Abstract
Background
Meditation is increasingly showing beneficial effects for psychiatric disorders. However, learning to meditate is not straightforward as there are no easily discernible outward signs of performance and thus no direct feedback is possible. As meditation has been found to correlate with posterior cingulate cortex (PCC) activity, we tested whether source-space EEG neurofeedback from the PCC followed the subjective experience of effortless awareness (a major component of meditation), and whether participants could volitionally control the signal.
Methods
Sixteen novice meditators and sixteen experienced meditators participated in the study. Novice meditators were briefly trained to perform a basic meditation practice to induce the subjective experience of effortless awareness in a progressively more challenging neurofeedback test-battery. Experienced meditators performed a self-selected meditation practice to induce this state in the same test-battery. Neurofeedback was provided based on gamma-band (40–57 Hz) PCC activity extracted using a beamformer algorithm. Associations between PCC activity and the subjective experience of effortless awareness were assessed by verbal probes.
Results
Both groups reported that decreased PCC activity corresponded with effortless awareness (P<0.0025 for each group), with high median confidence ratings (novices: 8 on a 0–10 Likert scale; experienced: 9). Both groups showed high moment-to-moment median correspondence ratings between PCC activity and subjective experience of effortless awareness (novices: 8, experienced: 9). Both groups were able to volitionally control the PCC signal in the direction associated with effortless awareness by practicing effortless awareness meditation (novices: median % of time =77.97, P=0.001; experienced: 89.83, P<0.0005).
Conclusions
These findings support the feasibility of using EEG neurofeedback to link an objective measure of brain activity with the subjective experience of effortless awareness, and suggest potential utility of this paradigm as a tool for meditation training.
Keywords: neurofeedback, EEG, posterior cingulate cortex, PCC, meditation, mindfulness
Introduction
Mindfulness meditation programs have beneficial effects on a number of psychiatric conditions. For example, mindfulness has been shown to have similar treatment-related effect sizes for anxiety and depression as antidepressants, but without the associated toxicities (for a recent review and meta-analysis see (Goyal et al., 2014). However, learning to meditate may not be straightforward. Unlike activities such as yoga or football, no immediate feedback to students is possible in meditation as there are no easily discernible outward signs of performance. In addition, a teacher’s feedback may be biased. Bias may come from the teacher’s ability to interpret the students’ verbal descriptions, which may be further influenced by several factors, including the ability of a student to describe internal states (Van Lutterveld and Brewer, 2015). Real-time neurofeedback based on signals that are associated with effective meditation practice may provide a possible solution to this issue.
A recent study found that meditation is associated with decreased activity in the default mode network (DMN)(Brewer et al., 2011). In addition, mind-wandering and self-referential processes, which may be considered the opposite of meditation, have been associated with increased activity in this network (Mason et al., 2007; Whitfield-Gabrieli et al., 2011). Building on these findings, a recent fMRI neurofeedback study showed that activity in a major hub of the DMN, the posterior cingulate cortex (PCC), correlated negatively with the subjective experience of increased effortless awareness, (which is a major component of meditation and consists of the factors “concentration”, “observing sensory experience”, “not ‘efforting’“ and “contentment” (Garrison et al., 2013a)) and positively with mind-wandering and self-referential processes (Garrison et al., 2013a). These results illustrate that activity in the PCC may provide an ideal target for neurofeedback training during meditation, as information in either direction (increased or decreased activity) relates to the quality of practice. However, fMRI is expensive and impractical to use. Moreover, its temporal resolution is relatively poor, making it challenging for participants to interpret the feedback. In contrast, electroencephalography (EEG) is relatively inexpensive, portable and has excellent temporal resolution. Given recent advances in spatial source-estimation for targeting specific brain regions, EEG neurofeedback from the PCC may provide a scalable methodology to facilitate individuals in identifying and fostering specific cognitive states associated with meditation practice. As electro- and magnetoencephalographic studies have shown task-related gamma-band power suppression in the PCC (Brookes et al., 2011; Jerbi et al., 2010; Ossandon et al., 2011), and pilot testing showed that the best results were found for low gamma (40–57 Hz) in a symptom-capture approach, we will focus on this frequency band.
Based on the above reviewed fMRI findings, in this proof-of-concept study we hypothesized that 1) local desynchronization of PCC activity (decreased PCC activity), as presented via source-space EEG real-time neurofeedback, would correspond with the subjective experience of effortless awareness, and that 2) participants can volitionally control the PCC feedback signal in the direction of increased effortless awareness. To test this prediction, novice meditators performed a basic meditation practice to induce the subjective experience of effortless awareness while being provided EEG real-time neurofeedback in a double-blind, step-wise protocol. Furthermore, neurofeedback was provided to experienced meditators who performed a self-selected meditation practice to induce effortless awareness as this population has extensive practice in introspection related to the subtle cognitive states of this state.
Methods and materials
Participants
Sixteen novice meditators (defined as having no meditation practice in the previous year and <20 entire lifetime hours) and 16 experienced meditators (defined as meditating ≥ 30 minutes per day for at least 5 days per week over the past 5 years) were matched for age, gender and handedness. Exclusion criteria for both novice meditators and experienced meditators were: (i) any neurological condition, including head injury or head trauma, (ii) any serious psychiatric, cognitive or medical disorder which could interfere with completion of the study (anxiety and depressive disorders in remission were not considered exclusion criteria), (iii) not being on a stable dose for the last 6 months if using anxiolytic or antidepressant medication, (iv) alcohol abuse, specified as drinking more than 14 alcoholic drinks per week at any one time or more than 4 drinks at any one time for a male, and drinking more than 7 alcoholic drinks per week at any one time or more than 3 drinks at any one time for a female, (v) illegal or recreational drug use in the past 6 weeks. Additional exclusion criteria for the novice meditators were (vi) practicing any meditation practice or yoga, Tai Chi or Qigong in the last year or over 20 hours ever in life, attendance of a meditation or yoga retreat, and participation in any meditation course. Demographics for both groups are shown in Table 1. Participants were paid 30 dollars for their participation in the study. The study was approved by the University of Massachusetts Medical School Institutional Review Board and all participants were provided a fact sheet before participation in the study.
Table 1.
Demographics. Differences in gender were tested using the chi-square test. As the assumptions of the chi-square test did not hold for work status, marital status and race, these variables were tested using Fisher’s exact tests. Highest completed level of education was tested using the Mann-Whitney test. Differences in age were tested using an independent samples t-test after testing for normality.
| Novice (N=16) | Experienced (N=16) | Test statistica | P | |
|---|---|---|---|---|
| Gender (male/female) | 11/5 | 10/6 | 0.139 | 0.710 |
| Age (mean with standard deviation in parentheses) | 51 (14) | 53 (12) | −0.521 | 0.606 |
| Handedness (right/non-right) | 13/3 | 13/3 | 0.000 | 1.000 |
| Highest level of completed education (college or university/graduate school) | 6/10 | 1/15 | 88.000 | 0.035 |
| Work status (full-time/part-time/homemaker/retired/unemployed) | 9/3/1/2/1 | 11/3/0/2/0 | 2.276 | 0.926 |
| Marital status (never married/married/living in permanent relationship/divorced) | 1/11/2/2 | 2/9/4/1 | 1.681 | 0.706 |
| Race (White/African American/Asian) | 13/1/2 | 16/0/0 | 2.865 | 0.226 |
| Meditation practice (Theravada/Vajrayana/Zen/Theravada, Zen and Vajrayana/Vedanta and Mindfulness/Zen and Catholic Contemplative/Theravada, Zen and Mindfulness) | 5/6/1/1/1/1/1 | |||
| Lifetime meditation practice hours (median, range) | 6164 (1527–50978) |
Effortless awareness
All participants were first taught the concept of effortless awareness. Effortless awareness is a major component of meditation practice and consists of the factors “concentration”, “observing sensory experience”, “not ‘efforting’“ and “contentment” (Garrison et al., 2013a). Novice meditators were taught “noting practice” meditation, which is theoretically thought to support and train effortless awareness. During noting practice, novices were instructed to silently label the sensory experience that was most predominant from moment-to-moment (i.e. seeing, hearing, feeling or thinking)(Fronsdal, 2008). Before the experiment, novices performed a short noting practice session (~30 s) in which they verbalized the noting practice out loud to confirm that the participants understood the instructions. Next, they completed a short silent practice session (~30 s). Exact instructions for the noting practice and the practice session are provided in Supplementary Text S1. To provide novice meditators with the highest temporal resolution of feedback, novice meditators performed noting practicewith their eyes open. Experienced meditators performed the meditation practice in which it was easiest for them to foster effortless awareness. Exact instructions for the experienced meditators are provided in Supplementary Text S1. In pilot experiments it was found that for experienced meditators who did not regularly meditate with their eyes open, doing so interfered with their sense of effortless awareness. For this reason, experienced meditators were allowed to choose whether they meditated with either eyes open or eyes closed (whichever they considered most effortless). Five out of sixteen experienced meditators meditated with eyes closed. They were instructed to open their eyes periodically (approximately every 5 seconds) during the visual neurofeedback runs to allow them to correlate their subjective experience with the feedback (see below).
Neurofeedback
Technical setup
The participants sat in a quiet room and watched a flat-panel monitor with a viewing distance of 70 cm. Electroencephalography data were recorded with a high-density EEG system using a cap with 128 active electrodes (BioSemi, Amsterdam, The Netherlands). For off-line horizontal electrooculography (EOG) assessment, two electrodes were placed at the outer canthus of the left and right eye (HEOGL and HEOGR, respectively. For off-line vertical EOG assessment two electrodes were placed infra- and supraorbitally at the right eye (VEOGI and VEOGS, respectively). Signals were digitized on-line by a computer at a rate of 2048 Hz.
Online data processing and source estimation
The real-time neurofeedback module applied an average reference to the incoming EEG signal, after which the EEG was band-pass filtered between 40 and 57 Hz using a 2-nd order Infinite Impulse Response (IIR) Butterworth filter. This frequency band was chosen based on electro- and magnetoencephalographic findings of task-related gamma-band power suppression in the posterior cingulate cortex (PCC)(Brookes et al., 2011; Jerbi et al., 2010; Ossandon et al., 2011) and pilot testing. PCC activity (MNI coordinates −6, −60, 18) was estimated using a spatial filter constructed in accordance with the linearly constrained minimum variance (LCMV) beamformer technique (Greenblatt et al., 2005; Sekihara et al., 2001) by means of EMSE suite (Source Signal Imaging, La Mesa, CA, USA) and neurofeedback was provided using in-house developed software. Beamformers have been successfully used in previous EEG studies assessing brain activity in the PCC (Hofle et al., 2013; Michels et al., 2013). The PCC coordinates were defined based on peak deactivation in our previous study of meditation (Brewer et al., 2011) and real-time fMRI neurofeedback from these coordinates has been shown to correlate with the subjective experience of effortless awareness (Garrison et al., 2013a). A realistic (average) head model with different electrical conductivities for skull, scalp and brain was employed (Salu et al., 1990). This approach has been shown to improve source estimation compared to similar spherical head models (Cuffin, 1996). Each second, PCC signal power was calculated as the root mean square of the 40–57 Hz band filtered PCC activity by averaging within 1 s segments. Each effortless awareness task started with a 30-second baseline task (see task design and experimental procedure below). During the effortless awareness part of each task, PCC signal power in each segment was baseline corrected by subtracting mean PCC activity during baseline and dividing by the standard deviation during baseline. After this, segments were smoothed by applying a half-Gaussian curve, multiplying the last data point by 0.57, the preceding data point by 0.35 and the second to last data point by 0.08. The feedback signal was visually presented as a bar graph filling in from left to right with a new bar appearing each second. Figure 1 shows an example of a feedback graph. No online artifact correction was implemented (e.g. eye movements). The paragraph “task design and experimental procedure” lists the length of each run on which the neurofeedback algorithm was applied (including the 30 s baseline).
Figure 1.

Example of a real-time feedback graph indicating PCC activity (40–57 Hz) relative to baseline.
Randomization and blinding
To avoid bias, the direction of the graph (upward/downward) corresponding to increased or decreased power in the PCC relative to baseline was randomized across participants. Thus, for 50% of participants in each group, increases in PCC power relative to baseline corresponded to the graph moving upward and decreases in PCC power to the graph moving downward, while for the other 50% the direction was inverted. The experiment was conducted in a double-blind design, i.e. both participants and researchers running the experiment were blind to the randomization.
Task design and experimental procedure
Each run started with a 30-second baseline task during which participants viewed trait-adjectives and assessed if the words described themselves (adapted from (Kelley et al., 2002)). Similar tasks that require evaluation of self-related stimuli have been shown to engage regions of the default mode network, including the PCC (Northoff et al., 2006). We have found that this task may provide a more consistent baseline across runs within individual subjects during neurofeedback compared to a resting-state baseline (Garrison et al., 2013b). After completing thecbaseline task, participants performed effortless awareness meditation in a series of progressively challenging step-wise blocks designed to allow participants to “discover” how a given feedback graph representing PCC activity corresponded with their subjective experience of effortless awareness in real-time (Garrison et al., 2013a). The steps progressed as follows:
Meditation without feedback (2 runs, 4 min each)
Meditation with offline feedback (feedback graph shown offline after each run; 4 runs, 1.5 minutes each)
Meditation with real-time feedback (3 runs, 1.5 minutes each)
“Free-play,” in which participants were allowed to experiment with the real-time feedback graph using mental strategies of their choosing (e.g. reliving past anxious moments, resting, mentally subtracting numbers, different types of meditation [experienced meditators only]). Novices performed 2 free-play runs. As several experienced meditators were curious to explore the relationship between the feedback signal and the subtle effects of meditation, they were allowed to have more than 2 free-play sessions (median 2.5, range 2–16). Free-play runs lasted up to 7 min each.
Volitional manipulation of the feedback graph in the direction that corresponded to effortless awareness (i.e. participants attempted to make the graph move in the direction that they considered to corresponded to their experience of effortless awareness) (3 runs, 1.5 min each). Individuals were not explicitly instructed to use a particular strategy, but to draw on their previous experience.
Volitional manipulation of the feedback graph in the direction of what the participant considered the opposite direction of effortless awareness (3 runs, 1.5 min each). Individuals were not explicitly instructed to use a particular strategy, but to draw on their previous experience.
Association between PCC activity and effortless awareness
We tested whether there was an association between effortless awareness and PCC activity in two ways: 1) the direction of the graph that participants reported as corresponding to effortless awareness was assessed. 2) the moment-to-moment correspondence between the graph and the subjective experience of effortless awareness was assessed. These approaches were performed in the following two ways:
Association between effortless awareness and direction of PCC activity
Participants were asked after each step of the experiment which direction of the graph (upward/downward) corresponded with their subjective experience of effortless awareness across all completed runs, and how confident they were about this assessment (11-point Likert scale). Exact wording of the questions is provided in Supplementary Text S2.
Moment-to-moment correspondence between effortless awareness and PCC activity
Participants were asked after each of the three real-time feedback runs (step iii) how well the feedback graph corresponded with their subjective experience of effortless awareness and its opposite (e.g., being caught up in experience) during each run on an 11-point Likert scale. Participants were also asked which direction of the graph (upward or downward) corresponded with their subjective experience of effortless awareness. Exact wording of the questions is provided in Supplementary Text S2.
Volitional control in the direction of effortless awareness runs
As individuals were not explicitly instructed to use a particular strategy to make the graph move in the direction they associated with effortless awareness, participants were asked what strategy they used after each run.
Eyes open vs eyes closed analysis
As 5 out of 16 experienced meditators practiced effortless awareness with their eyes closed and 11 with their eyes open, we assessed differences between these groups for all behavioral measures.
Confounders
As gamma-band activity has been associated with non-neuronal artifacts such as muscular activity and eye-movements (Whitham et al., 2007; Yuval-Greenberg et al., 2008), we assessed the influence of muscle contraction during effortless awareness practice in the temporalis muscle, eye blinks, horizontal eye movements and microsaccades on the PCC neurofeedback signal for both the real-time feedback runs (step iii) and volitional manipulation in the direction of effortless awareness runs (step v). In short, time-series were calculated for each of these confounders and correlated with the time-series from the PCC for the real-time neurofeedback (step iii) runs.
Muscle contraction
Muscle activity was detected by applying a 70–80 Hz 8th order bandpass filter to electrodes T7 and T8 as recently recommended (Salari and Rose, 2013). Identical to the neurofeedback module, an average reference was applied to the T7 and T8 time series, after which the data was downsampled to 1024 Hz and the bandpass filter was applied. For each channel, the detection threshold was set at 2 standard deviations from the mean, and the number of data values exceeding the threshold was calculated per segment of 1 s.
Eye blinks
Eye blinks were detected by creating a bipolar vertical EOG channel by subtracting activity in the infraorbitally placed electrode from the superorbitally placed electrode. The channel was 0.5 Hz high-pass filtered to remove DC offsets. A threshold of 80 μV was used to identify blinks in each segment of 1 s (Qian et al., 2012).
Horizontal eye movements
Horizontal eye movements were detected by creating a bipolar horizontal EOG channel by subtracting activity in the electrode placed at the outer canthus of the left eye from the electrode placed at the outer canthus of the right eye. The channel was 0.5 Hz high-pass filtered to remove DC offsets. Thresholds of −40 and 40 μV were used to determine horizontal eye movements in each segment (Qian et al., 2012), with the requirement that for the following 40 ms data values had to exceed the threshold value (Salari and Rose, 2013).
Microsaccades
Microsaccades were detected as recently recommended (Keren et al., 2010; Salari and Rose, 2013). A “radial” REOG channel was calculated as the average of all EOG channels referenced to Pz: REOG = (HEOGR+HEOGL+VEOGS+VEOGI)/4–Pz. The REOG channel was bandpass filtered with an 8th order Butterworth IIR filter (30–100 Hz). The detection threshold was set at 2 standard deviations from the mean, and the number of data values exceeding the threshold was calculated per segment.
Correlation source-estimation techniques
As an independent verification of our EEG source-estimation, we also computed an off-line independent estimate of source-estimated PCC activity using a different method. Specifically, we estimated PCC activity using the low-resolution brain electromagnetic tomography (LORETA) approach and then correlated this signal with the beamformer estimates that were generated in real-time (Pascual-Marqui, 1999; Pascual-Marqui et al., 1994). We used LORETA as it has been successfully used in EEG studies to assess brain activity in the PCC (e.g., (Frei et al., 2001; Gianotti et al., 2008; Laufs et al., 2003; Neuner et al., 2014; Vanneste et al., 2010)). LORETA PCC time series were calculated using the BrainVision Analyzer software suite (BrainProducts, Munich, Germany). LORETA calculates an estimated solution of the inverse problem based on the assumption that the smoothest of all possible activities is the most plausible one (Pascual-Marqui et al., 1994). LORETA uses a three-shell spherical head model registered to a standardized stereotactic space (Talairach and Tournoux, 1988) available as a digitized MRI from the Brain Imaging Centre (Montreal Neurological Institute, MNI305) (Collins, 1994; Collins et al., 1994; Evans et al., 1992a; Evans et al., 1992b; Towle et al., 1993). First, as with the beamformer calculation, an average reference was applied to the recorded data for the real-time neurofeedback runs (step iii) and the data were band-pass filtered between 40 and 57 Hz using a 2-nd order IIR Butterworth filter. Hereafter, electrical activity in the PCC (MNI coordinates −6, −60, 18, identical to those used by the neurofeedback module) was calculated using the LORETA algorithm as squared magnitude (i.e. power) of the computed current density. The beamformer time-series were calculated identically to during neurofeedback (described in “online data processing and source estimation” paragraph). The LORETA time series were averaged over consecutive 1 second bins, identical to the beamformer time-series.
Control region analysis
To establish the spatial specificity of the PCC signal, we analyzed the correlation between PCC activity with ROIs in the right lateral occipital cortex and left supplementary motor area off-line. These regions were chosen as meta-analysis showed that activity in these areas does not correlate with a variety of meditation practices and techniques (Tomasino et al., 2012). MNI coordinates of the right lateral occipital cortex were based on an EEG gamma-band neurofeedback training study in this structure (34, −73, −8)(Salari and Rose, 2013) and coordinates of the left supplementary motor area were based on an MEG study investigating memory encoding (Park et al., 2014). Control region time-series (40–57 Hz) were extracted using an identical procedure to the real-time PCC time-series. Correlations between PCC time-series and the control regions were calculated for the effortless awareness part of the real-time neurofeedback runs for both the real-time feedback runs (step iii) and volitional manipulation in the direction of effortless awareness runs (step v).
Frequency-band control analysis
To assess the specificity of the 40–57 Hz frequency band for PCC feedback, we analyzed the correlation between PCC activity in this frequency band with PCC time-series in 4 other frequency bands off-line (delta 1–4 Hz, theta 4–8 Hz, alpha 8–13 Hz, beta 13–30 Hz). Time-series were extracted using an identical procedure to the real-time PCC time-series. Correlations between PCC time-series and the control frequency time-series were calculated for the effortless awareness part of the real-time neurofeedback runs.
Statistical analysis
Statistical analyses were performed using SPSS (version 22.0; SPSS Inc, Chicago, IL, USA) and statistical significance was set at P<0.05.
Association between effortless awareness and PCC activity
One-sample Chi-square tests were performed for each step of the experiment to compare the number of participants choosing decreased PCC activity versus increased PCC activity as corresponding with effortless awareness. Bonferroni correction was used to correct for multiple comparisons. Differences between groups were assessed using a general estimating equation logistic regression model. Differences in confidence ratings across groups were assessed using repeated measures ANOVA. Confidence ratings were square root transformed to attain normality and homoscedasticity.
Moment-to-moment correspondence between effortless awareness and PCC activity
The association between the feedback signal and moment-to-moment subjective experience was investigated by assessing the median rating across all real-time neurofeedback runs (step iii) in which participants chose effortless awareness to correspond to decreased PCC activity. In the same way, the median rating across all runs in which participants chose effortless awareness to correspond to increased PCC activity per group was assessed. Differences between groups for runs in which participants chose effortless awareness to correspond to decreased PCC activity were tested using a repeated-measures ANOVA.
Volitional control in the direction of effortless awareness
We next tested whether individuals could volitionally control the signal in the direction of effortless awareness. First the percentage of time that the feedback signal showed decreased PCC activity versus baseline was calculated for each of the three volitional manipulation runs in the direction of effortless awareness runs (step v). Subjects who chose increased PCC activity to be associated with subjective experience of effortless awareness before the start of step (v) were excluded from this analysis (N=1 for each group). As individuals were not explicitly instructed to use a particular strategy, data was analyzed separately for runs in which a noting practice/effortless awareness meditation approach was used versus alternative approaches. Percentage of time that PCC activity was reduced compared to baseline was tested against random chance (i.e., 50%) using one-sample Wilcoxon signed rank tests. Differences between groups were calculated using a Mann-Whitney U test.
Volitional control in the opposite direction of effortless awareness
We next tested whether individuals could volitionally control the signal in the opposite direction of effortless awareness. First the percentage of time that the feedback signal showed decreased PCC activity versus baseline was calculated for each of the three volitional manipulation runs in the direction of effortless awareness runs (step vi). Subjects who chose increased PCC activity to be associated with subjective experience of effortless awareness before the start of step (vi) were excluded from this analysis (N=1 for each group). Percentage of time that PCC activity was reduced compared to baseline was tested against random chance (i.e., 50%) using one-sample Wilcoxon signed rank tests. Differences between groups were calculated using a Mann-Whitney U test.
Eyes open vs eyes closed
Differences in the number of experienced meditators practicing effortless awareness with eyes closed vs. eyes open who chose decreased PCC activity as corresponding with effortless awareness were tested using Chi-square tests for each step of the experiment and differences in confidence ratings between these two groups were tested using Mann-Whitney tests for each step of the experiment. Differences in moment-to-moment correspondence ratings between effortless awareness and PCC activity were tested for all real-time neurofeedback runs (step iii) for which participants chose effortless awareness to correspond to decreased PCC activity using a Mann-Whitney test. Differences in percentage of time that both groups were able to volitionally decrease PCC activity relative to baseline were tested using a Mann-Whitney test for the volitional control in the direction of effortless awareness (step v) runs. Differences in percentage of time that participants were able to volitionally increase PCC activity relative to baseline for the volitional control in the opposite direction of effortless awareness (step vi) were tested in the same way. Bonferroni correction was used to correct for multiple comparisons.
Confounders
We used Kendall’s tau-b to assess the relation between the confounder time series and PCC time series for the real-time neurofeedback (step iii) and the volitional manipulation in the direction of effortless awareness run (step v). The two time-series were considered to be significantly associated for a given participant when at least 2 out of their 3 runs showed a significant positive correlation (uncorrected for multiple comparisons).
Relations between source-estimation techniques
The relation between the beamformer PCC time series and LORETA estimated time series was assessed for the real-time neurofeedback runs (step iii) and volitional manipulation in the direction of effortless awareness runs (step v) using Kendall’s tau-b. Percentage of runs was assessed for which a significant correlation was observed.
Control region analysis
The relation between the beamformer PCC time series and the estimated time series in the control regions was assessed for the real-time neurofeedback runs (step iii) and volitional manipulation in the direction of effortless awareness runs (step v) using Kendall’s tau-b. Median correlation coefficients were assessed per group. In addition, the correlation between the electrode weights of the PCC beamformer and the control region beamformers was calculated using Kendall’s tau-b.
Frequency-band control analysis
The relation between the 40–57 Hz PCC time series and delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz) and beta (13–30 Hz) PCC time-series was investigated for the real-time neurofeedback runs (step iii) using Kendall’s tau-b. The number of significant correlations per group per frequency band was assessed.
Results
Association between effortless awareness and direction of PCC activity
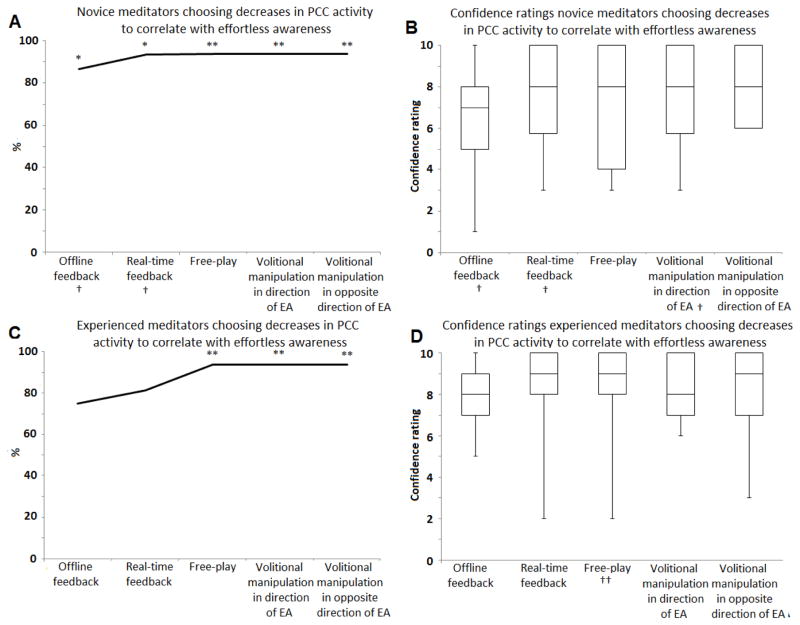
Data was missing for one novice meditator for both the offline and real-time feedback runs. For novice meditators, significantly more participants chose decreased versus increased PCC activity as corresponding with effortless awareness after each step in the experiment. For experienced meditators, this was observed after the free-play, the volitional manipulation in the direction of effortless awareness, and the volitional manipulation in the opposite direction of effortless awareness steps. Table 2 shows raw values, χ2 values and associated P-values, while Figure 2 provides a graphical representation of these results. No significant difference was observed between groups (Wald χ2 (1)=0.130, P=0.719) and no significant interaction between group and the steps of the experiment was observed (Wald χ2 (2) = 2.103, P=0.349). Taken together, these results show that both groups associated effortless awareness with decreased PCC activity and that the number of participants making this assessment plateaued after the free-play runs (step iv).
Table 2.
Statistics for assessment of number of participants choosing decreased PCC activity versus increased PCC activity to correspond with effortless awareness.
| Novice meditators | Experienced meditators | |||||
|---|---|---|---|---|---|---|
| ↓/↑ | χ2 | P(corrected) | ↓/↑ | χ2 | P(corrected) | |
| Off-line feedback | 13/2† | 8.067 | 0.025* | 12/4 | 4.000 | 0.23 |
| Real-time feedback | 14/1† | 11.267 | 0.005* | 13/3 | 6.250 | 0.06 |
| Free-play | 15/1 | 12.250 | < 0.0025* | 15/1 | 12.250 | < 0.0025* |
| Volitional manipulation in direction of effortless awareness | 15/1 | 12.250 | < 0.0025* | 15/1 | 12.250 | < 0.0025* |
| Volitional manipulation in the opposite direction of effortless awareness | 15/1 | 12.250 | < 0.0025* | 15/1 | 12.250 | < 0.0025* |
↓: Number of participants choosing decreased PCC activity as corresponding with effortless awareness ↑: Number of participants choosing increased PCC activity as corresponding with effortless awareness. P-values are Bonferroni corrected.
: Significant at P<0.05.
Missing data for one participant.
Figure 2.
(A) Percentage of novice meditators choosing decreased PCC activity to correlate with effortless awareness. (B) Box plots for the confidence ratings of novice meditators who chose decreased PCC activity to correlate with effortless awareness. (C) Percentage of experienced meditators choosing decreased PCC activity to correlate with effortless awareness. (D): Box plots for the confidence ratings of experienced meditators choosing decreased PCC activity to correlate with effortless awareness. * Significantly more participants choosing decreased PCC activity to correspond to effortless awareness than increased PCC activity at P<0.05. ** Same at P<0.0025. EA: effortless awareness. † Missing data for one participant. †† Missing data for two participants.
Confidence ratings
Confidence ratings were missing for one novice meditator for the offline feedback, real-time feedback, and volitional manipulation runs as well as for two experienced meditators for the free-play runs as this assessment was not made for these participants.
Confidence ratings were high. At the end of the experiment, the median confidence level for novice meditators who chose decreased PCC activity to be associated with effortless awareness was 8 (range=6–10) and for experienced meditators 9 (range=3–10). Figure 2 shows results for each step of the experiment. No significant differences in confidence ratings across groups were observed (F(1,26)=3.578, P=0.070) and no significant interaction between group and step of the experiment was observed (F(1,26)=0.202; P=0.937).
Moment-to-moment correspondence between effortless awareness and PCC activity
Novice meditators indicated that effortless awareness was associated with decreased PCC activity for 40 out of a total of 48 runs (83.3%) and increased PCC activity for 8 runs (16.7%) of the realtime neurofeedback runs (step iii). Median ratings of how well moment-to-moment subjective experience corresponded with the feedback signal for the former was 8 (range 4–10) and for the latter was 7 (range 4–8). Experienced meditators indicated that effortless awareness was associated with decreased PCC activity for 44 out of 48 runs (91.7%) and increased PCC activity for 4 runs (8.3%). Median rating of how well subjective experience corresponded with the former was 9 (range=1–10) and for the latter 8 (range=5–9). No significant differences between groups in moment-to-moment subjective ratings for the runs in which decreased PCC activity was chosen to be associated with effortless awareness was observed (F(1,21)=0.556, P=0.464) Taken together, these results show that the neurofeedback signal showed a strong moment-to-moment correspondence between decreased PCC activity and effortless awareness for the vast majority of runs for both groups.
Volitional control in the direction of effortless awareness
One novice and one experienced meditator chose increased PCC activity to be associated with subjective experience of effortless awareness before the start of the volitional control runs and were subsequently excluded from this analysis. Novice meditators practiced noting meditation to decrease PCC activity for 33 out of 45 runs (73.3%), and experienced meditators practiced effortless meditation to decrease PCC activity for all runs (45 out of 45; 100%). Novice meditators were able to volitionally move the graph in the direction of effortless awareness for the runs in which they performed noting practice (Z=471.500, P=0.001, median % of time=77.97, range 0.00–100.00), while they were not able to volitionally move the graph in the direction of effortless awareness for the runs in which noting practice was not performed (Z=56.000, P=0.182, median % of time=59.89, range 20.69–98.31). Experienced meditators were able to move the graph in the direction of effortless awareness (Z=1006.000, P<0.0005, median % of time=89.83, range=20.34–100.00). No difference between groups was found for the runs for which a meditation approach was used (U=622.500, P=0.224).
Volitional control in the opposite direction of effortless awareness
One novice and one experienced meditator chose increased PCC activity to be associated with subjective experience of effortless awareness before the start of the runs and were subsequently excluded from this analysis. Both groups were not able to volitionally move the graph in the opposite direction of effortless awareness (i.e. increased PCC activity, novice meditators: Z=53.000, P = 0.691, median % of time = 47.2, range 22.03–78.29); experienced meditators: Z=61.000, P=0.955, median % of time = 42.78, range 9.21–100.00). No significant difference between groups was observed (U=108.000, P=0.852).
Eyes open vs eyes closed
No differences were observed between experienced meditators practicing effortless awareness with eyes open vs. eyes closed in number of experienced meditators choosing decreased PCC activity to be associated with effortless awareness for any of the steps of the experiment and no differences in confidence ratings were observed for any of the steps of the experiment. No differences in correspondence ratings were observed for the real-time feedback runs (step iii) and no differences in ability to volitionally move the graph in the direction of effortless awareness as well as the ability to volitionally move the graph in the opposite direction of effortless awareness were observed. Supplementary data S3–S7 shows corresponding data and associated P-values for these analyses.
Confounders
Eye-movement related confounders could not be calculated for one novice meditator and one experienced meditator due to ocular electrode malfunction. Six out of sixteen novice meditators (38%) and seven out of sixteen experienced meditators (44%) showed at least one significant association between any of the 5 confounders and the PCC signal for the real-time neurofeedback (step iii) runs. Two novice meditators (13%) and two experienced meditators (13%) showed at least one significant association between the confounders and the PCC signal for the volitional manipulation runs. Table 3 shows the results per confounder, while Supplementary tables S8 to S12 show confounder results for each participant.
Table 3.
Percentage of participants showing a significant association between potential confounders and PCC activity, as determined by having at least 2 out of 3 runs having a significant correlation (P<0.05 uncorrected) between the confounder time series and the PCC time series for that participant.
| Left temporalis muscle | Right temporalis muscle | Horizontal eye-movements† | Eye blinks† | Microsaccades† | ||
|---|---|---|---|---|---|---|
| Real-time feedback (step iii) | Novice | 25.0 | 31.3 | 6.7 | 6.7 | 26.7 |
| Experienced | 25.0 | 25.0 | 6.7 | 0.0 | 6.7 | |
| Volitional manipulation into direction of effortless awareness (step v) | Novice | 6.3 | 0 | 6.7 | 0 | 0 |
| Experienced | 12.5 | 6.3 | 0 | 0 | 0 |
: Missing data for one participant in each group.
PCC beamformer and LORETA correlation
For novice meditators, 42 out of 48 runs (87.5%) showed a significant correlation between the beamformer and LORETA time series for meditation with real-time neurofeedback (step iii) and 42 out of 48 runs (87.5%) for volitional manipulation in the direction of effortless awareness (step v). For experienced meditators, 38 out of 48 runs (79.2%) showed a significant correlation for meditation with real-time neurofeedback (step iii) and 37 out of 48 runs (77.1%) for volitional manipulation in the direction of effortless awareness (step v). These results show that these source-estimation algorithms yield convergent results. Table 4 shows the correlation coefficients with associated P-values for each run.
Table 4.
Correlation coefficients between LORETA and beamformer source localization algorithms with associated P-values for each meditation with real-time neurofeedback run (step iii) and volitional manipulation in the direction of effortless awareness run (step v).
| Subject | Run 1 | Run 2 | Run 3 | MRTNF | VC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRTNF | VC | MRTNF | VC | MRTNF | VC | ||||||||||
| p-value | Cor. Coeff | p-value | Cor. Coeff | p-value | Cor. Coeff | p-value | Cor. Coeff | p-value | Cor. Coeff | ||||||
| Novice meditators | A | 0.260 | 0.081 | 0.007* | 0.194 | 0.197 | 0.093 | <0.0005** | 0.297 | 0.966 | 0.003 | <0.0005** | 0.339 | (+) | |
| B | <0.0005** | 0.288 | 0.011* | 0.183 | <0.0005** | 0.407 | 0.001** | 0.248 | <0.0005** | 0.285 | <0.0005** | 0.344 | (+) | (+) | |
| C | <0.0005** | 0.329 | 0.001* | 0.229 | <0.0005** | 0.498 | <0.0005** | 0.352 | 0.005* | 0.202 | <0.0005** | 0.346 | (+) | (+) | |
| D | 0.019* | 0.170 | 0.036* | 0.151 | <0.0005** | 0.263 | 0.205 | 0.091 | 0.009* | 0.187 | 0.023* | 0.164 | (+) | (+) | |
| E | 0.002** | 0.219 | 0.556 | 0.042 | <0.0005** | 0.258 | 0.004* | 0.206 | 0.190 | 0.094 | 0.202 | 0.092 | (+) | ||
| F | <0.0005** | 0.493 | <0.0005** | 0.382 | <0.0005** | 0.454 | <0.0005** | 0.368 | 0.002** | 0.227 | <0.0005** | 0.445 | (+) | (+) | |
| G | <0.0005** | 0.437 | 0.003* | 0.213 | <0.0005** | 0.499 | <0.0005** | 0.475 | <0.0005** | 0.523 | <0.0005** | 0.392 | (+) | (+) | |
| H | <0.0005** | 0.306 | <0.0005** | 0.353 | <0.0005** | 0.537 | 0.712 | −0.027 | <0.0005** | 0.316 | <0.0005** | 0.448 | (+) | (+) | |
| I | <0.0005** | 0.586 | <0.0005** | 0.497 | 0.001** | 0.241 | 0.038* | 0.150 | 0.075 | 0.128 | 0.027* | 0.160 | (+) | (+) | |
| J | <0.0005** | 0.467 | <0.0005** | 0.582 | <0.0005** | 0.400 | <0.0005** | 0.325 | 0.006* | 0.197 | <0.0005** | 0.296 | (+) | (+) | |
| K | <0.0005** | 0.516 | 0.010* | 0.186 | <0.0005** | 0.384 | 0.001* | 0.231 | 0.004** | 0.207 | <0.0005** | 0.370 | (+) | (+) | |
| L | <0.0005** | 0.531 | <0.0005** | 0.469 | <0.0005** | 0.544 | 0.001* | 0.231 | <0.0005** | 0.395 | <0.0005** | 0.378 | (+) | (+) | |
| M | <0.0005** | 0.472 | 0.001* | 0.241 | <0.0005** | 0.391 | <0.0005** | 0.295 | <0.0005** | 0.432 | 0.448 | 0.055 | (+) | (+) | |
| N | 0.004** | 0.207 | 0.001** | 0.243 | <0.0005** | 0.425 | <0.0005** | 0.416 | <0.0005** | 0.405 | <0.0005** | 0.279 | (+) | (+) | |
| O | 0.080 | 0.126 | <0.0005** | 0.597 | <0.0005** | 0.490 | 0.001* | 0.234 | <0.0005** | 0.331 | 0.002* | 0.224 | (+) | (+) | |
| P | 0.016* | 0.174 | <0.0005** | 0.459 | 0.002** | 0.225 | 0.144 | 0.105 | 0.01* | 0.186 | 0.004* | 0.205 | (+) | (+) | |
| Experienced meditators | AA | 0.053 | 0.139 | 0.650 | 0.033 | 0.105 | 0.117 | 0.051 | 0.140 | 0.470 | 0.052 | 0.001** | 0.231 | ||
| AB | <0.0005** | 0.345 | <0.0005** | 0.653 | <0.0005** | 0.448 | <0.0005** | 0.510 | <0.0005** | 0.615 | <0.0005** | 0.489 | (+) | (+) | |
| AC | <0.0005** | 0.527 | <0.0005** | 0.353 | <0.0005** | 0.455 | 0.217 | 0.089 | <0.0005** | 0.492 | 0.012* | 0.181 | (+) | (+) | |
| AD | 0.807 | −0.018 | 0.002* | 0.239 | 0.887 | −0.010 | 0.043* | 0.146 | 0.005* | 0.200 | 0.595 | 0.038 | (+) | ||
| AE | <0.0005** | 0.281 | 0.003* | 0.213 | <0.0005** | 0.306 | 0.024* | 0.163 | <0.0005** | 0.315 | 0.017* | 0.172 | (+) | (+) | |
| AF | <0.0005** | 0.460 | <0.0005** | 0.519 | <0.0005** | 0.358 | <0.0005** | 0.384 | <0.0005** | 0.309 | <0.0005** | 0.366 | (+) | (+) | |
| AG | <0.0005** | 0.532 | <0.0005** | 0.369 | 0.910 | −0.008 | <0.0005** | 0.500 | <0.0005** | 0.607 | <0.0005** | 0.588 | (+) | (+) | |
| AH | 0.005* | 0.201 | 0.001** | 0.231 | <0.0005** | 0.298 | 0.042 | 0.146 | 0.050* | 0.141 | <0.0005** | 0.313 | (+) | (+) | |
| AI | 0.001** | 0.247 | <0.0005** | 0.253 | <0.0005** | 0.348 | 0.212 | 0.090 | <0.0005** | 0.359 | <0.0005** | 0.449 | (+) | (+) | |
| AJ | 0.032* | 0.154 | <0.0005** | 0.397 | <0.0005** | 0.371 | <0.0005** | 0.463 | 0.013* | 0.180 | <0.0005** | 0.327 | (+) | (+) | |
| AK | 0.002** | 0.219 | <0.0005** | 0.384 | 0.002** | 0.222 | <0.0005** | 0.488 | <0.0005** | 0.327 | <0.0005** | 0.445 | (+) | (+) | |
| AL | <0.0005** | 0.732 | <0.0005** | 0.565 | <0.0005** | 0.318 | 0.009* | 0.187 | <0.0005** | 0.767 | 0.001** | 0.251 | (+) | (+) | |
| AM | <0.0005** | 0.475 | 0.002* | 0.222 | 0.028* | 0.158 | 0.026* | 0.161 | <0.0005** | 0.363 | <0.0005** | 0.285 | (+) | (+) | |
| AN | 0.605 | 0.037 | 0.105 | 0.117 | <0.0005** | 0.274 | 0.031* | 0.156 | 0.117 | 0.113 | 0.605 | 0.037 | |||
| AO | 0.070 | 0.131 | 0.407 | −0.060 | <0.0005** | 0.275 | 0.580 | 0.040 | <0.0005** | 0.484 | 0.112 | 0.114 | (+) | ||
| AP | <0.0005** | 0.351 | <0.0005** | 0.440 | <0.0005** | 0.482 | <0.0005** | 0.437 | <0.0005** | 0.385 | <0.0005** | 0.352 | (+) | (+) | |
The last column indicates whether at least 2 out of 3 runs for a participant showed a significant positive (+) or negative (−) correlation in the same direction.
p < 0.05,
p < 0.005.
MRTNF: meditation with real-time neurofeedback. VC: volitional manipulation in the direction of effortless awareness.
Control region analysis
Right occipital cortex
For the meditation with real-time neurofeedback runs (step iii), 39 out of a total of 48 data sets (81.3%) showed a significant correlation for novices (median correlation value of 0.339, range 0.184–0.666), while 41 out of a total of 48 data sets (85.4%) showed a significant correlation for experienced meditators (median correlation value of 0.340, range 0.177–0.712). For the volitional control in the direction of effortless awareness runs (step v), 41 out of a total of 48 data sets (85.4%) showed a significant correlation for novices (correlation value of 0.345, range 0.176–0.672), while 39 out of a total of 48 data sets (81.3%) showed a significant correlation for experienced meditators (median correlation value of 0.399, range 0.176–0.769). Supplementary table S13 shows the correlation coefficients with associated P-values for each run. The correlation between the electrode weights of the PCC beamformer and the right occipital cortex beamformer was statistically significant (P<0.0005) with a moderate to strong correlation value of 0.511.
Left supplementary motor area
For the meditation with real-time neurofeedback runs (step iii), 16 out of a total of 48 data sets (33.3%) showed a significant correlation for novices (median correlation value of 0.264, range −0.275–0.433), while 10 out of a total of 48 data sets (20.8%) showed a significant correlation for experienced meditators (median correlation value of 0.224, range −0.420–0.685). For the volitional control in the direction of effortless awareness runs (step v), 7 out of a total of 48 data sets (14.6%) showed a significant correlation for novices (correlation value of 0.271, range 0.205–0.433), while 17 out of a total of 48 data sets (35.4%) showed a significant correlation for experienced meditators (median correlation value of 0.261, range 0.182–0.514). Supplementary table S14 shows the correlation coefficients with associated P-values for each run. The correlation between the electrode weights of the PCC beamformer and the left supplementary motor area beamformer was statistically significant (P=0.002) with a weak correlation value of 0.188.
Frequency-band control analysis
For novices, 10 runs out of a total of 48 runs (20.8%) showed a significant correlation between delta (1–4 Hz) and 40–57 Hz PCC time-series (median 0.183, range −0.188–0.341), 16 runs (33.3%) showed a significant correlation between theta (4–8 Hz) and 40–57 PCC time-series (median 0.216, range −0.239–0.455), 12 runs (25.0%) showed a significant correlation between alpha (8–13 Hz) and 40–57 Hz PCC time-series (median 0.177, range −0.334–0.489), and 18 runs (37.5%) showed a significant correlation between beta (13–30 Hz) and 40–57 Hz PCC time-series (median 0.248, range 0.148–0.379). For experienced meditators, 8 runs out of a total of 48 runs (16.7%) showed a significant correlation between delta (1–4 Hz) and 40–57 Hz PCC time-series (median 0.192, range −0.313–0.329), 14 runs (29.2%) showed a significant correlation between theta (4–8 Hz) and 40–57 PCC time-series (median 0.177, range −0.278–0.388), 16 runs (33.3%) showed a significant correlation between alpha (8–13 Hz) and 40–57 Hz PCC time-series (median −0.001, range −0.287–0.358), and 19 runs (39.6%) showed a significant correlation between beta (13–30 Hz) and 40–57 Hz PCC time-series (median 0.219, range −0.162–0.409). Supplementary tables S15–S18 shows the correlation coefficients with associated P-values for each run.
Discussion
This is the first study assessing the coupling between real-time gamma-band EEG neurofeedback from the PCC and cognitive states related to effortless awareness, in novice and experiencedmeditators. As hypothesized, both groups consistently and with high confidence indicated that decreased PCC activity corresponded to their subjective experience of effortless awareness. In addition, both groups showed high ratings of moment-to-moment correspondence between the feedback signal and effortless awareness. Both groups were also able to volitionally move the feedback signal in the direction of effortless awareness by performing the noting practive or effortless awareness meditation. These results demonstrate the feasibility of using real-time EEG to link an objective measure of brain activity and subjective mind states related to meditation.
The present findings build upon recent fMRI neurofeedback studies showing that PCC activity correlated negatively with the subjective experience of meditation (Garrison et al., 2013a; Garrison et al., 2013b). Importantly, the current study utilized multiple methods to minimize subjective biases that may have yielded false positive results. These included the use of double-blinding participants and research staff and randomization of graph directionality. In the current study both novice and experienced meditators were able to volitionally decrease PCC activity, while in previous fMRI studies only the experienced meditators were able to do so. Potential explanations include that the fMRI setting, i.e., lying in a loud, confined space, makes it more difficult for novice meditators to perfirn noting practice meditation (and modulate PCC activity) than in a less confined, silent EEG room and that the noting practice performed by the novices in the current study may be easier than the breath awareness practice performed in the fMRI studies. Although experienced meditators had higher ratings than novice meditators for the moment-to-moment correspondence between the PCC signal and the subjective experience of effortless awareness, this finding suggests that even novice practitioners may benefit from neurofeedback-assisted meditation. Interestingly, both groups were not able to volitionally move the feedback signal into the opposite direction of effortless awareness. These results fit with earlier fMRI work by our group, in which it was found that after a long period of effortless awareness/meditation practice individuals find it difficult to do the opposite, thus producing variable results (Brewer et al., 2013).
The ability to volitionally decrease the feedback graph may be considered, in part, as confirming the high moment-to-moment correspondence ratings between subjective experience and the feedback graph. That is, the high ratings indicate that participants may be able to use insights about how their experience relates to the graph to manipulate the signal in real time (Garrison et al., 2013b). A cardinal difference between the fMRI studies and the current EEG study is the excellent temporal resolution of EEG. The fMRI BOLD signal peaks approximately 4–6 seconds after event onset (Buckner, 1998), whereas EEG allows for more temporally specific neurofeedback. However, a concern with EEG source estimation is its spatial resolution. A high number (>100) of electrodes has been shown to improve results (Michel et al., 2004). Thus, to minimize source estimation errors, we used a high-density 128-electrode set. To further aid source estimation, we used a realistic (average) head model with different electrical conductivities for skull, scalp and brain. This approach has been shown to improve source estimation compared to similar spherical head models (Cuffin, 1996). In addition, for spatial filtering we used a beamformer approach which has recently been shown to greatly reduce the effect of muscular and ocular artifacts in the gamma band (Hipp and Siegel, 2013). Recently, beamformer source-estimated EEG gamma band activity has been shown to co-localize with fMRI BOLD activity, showing that beamformers can provide spatially specific estimates of local cortical high-frequency signals (Smith et al., 2014). To further verify the signal estimates from the beamformer algorithm, the analysis was rerun offline using an independent source-localization approach (LORETA). For the vast majority of runs, significant correlations were observed between the two approaches, showing that both methods yielded convergent results. In line with this, we observed that only a minority of runs showed significant correlations between time-series from the PCC and the left superior motor area control region. However, for the right occipital cortex control region the vast majority of runs showed significant correlation values with PCC time-series. A potential explanation for these different findings is that the weighting of the right occipital cortex beamformer and the PCC beamformer showed a moderate to strong correlation, while the left superior motor area beamformer and the PCC beamformer hardly do. Although the median correlation values between for the right occipital cortex control region analysis were only weak to moderate, these findings point to a limitation in spatial specificity of beamformers for at least some brain regions.
A concern with gamma-band EEG activity is that it has been associated with non-neuronal artifacts including muscular activity and eye movements (Whitham et al., 2007; Yuval-Greenberg et al., 2008). For this reason, we specifically assessed the influence of temporalis muscle activity, eye blinks, horizontal eye movements, and microsaccades on the PCC gamma-band neurofeedback signal. For the real-time neurofeedback runs approximately 40% of participants showed at least one significant association between any of the confounders and the PCC signal, primarily with temporalis muscle activity. However, this dropped to low levels (13%) for the volitional control runs. Ideally, the effects of non-neuronal artifacts would be removed in real-time. Several techniques exist to minimize or remove non-neuronal artifacts from EEG, including independent component analysis (ICA) and a Laplacian montage approach (Makeig et al., 1996; Nunez and Pilgreen, 1991). However, these techniques have their own limitations. For example, ICA is currently not available for real-time implementation in EEG and Laplacian montages are ineffective in targeting non-superficial sources like the PCC (Fitzgibbon et al., 2013).
Limitations
In addition to the limitations noted above, there are several limitations that should be noted. Although correspondence ratings between subjective experience and the feedback graph were high and collected in a double-blinded manner, the rating of subjective experience was limited to a single dimension (effortless awareness). This did not allow for a refined assessment of which specific features of subjective experience related to specific aspects of the feedback (Garrison et al., 2013b). The rationale behind focusing on effortless awareness was that previous studies have found that limiting subjective assessment to a single dimension aids the feedback process (Garrison et al., 2013a; Garrison et al., 2013b). It should be noted that there were significant correlations between PCC time-series in the 40–57 Hz frequency band on one hand and the delta, theta, alpha, and beta frequency bands on the other hand for a substantial number of participants (16.7–39.6%). This finding highlights the limitation in frequency-band specificity of the 40–57 Hz frequency band. It should also be noted that neurofeedback studies usually employ more than a single neurofeedback session to successfully regulate brain activity (Arns et al., 2009; Tan et al., 2009). However, as we have found previously, when the cognitive paradigm is closely neurophenomenologically linked to brain activity, and the neurofeedback is not used as a training per se, single session “regulation” is possible (i.e. the cognitive task produces the corresponding change in brain activity) (Garrison et al., 2013b). Also, traditional or cultural meditation practices typically involve contextual components, such as intentions for practice, ethical consideration, background conceptual beliefs, and the support of a community, among others. Thus the interpretation of our results remains limited to that of effortless awareness practice in a decontextualized research setting (Garrison et al., 2013b). In our previous work, we have demonstrated a close neurophenomenological link specifically between effortless awareness and PCC deactivation (Garrison et al., 2013a). In particular, Garrison et al demonstrated that the effortless aspect of attention could be separated from attention itself (Garrison et al., 2013a). Additionally, we extended these findings, specifically showing that it is not just attention that produces this result (Garrison et al., 2015). This study utilized a control condition that deactivates the PCC, and found that meditation in fact deactivates PCC/precuneus activity relative to this active condition. Further, we have found specific instances where individuals try to “pay attention harder” (direct quote from fMRI neurofeedback participant), which paradoxically increases PCC activity (Brewer and Garrison, 2014; Brewer et al., 2013). Based on these findings, we attempted to specifically focus on the effortless aspect of meditation in experts, and train novices in a practice that would be least effortful. However, despite these, and our previous findings, it is still possible that another linked cognitive process could be producing these results. It should also be noted that meditative practices may vary in how much they emphasize aspects that define effortless awareness (concentration, observing sensory experience, not ‘efforting’ and contentment). As such, the present results should be interpreted within the framework of the specific instructions of the present study.
In sum, beamformer source-estimated PCC activity tracked effortless awareness in both novice and experienced meditators. Moreover, both groups were able to move the signal in the direction of effortless awareness. These findings highlight the potential utility for mechanistically based neurofeedback training programs. In light of the growing clinical utility of mindfulness based approaches for psychiatric disorders, the development and refinement of neurofeedback tools may improve treatment of these disabling and costly illnesses (Bowen et al., 2014; Brewer et al., 2011; Goyal et al., 2014; Kuyken et al., 2015). Future studies could focus on investigating the usefulness of 40–57 Hz PCC neurofeedback to aid effortless awareness practice.
Supplementary Material
Acknowledgments
This research was supported by NIH/NCCIH grant R01 AT007922-01 and UMASS Medical School institutional funds. Previous versions of this work were presented as a poster on the Organization for Human Brain Mapping 2015 conference and the Real-Time Functional Imaging and Neurofeedback 2015 conference.
Footnotes
Financial disclosures
Yale University has filed for patent protection of the application of providing neurofeedback from the PCC. Drs. Brewer and Pal own stock in a company that has rights to license this patent. No other authors have potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clinical EEG and Neuroscience. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, Carroll HA, Harrop E, Collins SE, Lustyk MK, Larimer ME. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry. 2014;71:547–556. doi: 10.1001/jamapsychiatry.2013.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA. The posterior cingulate cortex as a plausible mechanistic target of meditation: findings from neuroimaging. Annals of the New York Academy of Sciences. 2014;1307:19–27. doi: 10.1111/nyas.12246. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “Self” is Processed in the Posterior Cingulate Cortex? Frontiers in Human Neuroscience. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Wood JR, Stevenson CM, Zumer JM, White TP, Liddle PF, Morris PG. Changes in brain network activity during working memory tasks: a magnetoencephalography study. Neuroimage. 2011;55:1804–1815. doi: 10.1016/j.neuroimage.2010.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Event-related fMRI and the hemodynamic response. Human Brain Mapping. 1998;6:373–377. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<373::AID-HBM8>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL. Thesis. McGill University; Canada: 1994. 3D Model-Based Segmentation of Individual Brain Structures from Magnetic Resonance Imaging Data. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18:192–205. [PubMed] [Google Scholar]
- Cuffin BN. EEG localization accuracy improvements using realistically shaped head models. IEEE Transactions on Biomedical Engineering. 1996;43:299–303. doi: 10.1109/10.486287. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Milner B. An MRI-based stereotactic atlas from 250 young normal subjects. Abstracts - Society for Neuroscience 1992a [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992b;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon SP, Lewis TW, Powers DM, Whitham EW, Willoughby JO, Pope KJ. Surface Laplacian of central scalp electrical signals is insensitive to muscle contamination. IEEE Transactions on Biomedical Engineering. 2013;60:4–9. doi: 10.1109/TBME.2012.2195662. [DOI] [PubMed] [Google Scholar]
- Frei E, Gamma A, Pascual-Marqui R, Lehmann D, Hell D, Vollenweider FX. Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA) Human Brain Mapping. 2001;14:152–165. doi: 10.1002/hbm.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronsdal G. [accessed August 3, 2015];Mental Noting. 2008 http://www.insightmeditationcenter.org/books-articles/articles/mental-noting.
- Garrison KA, Santoyo JF, Davis JH, Thornhill TAt, Kerr CE, Brewer JA. Effortless awareness: using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators’ self-report. Frontiers in Human Neuroscience. 2013a;7:440. doi: 10.3389/fnhum.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Worhunsky PD, Elwafi HM, Thornhill TAt, Thompson E, Saron C, Desbordes G, Kober H, Hampson M, Gray JR, Constable RT, Papademetris X, Brewer JA. Real-time fMRI links subjective experience with brain activity during focused attention. Neuroimage. 2013b;81:110–118. doi: 10.1016/j.neuroimage.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Zeffiro TA, Scheinost D, Constable RT, Brewer JA. Meditation leads to reduced default mode network activity beyond an active task. Cognitive, Affective & Behavioral Neuroscience. 2015;15:712–720. doi: 10.3758/s13415-015-0358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti LR, Kunig G, Faber PL, Lehmann D, Pascual-Marqui RD, Kochi K, Schreiter-Gasser U. Rivastigmine effects on EEG spectra and three-dimensional LORETA functional imaging in Alzheimer’s disease. Psychopharmacology. 2008;198:323–332. doi: 10.1007/s00213-008-1111-1. [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, Berger Z, Sleicher D, Maron DD, Shihab HM, Ranasinghe PD, Linn S, Saha S, Bass EB, Haythornthwaite JA. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt RE, Ossadtchi A, Pflieger ME. Local linear estimators for the bioelectromagnetic inverse problem. IEEE Transactions on Signal Processing. 2005;53:3403–3412. [Google Scholar]
- Hipp JF, Siegel M. Dissociating neuronal gamma-band activity from cranial and ocular muscle activity in EEG. Frontiers in Human Neuroscience. 2013;7:338. doi: 10.3389/fnhum.2013.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofle M, Pomper U, Hauck M, Engel AK, Senkowski D. Spectral signatures of viewing a needle approaching one’s body when anticipating pain. European Journal of Neuroscience. 2013;38:3089–3098. doi: 10.1111/ejn.12304. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Vidal JR, Ossandon T, Dalal SS, Jung J, Hoffmann D, Minotti L, Bertrand O, Kahane P, Lachaux JP. Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Frontiers in Systems Neuroscience. 2010;4:27. doi: 10.3389/fnsys.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Keren AS, Yuval-Greenberg S, Deouell LY. Saccadic spike potentials in gamma-band EEG: characterization, detection and suppression. Neuroimage. 2010;49:2248–2263. doi: 10.1016/j.neuroimage.2009.10.057. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, Sutton H, Vicary R, Weaver A, Wilks J, Williams M, Taylor RS, Byford S. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386:63–73. doi: 10.1016/S0140-6736(14)62222-4. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Bell AJ, Jung T-P, TJS . Independent component analysis of electroencephalographic data. In: Mozer MT, Hasselmo M, editors. Advances in Neural Information Processing Systems. MIT Press; Cambridge, MA, USA: 1996. pp. 145–151. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clinical Neurophysiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Michels L, Muthuraman M, Luchinger R, Martin E, Anwar AR, Raethjen J, Brandeis D, Siniatchkin M. Developmental changes of functional and directed resting-state connectivities associated with neuronal oscillations in EEG. Neuroimage. 2013;81:231–242. doi: 10.1016/j.neuroimage.2013.04.030. [DOI] [PubMed] [Google Scholar]
- Neuner I, Arrubla J, Werner CJ, Hitz K, Boers F, Kawohl W, Shah NJ. The default mode network and EEG regional spectral power: a simultaneous fMRI-EEG study. PloS One. 2014;9:e88214. doi: 10.1371/journal.pone.0088214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Pilgreen KL. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. Journal of Clinical Neurophysiology. 1991;8:397–413. [PubMed] [Google Scholar]
- Ossandon T, Jerbi K, Vidal JR, Bayle DJ, Henaff MA, Jung J, Minotti L, Bertrand O, Kahane P, Lachaux JP. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. Journal of Neuroscience. 2011;31:14521–14530. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Lee DS, Kang E, Kang H, Hahm J, Kim JS, Chung CK, Jensen O. Blocking of irrelevant memories by posterior alpha activity boosts memory encoding. Human Brain Mapping. 2014;35:3972–3987. doi: 10.1002/hbm.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Review of methods for solving the EEG inverse problem. International Journal of Bioelectromagnetism. 1999;1:75–86. [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Qian C, Al-Aidroos N, West G, Abrams RA, Pratt J. The visual P2 is attenuated for attended objects near the hands. Cognitive Neuroscience. 2012;3:98–104. doi: 10.1080/17588928.2012.658363. [DOI] [PubMed] [Google Scholar]
- Salari N, Rose M. A brain-computer-interface for the detection and modulation of gamma band activity. Brain Sci. 2013;3:1569–1587. doi: 10.3390/brainsci3041569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salu Y, Cohen LG, Rose D, Sato S, Kufta C, Hallett M. An improved method for localizing electric brain dipoles. IEEE Transactions on Biomedical Engineering. 1990;37:699–705. doi: 10.1109/10.55680. [DOI] [PubMed] [Google Scholar]
- Sekihara K, Nagarajan SS, Poeppel D, Marantz A, Miyashita Y. Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE Transactions on Biomedical Engineering. 2001;48:760–771. doi: 10.1109/10.930901. [DOI] [PubMed] [Google Scholar]
- Smith MM, Weaver KE, Grabowski TJ, Rao RP, Darvas F. Non-invasive detection of high gamma band activity during motor imagery. Frontiers in Human Neuroscience. 2014;8:817. doi: 10.3389/fnhum.2014.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brai. Thieme Medical; New York: 1988. [Google Scholar]
- Tan G, Thornby J, Hammond DC, Strehl U, Canady B, Arnemann K, Kaiser DA. Meta-analysis of EEG biofeedback in treating epilepsy. Clinical EEG and Neuroscience. 2009;40:173–179. doi: 10.1177/155005940904000310. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Fregona S, Skrap M, Fabbro F. Meditation-related activations are modulated by the practices needed to obtain it and by the expertise: an ALE meta-analysis study. Frontiers in Human Neuroscience. 2012;6:346. doi: 10.3389/fnhum.2012.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle VL, Bolanos J, Suarez D, Tan K, Grzeszczuk R, Levin DN, Cakmur R, Frank SA, Spire JP. The spatial location of EEG electrodes: locating the best-fitting sphere relative to cortical anatomy. Electroencephalography and Clinical Neurophysiology. 1993;86:1–6. doi: 10.1016/0013-4694(93)90061-y. [DOI] [PubMed] [Google Scholar]
- Van Lutterveld R, Brewer J. Neurofeedback from the posterior cingulate cortex as a mental mirror for meditation. Biofeedback. 2015;43:117–120. [Google Scholar]
- Vanneste S, Plazier M, der Loo E, de Heyning PV, Congedo M, De Ridder D. The neural correlates of tinnitus-related distress. Neuroimage. 2010;52:470–480. doi: 10.1016/j.neuroimage.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castanon A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Broberg M, Wallace A, DeLosAngeles D, Lillie P, Hardy A, Fronsko R, Pulbrook A, Willoughby JO. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clinical Neurophysiology. 2007;118:1877–1888. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.