Abstract
Objectives
Biofilm acids contribute to secondary caries which is a reason for restoration failure. Previous studies synthesized nanoparticles of amorphous calcium phosphate (NACP) and dimethylaminohexadecyl methacrylate (DMAHDM). The objectives of this study were to develop DMAHMD-NACP nanocomposite for double benefits of antibacterial and remineralization capabilities, and investigate the DMAHMD mass fraction effects on fracture toughness and biofilm response of NACP nanocomposite for the first time.
Methods
DMAHDM was incorporated into NACP nanocomposite at mass fractions of 0% (control), 0.75%, 1.5%, 2.25% and 3%. A single edge V-notched beam method was used to measure fracture toughness Kic. A dental plaque microcosm biofilm model using human saliva as inoculum was used to measure the antibacterial properties of composites.
Results
Kic was about 1 MPa·m1/2 for all composite (mean ± sd; n = 6). Adding DMAHDM from 0% to 3% did not affect Kic (p > 0.1). Lactic acid production by biofilms on composite containing 3% DMAHDM was reduced to less than 1% of that on composite control. Metabolic activity of adherent biofilms on composite containing 3% DMAHDM was reduced to 4% of that on composite control. Biofilm colony-forming unit (CFU) counts were reduced by three orders of magnitude on NACP nanocomposite containing 3% DMAHDM.
Conclusions
DMAHDM-NACP nanocomposite had good fracture resistance, strong antibacterial potency, and NACP for remineralization (shown in previous studies). The DMAHDM-NACP nanocomposite may be promising for caries-inhibiting dental restorations, and the method of using double agents (DMAHDM and NACP) may have a wide applicability to other dental materials including bonding agents and cements.
Keywords: Calcium phosphate nanocomposite, quaternary ammonium monomer, antibacterial, dental plaque microcosm biofilm, mechanical properties, caries inhibition
1. Introduction
Dental caries is one of the most common bacterial infections in the human body, and it remains a prevalent problem worldwide.1 Resin composites have been increasingly used to fill tooth cavities and restore the decayed teeth.2 Composites have superior esthetics with direct-filling and photo-polymerization capabilities, and have shown improved clinical performance.3-6 However, composites tend to accumulate more biofilms and plaque than other restorative materials.7,8 Biofilm acids could lead to recurrent (secondary) caries at the tooth structure-composite margins.9,10 Secondary caries is one of the primary reasons for restoration failure.11-16 Half of all restorations fail within 10 years,11 and replacing the failed restorations accounts for 50%-70% of all the restorations performed.12
Therefore, there is a need to develop new composites with caries-inhibiting ability to prolong the durability in vivo. One strategy developed antibacterial composite. Quaternary ammonium salts (QAS) have a broad use due to their low toxicity and potent antimicrobial activity. Efforts were made to develop quaternary ammonium monomers (QAMs) and immobilize them into resin matrix by forming a covalent bond.17-19 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) was copolymerized into the polymer bulk, and the dental resin exhibited a strong antibacterial activity.20 The antibacterial potency of MDPB-containing resin was maintained after water-aging for 3 months and did not decrease over time.20 Several other antibacterial materials were recently reported, including quaternary ammonium polyethylenimine nanoparticles, methacryloxylethyl cetyl dimethyl ammonium chloride (DMAE-CB)-containing adhesive, antibacterial glass ionomer cements, and quaternary ammonium dimethacrylate-containing resins.21-25 New QAMs were prepared to yield strong antibacterial activities.26,27 These materials share a similar antimicrobial mechanism. QAS can cause cytoplasmic leakage through binding themselves to cell membrane.28 The positively-charged quaternary amine N+ on their structure can attract and interact with the negatively-charged cell membrane of bacteria, disrupt membrane functions, alter the balance of essential ions, interrupt protein activity and damage bacterial DNA.28 Long-chained quaternary ammonium compounds can penetrate into bacterial membrane like a needle, resulting in physical disruption.29 Recently, a novel dimethylaminohexadecyl methacrylate (DMAHDM) with an alkyl chain length of 16 was synthesized, which showed a strong antibacterial effect.30,31
Another important approach to inhibiting caries focused on development of calcium phosphate (CaP) particle-filled dental resins to achieve remineralization capability.29 CaP biomaterials have excellent bioactivity, biocompatibility and similarity to the minerals in teeth and bones.30 CaP-containing resins can release calcium (Ca) and phosphate (P) ions to remineralize tooth lesions.31 Nanoparticles of amorphous calcium phosphate (NACP) were synthesized and incorporated into dental resins, which released high levels of Ca and P ions, neutralized acids, and inhibited caries in a human in situ model.32-34 Furthermore, NACP nanocomposite had mechanical properties matching those of commercial control composites.35 However, studies have not been performed to combine DMAHDM with NACP in the same composite, and to investigate the fracture toughness Kic and antibacterial properties as a function of DMAHDM mass fraction in the NACP nanocomposite.
Therefore, the objectives of this study were to develop DMAHMD-NACP nanocomposite for double benefits of antibacterial and remineralization capabilities, and investigate the DMAHMD mass fraction effects on fracture toughness and biofilm response of NACP nanocomposite for the first time. The following hypotheses were tested: (1) Incorporating DMAHMD into NACP nanocomposite will not adversely affect composite facture toughness; (2) Incorporating DMAHMD into NACP nanocomposite will impart a strong antibacterial activity; (3) Biofilm growth and acid production on nanocomposite will monotonically decrease with increasing DMAHDM mass fraction.
2. Materials and Methods
2.1. Fabrication of NACP and DMAHDM
NACP [Ca3(PO4)2] was prepared by a spray-drying technique as described elsewhere.32 Briefly, calcium carbonate (CaCO3, Fisher, Fair Lawn, NJ) and dicalcium phosphate anhydrous (CaHPO4, Baker Chemical, Phillipsburg, NJ) were dissolved into an acetic acid solution to obtain Ca and P ionic concentrations of 8 mmol/L and 5.333 mmol/L, respectively, yielding a Ca/P molar ratio of 1.5. The solution was sprayed into a heated chamber, and an electrostatic precipitator (AirQuality, Minneapolis, MN) was used to collect the dried particles. This method produced NACP with a mean particle size of 116 nm.33
DMAHDM was synthesized via a modified Menschutkin reaction.36 Briefly, 10 mmol of 2-(dimethy- lamino) ethyl methacrylate (DMAEMA, Sigma, St. Louis, MO) and 10 mmol of 1-bromohexadecane (BHD, TCI America, Port-land, OR) were combined with 3 g of ethanol in a 20 mL scintillation vial.28,37,38 The vial was stirred at 70 °C for 24 h for the reaction to occur. The sample was then placed under vacuum to remove the solvent, any unreacted components and impurities. This yielded DMAHDM as a waxy solid at room temperature.28,37,38 Fourier transform infrared (FTIR) spectroscopy (Nicolet 6700, Thermo Scientific, Waltham, MA) spectra of the starting materials and the products were collected between two KBr windows in the 4000–400 cm−1 region. 1H NMR spectra (GSX 270, JEOL, Peabody, MA) were taken in deuterated chloroform at a concentration of about 3%.27,37 The chemical structure of the reaction product was confirmed via FTIR and NMR.27,37 FTIR and NMR examination revealed no detectable impurities, indicating a purity of close to 100%.
2.2. Preparation of DMAHDM-NACP nanocomposite
Bisphenol A glycidyl dimethacrylate (BisGMA, Esstech, Essington, PA) and triethylene glycol dimethacrylate (TEGDMA) were mixed at a mass ratio of 1:1. The mixture was rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoat. DMAHDM was added into the resin at the following mass fractions: 0% (control), 2.5%, 5%, 7.5% and 10%, respectively, yielding five different resins. Barium boroaluminosilicate glass with a median particle size of 1.4 μm (Caulk/Dentsply, Milford, DE) were silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine. A filler level of 70% was used for each resin, with 20% of NACP and 50% of glass fillers (all mass fractions), to form a cohesive paste. NACP was used for remineralization and the glass fillers were used for mechanical reinforcement, based on a previous study.33 Since the resin matrix mass fraction was 30% in the composite, the DMAHDM mass fraction in the final composite was: 0% (control), 0.75%, 1.5%, 2.25% and 3%, respectively.
2.3. Fracture toughness (Kic) testing
Each composite paste was placed into a stainless steel mold of 2 × 2 × 25 mm and photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each open side. Kic was measured using a single edge V-notched beam (SEVNB) method.39 A notch depth of approximately 500 μm was machined into the bar specimen using a 150 μm-thick diamond blade. Then, diamond polishing paste (3 μm, Buehler, Lake Bluff, IL) was placed into the notch tip, and a new razor blade was used to cut the notch further to a total depth of 700-800 μm. This method yielded a relatively sharp notch tip of about 20 μm as shown in a previous study.40 For each specimen, the notch length was measured on both sides of the bar using an optical microscope (TE2000-S Nikon, Japan) and averaged. Specimens were immersed in water at 37 °C for 24 h prior to testing. SEVNB specimens were tested at 0.5 mm/min displacement rate using 10 mm span with the notch on the tensile side and the loading pin aligned with the notch. Kic (MPa·m1/2) was calculated using a standard equation:39
where P is load at fracture, L is span, b is width, h is thickness, a is notch length, and f is stress intensity factor. Six specimens were measured per group (n = 6).
2.4. Saliva collection for dental plaque microcosm biofilm model
The dental plaque microcosm biofilm model was approved by the University of Maryland Baltimore Institutional Review Board. Saliva is ideal for growing biofilms to maintain much of the complexity and heterogeneity in vivo.41 Whole human saliva was used as an inoculum to provide multi-species biofilms consisting of organisms found in the oral cavity. To represent the diverse bacterial populations, saliva from ten healthy individuals was collected and combined together, following a previous study.42 Saliva was collected from healthy adult donors who had natural dentition without active caries or periopathology, and without the use of antibiotics in the last 3 months. The donors did not brush teeth for 24 h and abstained from food/drink intake for 2 h prior to donating saliva. Stimulated saliva was collected during parafilm gum chewing and was kept on ice. Saliva was diluted in sterile glycerol to a concentration of 70%, then stored at −80 °C for subsequent use.43
2.5. Live/dead staining of biofilms
To make composite specimens for the biofilm experiments, the cover of a sterile 96-well plate was used as molds following a previous study.38 Each composite paste was placed into a dent in the cover of the 96-well plate and light-cured for 30 s (Optilux VCL 401, Demetron Kerr, Danbury, CT) using a Mylar strip covering. This produced a composite disk of approximately 8 mm in diameter and 0.5 mm in thickness.44 The cured disks were immersed in water and agitated for 1 h to remove any diffusible species, following a previous study.45 Then the disks were sterilized with ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC).
The saliva-glycerol stock with 1:50 dilution was added into a growth medium as inoculum. The McBain artificial saliva growth medium contained mucin (type II, porcine, gastric) at a concentration of 2.5 g/L; bacteriological peptone, 2.0 g/L; tryptone, 2.0 g/L; yeast extract, 1.0 g/L; NaCl, 0.35 g/L; KCl, 0.2 g/L; CaCl2, 0.2 g/L; cysteine hydrochloride, 0.1 g/L; hemin, 0.001 g/L; vitamin K1, 0.0002 g/L, at pH7.46 1.5 mL of the inoculum was added to each well of a 24-well plate containing one composite disk per well, and incubated in 5% CO2 at 37 °C for 8 h. Then, the disks were transferred to a new 24-well plate filled with fresh medium and incubated. After 16 h, the disks were moved to another new 24-well plate with fresh medium and incubated for 24 h. This totaled 48 h of incubation, which was shown previously to form mature biofilms on resins.43
The 2-day biofilms on composites were rinsed with phosphate-buffered saline (PBS) and then stained using the Bac Light live/dead bacterial viability kit (Molecular Probes, Eugene, OR). Live bacteria were stained with Syto 9 to produce a green fluorescence. Bacteria with compromised membranes were stained with propidium iodide to yield a red fluorescence. Specimens were examined with an inverted epifluorescence microscope (Eclipse TE2000-S, Nikon, Japan).47 The area of green staining was measured using the NIS Elements imaging software (Nikon). The area fraction of live bacteria = green staining area/total area of the image. Six disks were evaluated for each group. Three images were taken at random locations on each disk, yielding 18 images per group.
2.6. MTT assay of metabolic activity of biofilms
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan.27,47 Disks with 2-day biofilms were moved to a new 24-well plate, then 1 mL of MTT dye (0.5 mg/mL MTT in PBS) was added to each well and incubated at 37 °C in 5% CO2 for 1 h. During this process, the metabolically active bacteria reduced the MTT to purple formazan. Disks were transferred to a 24-well plate, 1 mL of dimethyl sulphoxide (DMSO) was added to dissolve the formazan crystals, and the plate was incubated for 20 min with gentle mixing in the dark. 200 mL of the DMSO solution from each well was transferred to a 96-well plate, and the absorbance at 540 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). A higher absorbance indicates a higher formazan concentration, which means a higher metabolic activity in the biofilm.27,47
2.7. Lactic acid production of biofilms
Composite disks with 48 h biofilm were rinsed in cysteine peptone water (CPW) to remove loose bacteria. Each disk was placed in a new 24-well plate filled with 1.5 mL of buffered peptone water (BPW) supplemented with 0.2% sucrose. Disks with biofilms were incubated at 5% CO2 and 37 °C for 3 h to allow the biofilms to produce acid. Lactate concentrations of the BPW solutions were measured using an enzymatic (lactate dehydrogenase) method.27,47 A microplate reader (SpectraMax M5) was used to measure the absorbance at 340 nm (OD340) for the collected BPW solutions. Standard curves were prepared using a lactic acid standard (Supelco Analytical, Bellefonte, PA).27,47
2.8. Biofilm colony-forming unit (CFU) counts
Disks with 2-day biofilms were transferred into tubes with 2 mL CPW, and the biofilms were harvested by sonication (3510R-MTH, Branson, Danbury, CT) for 5 minutes, followed by vortexing at 2400 rpm for 30 seconds using a vortex mixer (Fisher, Pittsburgh, PA), as in previous studies.37,48 Three types of agar plates were used to assess the microorganism viability after serial dilution in CPW. Tryptic soy blood agar culture plates were used to determine total microorganisms. Mitis salivarius agar (MSA) culture plates containing 15% sucrose were used to determine total streptococci. MSA agar plates with 0.2 units of bacitracin per mL were used to determine mutans streptococci. MSA contains selective agents which inhibit many species of bacteria but streptococci, thus enables streptococci to grow. MSA plus bacitracin may inhibit other species of oral microflora, but cariogenic mutans streptococci are under no influence because of their resistance to bacitracin.37,48
2.9. Statistical analysis
One-way and two-way analyses-of-variance (ANOVA) were performed to detect significant effects of the variables. Tukey's multiple comparison tests were used at a p value of 0.05.
3. Results
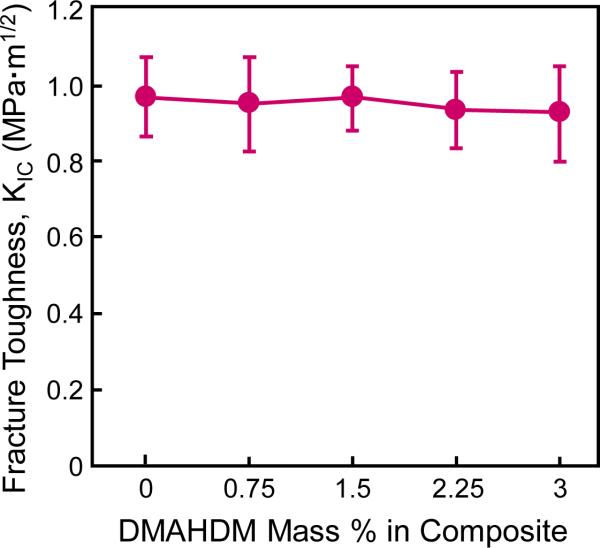
Kic values of the composites are plotted in Fig. 1 (mean ± sd; n = 6). Kic of NACP nanocomposite with various DMAHDM contents was not significantly different from that of control composite without DMAHDM (p > 0.1). These results showed that incorporation of DMAHDM from 0.75% to 3% into NACP nanocomposite caused no decrease in Kic.
Fig. 1.
Fracture toughness Kic of NACP nanocomposites as a function of DMAHDM mass fraction in the composite (mean ± sd; n = 6). All the values are significantly similar (p > 0.1). Incorporation of DMAHDM up to 3% into NACP nanocomposite did not adversely affect the Kic.
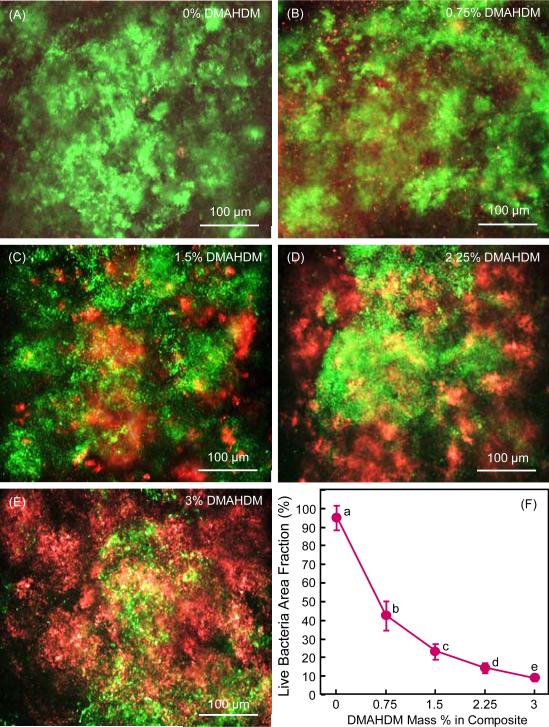
Representative live/dead staining images of 2-day biofilms grown on composites are shown in Fig. 2A-E. Live bacteria were stained green, and dead bacteria were stained red. Live and dead bacteria that were on the top of each other yielded orange or yellow staining colors. Biofilms on composite control with 0% DMAHDM were primarily alive. Increasing the DMADDM mass fraction in NACP nanocomposite increased the red or orange/yellow staining, indicating an increasing antibacterial potency of the composite. Fig. 2F plots the area fraction of live bacteria (mean ± sd; n = 6). The live bacteria coverage area monotonically decreased with increasing the DMAHDM mass fraction in the composite.
Fig. 2.
Biofilm viability: (A-E) Representative live/dead staining images of 2-day biofilms on composites, (F) Live bacteria area fraction (mean ± sd; n = 6). Live bacteria were stained green, and dead bacteria were stained red. Live and dead bacteria in close proximity of each other yielded yellow or orange colors. In (F), values with dissimilar letters are significantly different from each other (p < 0.05)
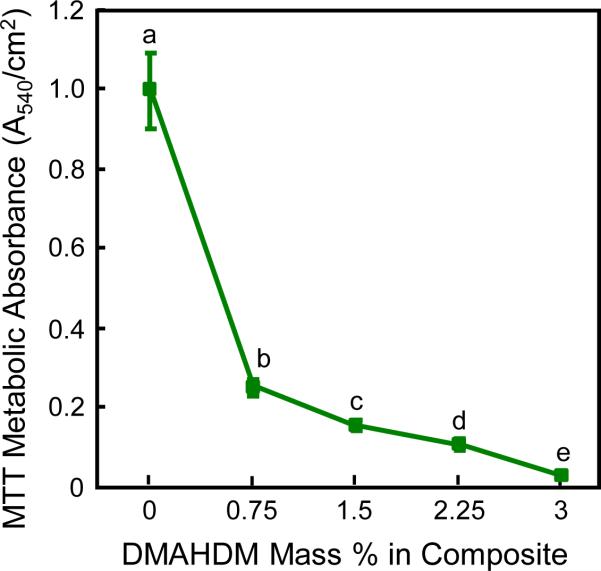
The biofilm metabolic activity is plotted in Fig. 3 (mean ± sd; n = 6). Biofilms on composite without DMAHDM had a relatively high metabolic activity. The metabolic activity 3% DMAHDM was reduced by 96%, compared to that of composite control.
Fig. 3.
MTT assay of biofilm metabolic activity grown for 2 days on composites (mean ± sd; n = 6). Values with dissimilar letters are significantly different from each other (p < 0.05).
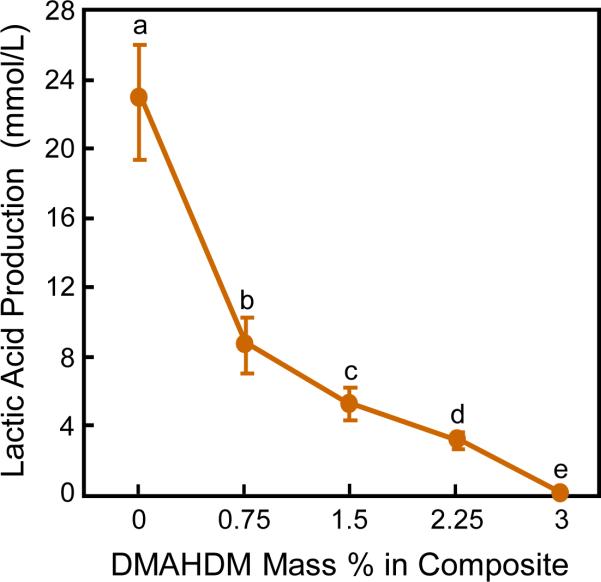
The lactic acid production biofilms on composites is plotted in Fig. 4 (mean ± sd; n = 6). The biofilms on composite control with 0% DMAHDM produced the most amount of acid. With the DMADHM concentration being increased, the lactic acid production decreased monotonically (p < 0.05). The NACP nanocomposite containing 3% of DMAHDM had biofilm acid that was less than 1% of that for composite control.
Fig. 4.
Lactic acid production of 2-day biofilms on composites (mean ± sd; n = 6). Values with dissimilar letters are significantly different from each other (p < 0.05).
The CFU counts of 2-day biofilms on composite disks are plotted in Fig. 5: (A) Total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). In each plot, the control composite with 0% DMAHDM had the highest CFU. Increasing the DMAHDM mass fraction in NACP nanocomposite enhanced the antibacterial activity and decreased the CFU (p < 0.05). Compared to control composite, all three CFU counts on NACP nanocomposite containing 3% DMAHDM were reduced by three orders of magnitude.
Fig. 5.
Colony-forming unit (CFU) counts of 2-day biofilms on composites: (A) Total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). In each plot, values with dissimilar letters are significantly different from each other (p < 0.05). Note the log scale for the y-axis.
4. Discussion
The present study developed DMAHMD-NACP nanocomposite with combined antibacterial and remineralizing capabilities, and determined the effect of DMAHDM mass fraction in NACP composite on fracture toughness and biofilm response for the first time. Previous studies showed that dental composites containing NACP had flexural strength matching commercial control composite.27,33,47 In thermal cycling, three-body wear, and water-aging experiment for 2 years, NACP nanocomposite had mechanical properties matching those of commercial non-remineralizing control composite.49 However, fracture toughness Kic of NACP nanocomposite had not been reported in previous studies. Kic is an important property which indicates a dental restorative material's fracture resistance and durability.50 In the present study, the control NACP nanocomposite without DMAHDM had a Kic of 0.97 MPa·m1/2. This value is within the reported Kic range of 0.77 to 2.40 MPa·m1/2 for dental commercial composites which had no NACP for remineralization.51 Furthermore, incorporation of DMAHDM into NACP nanocomposite from 0.75% to 3% had no adverse effect of Kic. Therefore, DMAHDM-NACP nanocomposite with antibacterial and remineralization capabilities can be developed with Kic still within the reported range for commercial composites in clinical use. Future efforts could likely further increase the Kic of the DMAHDM-NACP nanocomposite. For example, as a pilot study, the present study used 20% NACP and 50% glass with a total filler level of 70% which were readily mixed manually, using a plastic spatula, into a cohesive composite paste. The glass filler level could likely be further increased (perhaps to 55%) for enhanced reinforcement to further improve the Kic. Instead of hand mixing, further study should investigate the use of a mixing machine and the application of vacuum to increase the mixing thoroughness, raise the filler level, and reduce air bubbles in order to further increase the Kic.
DMAHDM mass fraction was a key property affecting the antibacterial potency of the composite. Increasing DMAHDM mass fraction from 0% to 3% decreased biofilm CFU by 3 log. Caries is a dietary carbohydrate-modified bacterial infectious disease caused by acid production by biofilms in vivo. Dental plaque microcosm biofilm is a complex oral microbial community that covers dental restorative materials and teeth in vivo.52 Bacteria play a central role in dental caries on enamel and root surfaces.53 Therefore, developing a composite with antibacterial property to inhibit biofilm growth is of utter most interest. The combination of QAS with methacrylate group to form an antibacterial monomer is proved to be an effective method.54 The QAS structure is responsible for the antibacterial ability while the aliphatic vinyl group allows for copolymerization with other conventional dental monomers. Hence, the QAMs are immobilized into the polymer backbone through chemical bonding, and will not be released or lost after curing, thus yielding a long-term antibacterial activity. QAM immobilization can overcome the negative effects of traditional agent-releasing antibacterial materials, including the decrease of antibacterial activity overtime, and toxic effects of the released agents.55 After the development of MDPB-containing dental resins,54,56 several different polymerizable QAMs were developed for use in antibacterial dental restorative materials.22,24,25,27,28,57
QAMs possess bacteriolysis effects, because the positively-charged ammonium group can interact with the negatively-charged bacterial membrane to disrupt the membrane functions, alter the balance of essential ions (i.e., K+, Na+, Ca2+, and Mg2+), interrupt protein activity, and damage bacterial DNA.28,29 Therefore, charge density is expected to affect the antibacterial potency, and charge density can be varied by varying the QAM mass fraction in the resin. In the present study, the DMAHDM mass fraction was increased from 0% to 3% in the NACP nanocomposite. Recent studies showed that when DMAHDM mass fraction was increased in adhesive, the charge density on adhesive resin increased, which was responsible for the stronger antibacterial function.28,38,44 Indeed, increasing the DMAHDM mass fraction from 0% to 3% in the NACP nanocomposite greatly decreased the lactic acid production and biofilm metabolic activity. Lactic acid production was reduced by two orders of magnitude, compared to that of the control. Metabolic activity of biofilms was reduced by 25 folds. Furthermore, the composite with 3% DMAHDM reduced the biofilm CFU by three orders of magnitude. These results were achieved without sacrificing the mechanical properties of the composite. Hence, NACP nanocomposite containing the new antibacterial DMAHDM is promising for applications in dental restorations to inhibit biofilm/plaque buildup and reduce caries occurrence.
The benefit of incorporating NCAP into the composite is Ca and P ion release and remineralization. Although many studies focused on developing QAMs, only a few recent reports incorporated QAM and calcium phosphate together into the same polymer matrix to obtain the double benefits of antibacterial and remineralization capabilities.27 The present study focused on fracture toughness and microcosm biofilm response, and did not investigate the remineralization aspect of the composite. However, several recent reports already showed that NACP nanocomposite was “smart” and released high levels of Ca and P ions at cariogenic pH when these ions were most needed to inhibit caries,33 and NACP nanocomposite could rapidly neutralize acid challenges to avoid demineralization.32 Furthermore, the NACP nanocomposite was able to remineralize enamel lesions in vitro,60 and inhibit secondary caries at the composite-enamel margins in an in situ study involving 25 participants.34 These results were achieved without an antibacterial agent in the NACP nanocomposite. Therefore, the novel DMAHDM-NACP nanocomposite of the present study, with both antibacterial and remineralization properties, has the potential to be used as a caries-inhibiting restorative material. The DMAHDM-NACP nanocomposite had three advantages: good fracture toughness, strong antibacterial potency to combat biofilm growth and acid production, and NACP for remineralization and caries inhibition (which were shown in previous studies). The DMAHDM-NACP nanocomposite may be promising for caries-inhibiting dental restorations, and the method of using double agents (DMAHDM and NACP) may have a wide applicability to other dental materials including flowable composites, bonding agents, sealants, orthodontic cements and crown cements.
This study focused on the effect of DMAHDM mass fraction in the composite on the Kic and anti-biofilm properties in the short-term. Further study is needed to investigate longer term properties, such as Kic and anti-biofilm properties vs. time for 3 months, 6 months and 1 year. In addition, the present study investigated the DMAHDM mass fraction of 0% to 3% in the composite, because mass% larger than 3% reduced Kic in preliminary study. Figs. 2-5 showed a decreasing trend in biofilm growth vs. increasing DMAHDM mass%. Further study is needed to investigate mechanical reinforcement methods so that the DMAHDM mass% and hence the antibacterial potency can be further increased, without compromising the Kic. Regarding the clinical importance of the results, the antibacterial NACP nanocomposite without compromising fracture toughness is promising for use in tooth cavity restorations to inhibit biofilm acids and minimize the occurrence of recurrent caries. In particular, tooth root caries and Class V restorations with subgingival margins may be difficult to clean with pockets for bacterial growth, leading to gingivitis and periodontitis. The antibacterial NACP nanocomposite can be beneficial in suppressing not only acidogenic bacteria but also periodontal pathogens, while NACP can neutralize acids and remineralize the tooth structure to protect the roots. Further studies are needed to improve the resin-filler particle mixing, increase the filler level and minimize porosity, and determine the antibacterial efficacy in the oral environment using a human in situ model.
5. Conclusions
This study developed DMAHDM-NACP nanocomposite for the double benefits of antibacterial and remineralizing functions, and determined the effects of DMAHDM content in NACP nanocomposite on Kic and biofilm response for the first time. Within the limitations of the present study, the following could be concluded. Increasing the DMAHDM mass fraction in NACP nanocomposite from 0% to 3% did not adversely affect Kic, which was within the reported range of Kic for commercial dental composites. Increasing the DMAHDM mass fraction in NACP nanocomposite from 0% to 3% decreased the biofilm CFU by three orders of magnitude, and reduced lactic acid by two orders of magnitude. Therefore, the hypotheses of this study were proven that incorporating DMAHMD into NACP nanocomposite did not adversely affect the facture toughness; incorporating DMAHMD exerted a strong antibacterial activity; and the biofilm growth and acid production monotonically decreased with increasing DMAHDM mass fraction.
Acknowledgments
We thank Esstech (Essington, PA) for generously donating the BisGMA and TEGDMA monomers, and Drs. Laurence C. Chow, Joseph M. Antonucci and Ashraf F. Fouad for fruitful discussions. This study was supported by NIH R01 DE17974 (HX), ZR2014HM073 (JLW) from Shandong Provincial Natural Science Foundation in China, and a Seed Grant (HX) from the University of Maryland School of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. American Journal of Dentistry. 2009;22:3–8. [PubMed] [Google Scholar]
- 2.Ferracane JL. Current trends in dental composites. Critical Reviews in Oral Biology & Medicine. 1995;6:302–18. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 3.Lynch CD. Successful posterior composites. Quintessence Publishing Co.; London: 2008. [Google Scholar]
- 4.Ferracane JL. Resin composite-State of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dental Materials. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. Journal of Dental Research. 2005;84:822–6. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 7.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Spencer P, Ye Q, Misra A, Goncalves SE, Laurence JS. Proteins, pathogens, and failure at the composite-tooth interface. Journal of Dental Research. 2014;93:1243–9. doi: 10.1177/0022034514550039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, et al. Adhesive/Dentin interface: the weak link in the composite restoration. Annals of Biomedical Engineering. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dental Materials. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Journal of Dental Research. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. Journal of the American Dental Association. 2011;142:612–20. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 14.Bohaty BS, Ye Q, Misra A, Sene F, Spencer P. Posterior composite restoration update: focus on factors influencing form and function. Journal of Clinical, Cosmetic and Investigational Dentistry. 2013;5:33–42. doi: 10.2147/CCIDE.S42044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mjor IA, Toffeneti F. Secondary caries: a literature review with caries reports. Quintessence International. 2000;31:165–79. [PubMed] [Google Scholar]
- 16.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Primary Dental Care. 2002;9:31–6. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 17.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 18.Gong SQ, Niu LN, Kemp LK, Yiu CK, Ryou H, Qi YP, et al. Quaternary ammonium silane-functionalized, methacrylate resin composition with antimicrobial activities and self-repair potential. Acta Biomaterialia. 2012;8:3270–82. doi: 10.1016/j.actbio.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Luo XJ, Niu LN, Liu SY, Zhu WC, Epasinghe J, et al. One-pot synthesis of antibacterial monomers with dual biocidal modes. Journal of Dentistry. 2014;42:1078–95. doi: 10.1016/j.jdent.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. Journal of Dental Research. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 21.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. Journal of Dentistry. 2009;37:289–96. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dental Materials. 2011;27:487–96. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:1511–62. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng L, Zhang K, Weir MD, Liu H, Zhou X, Xu HH. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dental Materials. 2013;29:462–72. doi: 10.1016/j.dental.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29:859–70. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou H, Li F, Weir MD, Xu HH. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. Journal of Dentistry. 2013;41:1122–31. doi: 10.1016/j.jdent.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melo MA, Guedes SF, Xu HH, Rodrigues LK. Nanotechnology-based restorative materials for dental caries management. Trends in Biotechnology. 2013;31:459–67. doi: 10.1016/j.tibtech.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. Journal of Dental Research. 1996;75:1679–86. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 31.Langhorst SE, O'Donnell JN, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dental Materials. 2009;25:884–91. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau JL, Sun L, Chow LC, Xu HH. Mechanical and acid neutralizing properties and inhibition of bacterial growth of amorphous calcium phosphate dental nanocomposite. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;98:80–8. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dental Materials. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melo MA, Weir MD, Rodrigues LK, Xu HH. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dental Materials. 2013;29:231–40. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, et al. Strong nanocomposites with Ca, PO4 and F release for caries inhibition. Journal of Dental Research. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:345–55. doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F, Weir MD, Xu HH. Effects of quaternary ammonium chain length on antibacterial bonding agents. Journal of Dental Research. 2013;92:932–8. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuebler J. Fracture toughness of ceramics using the SEVNB method; Round robin. EMPA; CH-8600 Duebendorf, Switzerland: 1999. VAMAS Report No. 37, TWA3, ESIS Report D2-99. [Google Scholar]
- 40.Xu HH, Quinn JB, Giuseppetti AA. Wear and mechanical properties of nano-silica-fused whisker composites. Journal of Dental Research. 2004;83:930–5. doi: 10.1177/154405910408301208. [DOI] [PubMed] [Google Scholar]
- 41.McBain AJ. In vitro biofilm models: an overview. Advances in Applied Microbiology. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 42.Pratten J, Wilson M, Spratt DA. Characterization of in vitro oral bacterial biofilms by traditional and molecular methods. Oral Microbiology and Immunology. 2003;18:45–9. doi: 10.1034/j.1399-302x.2003.180107.x. [DOI] [PubMed] [Google Scholar]
- 43.Cheng L, Exterkate RA, Zhou X, Li J, ten Cate JM. Effect of Galla chinensis on growth and metabolism of microcosm biofilms. Caries Research. 2011;45:87–92. doi: 10.1159/000324084. [DOI] [PubMed] [Google Scholar]
- 44.Li F, Weir MD, Xu HH. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dental Materials. 2014;30:433–41. doi: 10.1016/j.dental.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. Journal of Dentistry. 1998;26:267–71. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 46.McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. Development and characterization of a simple perfused oral microcosm. Journal of Applied Microbiology. 2005;98:624–34. doi: 10.1111/j.1365-2672.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- 47.Cheng L, Weir MD, Zhang K, Wu E, Xu SM, Zhou XD, et al. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dental Materials. 2012;28:853–62. doi: 10.1016/j.dental.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng L, Zhang K, Melo MA, Weir MD, Zhou XD, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau JL, Weir MD, Giuseppetti AA, Chow LC, Antonucci JM, Xu HH. Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:1264–73. doi: 10.1002/jbm.b.32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferracane JL, Antonio RC, Matsumoto H. Variables affecting the fracture toughness of dental composites. Journal of Dental Research. 1987;66:1140–5. doi: 10.1177/00220345870660060901. [DOI] [PubMed] [Google Scholar]
- 51.Ilie N, Hickel R, Valceanu AS, Huth KC. Fracture toughness of dental restorative materials. Clinical Oral Investigations. 2012;16:489–98. doi: 10.1007/s00784-011-0525-z. [DOI] [PubMed] [Google Scholar]
- 52.Tanner J, Robinson C, Soderling E, Vallittu P. Early plaque formation on fibre-reinforced composites in vivo. Clinical Oral Investigations. 2005;9:154–60. doi: 10.1007/s00784-005-0317-4. [DOI] [PubMed] [Google Scholar]
- 53.Clark J. On the bacterial factor in the aetiology of dental caries. British Journal of Experimental Pathology. 1992;5:141–7. [Google Scholar]
- 54.Imazato S, Ma S, Chen JH, Xu HH. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dental Materials. 2014;30:97–104. doi: 10.1016/j.dental.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28:11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 56.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 57.He J, Söderling E, Österblad M, Vallittu PK, Lassila LV. Synthesis of methacrylate monomers with antibacterial effects against S. Mutans. Molecules. 2011;16:9755–63. doi: 10.3390/molecules16119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simoncic B, Tomcis B. Structures of novel antimicrobial agents for textiles-a review. Textile Research Journal. 2010;80:1721–37. [Google Scholar]
- 59.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surface-2:How high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–9. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. Journal of Dental Research. 2012;91:979–84. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]