Abstract
Purpose
To evaluate tonometric outcomes of patients with primary angle closure glaucoma (PACG) who have undergone trabeculectomy with mitomycin C (MMC) with and without concurrent phacoemulsification and to identify risk factors for post-operative failure.
Patients and Methods
Retrospective cohort study of 44 eyes of 33 phakic patients who underwent trabeculectomy with MMC with or without combined phacoemulsification for PACG. The primary endpoint was qualified tonometric success at 12 months according to predefined criteria. LogMAR visual acuity, number of glaucoma medications, and postoperative complications were also evaluated. Cox proportional hazard regression analysis was performed to identify potential risk factors for trabeculectomy failure.
Results
Mean intraocular pressure (IOP) decreased from 21.3±7.9 mmHg to 12.2±3.9 mmHg at 12 months (p<0.001) in all patients. A significant reduction in mean number of glaucoma medications (p<0.001) was also seen. There was no change in logMAR visual acuity (p=0.39) after 12 months. There were no significant intergroup differences in mean IOP (p=0.42), number of glaucoma medications (p=0.85), or logMAR visual acuity (p=0.42) between the trabeculectomy versus combined surgery groups after 12 months. Increased age, greater baseline IOP, limbus-based conjunctival flaps, and MMC duration >1 minute were associated with decreased risk of surgical failure. Concurrent phacoemulsifcation at the time of trabeculectomy did not alter tonometric success or rate of complications.
Conclusions
In phakic patients with PACG, trabeculectomy with MMC significantly reduces IOP and number of glaucoma medications at 12 months without change in visual acuity. However, success rates are modest when based on more demanding tonometric criteria.
Keywords: Primary angle closure glaucoma, trabeculectomy, intraocular pressure, cataract
INTRODUCTION
Trabeculectomy remains the established gold-standard primary procedure for the treatment of medically refractory glaucoma in most cases.1 Studies comparing initial treatment with trabeculectomy versus medical therapy have demonstrated that trabeculectomy is more successful in achieving lower intraocular pressures (IOP).2,3 Furthermore, trabeculectomy has the added advantage of stabilizing IOP by minimizing diurnal fluctuation and decreasing dependence on patient compliance with medications.4,5
Previous studies have shown successful long-term outcomes and IOP reduction after trabeculectomy in the setting of open angle glaucoma.6,7 However, angle closure glaucoma (ACG) presents unique management challenges compared to primary open angle glaucoma (POAG). Unlike POAG, appositional contact at the iridocorneal angle in primary angle closure glaucoma (PACG) is believed to cause the formation of peripheral anterior synechiae, which subsequently leads to structural damage and dysfunction of the trabecular meshwork. This process causes a rise in IOP, which ultimately leads to the development of glaucomatous optic neuropathy.
As a result of the anatomic abnormalities that accompany ACG, there is an increased risk of severe post-operative complications, like aqueous misdirection, following filtration surgery in these patients.8,9 However, few studies have compared the long-term outcomes and complications associated with primary trabeculectomy with the effects of combined phacoemulsification and trabeculectomy in the setting of ACG.10–13 In addition, previous studies comparing these two procedures in ACG patients have been limited exclusively to East Asian populations and risk factor analyses for surgical failure have not been reported in these particular studies. The purpose of this study is to evaluate the tonometric outcomes and risk factors for failure of trabeculectomy with mitomycin C (MMC) or combined phacoemulsification with trabeculectomy (phaco/trab) in PACG with clinically relevant criteria to determine success rates across different ranges of IOP.
MATERIALS AND METHODS
This is a retrospective cohort study of adult patients who underwent trabeculectomy alone (trab) or combined phacoemulsification and trabeculectomy (phaco/trab) with adjunctive MMC between March 2000 and February 2013 for PACG. The study protocol was approved by the Human Subject Protection Committee and Institutional Review Board of the University of California Los Angeles, USA, and all study procedures adhered to the tenants of the Declaration of Helsinki. Data were obtained from a chart review of 44 eyes from 33 patients diagnosed with medically uncontrolled PACG as determined by clinical examination by a glaucoma specialist (JC, ALC, JAG, SKL) and subsequently underwent glaucoma filtration surgery. Eligibility criteria included age ≥ 40 years at the time of surgery, phakic lens status, and a diagnosis of PACG. Patients with a history of previous intraocular surgery or co-existing retinal or neurologic disease known to effect visual fields or optic nerve structure were excluded.
PACG was defined as the presence of glaucomatous optic neuropathy and a nerve fiber layer-related visual field deficit in the setting of ≥ 2 quadrants of synechial and/or appositional angle closure occluding the pigmented trabecular meshwork on gonioscopy. All gonioscopy was performed using a 4-mirror indirect gonioscopy lens and notated using the Spaeth Gonioscopic Grading System.14 The presence of medically uncontrolled glaucoma was determined by IOP ≥ 21 mmHg despite the use of topical glaucoma medications, evidence of visual field progression despite IOP < 21 mmHg, or the need for 3 or more glaucoma medications for intraocular pressure control. The decision to perform cataract surgery at the time of trabeculectomy was surgeon-specific but in general, phaco/trab was recommended when Snellen BCVA was 20/40 or worse or in the presence of self-reported difficulties with activities of daily living, such as reading and driving, due to visual impairment.
The following baseline data were collected from the medical records of eligible patients: race, sex, age at the time of surgery, eye laterality, preoperative best-corrected visual acuity (BCVA), refractive error, preoperative IOP, preoperative number of glaucoma medications, concurrent ocular conditions before or at the time of surgery, history of intraocular or extraocular surgery, previous laser surgery for glaucoma, date of surgery, type of conjunctival flap, concentration and duration of mitomycin C, and intraoperative complications. Postoperative data including BCVA, IOP, number of glaucoma medications, complications, suture lysis, and additional interventions (such as 5-Fluoruracil injection) or surgery were also noted. All IOP measurements were recorded using Goldmann applanation tonometry.
Surgical Technique
All surgery was performed by an attending glaucoma surgeon at the UCLA Stein Eye Institute (JC, ALC, JAG, SKL) following either a retrobulbar or peribulbar injection of 1% lidocaine for anesthesia. For limbus-based trabeculectomy, a limbal-based conjunctival peritomy was performed approximately 10 mm posterior to the limbus. After blunt dissection of the conjunctiva and Tenon’s capsule anteriorly, a thin cellulose sponge soaked in MMC (0.2 to 0.5 mg/ml) was placed over the intended site of the scleral flap for 1 to 3 minutes depending on the surgeon’s preference. A partial-thickness scleral flap was then dissected into clear cornea, and a sclerotomy was created followed by a basal iridectomy. The scleral flap was closed with 10-0 nylon sutures and the overlying Tenon’s capsule and conjunctiva closed with a running 9-0 polyglactin suture.
For fornix-based trabeculectomy, a limbal peritomy centered at the 12 o’clock position was created. The conjunctiva and Tenon’s capsule were dissected using Westcott scissors and a MMC-soaked cellulose sponge applied as determined by surgeon preference as above. A partial-thickness scleral flap was created followed by a sclerotomy and iridectomy in the same fashion as the limbus-based trabeculectomy. The scleral flap was closed using 10-0 nylon sutures, and conjunctiva and Tenon’s capsule were closed using two 9-0 or 10-0 polyglactin wing sutures at the limbus.
When performed in combination with cataract surgery, phacoemulsification with a temporal clear-corneal incision was performed prior to trabeculectomy. A 10-0 nylon suture was placed to close the clear-corneal wound prior to initiating the trabeculectomy. A posterior chamber intraocular lens was inserted in the capsular bag in all patients included in the study.
Post-operative care for all patients involved a topical antibiotic four times daily for one week and a topical corticosteroid four times daily, which was then tapered over a period of 6 to 8 weeks. Argon laser suture lysis was performed when the surgeon believed that filtration into the trabeculectomy bleb was inadequate or the IOP too high to achieve a successful outcome.
Definitions of Success
Four different criteria were established to define the primary outcome of qualified tonometric success with or without glaucoma medications (Table 1). For all criteria, final IOP was required to be less than or equal to baseline pre-operative IOP.
Table 1.
Criteria for Tonometric Success.
| Criteria for Success | Definition |
|---|---|
| Criteria A | Final IOP ≤ 21 mmHg and ≥ 15% IOP reduction from baseline |
| Criteria B | Final IOP ≤ 18 mmHg and ≥ 20% IOP reduction from baseline |
| Criteria C | Final IOP ≤ 15 mmHg and ≥ 25% IOP reduction from baseline |
| Criteria D | Final IOP ≤ 12 mmHg and ≥ 30% IOP reduction from baseline |
IOP, intraocular pressure.
Secondary outcomes measures were mean IOP, BCVA, number of glaucoma medications required, postoperative complications, and need for further surgical intervention. All visual acuities were converted to logMAR for the purpose of our analysis.15
Statistical Analysis
Pre-operative and post-operative values of IOP, logMAR BCVA, and number of glaucoma medications required were compared using a Wilcoxon signed-rank test. Intergroup comparisons were performed using the Wilcoxon rank-sum test, Fisher exact test, or Chi-square test. Kaplan-Meier survival analyses were plotted and analyzed with a log-rank test to compare long-term tonometric outcomes between groups using R (version 3.0.3, R Development Core Team, Vienna, Austria). Stata (version 12.1, Stata Corporation, College Station, TX, USA) was used to perform Cox’s proportional hazard regression analysis to determine predictive factors for surgical failure. Potential predictors analyzed in the model included age, gender, baseline IOP, number of pre-operative glaucoma medications, pre-operative laser procedures (argon or selective laser trabeculoplasty, or laser iridotomy), type of conjunctival flap (fornix versus limbus based), duration of MMC application, concurrent cataract surgery at the time of trabeculectomy, and performance of argon laser suture lysis or 5-Fluorouracil injection post-operatively. A p-value <0.05 was considered statistically significant for all tests.
RESULTS
Demographic and Clinical Characteristics
Between March 2000 and February 2013, 131 eyes underwent trabeculectomy with MMC for ACG. Of these, 87 were excluded: 16 eyes had a history of a prior trabeculectomy, 5 had a diagnosis of secondary angle closure glaucoma, 1 had prior retinal detachment and repair, and the remaining 65 eyes did not have the necessary 12 months of post-operative follow-up. Baseline demographic characteristics of the final study group are summarized in Table 2. The phaco/trab group was significantly older than the trab group (p=0.004). There were no other significant baseline differences between the trab (n=29 eyes) and phaco/trab (n=15 eyes) groups.
Table 2.
Baseline Demographic Characteristics of Study Patients.
| Variable | All Patients | Trab Only | Phaco/Trab | P-value* |
|---|---|---|---|---|
| Number of eyes (patients) | 44 (33) | 29 (20) | 15 (13) | |
| Age (years) | 0.004 | |||
| • Mean (± SD) | 67.7 (±10.2) | 64.3 (±8.9) | 74.3 (±9.7) | |
| • Range | 44.1–84.9 | 44.1–84.5 | 56.5–84.9 | |
| • Median | 66 | 65 | 77 | |
| Gender (n) | 0.92 | |||
| • Male | 14 (31.8%) | 10 (34.5%) | 4 (26.7%) | |
| • Female | 30 (68.1%) | 19 (65.5%) | 11 (73.3%) | |
| Ethnicity (n) | 0.44 | |||
| • Asian | 11 (25.0%) | 6 (20.7%) | 5 (33.3%) | |
| • Black | 9 (20.5%) | 6 (20.7%) | 3 (20.0%) | |
| • Hispanic | 4 (9.1%) | 4 (13.8%) | 0 (0%) | |
| • White | 20 (45.5%) | 13 (44.8%) | 7 (46.7%) | |
| Eye (n) | 0.81 | |||
| • Right | 18 (40.9%) | 17 (58.6%) | 9 (60.0%) | |
| • Left | 26 (59.1%) | 12 (41.4%) | 6 (40.0%) | |
| Visual Acuity (logMAR) | 0.05 | |||
| • Mean (± SD) | 0.29 (±0.37) | 0.21 (±0.27) | 0.44 (±0.49) | |
| • Range | 0.0–2.0 | 0.0–1.3 | 0.0–2.0 | |
| Preoperative IOP (mmHg) | 0.39 | |||
| • Mean (± SD) | 21.3 (±7.9) | 22.0 (±7.7) | 19.8 (±8.3) | |
| • Range | 10–46 | 10–42 | 11.5–46 | |
| Preoperative Medications (n) | 0.05 | |||
| • Mean (± SD) | 3.1 (±1.0) | 3.3 (±0.8) | 2.7 (±1.2) | |
| • Range | 0–5 | 2–5 | 0–4 | |
| Previous Laser Procedures (n) | 0.19 | |||
| • ALT | 6 (13.6%) | 5 (17.2%) | 1 (6.7%) | |
| • SLT | 2 (4.6%) | 2 (6.9%) | 0 (0.0%) | |
| • LPI | 23 (52.3%) | 16 (55.2%) | 7 (46.7%) | |
| Conjunctival Flap (n) | 0.51 | |||
| • Limbus-based | 23 (52.3%) | 18 (62.1%) | 7 (46.7%) | |
| • Fornix-based | 21 (47.7%) | 11 (37.9%) | 8 (53.3%) | |
| Dose of MMC | 0.21 | |||
| • 0.2 mg/ml | 2 (4.5%) | 2 (6.9%) | 0 (0.0%) | |
| • 0.25 mg/ml | 4 (9.1%) | 4 (13.8%) | 0 (0.0%) | |
| • 0.3 mg/ml | 36 (81.8%) | 22 (75.9%) | 14 (93.3%) | |
| • 0.4 mg/ml | 1 (2.3%) | 1 (3.4%) | 0 (0.0%) | |
| • 0.5 mg/ml | 1 (2.3%) | 0 (0.0%) | 1 (6.7%) | |
| Duration of MMC (minutes) | 0.54 | |||
| • Mean (± SD ) | 1.2 (±0.5) | 1.2 (±0.6) | 1.1 (±0.2) | |
| • Range | 1–3 | 1–3 | 1–1.5 |
SD, standard deviation; ALT, argon laser trabeculoplasty; SLT, selective laser trabeculoplasty; LPI, laser peripheral iridotomy.
P-value comparing trab only versus phaco/trab groups. Statistically significant p-values (<0.05) are shown in bold.
Intraocular Pressure Control
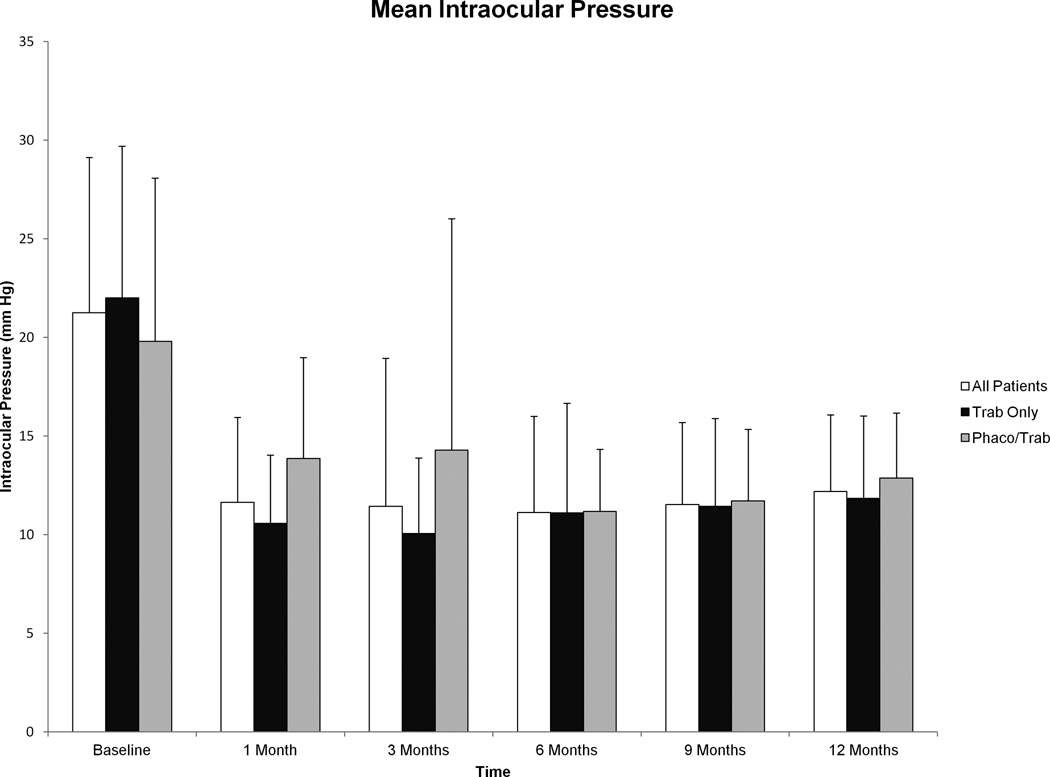
Mean IOP for all patients prior to surgery was 21.3±7.9 mmHg, which subsequently decreased after surgery to 12.2±3.9 mmHg at 12 months (p<0.001). Interval IOP data are shown graphically in Figure 1, and a significant reduction in IOP was seen post-operatively (p<0.001 at all time points for all groups compared to baseline). There were no significant differences in post-operative IOP between the trab and phaco/trab groups (Table 3).
Figure 1.
Mean intraocular pressure (IOP) before and after trabeculectomy with mitomycin C from baseline to 12 months. Post-operative reduction in IOP compared to baseline was significant for all groups at all time points (p<0.001 at all time points for all groups). Error bars represent standard deviation.
TABLE 3.
Post-Operative Outcomes of Trab versus Phaco/Trab Groups at 6 and 12 Months.
| Trab (n=29) |
Phaco/Trab (n=15) |
P-Value | |
|---|---|---|---|
| Mean IOP at 6 Months (mmHg) | 11.10±5.57 | 11.18±3.15 | 0.96 |
| Mean IOP at 12 Months (mmHg) | 11.85±4.17 | 12.87±3.30 | 0.42 |
| Mean LogMAR BCVA at 6 Months | 0.20±0.23 (Snellen 20/32) |
0.36±0.42 (Snellen 20/46) |
0.11 |
| Mean LogMAR BCVA at 12 Months | 0.20±0.23 (Snellen 20/32) |
0.27±0.34 (Snellen 20/37) |
0.42 |
| Mean Number of Glaucoma Medications at 6 Months | 0.28±0.75 | 0.07±0.27 | 0.30 |
| Mean Number of Glaucoma Medications at 12 Months | 0.35±0.86 | 0.40±0.74 | 0.85 |
All values are shown as mean ± standard deviation. Trab, trabeculectomy; Phaco/trab, combined phacoemulsification-trabeculectomy; IOP, intraocular pressure; BCVA, best corrected visual acuity.
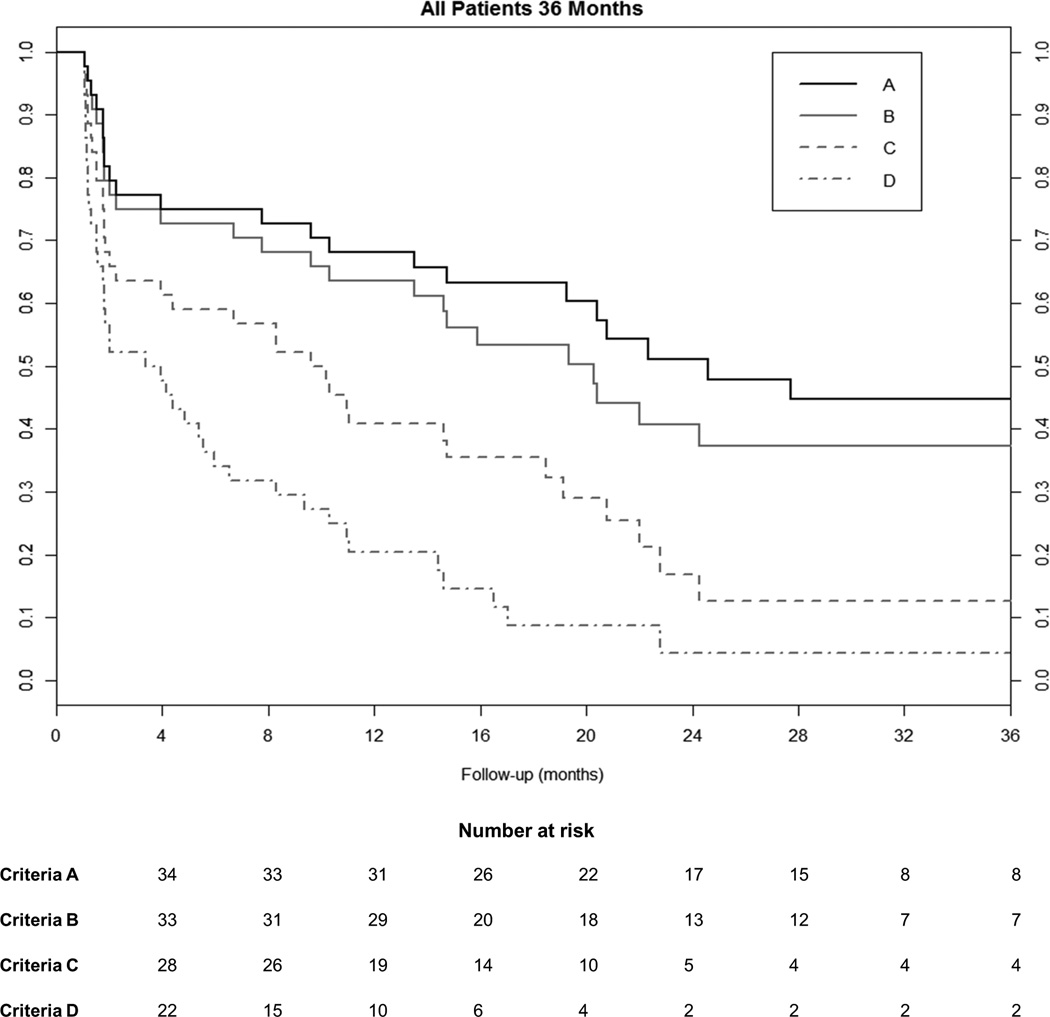
Figure 2 shows the results of Kaplan-Meier survival analysis for all patients according to criteria A-D. For criteria A, the overall success rate (± standard error) at 6 months and 12 months following surgery were 72.7%±6.7% and 65.7%±7.2%, respectively. For criteria B, the respective success rates were 70.5%±6.9% and 61.1%±7.4%. For criteria C, the success rates at 6 months and 12 months were 56.8%±7.5% and 38.2%±7.4%, respectively. For criteria D, the corresponding success rates were 31.8%±7.0% and 17.5%±5.9%, respectively. There were no significant differences in the cumulative probability of tonometric success between the trab versus phaco/trab groups (criteria A, p=0.41; criteria B, p=0.39; criteria C, p=0.24; and criteria D, p=0.30).
Figure 2.
Kaplan-Meier survival analysis showing success according to criteria A, B, C, and D for all subjects who underwent trabeculectomy with or without combined cataract surgery. Number of eyes at risk is shown for each of the 4 criteria used to define tonometric success at the appropriate corresponding time points.
Number of Glaucoma Medications
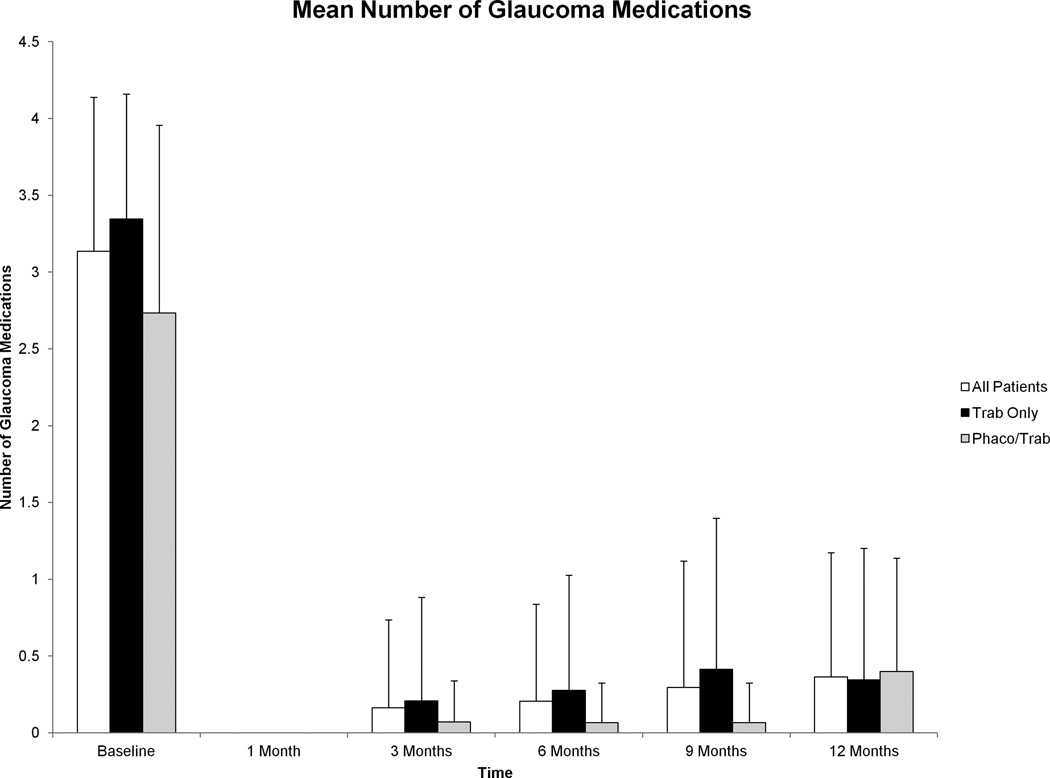
Mean number of glaucoma medications for all patients decreased from 3.1±1.00 at baseline to 0.4±0.81 at 12 months (p<0.001). This reduction in glaucoma medications from baseline was significant at each time point post-operatively (p<0.001 at all time points for all groups) and these data are shown graphically in Figure 3. There were no significant differences in the number of glaucoma medications used post-operatively between groups (Table 3).
Figure 3.
Mean number of glaucoma medications before and after trabeculectomy with mitomycin C from baseline to 12 months. Reduction in mean number of glaucoma medications compared with baseline was significant at all visits for all groups after surgery (p<0.001 at all time points for all groups). Error bars represent standard deviation.
Visual Acuity
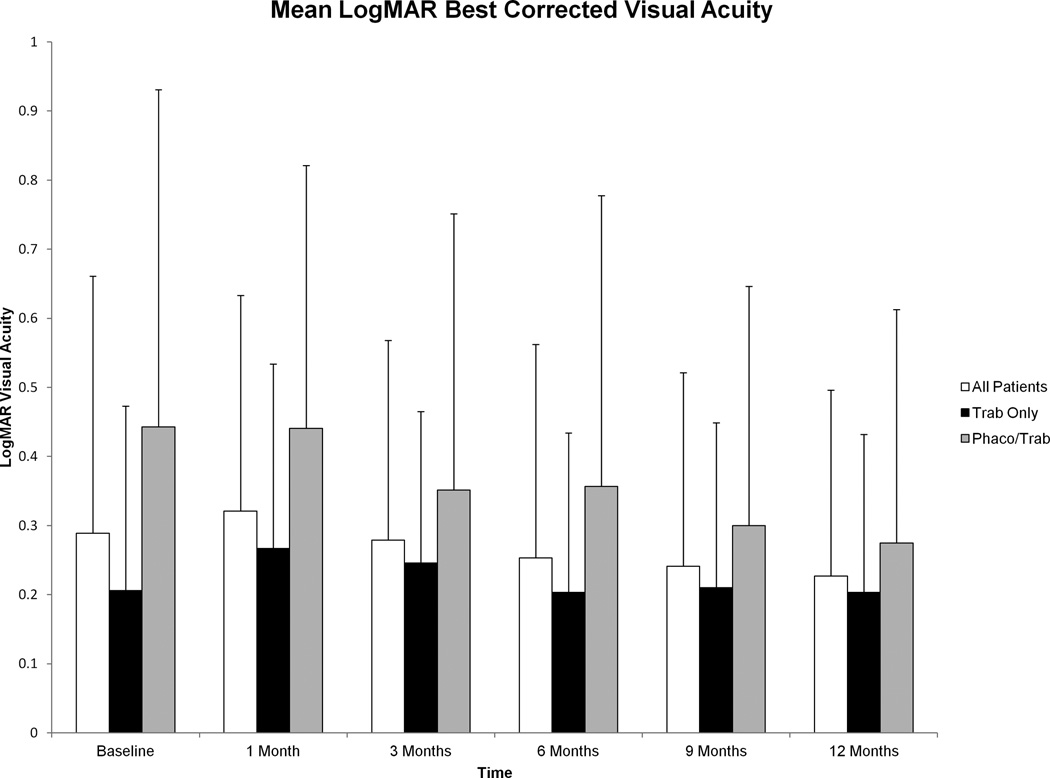
Mean logMAR BCVA for all patients at baseline was 0.29±0.37 (Snellen 20/39) at baseline and 0.23±0.27 (Snellen 20/34) at 12 months. This difference was not statistically significant (p=0.39). These data are summarized in Figure 4.
Figure 4.
Mean logMAR best corrected visual acuity (BCVA) before and after trabeculectomy with mitomycin C from baseline to 12 months. No significant differences were seen in logMAR BCVA from baseline to 12 months after surgery in a comprehensive analysis of all patients (p=0.39) or in a stratified analysis of the trab subgroup (p=0.78). A statistically significant improvement in logMAR BCVA was seen in the phaco/trab group at 12 months compared to baseline (p=0.04). Error bars represent standard deviation.
Baseline mean logMAR BCVA was 0.21±0.27 (Snellen 20/32) in the trab group and 0.44±0.49 (Snellen 20/55) in the phaco/trab group. This difference between groups approached statistical significance (p=0.05).
Subgroup analysis of those 15 patients who underwent phaco/trab showed a statistically significant improvement in logMAR BCVA at 12 months post-operatively (0.27±0.34, Snellen 20/37; p=0.04). In contrast, there was no statistically significant difference in logMAR BCVA at 12 months post-operatively in the trab group (0.20±0.23, Snellen 20/32) compared to baseline (p=0.99). There were no significant intergroup differences in post-operative BCVA at 6 and 12 months (Table 3).
Surgical Complications
There were no intraoperative complications in the study sample. Four eyes (13.8%) in the trab group and 2 eyes (13.3%) in the phaco/trab group experienced a post-operative complication within 12 months of surgery (p=0.84). These findings are described in Table 4. One eye of the same patient in the trab group developed endophthalmitis at post-operative month 7 followed by late cystoid macular edema at post-operative month 9. There were no cases of aqueous misdirection.
Table 4.
Post-Operative Complications within 12 Months of Surgery.
| Complication | Number of Complications (%) | |
|---|---|---|
| Trab (n=29) | Phaco/Trab (n=15) | |
| Hyphema | 2 (6.9%) | 0 (0.0%) |
| Cystoid Macular Edema | 1 (3.4%)† | 1 (6.7%) |
| Choroidal Effusion | 0 (0.0%) | 1 (6.7%)* |
| Bleb Leak | 1 (3.4%) | 0 (0.0%) |
| Endophthalmitis | 1 (3.4%)† | 0 (0.0%) |
Required secondary surgical intervention.
Occurred in the same eye of the same patient.
Predictive Factors for Failure
Cox proportional hazard ratio was used to identify potential risk factors for surgical failure. For criteria A and B, increasing age was found to be protective with a hazard ratio (HR) of 0.92 and p=0.03 for every 1 year of age by both criteria. Increasing baseline IOP was also protective for criteria A and B (HR 0.86, p=0.02 for criteria A and HR 0.87, p=0.01 for criteria B) for every 1 mmHg increase in IOP. Fornix-based conjunctival flaps were a significant risk factor for failure for both criteria A (HR 5.26, p=0.02) and criteria B (HR 3.78, p=0.04). There were no significant predictive factors for criteria C. For criteria D, duration of MMC for greater than 1 minute was associated with a decreased risk of failure (HR 0.19, p=0.04). Neither MMC concentration or concurrent cataract surgery at the time of trabeculectomy significantly affected tonometric failure.
DISCUSSION
In this retrospective cohort study of PACG patients, overall success rates of trabeculectomy with MMC ranged from 31.8% to 72.7% at 6 months and 17.5% to 65.7% at 12 months, depending on the tonometric criteria used. Previously, we showed that trabeculectomy with MMC for POAG effectively reduces IOP in both phakic and pseudophakic eyes with about half of all patients maintaining therapeutic IOP levels after 36 months without the need for additional surgical intervention.6,7 In the current study however, over 50% of trabeculectomies failed using these same tonometric criteria by 24 months post-operatively (criteria A) and overall success with the most stringent criteria (criteria D) was only 4% at the same time point.
Few studies have compared the long-term outcomes of trabeculectomy with MMC with and without simultaneous lens extraction in the setting of ACG.10–12 Findings from these studies report success rates ranging from 54 to 78% for trabeculectomy in ACG and 56 to 88% for phacotrabeculectomy at follow-up intervals of 10 months to 3 years. A large retrospective study by Hong et al. reported overall success rates of 61% for trabeculectomy and 73% for phacotrabeculectomy at 15 years in a Korean patient population, but this study included cases of both POAG and PACG.13 We found more modest success rates of trabeculectomy in ACG patients, and it is feasible that this may be due in part to racial differences in our cohort compared to these previous studies which were carried out exclusively in East Asian patients. Similar to these studies however, we found that concurrent phacoemulsification at the time of trabeculectomy did not have a significant effect on tonometric outcomes. Lens extraction has been reported to be effective in lowering intraocular pressure in PACG and has even been advocated as an alternative to filtration surgery in select cases as a result.16–19 While studies comparing phaco/trab versus trabeculectomy in POAG have shown a tendency toward better IOP control when performing trabeculectomy alone, it is unclear if these results hold true in PACG where the anatomical benefit of lens extraction may offset any adverse effects of intraocular inflammation on bleb function induced from cataract surgery.20,21 Cox hazard regression analysis found no benefit of concurrent lens extraction on long-term IOP control after trabeculectomy in PACG patients and there was also no difference in cumulative tonometric success in our subgroup analysis. As a result, any anatomical benefit achieved by lens extraction is unlikely to offset the deleterious effects of combination surgery on long-term bleb function.
Older age was a protective factor in our analysis, which is consistent with findings from the Advanced Glaucoma Intervention Study.22 Duration of MMC for greater than one minute at the time of surgery was also associated with a decreased risk of failure . While the use of anti-fibrotic agents to improve tonometric outcomes is well-established,23,24 one study found that duration of MMC exposure appeared to be more important than concentration in affecting long-term outcomes.25 Elevated baseline IOP was also associated with a lower risk of failure, and we postulate that the creation of a fistula results in a greater reduction in IOP in these patients who likely have more significant impairment of physiologic trabecular outflow than those with lower baseline IOP. Fornix-based trabeculectomies were associated with a greater risk of failure in our analysis, and while a common concern of fornix-based trabeculectomies is their greater propensity to develop bleb leaks in the early post-operative period,26 only one (4.8%) of the 21 eyes that underwent a fornix-based trabeculectomy in our study experienced this complication, which resolved spontaneously.
Limitations of the current study include the retrospective nature of our analysis as well as the significant loss of patients who but did not meet the minimum required 12 months of follow-up (65 eyes). The high loss to follow-up inherently introduces bias into the analysis as it is possible that the subjects lost may have had different outcomes from those who continued to return for follow-up. However, it should be noted that all patients in our cohort underwent surgery at a tertiary care academic referral center and many return to their referring physicians for follow-up care after surgery. The retrospective nature of this study also limits our ability to control for the inherent variability that is introduced by differences among surgeons in surgical technique as well as subjective differences in post-operative management, including secondary interventions such as injection of 5-Fluoruracil. In addition, the small sample size of the two subgroups in our study is admittedly a limiting factor in performing a more robust comparison of outcomes between trabeculectomy alone versus combined phaco/trab.
Six eyes (13.6%) experienced a post-operative complication during the study period. While cataract progression is a concern following intraocular surgery, our study found no significant change in BCVA at 12 months after trabeculectomy alone. As expected, subgroup analysis showed a statistically significant improvement in BCVA at 12 months from baseline in those patients who underwent combined phaco/trab. However, final BCVA at 12 months was similar in those patients undergoing trabeculectomy alone versus phaco/trab surgery. Based on these data, it is reasonable to consider trabeculectomy alone in phakic patients with little concern for significant media-related visual deterioration over the first 12 months after surgery.
This retrospective cohort demonstrates that trabeculectomy with MMC achieves reasonable success rates with an acceptable complication rate in patients with PACG. As in other studies, increased age and duration of MMC were associated with a decreased risk of failure. Concurrent cataract surgery at the time of trabeculectomy was not associated with a difference in IOP outcomes or rate of complications after 12 months. These data do suggest, however, that trabeculectomy outcomes in PACG are less successful than for POAG. Further investigation is required to better evaluate those factors which may affect surgical success and to enhance methods for improving long-term visual and tonometric stability in this challenging patient population.
Supplementary Material
Acknowledgments
The authors would like to thank Fei Yu (Department of Biostatistics, UCLA) for his assistance with statistical analysis. This research was supported by an unrestricted departmental grant from Research to Prevent Blindness, Inc. The funding organization had no role in the design or conduct of the study. Brian J. Song currently receives grant support through an institutional K12 clinician-scientist award, the Harvard-Vision Clinical Scientist Development Program through the National Institute of Health and the National Eye Institute (5K12EY016335). Simon K. Law has received lecture fees from Allergan Inc. JoAnn Giaconi is a consultant for Allergan Inc. Anne L. Coleman is a consultant for Allergan Inc. and Reichert Inc. Joseph Caprioli is a consultant for Allergan Inc. and has received grant support from Alcon Inc., Allergan Inc., and New World Medical Inc.
Footnotes
Meera Ramanathan and Esteban Morales have no financial disclosures.
REFERENCES
- 1.Schmier JK, Covert DW, Lau EC, et al. Trends in annual Medicare expenditures for glaucoma surgical procedures from 1997 to 2006. Arch Ophthalmol. 2009;127:900–905. doi: 10.1001/archophthalmol.2009.122. [DOI] [PubMed] [Google Scholar]
- 2.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 3.Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology. 1994;101:1651–1656. doi: 10.1016/s0161-6420(94)31120-1. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz K, Budenz D. Current management of glaucoma. Curr Opin Ophthalmol. 2004;15:119–126. doi: 10.1097/00055735-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Wilensky JT, Zeimer RC, Gieser DK, et al. The effects of glaucoma filtering surgery on the variability of diurnal intraocular pressure. Trans Am Ophthalmol Soc. 1994;92:377–381. discussion 381–383. [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana H, Nouri-Mahdavi K, Lumba J, et al. Trabeculectomy with Mitomycin C: outcomes and risk factors for failure in phakic open-angle glaucoma. Ophthalmology. 2006;113:930–936. doi: 10.1016/j.ophtha.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 7.Fontana H, Nouri-Mahdavi K, Caprioli J. Trabeculectomy with mitomycin C in pseudophakic patients with open-angle glaucoma: outcomes and risk factors for failure. Am J Ophthalmol. 2006;141:652–659. doi: 10.1016/j.ajo.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Katz LJ, Costa VP, Spaeth GL. Filtration surgery. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. 2nd. St. Louis: CV Mosby; 1996. pp. 1661–1702. [Google Scholar]
- 9.Greenfield DS, Tello C, Budenz DL, et al. Aqueous misdirection after glaucoma drainage device implantation. Ophthalmology. 1999;106:1035–1040. doi: 10.1016/S0161-6420(99)00530-8. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Fang M, Bai YJ, et al. Comparison of combined phacotrabeculectomy with trabeculectomy only in the treatment of primary angle-closure glaucoma. Chin Med J. 2012;125:1429–1433. [PubMed] [Google Scholar]
- 11.Tsai HY, Liu CJ, Cheng CY. Combined trabeculectomy and cataract extraction versus trabeculectomy alone in primary angle-closure glaucoma. Br J Ophthalmol. 2009;93:943–948. doi: 10.1136/bjo.2008.151803. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Teng L, Li A, Du S, Zhu Y, Ge J. The clinical outcomes of three surgical managements on primary angle-closure glaucoma. Yan Ke Xue Bao. 2007;23:65–74. [PubMed] [Google Scholar]
- 13.Hong S, Park K, Ha SJ, et al. Long-term intraocular pressure control of trabeculectomy and triple procedure in primary open angle glaucoma and primary angle closure glaucoma. Ophthalmologica. 2007;221:395–401. doi: 10.1159/000107499. [DOI] [PubMed] [Google Scholar]
- 14.Spaeth GL. The normal development of the human anterior chamber angle: a new system of descriptive grading. Trans Ophthalmol Soc UK. 1971;91:709–739. [PubMed] [Google Scholar]
- 15.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–391. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 16.Tham CC, Kwong YY, Baig N, et al. Phacoemulsification versus trabeculectomy in medically uncontrolled chronic angle-closure glaucoma without cataract. Ophthalmology. 2013;120:62–67. doi: 10.1016/j.ophtha.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Gunning FP, Greve EL. Lens extraction for uncontrolled angle-closure glaucoma: long-term follow-up. J Cataract Refract Surg. 1998;24:1347–1356. doi: 10.1016/s0886-3350(98)80227-7. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, Hayashi H, Nakao F, et al. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg. 2001;27:1779–1786. doi: 10.1016/s0886-3350(01)01036-7. [DOI] [PubMed] [Google Scholar]
- 19.Lai JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction by phacoemulsification in eyes with primary angle closure glaucoma (PACG) and co-existing cataract: a prospective case series. J Glaucoma. 2006;15:47–52. doi: 10.1097/01.ijg.0000196619.34368.0a. [DOI] [PubMed] [Google Scholar]
- 20.Kleinmann G, Katz H, Pollack A, et al. Comparison of trabeculectomy with mitomycin C with or without phacoemulsification and lens implant. Ophthalmic Surg Lasers. 2002;33:102–108. [PubMed] [Google Scholar]
- 21.Noben KJ, Linsen MC, Zeyen TG. Is combined phacoemulsification and trabeculectomy as effective as trabeculectomy alone? Bull Soc Belge Ophthalmol. 1998;270:85–90. [PubMed] [Google Scholar]
- 22.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 11. Risk factors for failure of trabeculectomy and argon laser trabeculoplasty. Am J Ophthalmol. 2002;134:481–498. doi: 10.1016/s0002-9394(02)01658-6. [DOI] [PubMed] [Google Scholar]
- 23.Singh K, Mehta K, Shaikj NM, et al. Trabeculectomy with intraoperative mitomycin C versus 5-fluorouracil. Prospective randomized clinical trial. Ophthalmology. 2000;107:2305–2309. doi: 10.1016/s0161-6420(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 24.Chen CW, Huang HT, Bair JS, et al. Trabeculectomy with topical application of mitomycin-C in refractory glaucoma. J Ocul Pharmacol. 1990;6:175–182. doi: 10.1089/jop.1990.6.175. [DOI] [PubMed] [Google Scholar]
- 25.Robin AL, Ramakrishnan R, Krishnadas R, et al. A long-term dose-response study of mitomycin in glaucoma filtration surgery. Arch Ophthalmol. 1997;115:969–974. doi: 10.1001/archopht.1997.01100160139001. [DOI] [PubMed] [Google Scholar]
- 26.Solus JF, Jampel HD, Tracey PA, et al. Comparison of limbus-based and fornix-based trabeculectomy: success, bleb-related complications, and bleb morphology. Ophthalmology. 2012;119:703–711. doi: 10.1016/j.ophtha.2011.09.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.