ABSTRACT
Extremity amputation or traumatic injury can often lead to the formation of heterotopic ossification (HO). Studies to induce HO in rat muscle using cell‐based gene therapy show that this process appears to be location dependent. In the present study, HO was induced in mice and rats through injection of immunologically matched cells transduced with either a replication‐defective adenovirus possessing bone morphogenetic protein 2 (BMP2) or an empty adenovirus vector (control). Injection in rat near the skeletal bone resulted in HO, whereas cells injected into the same muscle group but distal from the bone did not result in bone formation. When cells were injected in the same limb at both locations at the same time, HO was formed at both sites. Characterization of the bone formation in rats versus mice demonstrated that different sources of osteogenic progenitors were involved, which may account for the location dependent bone formation observed in the rat. Further experimentation has shown that a potential reason for this difference may be the inability of rat to activate matrix metalloproteinase 9 (MMP9), an essential protease in mice necessary for recruitment of progenitors. Inhibition of active MMP9 in mice led to a significant decrease in HO. The studies reported here provide insight into the mechanisms and pathways leading to bone formation in different animals and species. It appears that not all animal models are appropriate for testing HO therapies, and our studies also challenge the conventional wisdom that larger animal models are better for testing treatments affecting bone. © 2016 The Authors. Journal of Orthopaedic Research published by Wiley Periodicals, Inc. on behalf of the Orthopaedic Research Society. J Orthop Res 34:1894–1904, 2016.
Keywords: Heterotopic ossification, BMP2, matrix metalloproteinase‐9
Heterotopic ossification (HO) is the formation of bone in the soft tissue outside the skeletal bone. HO is induced by traumatic injuries or musculoskeletal diseases, including spinal cord and brain trauma, fractures, muscle contusions, lower motor neuron disorders, genetic disorders, and joint arthroplasty.1, 2, 3, 4 HO may occur proximal to the site of trauma; as seen in amputations. However, HO occasionally forms remotely from the site of injury. Cutaneous burns typically incite HO that is distant from the site of the burn, suggesting that the burn increases osteogenic potential.5, 6
There are two main approaches to induce HO in a rat model. One way to induce HO is by bone morphogenetic protein 2 (BMP2).7, 8 HO can also be induced in vivo with an Achilles tenotomy.9 Recombinant human bone morphogenetic protein 2 (rhBMP2) serves as an alternative to autologous bone graft because it avoids using a donor, and it avoids blood loss and operative time that a bone graft requires.10 The use of rhBMP2 is also problematic, in that it requires large doses of the recombinant protein well above the endogenous levels of the protein, which can lead to deleterious effects. Also the use of rhBMP2 requires the inclusion of a foreign carrier and the resultant bone formation is often highly variable.11 However, new approaches to deliver the rhBMP2 are being tested to limit any possible complications.11, 12 In a rat model described here, cells were transduced with a replication‐defective adenovirus encoding for hBMP2 are injected into the soft tissue to form HO.
The results reveal that mechanisms of HO in the rat do not parallel those in the mouse. In the rat, when AdBMP2 transduced cells are injected into skeletal muscle at a location distal to the skeletal bone, de novo bone was not formed reproducibly. For these experiments the locations, proximal, and distal, are with respect to skeletal bone. Alternatively, when injected proximal to the skeletal bone, HO formed consistently without fusing with the adjacent skeletal bone. Only when the transduced cells were injected both proximal and distal, did bone form consistently at the distal site. Our focus was to discover the mechanistic stages where the rat and mouse models vary. Our data indicates that although the progenitors in both animal models appear similar, they are recruited from two different locations. Further, a key protein in recruitment of the mouse progenitors, active matrix metalloproteinase 9 (MMP9), was absent in the rats. Interestingly, total MMP9, both active and inactive forms, is found at significantly elevated levels in both species. This finding prompted us to investigate whether active MMP9 is present in soft tissues associated with HO in humans. The data suggests a potential novel role for active MMP9 in heterotopic ossification.
MATERIALS AND METHODS
Cell Culture
Primary skin fibroblast cell lines were created from skin biospies from a Wistar rat (WSF) and a C57BL/6 mouse (CSF).
BMP2 Delivery In Vivo
Replication defective E1‐E3 deleted first generation human type 5 adenovirus possessing cDNA for BMP2 (AdBMP2) or no transgene (AdEmpty) were constructed as previously described.13 The control vector lacks a transgene cassette in the E1 region (AdEmpty).13 The virus particle (vp) to plaque‐forming unit (pfu) ratios of AdBMP2 and AdEmpty cassette were 1:120 and 1:106, respectively. All viruses proved to be negative for replication‐competent adenovirus.
Cells were then transduced with AdBMP2 or the Adempty control virus at 7,500 vp/cell with 0.75% GeneJammer14 which provides a transduction efficiency of greater than 90%. Where indicated transduced cells were microencapsulated in poly(ethylene glycol) diacrylate (PEGDA) as described.15 In each experiment, BMP2 expression confirmed13 to be 20 ng/ml/5 × 106 cells. Transduced cells were resuspended at a concentration of 1 × 107 or 5 × 106 cells/100ul of PBS and delivered through an intramuscular injection into the hind limb quadriceps muscle of Wistar rats or C57/BL6 mice, respectively. After euthanasia, hind limbs tissues were harvested and either placed in formalin or snap frozen. All animal studies were conducted under an IACUC approved protocol in accordance with OLAW. All animals were housed in ALAACA accredited vivarium under standard conditions as described by OLAW. Animals were randomly selected based on age and health. Experimental groups were selected randomly, and animals coded to avoid bias. Group sizes are based on historical data; however, power analysis was repeated after data collection to ensure appropriate group sizes.
Minocycline Treatment
Drug was given by intraperitoneal injection 24 h prior to the induction of HO with 65 mg/kg minocycline in pharmaceutical grade saline or saline and then twice daily with 45 mg/kg for 6 days. Minocycline suppression of MMP9 was quantified by immunostaining of tissue sections for active, total MMP9, and total cells. Isolated tissues (n = 4) were sectioned throughout the region of new bone formation. Every 5th slide (∼4–5 slides) was immunostained and three microscopic fields per section were counted using standard software. The percentage of cells expressing active MMP9/total MMP9 (pro versus active) and standard error of the mean (SEM) is reported. A student t‐test was used to determine significance between groups with a 95% confidence interval (p < 0.05).
Tissues Processing
Paraffin
Mouse and rat hind limbs were formalin fixed, decalcified before processing. Human tissues were obtained from early heterotopic ossification, from patients undergoing surgeries at Walter Reed National Military Medical Center (WRNMMC), through approved IRB protocols. All human tissue transfers to Baylor College of Medicine (Olmsted‐Davis) from WRNMMC (Forsberg) followed the approved Cooperative Research and Development Agreement (CRADA) between BCM and the Department of the Navy. Tissues were shipped in formalin and processed as previously described.16
Frozen
Soft tissues encompassing the site of new bone formation were isolated from the rear hind limb and flash frozen. All tissues were serially sectioned (3–4 μm) throughout the entire region of interest and each sample ranged from 50–150 sections. Hematoxylin and eosin staining was performed on every tenth slide in order to locate the entire region of new bone formation.
Immunohistochemistry
Immunohistochemistry was performed as previously described.16 Primary antibodies used at a dilution of 1:100–1:200 and secondary antibodies (Alexa Fluor 488, 594, or 647; Invitrogen Life Technologies, Carlsbad, CA) at a 1:500 dilution. Primary antibodies used were as follows: Claudin 5 (Novus Biological, Littleton, CO), CD31 (BD Pharmingen, Franklin Lakes, NJ), neurofilament (Sigma‐Aldrich, St. Louis, MO), osterix (R&D Systems, Minneapolis, MN), human mitochondria (Chemicon International, Concord, MA), periostin (Abcam, Cambridge, MA), MMP9 (Millipore, Billerica, MA), MMP9 (Active) clone 4A3 (Millipore). Primary and secondary antibodies were diluted in 2% bovine serum albumin. Tissues were counterstained and covered with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). Stained tissue sections were examined by confocal microscopy (Zeiss Inc, Thornwood, NY, LSM 510 META).
Quantification of MMP9 Protein
Protein extracts were prepared from muscle (Total Protein Extraction Kit; Millipore, Billerica, MA). Protein concentrations were determined (Bio‐Rad Protein Assay kit® Bio‐Rad Corp, Hercules, CA). Active and total MMP9 protein was determined (MMP9 Biotrak Activity Assay System; GE Healthcare, Piscataway, NJ) and (Quantikine ELISA; R&D Systems) respectively. Sample analysis was done in triplicate, and the final values were calculated as the total MMP9 protein within the tissue, as the average of the sample population (n = 6), with standard deviation represented with error bars. A paired one‐way analysis of variance (ANOVA) with Tukey post hoc correction for multiple comparisons with 95% confidence interval (p < 0.05) was used for comparisons between days. A student t‐test was used to determine significance between the treated and control groups.
Microcomputed Tomography
MicroCT of whole hind limb specimens was performed at 7 μm resolution (SkyScan 1174; Micro Photonics Inc, Allentown, PA). A three‐dimensional (3D) region of interest (ROI) was defined for each specimen to isolate the new mineralized tissue from the skeletal musculature of the injection site or skeletal bone. HO bone volume was calculated using (CTAn; Micro Photonics Inc, Allentown, PA). Thresholds were set to exclude tissue with a density less than 0.1 g/cm3. The 3D microCT reconstructions were created using a Mimics & Magics software package (Materialise; Leuven, Belgium). All quantitative data were analyzed using an ANOVA with Tukey adjustment for multiple comparisons and alpha of 0.05 (SPSS 20; IBM, Armonk, NY).
RESULTS
AdBMP2 Transduced Cells Appear to Induce Heterotopic Ossification in a Location Dependent Manner
Previous experiments show mice injected with AdBMP2‐transduced cells produce bone within 7 days.13 Similar experiments performed in the rat failed to produce HO. This was observed in both wild‐type and immune compromised (athymic) rats. Radiological and histological analysis of tissues isolated 2 weeks after delivery of the Ad5BMP2 transduced cells showed normal muscle tissue with no evidence of HO (Fig. 1B panels c and d, respectively). However, when the injection site was varied (Fig. 1A) within the muscle, results revealed that bone consistently formed when the cells were placed proximal to the skeletal bone (Fig. 1B panels a and b, respectively). The location‐dependent HO was consistent even in animals that received a proximal injection of the AdBMP2 cells in one leg (Fig 1C panels a and b) and a distal injection in the opposite limb (Fig. 1C panels c and d). Interestingly, when the cells were injected in the same leg in both a proximal and distal location with respect to the skeletal bone, bone formation was observed at both sites (Fig. 1D panels a–d). To ensure that the cells did not mix, the cells were encapsulated in non‐degradable PEGDA hydrogels that allowed the BMP2 to freely diffuse.15 MicroCT analysis was done on all proximal samples to confirm that the bone was both present as well as distinct from the skeletal bone. Representative microCT images are shown for the animals receiving two injections in the same hind limb (Fig. 1D panel b). The data suggests that the distal site could support bone formation. In all cases delivery of AdEmpty‐transduced cells were used as a control and did not result in bone formation (Fig. 1E).
Figure 1.
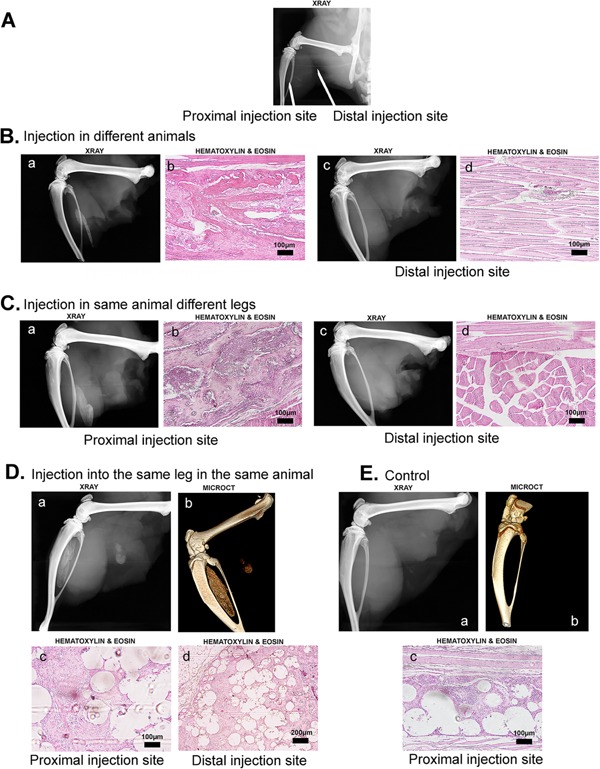
Detection of Heterotopic Ossification (HO). Radiographic and histologic analysis of tissues isolated 2 weeks after induction of HO. (A) Representative radiograph showing the location of the distal versus proximal injection sites. (B) Representative radiographs and photomicrographs of tissues isolated from Wistar rats 2 weeks after receiving either a proximal or distal injection of AdBMP2 transduced cells. (C) Representative radiographs and photomicrographs of tissues isolated from a single Wistar rat 2 weeks after receiving a proximal injection of AdBMP2 transduced cells and a distal injection of the same cells in the contralateral limb. (D) Representative radiograph, microCT image, and photomicrographs of tissue isolated from a single Wistar rat 2 weeks after receiving a proximal and distal injection in the same limb of microencapsulated AdBMP2 transduced cells. (E) Representative radiograph, microCT image and photomicrograph of tissue isolated 2 weeks after injection of Adempty transduced cells.
Addition of Stem Cells Did Not Rescue the Bone Formation
To test whether this was a lack of progenitor recruitment, AdBMP2‐transduced cells encapsulated in PEGDA hydrogel were co‐injected with human bone marrow mesenchymal stem cells (hMSC)17 into the distal location of athymic rats. To ensure that the AdBMP2 cells were capable of inducing HO, they were also injected at a location next to skeletal bone (Fig 2 panel a and b). Addition of hMSCs when injected with the AdBMP2‐transduced cells (Fig 2 panel c) or alone (Fig 2 panel d) did not result in HO. To confirm the stem cell phenotype, they were co‐injected with AdBMP2‐transduced cells into NOD/Scid mice and their differentiation potential tracked using an antibody that detects human cells. Immunohistochemical analysis showed that the cells had incorporated into multiple mesenchymal structures including cartilage and bone (Fig. 2B). The data collectively suggests that these cells were unable to rescue HO.
Figure 2.
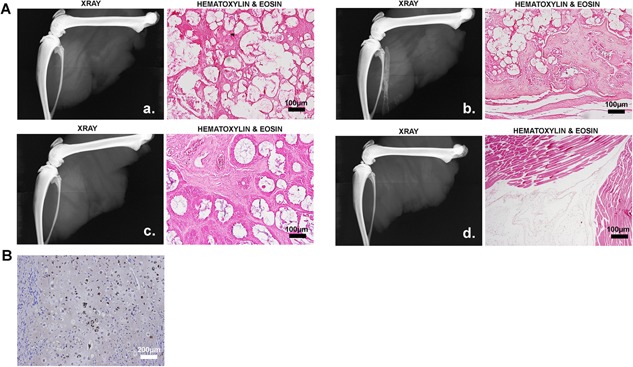
Human mesenchymal stem cells (hMSCs) were unable to rescue HO. (A) Radiographic and histological analysis of tissues isolated 2 weeks after induction of HO in the presence and absence of hMSCs. Wistar rats were injected at the distal (a) or proximal (b) location with AdBMP2 transduced cells and the distal location with hMSCs + AdBMP2 transduced cells (c) or hMSCs + PBS (d). (B) Photomicrograph of mouse tissue isolated 2 weeks after induction of HO by delivery of AdBMP2 transduced cells in the presence of hMSCs. The differentiation of the hMSCs into mesenchymal tissues of bone was confirmed through immunostaining with an antibody that specifically detects the human cells (brown). The representative section shows the presence of human chondrocytes within the mouse bone.
Osteoprogenitors Are Similar in Rat and Mouse, but Are Derived From Different Tissues
A histological comparison of the tissues was performed. One of the first changes observed in the mouse tissues is the expression of osterix in adjacent peripheral nerves.18, 19 However, unlike the mouse tissues, rat tissues isolated 1 day after induction showed only a handful of osterix+ cells (Fig. 3A). Mouse and rat innervation is very similar, and peripheral nerves were observed at both locations, with no correlation to the presence of absence of HO. Also unlike in the mouse, osterix expression does not disappear, but remains within the nerve (Fig. 3A). Claudin 5, a tight‐junction protein co‐expressed on osterix+ cells in mouse,19 also appeared to be expressed on the osterix+ cells. Further, the number of positive cells appeared to be much larger than the claudin 5+ cells observed in nerves within the days 1 or 2 tissues (Fig. 3A). The claudin 5+ osterix + cells appear to be connected in an organized manner with the fibula and connecting to the HO at all times analyzed during HO formation at the proximal site (Fig. 3B). These cells are positive for the periosteal marker periostin suggesting they may be derived from this tissue (Fig. 3B). Claudin 5 expression did not CD31+ vessels (Fig. 3B), suggesting that they are not a component of local vasculature. The data suggests a block in the ability of osteoprogenitors made in the nerve to exit and contribute to HO.
Figure 3.
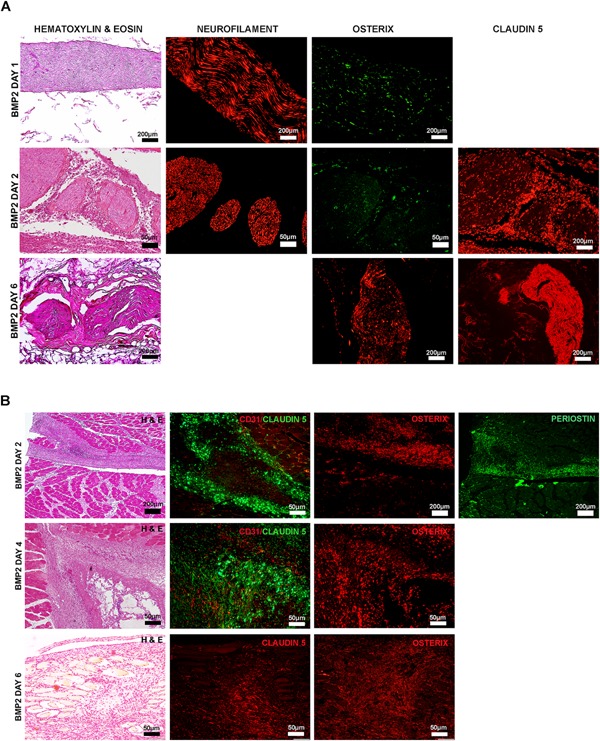
Representative photomicrographs of immunohistochemical staining of tissues isolated 1, 2, and 6 days after a proximal injection of AdBMP2 transduced cells. (A) Osterix and claudin 5 expression in peripheral nerves within the area of HO. Serial paraffin sections, every 10th slide, were hematoxylin and eosin stained to locate the region of bone formation. Unstained serial sections were then co‐immunostained for neurofilament to detect the nerve and the osteogenic factor osterix. Additional sections were also immunostained for claudin 5 an osteogenic marker in mice. (B) Osterix and claudin 5 expression in the periosteum of the fibula near site of HO. Serial paraffin sections, every 10th slide, were hematoxylin and eosin stained to locate the region of bone formation. Unstained serial sections were then immunostained for osterix (osteoprogenitors). Serial sections were also co‐immunostainned with claudin 5 and CD31 to determine if the cells expressing claudin 5 were associated with the vasculature and periostin to confirm that the cells were associated with the periosteum.
Active MMP9 Is Absent in the Rat Model
MMP9 has been shown to play a key role in opening the blood‐nerve barrier in mouse peripheral nerves during HO,18, 19, 20 which is a requirement for the entrance of osteoblast progenitors into endoneurial vessels so that they can exit the nerve through the circulation, since endoneurial endothelial cells maintain the tight and adherens junctions comprising this barrier. MMP9 activity has been shown to be significantly elevated in tissues within 24 h after induction of HO in the mouse model.20 Surprisingly, MMP9 expression was absent in the rat nerve at similar times, but immunostains to detect the active and precursor form of the protein (total MMP9) showed positive staining in a number of cells within the reaction site (Fig. 4). To determine if active MMP9 was present, tissues were next immunostained with an antibody that selectively detects only the active form of MMP9. The results showed only a few cells that were positive for the active form at 4 days after induction of HO with delivery of the AdBMP2 transduced cells (Fig. 4A). Alternatively, the majority of mouse MMP9 was observed to be in the active form, 2, 4, and 6 days after BMP2‐induction as confirmed by positive immunostaining (Fig. 4B), validating previous conclusions.20 To determine whether active MMP9 expression was associated with heterotopic ossification in humans, tissues were obtained from patients undergoing active bone formation, and serial sections were immunostained for both the active MMP9 and total MMP9 protein (Fig. 4C). The results show positive staining for both antibodies, and suggest that the majority of the protein is activated in these human tissues.
Figure 4.
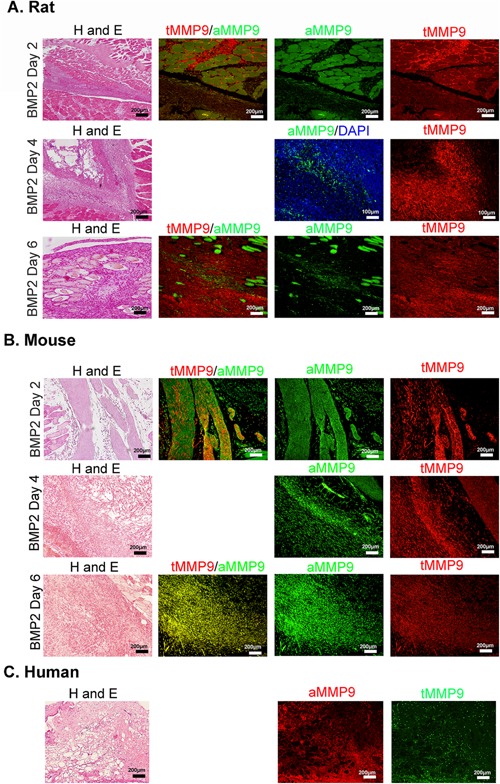
Expression of MMP9 active and inactive forms within the area of HO. Representative photomicrographs of paraffin sections serially cut from rat (A), mouse (B), or human (C) tissues. Rat and mouse tissues were isolated 2, 4, and 6 days after delivery of AdBMP2 transduced cells. Human tissues depict soft tissues immediately surrounding the newly forming heterotopic bone. Tissue sections were stained with hematoxylin and eosin or co‐immunostained with antibodies that detect total MMP9 (red) or the active form (green), or for human tissues total MMP9 (green) and active form (red) as indicated.
MMP9 activity was quantified in rat tissues isolated at 2, 4, and 6 days after induction of HO. Low levels of MMP9 activity were detected, with no significant changes between the tissues receiving AdBMP2‐transduced cells and those receiving AdEmpty‐transduced cells at 2 and 6 days after induction of HO (Fig. 5A). There was a slight but significant (p < 0.01) elevation in MMP9 activity at 4 days after induction of HO (Fig 5A). There was a small but significant increase in MMP9 activity 4 days after induction of HO when compared to the activity on days 2 and 6 (Fig. 5A). Previous findings in mice showed that MMP9 activity was significantly elevated in the tissues surrounding the site of bone formation on all days (1–6) after delivery of the AdBMP2‐transduced cells, confirming the variation between the two species.20 Total MMP9 protein was quantified in these tissues to confirm the presence of proMMP9 in the reaction site (Fig. 5B). Interestingly, MMP9 total protein was elevated in both the tissues receiving AdBMP2‐ and AdEmpty‐transduced cells that were isolated 2 days after induction of HO as compared to the other days (Fig. 5B). However, when comparisons of MMP9 total protein levels were made between days, both AdBMP2‐ and AdEmpty‐transduced cells were found to be significantly elevated over the day 6 tissue counterparts respectively (p < 0.028 and p < 0.0086) suggesting that perhaps the adenovirus‐transduced cells are leading to an inflammatory response and production of the protein. However, the MMP9 total protein levels in the control tissues appears to resolve by 4 days, which was significantly decreased as compared to the control day 2 samples (p < 0.014). MMP9 total protein levels in tissues receiving the AdBMP2‐transduced cells remain elevated (no statistically significant difference between day 2) but now was significantly different from the control tissue (p < 0.0056; Fig. 5B). By 6 days after induction of HO, MMP9 protein level was significantly lower in tissues receiving the AdBMP2‐transduced cells (p < 0.025) as compared to the tissues isolated on day 4, but the control tissues were not significantly different from the day 4 control tissues, again suggesting that MMP9 is not normally expressed at these levels in the tissues (Fig. 5B). In contrast, MMP9 total protein expression and activity in the mouse model were significantly elevated at this time period, and remained elevated throughout the remainder of HO.20 The data collectively suggests that MMP9 is not activated in the rat, and its expression is quickly down‐regulated compared to the mouse.
Figure 5.
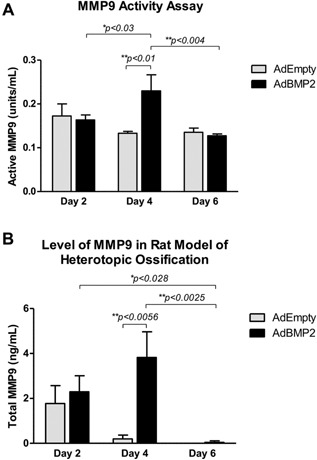
Quantification of MMP9 protein and functional activity by ELISA. Tissues were isolated from rats at 2, 4, and 6 days after proximal injection of AdBMP2 and AdEmpty transduced cells and protein extracts generated. (A) MMP9 protein was bound through ELISA, and then substrate added, to quantify MMP9 functional activity. (B) MMP9 total protein was bound through ELISA and quantified directly. Standard amounts of active MMP9 or total MMP9 protein respectively were assayed in order to calculate active protein amount from the activity measurements. Statistical significance was calculated using a standard t‐test, with n = 6. *Denotes statistical significance. Sample comparisons between the various days were calculated using a paired one‐way ANOVA with Tukey post‐hoc correction for multiple comparisons with 95% confidence interval (p < 0.05). A student t‐test was used to determine significance between the treated and control groups.
Suppression of MMP9 Significantly Reduces HO in Mouse
The data suggests that active MMP9 plays a critical function in the mechanism of heterotopic bone formation. Thus, suppression of MMP9 activation and/or activity in the mouse should prevent heterotopic bone formation. To test this hypothesis mice were treated with the MMP9 inhibitor minocycline21, 22 or vehicle and resultant heterotopic bone formation assessed by microCT. Results of radiographic analysis show bone formation in all mice; however, there was significantly (p < 0.057) less total bone formed in the group receiving the MMP9 inhibitor (Fig. 6C and D). Immunohistochemical staining for active and total MMP9 protein in tissues isolated 4 days after induction of bone formation showed the presence of MMP9 protein in tissues isolated from both vehicle and minocycline treated mice but a lack of activated protein in tissues derived from mice treated with minocycline, suggesting that it inhibited activation (Fig. 6A). The data suggests that MMP9 plays a key role in the process of heterotopic ossification and its lack of activation in the rat could potentially be responsible for the differences observed between the rat and the mouse.
Figure 6.
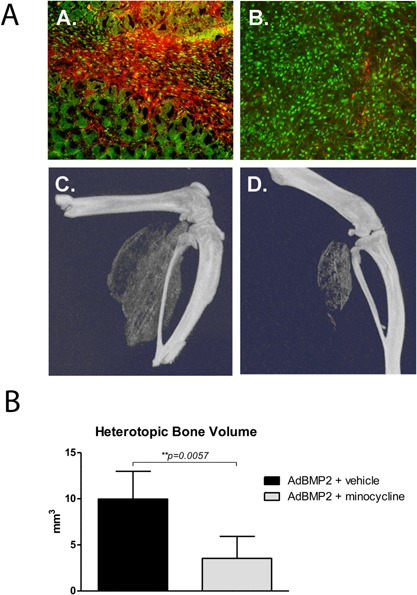
Quantification of HO in mice treated with minocycline or vehicle. Mice were treated with minocycline or vehicle and HO induced through delivery of AdBMP2 transduced cells. (A) Tissues isolated at 4 days were immunostained for the presence of both total MMP9 (green color) and active MMP9 (red color) expression. Percentage of cells expressing active MMP9/total MMP9 (n = 3 mice per group); 4.1% ± 0.56 S.E.M. and 95.5% ± 1.75 S.E.M. for minocycline and vehicle treated group respectively. Comparison of the groups using a student t‐test confirmed difference between groups to be highly significant (p < 0.001). (C and D) Bone formation was quantified in tissues isolated 14 days after induction of HO and three dimensional reconstruction of the resultant bone is depicted. (E) Bone volume was then calculated from each animal (n = 4) and the average volume and standard error of the mean depicted. Statistical significance was determined by student t‐test with confidence interval of 95% (p < 0.05). *Denotes statistical significance.
DISCUSSION
The translation of BMPs into larger animal models has been inconsistent and difficult. Investigators have been unable to see similar effects observed in small animal studies of the BMPs in larger animals such as sheep.23 The goal of this study was determine why the AdBMP2‐transduced cell delivery system was unable to produce HO in the rat model.
Several differences were noted between HO in rats versus mouse. One major difference was that no bone formation was observed when the cells were delivered at a distal location to skeletal bone. When the AdBMP2 cells were delivered proximally, bone formation occurred reliably. Further, when the AdBMP2‐transduced cells were delivered simultaneously into both proximal and distal locations, bone formation at the distal site was restored. To ensure that the cells remained at the two injection site, they were encapsulated in PEGDA hydrogel microspheres. MicroCT suggests that in both cases the newly forming bone is distinct not only from each other, but also from the skeletal bone. To clarify whether bone formation correlated with delivery of the cells near adjacent structures such as nerve or vessels tissues were visualized with hematoxylin and eosin. The placement site where the AdBMP2 transduced cells were localized could readily be detected when they were encapsulated in the nondegradble PEGDA hydrogel material. Presumably the BMP2 expression in this stepwise approach may be more effective at inducing expansion and mobilization of the tentative periosteal progenitors. The BMP2 may not otherwise reach them, when only placed distal to the bone. Mobilization of these progenitors may not be dependent on MMP9 activation.
However, addition of hMSCs did not rescue bone formation, although they could differentiate into cartilage and bone in mice. This suggests that rescuing the BMP2‐induced bone formation in the rat is more complex than delivery of progenitors, and likely involves environmental factors directing progenitor differentiation. To further identify the differences in the rat that could inhibit bone formation at the distal location, tissues were isolated and examined at early stages of the process. In all cases, we did not observe bone formation in the distal injections, and the delivered cells were rapidly cleared. Therefore, we focused on the tissues isolated from rats that received the proximal injection and were able to undergo HO.
Immunostaining for osterix and claudin 5, two markers expressed on osteoprogenitors in mouse showed the presence of cells positive for both markers at the site of new bone formation. In the mouse, the majority of cells within the endoneurium of peripheral nerves adjacent to the site of new bone formation, expressed osterix for 24 h after delivery of the AdBMP2‐transduced cells then disappeared from the nerve prior to the appearance of bone matrix.19 In rat, there were a handful of the osterix+ cells within the endoneurium of the nerve between 24 and 48 h after induction of HO, but by day 6 there were a substantial number of osterix+ cells in the endoneurium, similar to the amount observed in mouse tissues on day 1.19 The mouse progenitors were found to be exiting the nerve through endoneurial vessels and circulated to the site of new bone formation.19 These progenitors could be identified in the circulation as well as in the area of new bone formation by expressing the tight junction molecule claudin 5. In the rat, cells within the endoneurium were negative for claudin 5 until day 6, after which all the cells within the perineurium appeared to be expressing this molecule. The results suggest that the same progenitors as observed in the mouse may be present by they are unable to exit the nerve in the rat.
A further surprise was the presence of claudin 5+ osterix+ cells within the periosteum of the adjacent fibula in tissues isolated from the rat at 2 days after induction of HO. These cells were positive for the periosteal marker periostin. On later days (4 and 6) after induction of HO, these osterix+ claudin 5+ periostin+ cells were found in an organized structure leading from the fibula to the newly forming bone. Since claudin 5 is also an endothelial cell marker, we next co‐immunostained for CD31+ claudin 5+ cells. The data collectively suggest that claudin 5, the endothelial tight junction molecule found in peripheral nerves, may be expressed on periosteum‐derived progenitors in the rat. Further, the data presented in this report indicate that the rat utilizes similar progenitors, but they are ultimately recruited from a distinct niche.
The term HO refers to de novo bone formation in soft tissues but without reference to the distance form skeletal bone. The results presented here are intriguing because the cells delivered proximal to the skeletal bone appeared to generate HO, follow a mechanism similar to a periosteal reaction or orthotopic bone formation through stimulation of the periosteal cells. This raises the question as to whether this is truly HO. Further, the mechanism that prevents periosteal cells from forming new bone contiguous with the skeleton is unclear but warrants future study.
The study results indicate that the recruitment of cells from peripheral nerves in the rat may be problematic. Cells entering or exiting the nerve must pass through the highly regulated blood‐nerve barrier, which is controlled by several factors. First, claudin 5+ endothelial cells within the endoneurium have been shown to regulate this barrier. However, the barrier is also regulated at the perineurial layer, through claudin 1. Interestingly, one of the only factors known to open this tight junction within the barrier is MMP9.24 The opening of the barrier by MMP9 likely occurs through binding and regulation of claudin 1.24
Examination of total MMP9 protein in rat revealed its presence, but lack of activation. In experiments where the MMP9 inhibitor minocycline was delivered to mice during the formation of HO, the bone formation was significantly decreased with approximately 3‐fold lower bone volume. Bone formation was not completely suppressed in the presence of the MMP9 inhibitor, which is similar to previous studies using other inhibitors that affect this pathway.18 Because the mouse limb is very small, it is difficult to inject the cells distally without having some reside locally near the bone. Potentially the lack of total suppression of bone formation may in part be due to the activation of the periosteum, which is not dependent on this pathway for bone formation. Alternatively, the large amount of active MMP9 associated with early HO in humans suggests that MMP9 may play a key role in this process. If the partial ablation of HO observed in mice is due to inhibition of the neural pathway without affecting the periosteum, then conceivably targeting MMP9 activation and expression could be more effective in humans, where the entire bone may be of neural origin.
This is the first step in defining the functional role of MMP9 in HO. Understanding the essential role of MMP9 in this process is not only important for developing prophylactic and/or therapeutic interventions against HO, but also may provide new insights to varying efficacies of BMP2 in large animals. Thus, understanding MMP9's functional role in the process of de novo bone formation may provide valuable information to harness this mechanism for the regeneration of bone defects and healing fractures.
AUTHORS’ CONTRIBUTIONS
ELD, CS, EAS, TAD, JAF, ARD, EAO‐D conducted experiments, evaluated the results, and drafted the manuscript. ZWL, GH, and EVS conducted experiments and evaluated the results. All authors have read and approved the final version of the submitted manuscript.
ACKNOWLEDGMENTS AND DISCLAIMERS
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. Some of the authors are military service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. § 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. The study protocol was approved by the Naval Medical Research Center Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects.
REFERENCES
- 1. Baird EO, Kang QK. 2009. Prophylaxis of heterotopic ossification—an updated review. J Orthop Surg Res 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alfieri KA, Forsberg JA, Potter BK. 2012. Blast injuries and heterotopic ossification. Bone Joint Res 1:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salisbury E, Sonnet C, Heggeness M, et al. 2010. Heterotopic ossification has some nerve. Crit Rev Eukaryot Gene Expr 20:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zotz TG, Paula JB, Moser AD. 2012. Experimental model of heterotopic ossification in Wistar rats. Braz J Med Biol Res 45:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ipaktchi K, Mattar A, Niederbichler AD, et al. 2006. Attenuating burn wound inflammatory signaling reduces systemic inflammation and acute lung injury. J Immunol 177:8065–8071. [DOI] [PubMed] [Google Scholar]
- 6. Wu X, Rathbone CR. 2013. Satellite cell functional alterations following cutaneous burn in rats include an increase in their osteogenic potential. J Surg Res 184:e9–16. [DOI] [PubMed] [Google Scholar]
- 7. Shi S, de Gorter DJ, Hoogaars WM, et al. 2013. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci 70:407–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang M, Abbah SA, Hu T, et al. 2013. Minimizing the severity of rhBMP‐2‐induced inflammation and heterotopic ossification with a polyelectrolyte carrier incorporating heparin on microbead templates. Spine (Phila Pa 1976) 38:1452–1458. [DOI] [PubMed] [Google Scholar]
- 9. O'Brien EJ, Frank CB, Shrive NG, et al. 2012. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol 93:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campana V, Milano G, Pagano E, et al. 2014. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 25:2445–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang M, Abbah SA, Hu T, et al. 2015. Polyelectrolyte complex carrier enhances therapeutic efficiency and safety profile of BMP‐2 in porcine lumbar interbody fusion model. Spine (Phila Pa 1976) 40:964–973. [DOI] [PubMed] [Google Scholar]
- 12. Olabisi RM, Lazard Z, Heggeness MH, et al. 2011. An injectable method for noninvasive spine fusion. Spine J 11:545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olmsted‐Davis EA, Gugala Z, Gannon FH, et al. 2002. Use of a chimeric adenovirus vector enhances BMP2 production and bone formation. Hum Gene Ther 13:1337–1347. [DOI] [PubMed] [Google Scholar]
- 14. Fouletier‐Dilling CM, Bosch P, Davis AR, et al. 2005. Novel compound enables high‐level adenovirus transduction in the absence of an adenovirus‐specific receptor. Hum Gene Ther 16:1287–1297. [DOI] [PubMed] [Google Scholar]
- 15. Olabisi RM, Lazard ZW, Franco CL, et al. 2010. Hydrogel microsphere encapsulation of a cell‐based gene therapy system increases cell survival of injected cells, transgene expression, and bone volume in a model of heterotopic ossification. Tissue Eng Part A 16:3727–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olmsted‐Davis E, Gannon FH, Ozen M, et al. 2007. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol 170:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramos CA, Asgari Z, Liu E, et al. 2010. An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells 28:1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salisbury E, Rodenberg E, Sonnet C, et al. 2011. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem 112:2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazard ZW, Olmsted‐Davis EA, Salisbury EA, et al. 2015. Osteoblasts have a neural origin in heterotopic ossification. Clin Orthop Relat Res 473:2790–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodenberg E, Azhdarinia A, Lazard ZW, et al. 2011. Matrix metalloproteinase‐9 is a diagnostic marker of heterotopic ossification in a murine model. Tissue Eng Part A 17:2487–2496. [DOI] [PubMed] [Google Scholar]
- 21. Hu F, Ku MC, Markovic D, et al. 2014. Glioma‐associated microglial MMP9 expression is upregulated by TLR2 signaling and sensitive to minocycline. Int J Cancer 135:2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chaturvedi M, Kaczmarek L. 2014. Mmp‐9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egermann M, Lill CA, Griesbeck K, et al. 2006. Effect of BMP‐2 gene transfer on bone healing in sheep. Gene Ther 13:1290–1299. [DOI] [PubMed] [Google Scholar]
- 24. Hackel D, Krug SM, Sauer RS, et al. 2012. Transient opening of the perineurial barrier for analgesic drug delivery. Proc Natl Acad Sci USA 109:E2018–E2027. [DOI] [PMC free article] [PubMed] [Google Scholar]


