Abstract
Objective
Despite considerable advances in the understanding of systemic lupus erythematosus (SLE), there is still an urgent need for new and more targeted treatment approaches. We previously demonstrated that small molecule-blockade of Gβγ signaling inhibits acute inflammation through inhibition of chemokine receptor signal transduction. Here we examined whether inhibition of G protein βγ subunit signaling ameliorates disease in a mouse model of SLE.
Methods
Lupus prone NZB/NZW F1 female mice were prophylactically or therapeutically treated with the small molecule Gβγ inhibitor, gallein. Tissue samples were analyzed by flow cytometry and immunohistochemistry (IHC). The development and extent of nephritis were assessed by monitoring proteinuria and by IHC analysis. Serum immunoglobulin levels were measured by enzyme-linked immunosorbent assay (ELISA) and total IgG and dsDNA antibody-secreting cells (ASCs) by enzyme-linked immunospot assay.
Results
Gallein inhibited accumulation of T cells and germinal center (GC) B cells in the spleen. Both prophylactic and therapeutic treatment reduced GC size, decreased ASC production in spleen and markedly decreased accumulation of dsDNA specific auto-reactive ASCs in kidneys. Gallein also reduced immune complex deposition in kidneys. Finally, gallein therapy dramatically inhibited kidney inflammation, prevented glomerular damage and decreased proteinuria. Mechanistically, gallein inhibited immune cell migration and signaling in response to chemokines, in vitro, suggesting the mechanism of action in vivo is inhibition of migration of immune cells to sites of inflammation and immune cell maturation.
Conclusion
Overall these data demonstrate the potential use of gallein or novel inhibitors of G protein βγ signaling in SLE treatment.
Systemic lupus erythematosus (SLE) is a complex auto-immune disease that affects 1.5-2 million people in the United States. It is characterized by dysregulation of both the innate and adaptive arms of the immune system (1,2). Although abnormal activation of innate immune cells significantly contributes to lupus pathogenesis, spontaneous formation of germinal centers (GCs) and production of auto-reactive plasma cells are central events in the formation of immune complexes and their deposition in inflamed kidneys (3,4). Immune complexes are key in activation of the complement cascade and the production of inflammatory chemokines that are critical in lupus nephritis progression (5). GC formation, migration of antibody secreting cells (ASC), and other inflammatory cells to inflamed kidneys of lupus prone mice are coordinated by local chemokine gradients and the differential expression of chemokine receptors on immune cells.
There is substantial evidence showing complex and spatiotemporal changes in the expression of chemokine and chemokine receptors during lupus nephritis progression. Multiple chemokines (CCL2, CXCL9, CXCL10, CXCL11) and chemokine receptors (CCR2, CXCR3, CCR1, CCR5) have been identified as promising targets in lupus. However; the redundancy of chemokines, the remarkable complexity and variety of chemokine-receptors involved in recruitment of multiple cell populations to different compartments in inflamed kidneys, and the potential exclusion of populations with anti-inflammatory functions complicates the design of chemokine or chemokine receptor blocking therapies (6-8). Thus, modulating immune cell migration in the spleen and to inflammatory sites through blockade of multiple chemokine receptors is an attractive approach to ameliorate lupus.
Chemokine receptors are seven transmembrane proteins coupled to heterotrimeric G proteins that mediate cell signaling. Heterotrimeric G proteins are composed of an α subunit and a constitutive dimer of β and γ subunits (9-11). Chemokine receptors are coupled to the Gi family of G protein heterotrimers, and it is well established that the βγ dimer released from Gi plays a dominant role in chemokine receptor signaling in immune cells (12). In particular, Gβγ directly binds to phosphoinostide 3 kinase-γ (PI3Kγ) (13,14) to generate phosphatidylinositol 3,4,5 trisphosphate (PIP3) at the leading edge of chemokine stimulated immune cells, a key factor directing cell migration (15,16).
Due to the complexity of chemokine signaling in autoimmune diseases it has been proposed that inhibition of common signaling processes downstream might have a higher level of efficacy than targeting individual chemokine receptors (17). Indeed, inhibitors of PI3Kγ show strong efficacy in animal models of rheumatoid arthritis and lupus (18). Our laboratory has identified a class of small molecule inhibitors of Gβγ signaling, M119/gallein, that blocks Gβγ-dependent PI3Kγ activation in vitro (19), PIP3 production in cells, and chemokine-/chemoattractant-dependent neutrophil migration (20). These molecules show efficacy in mouse models of various diseases including heart failure, pain management and acute models of inflammation (20-23). Based on these data we hypothesized that gallein-dependent βγ inhibition would represent an alternative strategy for treatment of chronic inflammation of autoimmune disease, including SLE, by preventing migration of immune cells and effector lymphocytes to sites of inflammation.
Materials and Methods
Mice and experimental therapies
Aged matched NZB/NZW F1 female mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in the animal facilities at the University of Rochester and received chow and water ad libitum. Experiments were conducted in compliance with the approved University of Rochester Committee on Animal Resources protocol.
Prophylactic regimen
18 week old NZB/NZW F1 female mice were intraperitoneally injected 3 times/week with either 20 mg/kg or 35 mg/kg gallein over the course of 20 weeks. Untreated mice received a similar volume of phosphate buffered saline (PBS).
Therapeutic regimen
28 week old NZB/NZW F1 female mice with established nephritis (sustained proteinuria: 100 mg/dL) were intraperitoneally inoculated daily (5 days/week) with gallein for 4 weeks. Cohorts of experimental mice received 35 mg/kg gallein or a similar volume of PBS.
Post Treatment analysis
At the end of the therapy, mice were euthanized and spleen, bone marrow and kidneys were collected to enumerate lymphocytes by flow cytometry and antibody secreting cells (ASC) by ELISpot. Blood was collected by severing the renal artery and serum was used to determine levels of dsDNA specific IgG auto-antibodies by ELISA. Kidney and spleen sections were stained by immunofluorescence to visualize deposition of immune-complexes and germinal center formation/organization, respectively. Kidney sections were stained with Haematoxilin and eosin to measure perivascular cell infiltrates and blindly evaluate severity of glomerular damage (24).
Measurement of glomerular area covered by Ig G deposits
5 μm formalin sections were stained with FITC-anti mouse IgG. 200X fluorescent pictures were taken with an Axioplan Zeiss microscope and saved as JPEG files. Individual glomeruli were cropped with an automated tool from preview. Area covered by IgG deposition in each glomerulus was determined under the same conditions (Hue: Pass=143, Saturation: Pass 255, Brightness: Pass: 58-255) for all groups in a blinded fashion and is expressed in square pixels. n= 4-21 glomerulus per group. *, p=0.05, ***, p≤ 0.0005.
Proteinuria
Protein concentration in the urine was monitored every week with commercial urine dipsticks (Uristix, Bayer).
Enumeration of effector lymphocytes by flow cytometry
Antibodies
APCeFluor780-CD3-e (17A2, eBioscience), APC-CD4 (RM4-5, BD Pharmingen), V500-CD45R/B220 (RA3-6B2, BD Horizon), V500-CD8(53-6.7, BD Biosciences), biotin-CXCR5 (2G8, BD Pharmingen), FITC-PD1 (J43, eBioscience), PerCP Cy5.5-ICOS (C398.4A, Biolegend), PECy7-CD44 (IM7, Biolegend), FITC-CD62L (MEL-14, BD Biosciences), APC-CD19 (1D3, BD Biosciences), FITC-Peanut agglutinin (L7381-1MG, Sigma), CD95-PECy7(Jo2, BD Biosciences), PE-CD138 (281-2, BD Pharmingen), FITC-kappa light chain (187.1, BD Pharmingen), MHC CII (I-A/I-E, M5/114.15.2, eBisocience), PE-Streptavidin (554061, BD Pharmingen).
To block non-specific binding, cells isolated from spleen were incubated with antibodies against Fc receptors at 4 μg/mL (anti-mouse CD16/CD32, clone 2.4G2, BioXCell) for 5 minutes on ice. To detect surface markers, cells were incubated with fluorescently labeled antibodies for 15 minutes on ice and were washed twice with FACS media. To detect plasmablasts and plasma cells, cells were fixed/permeabilized for 15 minutes after surface staining. Cell suspensions were incubated with FITC conjugated antibodies against mouse kappa light chain for 10 minutes and washed twice with FACS media. 100,000 live cells were collected in a LSRII 12 color flow cytometer (BD Biosciences) and analyzed with Flow Jo software (Tree Star Inc, Ashland, OR).
Cell populations were defined as CD3+CD4+CD44-CD62L+ naïve T cells; CD3+CD4+CD44+CD62L- effector memory T cells; CD3+CD4+CD44+CD62L+ central memory T cells; CD3+CD8-B220−CD4+CXCR5+PD1+ICOS+ T follicular helper cells; and CD19+PNA+CD95+ germinal center B cells.
dsDNA ELISA
Blood was collected in microfuge tubes and centrifuged at 5,000 rpm for 5 minutes to separate serum. Serum was transferred to a clean microfuge tube and stored at -20oC until analysis. Levels of anti-dsDNA auto-antibodies were determined with an anti-dsDNA ELISA kit (Alpha Diagnostic International) according to manufacturers instructions.
dsDNA antibody secreting cell ELISpot
For detecting dsDNA specific ASC, 96 well multiscreen nitrocellulose plates (MAHAS4510, Millipore) were coated with 0.001% Poly-L-Lysine (P4707, Sigma) for 1 hour at 37°C. Without washing, calf thymus DNA was added to the plates and incubated for 3 hours at 37°C. To detect IgG+ ASC, 96 well multiscreen nitrocellulose plates were coated with goat anti-mouse IgG (115-005-008, Jackson Immunoresearch Laboratories) for 1 hour at 37°C. After coating with IgG or dsDNA, non-specific binding was blocked with 10% fetal bovine serum in RPMI media. Cell suspensions from spleen or BM were plated at 1×106 cells in the top well and two-fold dilutions were performed going down the plate. Cell suspensions were incubated at 37°C, overnight, under a 5% CO2 atmosphere. The next day, plates were washed several times with PBS-0.1% Tween 20 and incubated with horseradish peroxidase conjugated to goat anti-mouse IgG (1030-05, Southern Biotech) for 3 hours at 37°C. Plates were developed with peroxidase substrate kit (SK-4100, Vector Laboratories) and spots counted with a stereoscopic microscope.
Inmunofluorescence
Primary antibodies
PE-rat anti-mouse IgD (clone 11-26, eBioscience), biotin-donkey anti mouse IgG (715-066-150, Jackson Immunoresearch Laboratories), FITC-PNA (L7381-1MG, Sigma), goat anti-proliferating cell nuclear antigen (C-20, Santa Cruz Biotechnology), APC-CD45R/B220 (RA3-6B2, BD Biosciences).
Secondary antibodies and streptavidin
Alexa fluor 568-Donkey anti-goat (Life technologies), Rabbit-anti PE (200-4199, Rockland Immunochemicals), Cy3-donkey anti-rabbit (711-165-152, Jackson Immunoresearch Laboratories), Cy5-Streptavidin (19-4317-82, eBioscience). For both Spleen frozen sections and Paraffin embedded sections, non-specific binding was blocked with 5% normal donkey serum and Fc Block at 4 μg/mL (Anti mouse CD16/CD32, clone 2.4G2, BioXCell) dissolved in PBS with 0.1% Tween 20 and 0.1% Triton X 100. After incubation with the indicated antibodies, slides were washed and mounted with prolong gold antifade with DAPI. Images were visualized with a Zeiss Axioplan microscope and recorded with a Hamamatsu camera. Morphometric analysis was performed with an automated tool of the Zeiss Axiovision software.
Preparation of mouse neutrophils from bone marrow
Mice were sacrificed, the femur and the tibia from both hind legs were removed. The ends of the bones were cut and the bone marrow was flushed using PBS in a syringe. The cell suspension was centrifuged at 1400 rpm for 3 min, 4°C. Red blood cells were lysed using RBC lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). The cell suspension was strained through a 70 μm nylon cell strainer (352350, Falcon), centrifuged at 1400 rpm for 3 min, 4°C and resuspended in PBS. The crude BM population was counted using a hemocytometer. The cell suspension was prepared for neutrophil enrichment using the EasySep Negative Selection kit (19762, Stemcell Technologies) according to the manufacturer’s instructions.
Preparation of mouse T-cells from the spleen
Spleens were harvested from mice and mashed through a 70 μm nylon cell strainer and washed in PBS. Red blood cells were lysed using RBC lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). The splenocyte population was prepared for CD4+ T cell enrichment using the EasySep Mouse CD4+ T cell Enrichment Kit (19752, Stemcell Technologies) according to the manufacturer’s instructions.
Preparation of human T-cells from peripheral blood
Blood was collected from healthy volunteers and separated by layering over PolymorphPrep (AN1114683, Accurate Chemical) and centrifuged at 1200 rpm for 45 min, 20°C. The separated peripheral blood mononuclear cells were collected and washed in PBS. Mononuclear cells were finally resuspended and cultured overnight in RPMI containing 1μg/mL of PHA (L8902, Sigma). The next day, the media was collected and transferred to fresh media. At the fourth day, the media was changed into RPMI containing 2 ng/mL of IL-15 (247-IL-005, R&D Systems) for 4-10 days.
Cell Migration Analysis
Chemotaxis was measured in a Boyden chamber (AA12, Neuro Probe) using 3 μm polycarbonate membranes (PFB3, Neuro Probe). Murine neutrophils were resuspended in HBSS w/calcium, magnesium, and 0.1% BSA to a final concentration of 6×106 cells/mL. Cells were pretreated with small molecule Gβγ inhibitors for 10 minutes before stimulation with chemoattractant. Neutrophil suspensions (0.3×106 cells/well) were added to the upper chamber and allowed to migrate towards the bottom chamber in response to either 250 nM fMLP or 10 ng/mLCXCL2 for 15 min, or 100 ng/mL GM-CSF for 1 hour at 37°C. Human T-cell suspensions (0.5×106 cells/well) were added to the upper chamber and allowed to migrate towards the bottom chamber in response to 50 ng/mL CXCL12 for 3 hours, 37°C. Filters were stained according to the manufacturer’s recommendations using Diff Quik (CA53000-052, VWR International). Migrated neutrophils were analyzed by counting cells from three microscope fields. The number of cells that basally migrated towards vehicle was subtracted from the number of cells that migrated towards the chemoattractant.
Measuring PI3Kγ activity in HL-60 cells
HL-60 cells stably expressing the PH domain of Akt fused to GFP (PH-AKT-GFP) were differentiated in RPMI 1640, 10% FBS and 1% penicillin/streptomycin containing 1.2% DMSO for 5 days and serum starved overnight before confocal imaging. Cells were allowed to adhere on glass coverslips and treated with small molecule Gβγ inhibitors for 10 minutes at room temperature. Images were taken every 10 sec and 250 nM fMLP was added at 60 sec. Relative fluorescence was analyzed by obtaining the ratio of membrane to cytoplasmic fluorescence over time.
Statistical analysis
Statistical significance was calculated with Graph Pad Prism as indicated in the figure legends. Differences with a p value <0.05 were considered statistically significant.
Results
Gβγ inhibition with Gallein inhibits chemokine/chemoattractant-dependent migration and PI3K activation in immune cells
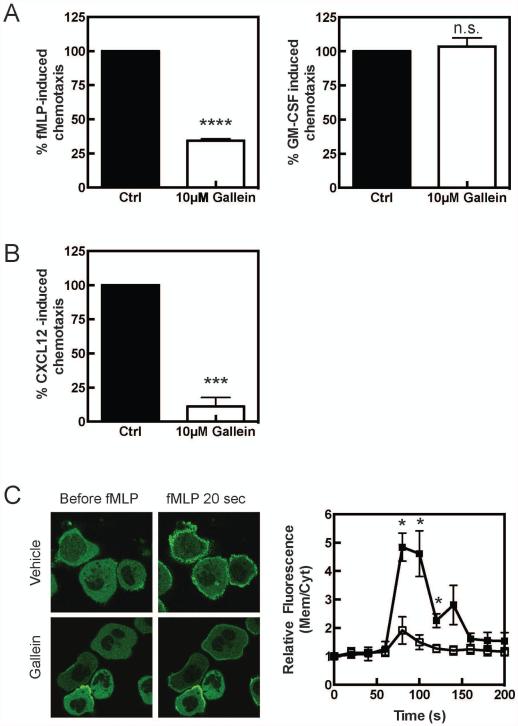
As we have previously demonstrated, acute inhibition of Gβγ subunit signaling with gallein inhibits fMLP-dependent chemotaxis of primary neutrophils (Figure 1A). GM-CSF-dependent chemotaxis, which does not couple to G protein signaling was unaffected by this treatment (Figure 1A). Chemokines direct signaling of various types of immune cells through Gβγ so we predict that the effects of gallein would generally inhibit chemokine dependent migration of other immune cells relevant to inflammatory disease. As shown in figure 1B gallein strongly inhibits CXCL-12 (SDF-1)-dependent chemotaxis of human T cells supporting this idea.
Figure 1. Gallein inhibits migration of mouse neutrophils and human T cells in response to GPCR-coupled chemoattractants.
A) Neutrophils were isolated from the bone marrow and pretreated with 10 μΜ gallein for 10 minutes and allowed to migrate in response to either 250 nM fMLP or 100 ng/mL GM-CSF. The filter was stained and cells attached to the bottom were counted. Background migration in response to vehicle was subtracted from migration in response to chemoattractant. B) Gallein inhibits migration of human T cells in response to CXCL12. Human T cells were pretreated with 10 μM gallein for 10 minutes and allowed to migrate in response to 100ng/mL CXCL12 for 3 hours, 37°C. The filter was stained and cells attached to bottom were counted. Background migration was subtracted. C) Gallein inhibits membrane PIP3 production in response to fMLP in differentiated HL-60 cells. Differentiated HL-60 cells stably expressing PH-AKT GFP were pretreated with 10 μΜ gallein for 10 minutes prior to stimulation with 250 nM fMLP. Images were taken every 10 sec and the relative fluorescence was determined by using the ratio of membrane over cytoplasmic fluorescence over time. Three cells were averaged per experiment and each experiment repeated three times. Data is expressed as mean ± standard error of the mean. Statistical significance was analyzed with a Student’s T-test: *, p<0.05, ***, p<0.001, ****, p<0.0001.
To examine the mechanistic basis for gallein-dependent inhibition of migration we focused on effects of gallein on fMLP-dependent PI3Kγ activation because Gβγ directly binds to and activates PI3Kγ and gallein blocks this interaction in vitro. To assay PI3K activation in cells we utilized HL-60 cells stably transfected with the PH domain of Akt fused to GFP (GFP-Akt-PH). As has been extensively documented (15), stimulation of HL-60 cells, differentiated to neutrophils, with fMLP, led to a rapid recruitment of GFP-Akt-PH to the plasma membrane reflecting an increase in PIP3 production at the plasma membrane due to PI3K activation (Figure 1C). Pretreatment with gallein strongly inhibited both the extent of translocation, and the number of cells responding to fMLP, supporting the idea that gallein inhibits Gβγ-dependent PI3K activation in immune cells.
To determine the effectiveness of gallein in inhibiting the migratory capacity of neutrophils in vivo, C5BL/6 mice were injected i.p. with 35 mg/kg gallein. Neutrophils were then isolated from the bone marrow and examined for their ability to respond to either fMLP or GM-CSF. Interestingly, neutrophils from gallein treated mice were impaired with respect to fMLP-dependent migration for up to 48 hours after the initial injection (Supplementary Figure 1 A). GM-CSF-dependent chemotaxis was unimpaired indicating the general health and migratory capacity of these cells was unaffected by gallein treatment (Supplementary Figure 1B). The molecular basis for this sustained effect on neutrophil migration is unclear but the result suggests that daily dosing with gallein has the potential to significantly inhibit migration during long-term administration.
Inhibition of G protein βγ subunit signaling targets germinal center reactions in lupus prone mice
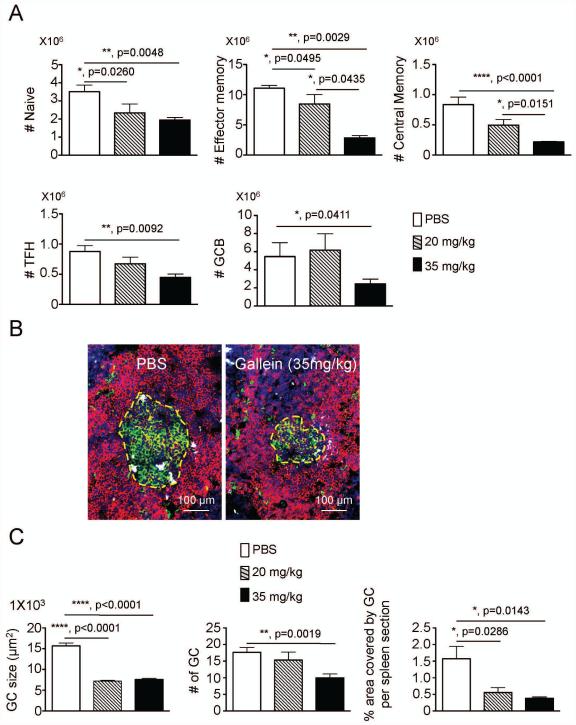
Chemokines are involved in recruiting T lymphocytes to the spleen where they participate in B cell activation and production of antibody secreting cells suggesting inhibition of chemokine signaling may decrease T-cell accumulation and GC formation in the spleen. Therefore, we enumerated different populations of lymphocytes in spleens of lupus prone mice treated with gallein compared to mice receiving PBS, i.p. injected 3 times weekly for 20 weeks during the development of lupus. Gallein treatment caused a dose-dependent and significant reduction in the number of naïve T cells, effector memory T cells, central memory T cells, T follicular helper cells, and GC B cells (Figure 2A) consistent with the idea that blocking Gβγ-dependent chemokine signaling prevents T cell migration to the spleen.
Figure 2. Prophylactic administration of gallein blocks lymphocyte recruitment and impairs germinal center responses in the spleen of lupus prone mice.
Groups of 18 week old NZB/NZW female mice were treated with vehicle, 20 mg/kg or 35 mg/kg gallein for 20 weeks (n=8 mice/group, 3 times a week intraperitoneal administration). A) At the end of the therapeutic regimen, spleens were collected and different cell populations were enumerated by flow cytometry. Total numbers of CD3+CD4+CD44−CD62+ naïve-, CD3+CD4+CD44+CD62L− effector- and CD3+CD4+CD44+CD62L+ central memory CD4 T cells are shown in top panels. Numbers of CD3+CD4+CXCR5+ICOS+PD1+ T follicular helper cells and CD19+PNA+FAS+ germinal center B cells are depicted in bottom panels. B) Spleens were collected at the end of the prophylactic therapy and embedded in OCT. 5 μm frozen sections were stained with antibodies against IgD (red) and IgG (white), in combination with fluorescein isothiocyanate-labeled peanut agglutinin (Green). Representative pictures taken at 200 x magnification are shown. C) All PNA+ germinal centers in individual spleen frozen sections (n = 8 spleen sections per group) were outlined with an automated tool of the Zeiss microscope to calculate their size (left panel), number (center panel) and percentage covered by germinal centers per spleen section (right panel). Representative results from two independent experiments with similar results are shown. Data is expressed as mean ± standard error of the mean. Statistical significance was calculated by non-parametric Mann Whitney test. *, p<0.05, **, p<0.005, ****, p<0.0001.
To evaluate the impact of gallein on GC structures, we stained spleen frozen sections with antibodies against mouse IgD and mouse IgG, in combination with peanut agglutinin (PNA). Large PNA+ GCs surrounded by IgD+ follicular naïve B cells and IgG+ producing PCs were detected in spleens of PBS treated mice (Figure 2B, left panel). In contrast, mice treated with gallein showed a significant reduction in the size of the PNA+ GCs and number of IgG+ ASC PCs, compared to untreated group (Figure 2B, right panel). Morphometric analysis revealed a dose-dependent and significant reduction in the size and area covered by GC in individual spleen sections after administration of gallein (Figure 2C). CCR7 and CXCR5 chemokine receptors are expressed on mature antigen presenting cells (APC), naïve, central memory and recently activated T cells suggesting that the impaired T cell priming and GC formation in this lupus model are due to inhibition of chemotactic signaling by chemokines.
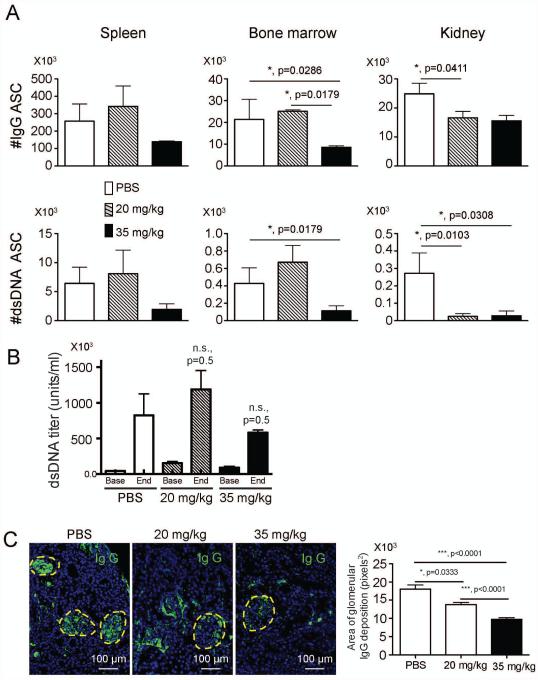
Gallein treatment inhibits accumulation of auto-reactive dsDNA specific antibody secreting cells in the kidney, and reduces immune complex deposition in kidneys of lupus prone mice
In experimental lupus, GCs are a main source of antibody secreting cells (ASC) that produce pathogenic auto-antibodies against dsDNA (3). Antibodies against dsDNA in lupus prone mice are critical in the formation of immune complexes and the development of lupus nephritis. Therefore, we next enumerated isotype switched and dsDNA specific auto-reactive PCs specific for dsDNA that were produced in GCs. Gallein reduced the accumulation of isotype switched and dsDNA specific auto-reactive ASC in the bone marrow (BM) at 35 mg/kg but the effect of gallein on accumulation of dsDNA+ ASC was much more pronounced in the kidney (Figure 3A). There were small increases in dsDNA+ASCs in the spleen and bone marrow, and in the concentration of dsDNA specific IgG auto-antibodies in the serum in mice treated with 20 mg/kg gallein. Treatment with 35 mg/kg trended toward a reduction compared to untreated lupus mice, but none of these effects was statistically significant possibly due to a small sample size (Figure 3B). However, there was a dose dependent reduction in the deposition of IgG antibodies on glomeruli of lupus prone mice treated with gallein (Figure 3C). These results indicate that a key site of gallein action is on migration and accumulation of dsDNA+ ASC to inflamed kidneys, and immune complex deposition in glomeruli of lupus prone mice.
Figure 3. Gallein decreases accumulation of auto-reactive antibody secreting cells in lupus prone mice.
Mice were treated as in figure 2. Spleen, BM and kidney were collected to isolate leukocytes. A) White cells were added to ELISpot plates coated with anti IgG or double stranded DNA (dsDNA) to enumerate isotype-switched (top panels) and dsDNA specific antibody secreting cells (ASC) (bottom panels) in different organs. n=8 mice/group B) Gallein therapy does not significantly reduce serum levels of dsDNA IgG antibodies. n=8 mice/group C) Prophylactic administration of gallein decreased IgG deposition in the kidneys of lupus prone mice. Representative pictures were taken at a 200 x magnification (n= 8 kidney sections/group). The area of IgG deposition was measured with Image J in a blinded manner. Representative results from two independent experiments with similar results are shown. Data is expressed as mean ± standard error of the mean. Statistical significance was calculated by non-parametric Mann Whitney test. *, p<0.05, ***, p<0.001.
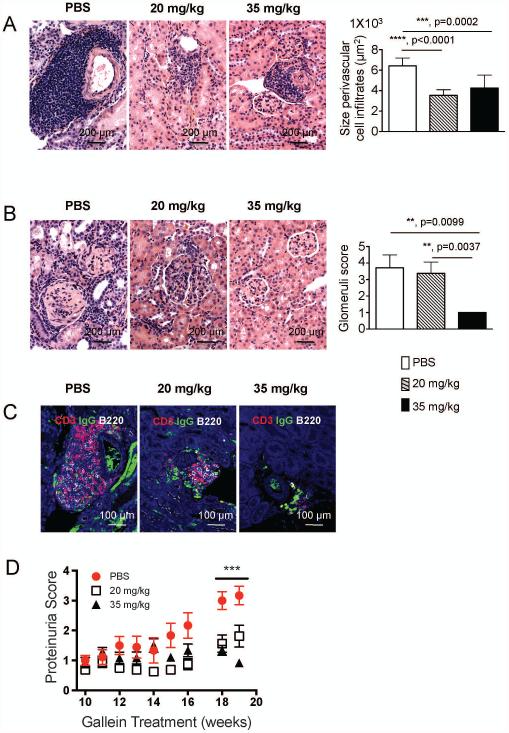
Inhibition of G protein βγ signaling inhibits inflammation and prevents damage in kidneys from lupus prone mice
Immune complexes are key in the activation of the complement cascade and production of soluble mediators by inflammatory cells, which play an active role in the process of lupus nephritis (5). Thus, based on the reduced immune complex deposition in the kidney of gallein treated mice, we evaluated inflammatory and pathological changes in kidney sections stained with H and E. Representative sections show massive accumulation of inflammatory cells around medium sized blood vessels in the kidneys of untreated lupus mice, contrasting with the greatly reduced size of perivascular cell infiltrates in kidneys of gallein treated mice (Figure 4A). Quantitative morphometric analysis confirmed these observations and clearly shows a statistically significant reduction in the size of perivascular cell infiltrates in gallein treated mice (Figure 4A, right panel). In PBS injected mice, glomeruli of lupus mice show inflammatory cell infiltration, local fibrosis and engrossment of the basement membrane. In sharp contrast, mice treated with 35 mg/kg gallein show almost normal glomerular architecture (Figure 4B). To characterize the cellular composition of infiltrates around blood vessels, we stained paraffin kidney sections with antibodies against mouse CD3-epsilon (T cells), CD45R/B220 (B cells) and IgG (IgG deposition and isotype switched ASC). In vehicle treated mice, perivascular inflammatory cell infiltrates contained large numbers of B220+ B cells, CD3+ T cells, and IgG+ ASC (Figure 4C, left panel) all of which are substantially reduced in dose-dependent manner by gallein treatment (Figure 4C, middle and right panels).
Figure 4. Inhibition of G protein βγ signaling prevents accumulation of immune cells in inflamed kidneys at early stages of experimental lupus.
After 20 weeks of gallein administration kidneys were fixed in formalin and embedded in paraffin. A) 5 μm kidney sections were stained with hematoxylin and eosin. Representative 200 x magnification pictures from kidneys showing inflammatory cell infiltrates around blood vessels are shown. Gross histological findings were confirmed by measurement of all perivascular cell infiltrates (right panel, n=8 mice/group). B) 5 μm kidney sections were stained with hematoxylin and eosin. Representative 200 x magnification pictures from kidneys showing glomerular inflammation are shown. Right panel shows quantitative blinded evaluation of glomerular inflammation by a certified pathologist (right graph) in individual kidney sections (n = 8 kidney sections/group). C) Effects of gallein treatment on accumulation of CD3+ and B220+ cells around blood vessels in the kidneys of lupus prone mice. 5 μm kidney sections were stained with primary antibodies against CD3 (red), IgG (green) and B220 (white). Representative 200 x magnification pictures from two independent experiments with similar results are shown. D) Protein levels in the urine of mice were monitored. n=8 mice/group Data is expressed as mean ± standard error of the mean. Statistical significance was calculated by non-parametric Mann Whitney test. **, p=<0.005, ***, p<0.0005, ****, p<0.0001.
Finally, we measured the effects of gallein treatment on proteinuria. Proteinuria was low in all groups until week 15. From week 15 to week 20 untreated mice start to excrete greater amounts of protein as lupus progresses, while gallein treated mice maintained a stable concentration of urine protein until the endpoint of our experiment (Figure 4D). At the end of the treatment, there was a clear and significant dose-dependent reduction in proteinuria due to gallein administration at either 20 mg/kg or 35 mg/kg (Figure 4D). The effectiveness of 20 mg/kg gallein was somewhat surprising given the lack of effect on glomerular score (Figure 4B). However, the glomerular score is quantitated based on mesangial proliferation and crescent formation, whereas proteinuria is often mediated by sub-epithelial and sub-endothelial immune deposits-very likely impacted by gallein. These results show that gallein therapy, at early stages of experimental lupus, modulates perivascular inflammation, prevents glomerular damage, impairs attraction of immune cells (T cells, B cells and ASC) to the inflamed kidney and significantly halts proteinuria in lupus prone mice.
Therapeutic administration of gallein is effective in lupus prone mice with established nephritis
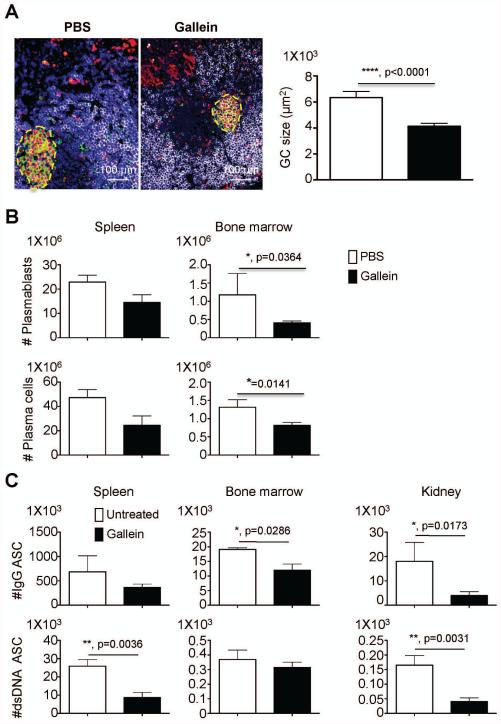
To test the potential of gallein to modulate the autoimmune response in lupus prone mice with established proteinuria, we treated 28 week old lupus prone mice 5 times weekly with gallein. We used 35 mg/kg gallein as the therapeutic dose because this was the most effective dose for many endpoints in the prophylactic model and therefore predicted to have the greatest chance of success in reversing active disease. At the end of the therapy, spleen paraffin sections were stained with antibodies against proliferating cell nuclear antigen (PCNA) and anti mouse CD45R/B220, in combination with PNA. Large GC containing proliferating B blasts that bind PNA and have down-regulated B220 expression were easily detected in the spleens of untreated lupus prone mice (Figure 5A). In gallein treated mice, GC were significantly smaller than those in the untreated group (Figure 5A) which was confirmed by morphometric analysis (Figure 5A, right graph).
Figure 5. Germinal centers and auto-reactive ASC are major targets of gallein in mice with active experimental lupus.
Groups of 28 weeks old NZB/NZW female mice, with established proteinuria, were treated with vehicle or 35 mg/kg gallein (n=8/group, 5 times a week administration). A) At the end of the therapy, mice were sacrificed and spleens were fixed in neutral buffered formalin and embedded in paraffin. 5 μm paraffin spleen sections were stained with FITC-labeled peanut agglutinin, in combination with primary antibodies against proliferating cell nuclear antigen (red) and B220 (white). 200 x magnification pictures show germinal centers (GC) that are outlined with a yellow dashed line. Morphometric analysis is shown on the right. B) CD19+kappalight+MHC CII+ plasmablasts and CD19+Kappalight+MHC CII− plasma cells were enumerated in red blood cell-free cell suspensions by flow cytometry. C) Spleen, BM and kidney were collected to isolate leukocytes. White cells were added to ELISpot plates coated with anti IgG or double stranded DNA (dsDNA) to enumerate isotype-switched and dsDNA specific antibody secreting cells (ASC) in different organs. Representative results from two independent experiments with similar results are shown. Data is expressed as mean ± standard error of the mean. Statistical significance was calculated by non-parametric Mann Whitney test. *, p<0.05, **, p=<0.005, ****, p<0.0001.
Given that GC are the main source of ASC, we enumerated CD19+CD138+Kappalight+MHCCII+ plasmablasts and CD19+CD138+Kappalight+MHCCII− plasma cells in spleen and BM of lupus prone mice by flow cytometry. Although gallein treatment reduced both the numbers of plasmablasts and plasma cells in the spleen, they were more dramatically reduced in the BM (Figure 5B). We also examined isotype-switched and dsDNA specific plasma cells in the spleen, BM and kidney. Therapeutic administration of gallein significantly impaired the production of dsDNA+ auto-reactive ASC in the spleen and markedly affected the accumulation of isotype-switched and dsDNA specific ASC in the inflamed kidney (Figure 5C).
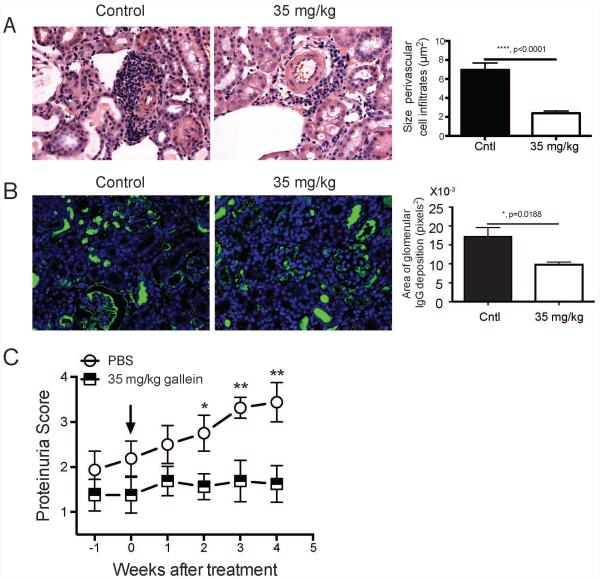
Gallein treatment significantly inhibited perivascular immune cell infiltration (Figure 6A) and IgG deposition (Figure 6B) in the kidney and prevented development of proteinuria in lupus prone mice with established nephritis (Figure 6C). Gallein treatment did not significantly affect the glomerular score, but as discussed earlier this score may not correlate directly with proteinuria. These findings confirm that gallein mainly targets GC reaction, impairing the production of auto-reactive dsDNA specific ASC in the spleen and modulating the migration of auto-reactive ASC to the BM and inflamed kidney. In addition, our results indicate that reduced accumulation of auto-reactive ASC in the kidney is likely contributing to abrogation of proteinuria in lupus prone mice with established nephritis.
Figure 6. Gallein halts deterioration of kidney function in mice with active lupus.
NZB/NZW mice with established lupus were treated as in figure 5. A) Kidneys were fixed in neutral buffered formalin and embedded in paraffin and 5 μm kidney sections were stained with hematoxylin and eosin. Representative 200 x magnification pictures from kidneys showing inflammatory cell infiltration around blood vessels and the effects of gallein treatment are shown. Gross histological findings were confirmed by measurement of all perivascular cell infiltrates (right panel). Glomerular scores were: Cntl: 2±0.29 n=3, Gallein: 1±0.29 n=6, these values were not statistically different. B) Effects of gallein treatment on accumulation of CD3+ and B220+ cells around blood vessels in the kidneys of lupus prone mice. 5 μm kidney sections were stained with primary antibodies against CD3 (red), IgG (green) and B220 (white), followed by detection with fluorescently-labeled secondary antibodies. Representative 200 x magnification pictures from two independent experiments with similar results are shown. Quantitation of IgG deposition in 8 kidney sections for each group are shown on the right. C) Proteinuria score at different time-points after treatment of NZB/NZW mice with established lupus. Arrow indicates the beginning of the treatment. n=8 mice/group. Data is expressed as mean ± standard error of the mean. Statistical significance was calculated by non-parametric Mann Whitney test. *, p<0.05; **, p=<0.005.
Discussion
In this study, we show that inhibition of G protein βγ subunit signaling ameliorates pathological nephritis in a mouse model of lupus when given either prophylactically or after the development of disease. There was decreased accumulation of effector lymphocytes and GC formation in the spleen and a marked abrogation of autoreactive PC and inflammatory cell infiltration in the kidney of lupus prone mice. These results suggest that inhibition of chemokine signaling mediates a potent synergistic effect on immune cell activation in peripheral lymphoid organs and migration to target organs.
In reactive secondary lymphoid organs, CCL19 and CCL21 are produced by stromal cells in the T cell zone and are critical for maximizing the encounters between CCR7+ mature antigen presenting cells and rare populations of naïve T cells, thus facilitating T cell priming (25,26) (27). In addition, CXCL13 is mainly produced by follicular dendritic cells and B cells and is important for the attraction of CXCR5+ T follicular helper cells (TFH) to the B cell follicle, where they contribute to the formation of GCs and production of high affinity ASCs against antigens or auto-antigens in the case of auto-immune diseases (28) (29,30). Our results are consistent with gallein targeting chemokine signaling in CCR7+ and CXCR5+ expressing populations (T cells, APC), which are actively participating in T cell priming and GC formation.
Another chemokine receptor important in lupus is CXCR3. Interestingly, recent evidence indicates that IFNγ produced by Th1 effector memory cells is critical in the aberrant production and expansion of TFH, which lead to abnormal GC formation in the sanroque lupus mouse model (31). Given that Th1 cells characteristically express CXCR3, the receptor for IFNγ induced chemokines, it is likely that gallein therapy regulates the accumulation of Th1 cells in the spleens of lupus prone mice, impairing the production of TFH and consequently affecting the formation/organization of auto-immune GC.
Freshly differentiated plasma cells also express CXCR3 and are attracted into inflamed kidneys of lupus prone mice by IFNγ induced chemokines CXCL9, CXCL10, and CXCL11 (32). Gallein had a strong effect on the accumulation of auto-reactive dsDNA specific ASC in the BM and inflamed kidney with a modest effect in the spleen. This suggests that the subtle effect of gallein on modulating the production of auto-reactive ASC in the spleen, combined with pronounced impact on the attraction to the BM and especially to the inflamed kidney are contributing to abrogate lupus nephritis.
G protein-coupled receptors participate in multiple physiological processes, which are potentially affected by gallein. However, gallein was identified as a compound that selectively interferes with subsets of Gβγ signaling pathways, many of which are enriched in immune cells and are downstream of chemokine and chemoattractant receptor signaling. For example, although gallein blocks the activation of phospholipase C β2 and PI3Kγ by Gβγ, this activation is rarely seen in other tissue types due to enrichment of these effector enzymes in immune cells (33). Thus, gallein effects are restricted to certain subsets of tissues and may explain why inhibition of this highly ubiquitous and important protein has only minimal apparent obvious side effects.
It has been previously demonstrated that inhibition of a common signaling component downstream of chemokine receptors, PI3Kγ, inhibits development of lupus (18) and rheumatoid arthritis (34) in mouse models. In these studies the general principle was similar to our study with the idea that inhibiting a common signal downstream of the complex repertoire of chemokine receptors may have more efficacy in treatment of a complex auto-inflammatory disease than inhibition of individual chemokine receptors. Targeting Gβγ may have alternative benefits relative to direct PI3Kγ inhibition including specificity for Gβγ-regulation of PI3Kγ, and also blocking a broader range chemotactic signaling pathways downstream of Gβγ that may lead to greater efficacy. Overall, Gβγ targeting with gallein shows strong efficacy both therapeutically and prophylactically for inhibition SLE pathology without major toxic side effects. Such an approach may be useful for other chronic inflammatory diseases such as rheumatoid arthritis.
Supplementary Material
Acknowledgements
The project described in this publication was supported by a pilot award under the University of Rochester CTSA award number UL1 TR000042 from the National Center for Advancing Translational Sciences of the National Institutes of Health (JA and AVS) and NIH GM081772 (AVS). Dr. Anolik is also supported by the NIH Rochester Autoimmunity of Excellence U19 AI563262, P01 AI078907, the NIAMS Accelerated Medicines Partnership (1UH2AR067690) and the Bertha and Louis Weinstein research fund.
Abbreviations used
- SLE
Systemic Lupus Erythematosus
- GPCR
G protein-coupled receptor
- PIP3
Phosphoinositide 3,4,5 trisphosphate
- PI3K
phosphoinositide 3-kinase
- GC
germinal center
- BM
bone marrow
- fMLP
formyl-Met-Leu-Phe
- GM-CSF
granulocyte macrophage colony stimulating factor
- PNA
peanut agglutinin
- dsDNA
double stranded DNA
- ASC
antibody secreting cells
Footnotes
There is no financial conflict of interest
REFERENCES
- 1.Marian V, Anolik JH. Treatment targets in systemic lupus erythematosus: biology and clinical perspective. Arthritis Res Ther. 2012;14(Suppl 4):S3. doi: 10.1186/ar3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anolik JH. B cell biology: implications for treatment of systemic lupus erythematosus. Lupus. 2013;22:342–349. doi: 10.1177/0961203312471576. [DOI] [PubMed] [Google Scholar]
- 3.Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, et al. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578–584. [PubMed] [Google Scholar]
- 4.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 5.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 6.Vielhauer V, Anders HJ, Schlondorff D. Chemokines and chemokine receptors as therapeutic targets in lupus nephritis. Semin Nephrol. 2007;27:81–97. doi: 10.1016/j.semnephrol.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Vielhauer V, Anders HJ. Chemokines and chemokine receptors as therapeutic targets in chronic kidney disease. Front Biosci (Schol Ed) 2009;1:1–12. doi: 10.2741/s1. [DOI] [PubMed] [Google Scholar]
- 8.Anders HJ, Sayyed SA, Vielhauer V. Questions about chemokine and chemokine receptor antagonism in renal inflammation. Nephron Exp Nephrol. 2010;114:e33–38. doi: 10.1159/000254389. [DOI] [PubMed] [Google Scholar]
- 9.Gilman AG. G proteins: transducers of receptor-generated signals. Ann Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 10.Clapham DE, Neer EJ. G Protein βγ subunits. Ann Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 11.Oldham WM, Hamm E. Structural basis of function in heterotrimeric G proteins. Quarterly Reviews of Biophysics. 2006;39:117–166. doi: 10.1017/S0033583506004306. [DOI] [PubMed] [Google Scholar]
- 12.Rossi D, Zlotnik A. The Biology of Chemokines and Their Receptors. Ann Rev Immunol. 2000;18:217–248. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 13.Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. A novel, phosphoinositide 3-kinase activity in myeloid-derived cells is activated by G-protein βγ -subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 14.Stephens LR, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AV, et al. The Gβγ Sensitivity of a PI3K is Dependent upon a Tightly Associated Adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 15.Servant G, Weiner OD, Hezmark P, Balla T, Sedat JW, Bourne HR. Polarization of Chemoattractant Receptor Signaling During Neutrophil Chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franca-Koh J, Kamimura Y, Devreotes PN. Leading-edge research: PtdIns(3,4,5)P3 and directed migration. Nat Cell Biol. 2007;9:15–17. doi: 10.1038/ncb0107-15. [DOI] [PubMed] [Google Scholar]
- 17.Johnson Z, Power CA, Weiss C, Rintelen F, Ji H, Ruckle T, Camps M, Wells TNC, Schwarz MK, Proudfoot AEI, Rommel C. Chemokine inhibition – why, when, where, which and how? Biochemical Society transactions. 2004;32:366–377. doi: 10.1042/bst0320366. [DOI] [PubMed] [Google Scholar]
- 18.Barber DF, Bartolome A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, et al. PI3Kγ inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 19.Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, et al. Differential targeting of Gβγ-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann DM, Seneviratne AMPB, Smrcka AV. Small Molecule Disruption of G Protein βγ Subunit Signaling Inhibits Neutrophil Chemotaxis and Inflammation. Mol Pharmacol. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathews JL, Smrcka AV, Bidlack JM. A novel Gβγ-subunit inhibitor selectively modulates μ-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J Neurosci. 2008;28:12183–12189. doi: 10.1523/JNEUROSCI.2326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, et al. Small molecule disruption of Gβγ signaling inhibits the progression of heart failure. Circ Res. 2010;107:532–539. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamal FA, Mickelsen DM, Wegman KM, Travers JG, Moalem J, Hammes SR, et al. Simultaneous adrenal and cardiac GPCR-Gβγ inhibition halts heart failure progression. J Am Coll Cardiol. 2014;63:2549–2557. doi: 10.1016/j.jacc.2014.02.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T, et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis and rheumatism. 2012;64:493–503. doi: 10.1002/art.33333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luther SA, Vogt TK, Siegert S. Guiding blind T cells and dendritic cells: A closer look at fibroblastic reticular cells found within lymph node T zones. Immunol Lett. 2011;138:9–11. doi: 10.1016/j.imlet.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013;24:269–283. doi: 10.1016/j.cytogfr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, et al. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Lacotte S, Decossas M, Le Coz C, Brun S, Muller S, Dumortier H. Early differentiated CD138(high) MHCII+ IgG+ plasma cells express CXCR3 and localize into inflamed kidneys of lupus mice. PLoS One. 2013;8:e58140. doi: 10.1371/journal.pone.0058140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smrcka AV, Lehmann DM, Dessal AL. G protein βγ subunits as targets for small molecule therapeutic development. Comb Chem High Throughput Screen. 2008;11:382–395. doi: 10.2174/138620708784534761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11 doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.