which further obscures causality.
Consistent with previous research15, e-cigarettes were rated as less harmful than tobacco products and prescription cessation medications, and similarly harmful as nicotine replacement therapies. It is difficult to evaluate the accuracy of these perceived differences in safety given that data on the safety of e-cigarettes is still emerging. However, existing data suggest that patients are likely accurate in their perception that e-cigarettes are less harmful that tobacco products, but may be inaccurate in their perception that e-cigarettes are similarly (in the case of nicotine replacement therapies) or less harmful (in the case of prescription cessation medications) than FDA approved medications.
When asked specifically about one year heart attack risk, participants reported that exclusive use of e-cigarettes would reduce risk relative to tobacco use, which is consistent with perceptions of general cardiac harm. However, participants also reported that e-cigarettes significantly increased the risk of heart attack relative to no nicotine use, suggesting that even e-cigarette users believe e-cigarettes have some risk.
These findings have implications for providers, particularly cardiac rehabilitation programs which aim to provide comprehensive patient health education post-ACS. National guidelines for rehabilitation16 emphasize the importance of smoking as a risk factor, but do not yet provide guidance regarding e-cigarettes. The current data indicates that about a quarter of smoking patients are using e-cigarettes post-ACS. Thus, rehabilitation providers should routinely assess e-cigarette use (particularly among smokers and recent quitters). Providers should also be prepared to educate patients regarding e-cigarettes and discuss potential discrepancies between patient beliefs about the safety of e-cigarettes and the current state of the science. The American Heart Association recently released a policy statement on the use of e-cigarettes11 (which is generally consistent with the current policy statements of other groups12) that should guide efforts to address e-cigarette use among post-ACS patients. Key conclusions of this statement include 1) that there is currently insufficient evidence “for clinicians to counsel their patients who are using tobacco products to use e-cigarettes as a primary cessation aid” and 2) “If a patient has failed initial treatment, has been intolerant to or refuses to use conventional smoking cessation medication, and wishes to use e-cigarettes to aid quitting, it is reasonable to support the attempt.” Given that many post-ACS patients do not attend rehabilitation, it is important that other post-ACS providers can also provide the above education.
This study has some limitations. In particular, the prevalence rates of e-cigarette use among ACS patients reported in this study should be interpreted within the context of our modest sample size and known regional differences in prevalence rates8. Second, we did not distinguish between generations of e-cigarettes in our survey (a limitation because recent research has shown substantial differences between generation, including nicotine dose and effectiveness for smoking cessation; e.g.,15) or collect data on frequency of e-cigarette use. Future work should address these limitations and explore methods of educating both providers and ACS patients regarding e-cigarettes.
Conclusion
A significant minority of patients are using e-cigarettes post-ACS. Post-ACS patients believe that e-cigarettes are similarly or less harmful than FDA approved medications. Providers should be prepared to discuss potential discrepancies between patient beliefs about the safety of e-cigarettes and the current state of the science.
Acknowledgments
Funding: Data collection for this study was supported by K23-HL107391. Dr. Tooley’s time on this study was supported by T32HL076134. Both awards were from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Conflicts of interest: No conflicts declared by the authors.
All authors have read and approved this manuscript.
References
- 1.King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine & Tobacco Research. 2015 Feb;17(2):219–227. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camenga DR, Delmerico J, Kong G, et al. Trends in use of electronic nicotine delivery systems by adolescents. Addict Behav. 2014 Jan;39(1):338–340. doi: 10.1016/j.addbeh.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine & Tobacco Research. 2013 Sep;15(9):1623–1627. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepper JK, Emery SL, Ribisl KM, Rini CM, Brewer NT. How risky is it to use e-cigarettes? Smokers’ beliefs about their health risks from using novel and traditional tobacco products. J Behav Med. 2015 Apr;38(2):318–326. doi: 10.1007/s10865-014-9605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farsalinos KE, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: Comparison with the effects of regular cigarettes. BMC Cardiovascular Disorders. 2014;14:78. doi: 10.1186/1471-2261-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington KF, Hull NC, Akindoju O, et al. Electronic cigarette awareness, use history, and expected future use among hospitalized cigarette smokers. Nicotine & Tobacco Research. 2014 Nov;16(11):1512–1517. doi: 10.1093/ntr/ntu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadimpati S, Nolan M, Warner DO. Attitudes, beliefs, and practices regarding electronic nicotine delivery systems in patients scheduled for elective surgery. Mayo Clin Proc. 2015 Jan;90(1):71–76. doi: 10.1016/j.mayocp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Rigotti NA, Harrington KF, Richter K, et al. Increasing prevalence of electronic cigarette use among smokers hospitalized in 5 US cities, 2010–2013. Nicotine & Tobacco Research. 2015 Feb;17(2):236–244. doi: 10.1093/ntr/ntu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer. 2014 Nov 15;120(22):3527–3535. doi: 10.1002/cncr.28811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 11.Bhatnagar A, Whitsel LP, Ribisl KM, et al. Electronic cigarettes: A policy statement from the American Heart Association. Circulation. 2014 Oct 14;130(16):1418–1436. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandon TH, Goniewicz ML, Hanna NH, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015 Feb 1;21(3):514–525. doi: 10.1158/1078-0432.CCR-14-2544. [DOI] [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991 Sep;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews. 2014;12:CD010216. doi: 10.1002/14651858.CD010216.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Tackett AP, Lechner WV, Meier E, et al. Biochemically verified smoking cessation and vaping beliefs among vape store customers. Addiction. 2015 May;110(5):868–874. doi: 10.1111/add.12878. [DOI] [PubMed] [Google Scholar]
- 16.American Association of Cardiovascular and Pulmonary Rehabilitation. AACVPR Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th. Champaign, IL: Human Kinetics; 2013. [Google Scholar]


