Abstract
We have measured the alkane and benzene-based molecules with aldehyde and carboxylic acid as anchoring groups by using the electrochemical jump-to-contact scanning tunneling microscopy break junction (ECSTM-BJ) approach. The results show that molecule with benzene backbone has better peak shape and intensity than those with alkane backbone. Typically, high junction formation probability for same anchoring group (aldehyde and carboxylic acid) with benzene backbone is found, which contributes to the stronger attractive interaction between Cu and molecules with benzene backbone. The present work shows the import role of backbone in junction, which can guide the design molecule to form effective junction for studying molecular electronics.
Keywords: Single-molecule junctions, ECSTM-BJ, Junction formation probability, Carboxylic acid, Aldehyde
Background
In recent years, single-molecule junctions have attracted wide attention because of its potential application in nano-electronic and molecular electronic device [1–10]. At present stage, it is important to fully understand the electron transport of single-molecule junctions and its influence factors [11]. Many factors can affect the conductance of single-molecule junctions, such as anchoring group, molecule structure, the contact configuration between molecule and electrode, and temperature [5, 7, 12–17]. Among them, anchoring group is very important in forming the molecular junction, and it was found that different anchoring groups have different junction formation probabilities [14, 18–20]. However, another interesting question is still unclear that how molecular structure would influence the junction formation probability for same anchoring group.
In this work, we will focus on the junction formation probability of same anchoring group with different molecular structures (saturated and conjugated structure) by using electrochemical jump-to-contact scanning tunneling microscopy break junction (ECSTM-BJ) approach (Fig. 1a) [21, 22]. Aldehyde and carboxylic acid anchoring groups binding to Cu electrode are used in the current study, for they have been demonstrated to form effective junctions [23–25]. We use 1,4-benzenedicarboxaldehyde, glutaraldehyde, 1,4-benzenedicarboxylic acid and pentanedioic acid as target molecules (Fig. 1b) to study the influence of different structures on the junction formation probability. Those molecules have different backbones with saturated (alkane) or conjugated (benzene) structure.
Fig. 1.
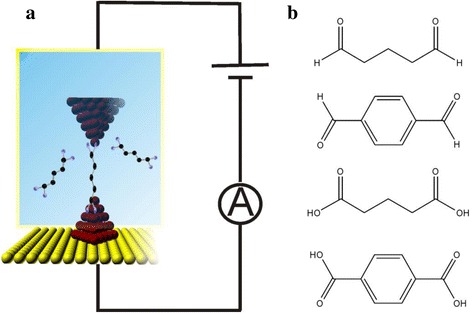
The schematic diagram of ECSTM-BJ and molecular structure. a Schematic diagram of electrochemical jump-to-contact scanning tunneling microscopy break junction (ECSTM-BJ) approach in solution containing target molecule. b Molecular structures of glutaraldehyde, 1,4-benzenedicarboxaldehyde, pentanedioic acid and 1,4-benzenedicarboxylic acid
Methods
Na2SO4 (99.995 %) and CuSO4 (99.999 %) were purchased from Alfa-Aesar, while petanedioic acid, 1,4-benzenedicarboxylic acid, glutaraldehyde, and 1,4-benzenedicarboxaldehyde were purchased from Sigma-Aldrich. Ultrapure water (≥18.2 MΩ cm) was used for preparing aqueous solutions. Naturally formed Au(111) was used as the substrate, and cut Pt-Ir STM tip was covered with thermosetting glue to reduce the electrochemical current. Meantime, Pt and Cu wires were used as the counter and reference electrodes, respectively.
The conductance measurement was performed by ECSTM-BJ approach on the modified Nanoscope IIIa STM (Veeco, USA); especially, preamplifier with four-out linear current-to-voltage converters was used [23]. The experiment was carried out in aqueous solution containing saturated target molecule + 1 mM CuSO4 + 50 mM Na2SO4 as following: Firstly, the tip potential was set at −5 mV to allow the bulk deposition of Cu. Secondly, after applying the pulse voltage on z-piezo, the deposited Cu on the tip would transfer to the substrate and build a metallic contact due to the tip closed to the surface. Thirdly, Cu atomic wire could be formed during the separation of tip and substrate with 20 nm/s, and then, molecule could simultaneously bridge to the both electrodes upon the breaking of metal atomic wire. The tip current vs. distance curves were recorded with sampling frequency of 20 kHz. More detailed procedure can be seen in our previously reports [22, 23].
Results and Discussion
Comparative Study on Single-Molecule Conductance of Glutaraldehyde and 1,4-Benzenedicarboxaldehyde with Cu Electrode
We firstly measure the conductance of Cu-glutaraldehyde-Cu junction. Conductance curves with obvious step can be seen in Fig. 2a and then were treated by logarithm and binning to construct the histogram (Fig. 2c). A peak at 10−3.45 G0 (27 nS) is found in the Fig. 2c. Comparing with glutaraldehyde, pronounced peak at 10−3.6 G0 (19 nS) is found for 1,4-benzenedicarboxaldehyde (Fig. 2b, d), and this value is consistent with our previously report [23]. Obviously, the peak intensity of 1,4-benzenedicarboxaldehyde is higher than that of glutaraldehyde. And the different intensity of peaks between glutaraldehyde and 1,4-benzenedicarboxaldehyde in the histograms may show internal property of benzene and alkane backbone.
Fig. 2.
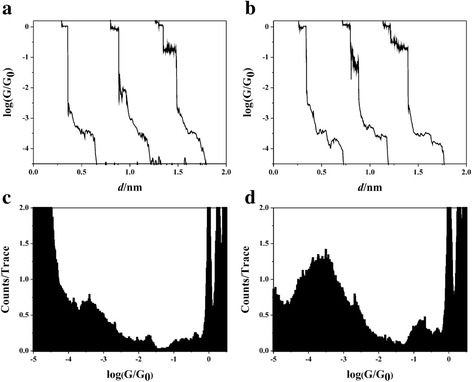
Single-molecule conductance histograms of pentanedioic acid and glutaraldehyde. A log-scale conductance curves of a Cu-glutaraldehyde-Cu junctions and b Cu-1,4-benzenedicarboxaldehyde-Cu junctions. Log-scale conductance histogram of c Cu-glutaraldehyde-Cu junctions and d Cu-terephthalaldehyde-Cu junctions
Then, we also construct histograms by using linear bin-size. Obvious difference is again observed for those molecules in Fig. 3. While rather weak peak can be found for petanedioic acid (Fig. 3a), pronounced peak is shown for 1,4-benzenedicarboxaldehyde (Fig. 3b). Conductance values of 4 and 11 nS are found for 1,4-benzenedicarboxaldehyde, which is different from the conductance value shown in log-scale histogram (Fig. 2d). This is caused by the different statistical methods between linear and logarithm bin-size, and different molecule-electrode configurations are shown in those histograms [23]. As our previously report, we can also obtain all conductance values of 4, 11, and 20 nS using data selection with linear bin-size [23]. However, the linear-bin histograms show even large difference of intensity between molecules with benzene and alkane backbone.
Fig. 3.
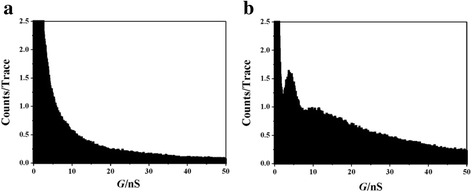
Comparison of the linear-scale conductance histograms of glutaraldehyde and 1,4-benzenedicarboxaldehyde. a The linear-scale conductance histogram of a Cu-glutaraldehyde-Cu junctions and b Cu-1,4-benzenedicarboxaldehyde-Cu junctions
Return back to the difference histograms between benzene and alkane backbone, the weak peak can be caused by the less probability in the forming the molecular junctions. Usually, the junction formation probability can be analyzed by stretched distance distribution [26, 27] or counting the number of curves with step [28, 29] as previously reports. Here, we manually analyze the opportunity of step (typically, the curve with step length longer than 0.05 nm) in conductance curves showing step value smaller than 10−2 G0, it is found that step opportunity is around 40 % in forming junction of 1,4-benzenedicarboxaldehyde, while around 22 % for glutaraldehyde. From above, we can conclude that the anchoring group of aldehyde with benzene backbone has high junction formation probability than that with alkane backbone connecting with Cu electrode, and we will discuss it later.
Comparative Study on Single-Molecule Conductance of Pentanedioic Acid and 1,4-Benzenedicarboxylic Acid with Cu Electrode
In order to prove the role of backbone in forming molecular junction, we also use carboxylic acid as the anchoring group to comparing difference between petanedioic acid and 1,4-benzenedicarboxylic acid. As shown in Fig. 4, similar behavior is also found that 1,4-benzenedicarboxylic acid shows more pronounced peak comparing with petanedioic acid. Again, different conductance values are found in different statistical method between linear and log bin-size. According to Fig. 4, the junction formation probability of 1,4-benzenedicarboxylic acid is higher than that of petanedioic acid in both linear-scale and log-scale statistical histograms. We found that the step opportunity of 1,4-benzenedicarboxylic acid and petanedioic acid is around 51 % and 33 %, respectively, which illustrates the similar results as the 1,4-benzenedicarboxaldehyde and glutaraldehyde. However, molecules with carboxylic acid have larger junction formation probability than those with aldehyde anchoring group; this may be caused by that carboxylic acid can also bind to the Cu through carboxylate form with two O atoms binding to the electrode, while only one O atom can bind to the electrode for aldehyde group.
Fig. 4.
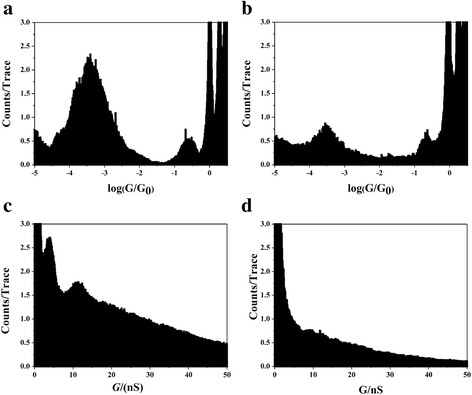
Comparison of linear-scale and log-scale conductance histogram of 1,4-benzenedicarboxylic acid and petanedioic acid. The log-scale conductance histograms of a Cu-1,4-benzenedicarboxylic acid-Cu junctions and b Cu-petanedioic acid-Cu junctions. The linear-scale conductance histograms of c Cu-1,4-benzenedicarboxylic acid-Cu junctions and d Cu-petanedioic acid-Cu junctions
The Role of Backbone in Forming Molecular Junction
According to above results, those molecules with benzene backbone have higher junction formation probabilities than those with alkane backbone connecting with Cu electrode, which should be caused by the stronger interaction between anchoring group and Cu in 1,4-benzenedicarboxaldehyde and 1,4-benzenedicarboxylic acid.
Taking carboxylic acid as example, carboxylic acid binds to the Cu electrode through carboxylate group [30]. It was reported that the bond length of Cu-O for benzene system is shorter than that of alkane system, which may reveal that benzene-based molecule and Cu system has stronger attractive interaction than that of alkane-based molecule and Cu system [30, 31]. This can explain our result that the junction formation probability of 1,4-benzenedicarboxylic acid is higher than that of petanedioic acid. We deduce the similar situation for molecules with aldehyde anchoring group, since similar Cu–O bond is formed in the junctions [23]. The current work shows the import role of backbone in forming molecular junctions and may help the design of molecule in studying the electron transport of single-molecule junction.
Conclusions
In this work, we have measured the single molecular junction conductance of molecules with aldehyde and carboxylic acid anchoring groups. It has been found that the structure of backbone can influence the junction formation probability for same anchoring group (aldehyde and carboxylic acid), which contributes to the stronger attractive interaction between Cu and molecules with benzene backbone. Those results can guide the design molecule to form effective junction for studying molecular electronics.
Acknowledgements
We gratefully thank Zhejiang Provincial Natural Science Foundation of China (No. LR15B030002) and the financial support by the National Natural Science Foundation of China (Nos. 21573198 and 21273204).
Authors’ Contributions
FC, LLP, and ZWH carried out the experiments; JCM and JFZ analyzed the results. FC, YS, ZJN, and XSZ conceived and designed the experiments, analyzed the results, and wrote the manuscript. All authors read and approved the final manuscript.
Authors’ Information
FC is a Master’s degree student under the supervision of XSZ in the Institute of Physical Chemistry, Zhejiang Normal University, China.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Xiang D, Wang X, Jia C, Lee T, Guo X. Molecular-scale electronics: from concept to function. Chem Rev. 2016;116:4318–4440. doi: 10.1021/acs.chemrev.5b00680. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Rudnev AV, Hong W, Wandlowski T. Break junction under electrochemical gating: testbed for single-molecule electronics. Chem Soc Rev. 2015;44:889–901. doi: 10.1039/C4CS00242C. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo CR, Frisenda R, Moth-Poulsen K, Seldenthuis JS, Bjornholm T, van der Zant HSJ. Quantum interference effects at room temperature in OPV-based single-molecule junctions. Nanoscale Res Lett. 2013;8:1–6. doi: 10.1186/1556-276X-8-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkataraman L, Klare JE, Nuckolls C, Hybertsen MS, Steigerwald ML. Dependence of single-molecule junction conductance on molecular conformation. Nature. 2006;442:904–907. doi: 10.1038/nature05037. [DOI] [PubMed] [Google Scholar]
- 5.Sedghi G, Garcia-Suarez VM, Esdaile LJ, Anderson HL, Lambert CJ, Martin S, Bethell D, Higgins SJ, Elliott M, Bennett N, et al. Long-range electron tunnelling in oligo-porphyrin molecular wires. Nat Nanotechnol. 2011;6:517–523. doi: 10.1038/nnano.2011.111. [DOI] [PubMed] [Google Scholar]
- 6.Guo CL, Wang K, Zerah-Harush E, Hamill J, Wang B, Dubi Y, Xu BQ. Molecular rectifier composed of DNA with high rectification ratio enabled by intercalation. Nat Chem. 2016;8:484–490. doi: 10.1038/nchem.2480. [DOI] [PubMed] [Google Scholar]
- 7.Darwish N, Aragones AC, Darwish T, Ciampi S, Diez-Perez I. Multi-responsive photo- and chemo-electrical single-molecule switches. Nano Lett. 2014;14:7064–7070. doi: 10.1021/nl5034599. [DOI] [PubMed] [Google Scholar]
- 8.Fujii S, Tada T, Komoto Y, Osuga T, Murase T, Fujita M, Kiguchi M. Rectifying electron-transport properties through stacks of aromatic molecules inserted into a self-assembled cage. J Am Chem Soc. 2015;137:5939–5947. doi: 10.1021/jacs.5b00086. [DOI] [PubMed] [Google Scholar]
- 9.Manrique DZ, Huang C, Baghernejad M, Zhao X, Al-Owaedi OA, Sadeghi H, Kaliginedi V, Hong W, Gulcur M, Wandlowski T, et al. A quantum circuit rule for interference effects in single-molecule electrical junctions. Nat Commun. 2015;6:6389. doi: 10.1038/ncomms7389. [DOI] [PubMed] [Google Scholar]
- 10.Zhou XS, Mao BW, Amatore C, Compton RG, Marignier J-L, Mostafavi M, Nierengarten J-F, Maisonhaute E. Transient electrochemistry: beyond simply temporal resolution. Chem Commun. 2016;52:251–263. doi: 10.1039/C5CC07953E. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Diaz-Fernandez YA, Gschneidtner TA, Westerlund F, Lara-Avila S, Moth-Poulsen K. Single-molecule electronics: from chemical design to functional devices. Chem Soc Rev. 2014;43:7378–7411. doi: 10.1039/C4CS00143E. [DOI] [PubMed] [Google Scholar]
- 12.Tao NJ. Electron transport in molecular junctions. Nat Nanotechnol. 2006;1:173–181. doi: 10.1038/nnano.2006.130. [DOI] [PubMed] [Google Scholar]
- 13.Rascón-Ramos H, Artés JM, Li Y, Hihath J. Binding configurations and intramolecular strain in single-molecule devices. Nat Mater. 2015;14:517–522. doi: 10.1038/nmat4216. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Li XL, Hihath J, Huang ZF, Tao NJ. Effect of anchoring groups on single-molecule conductance: comparative study of thiol-, amine-, and carboxylic-acid-terminated molecules. J Am Chem Soc. 2006;128:15874–15881. doi: 10.1021/ja065864k. [DOI] [PubMed] [Google Scholar]
- 15.Aradhya SV, Meisner JS, Krikorian M, Ahn S, Parameswaran R, Steigerwald ML, Nuckolls C, Venkataraman L. Dissecting contact mechanics from quantum interference in single-molecule junctions of stilbene derivatives. Nano Lett. 2012;12:1643–1647. doi: 10.1021/nl2045815. [DOI] [PubMed] [Google Scholar]
- 16.Capozzi B, Xia J, Adak O, Dell EJ, Liu Z-F, Taylor JC, Neaton JB, Campos LM, Venkataraman L. Single-molecule diodes with high rectification ratios through environmental control. Nat Nanotechnol. 2015;10:522–527. doi: 10.1038/nnano.2015.97. [DOI] [PubMed] [Google Scholar]
- 17.Zhou XY, Wang YH, Qi HM, Zheng JF, Niu ZJ, Zhou XS. Single-molecule conductance of dipyridines binding to Ag electrodes measured by electrochemical scanning tunneling microscopy break junction. Nanoscale Res Lett. 2014;9:77. doi: 10.1186/1556-276X-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaliginedi V, Rudnev AV, Moreno-Garcia P, Baghernejad M, Huang C, Hong W, Wandlowski T. Promising anchoring groups for single-molecule conductance measurements. Phys Chem Chem Phys. 2014;16:23529–23539. doi: 10.1039/C4CP03605K. [DOI] [PubMed] [Google Scholar]
- 19.Cheng ZL, Skouta R, Vazquez H, Widawsky JR, Schneebeli S, Chen W, Hybertsen MS, Breslow R, Venkataraman L. In situ formation of highly conducting covalent Au-C contacts for single-molecule junctions. Nat Nanotechnol. 2011;6:353–357. doi: 10.1038/nnano.2011.66. [DOI] [PubMed] [Google Scholar]
- 20.Mishchenko A, Zotti LA, Vonlanthen D, Bürkle M, Pauly F, Cuevas JC, Mayor M, Wandlowski T. Single-molecule junctions based on nitrile-terminated biphenyls: a promising new anchoring group. J Am Chem Soc. 2011;133:184–187. doi: 10.1021/ja107340t. [DOI] [PubMed] [Google Scholar]
- 21.Zhou XS, Wei YM, Liu L, Chen ZB, Tang J, Mao BW. Extending the capability of STM break junction for conductance measurement of atomic-size nanowires: an electrochemical strategy. J Am Chem Soc. 2008;130:13228–13230. doi: 10.1021/ja8055276. [DOI] [PubMed] [Google Scholar]
- 22.Zhou XS, Liang JH, Chen ZB, Mao BW. An electrochemical jump-to-contact STM-break junction approach to construct single molecular junctions with different metallic electrodes. Electrochem Commun. 2011;13:407–410. doi: 10.1016/j.elecom.2011.02.005. [DOI] [Google Scholar]
- 23.Hong ZW, Chen F, Wang YH, Mao JC, Li DF, Tang Y, Shao Y, Niu ZJ, Zhou XS. The binding sites of carboxylic acid group contacting to Cu electrode. Electrochem Commun. 2015;59:48–51. doi: 10.1016/j.elecom.2015.07.003. [DOI] [Google Scholar]
- 24.Peng ZL, Chen ZB, Zhou XY, Sun YY, Liang JH, Niu ZJ, Zhou XS, Mao BW. Single molecule conductance of carboxylic acids contacting Ag and Cu electrodes. J Phys Chem C. 2012;116:21699–21705. doi: 10.1021/jp3069046. [DOI] [Google Scholar]
- 25.Wang YH, Hong ZW, Sun YY, Li DF, Han D, Zheng JF, Niu ZJ, Zhou XS. Tunneling decay constant of alkanedicarboxylic acids: different dependence on the metal electrodes between air and electrochemistry. J Phys Chem C. 2014;118:18756–18761. doi: 10.1021/jp505374v. [DOI] [Google Scholar]
- 26.Hong W, Manrique DZ, Moreno-García P, Gulcur M, Mishchenko A, Lambert CJ, Bryce MR, Wandlowski T. Single molecular conductance of tolanes: experimental and theoretical study on the junction evolution dependent on the anchoring group. J Am Chem Soc. 2012;134:2292–2304. doi: 10.1021/ja209844r. [DOI] [PubMed] [Google Scholar]
- 27.Yoo PS, Kim T. High probability of single molecule junction formation with Ag electrodes. Curr Appl Phys. 2015;15:124–128. doi: 10.1016/j.cap.2014.11.016. [DOI] [Google Scholar]
- 28.Li XL, He J, Hihath J, Xu BQ, Lindsay SM, Tao NJ. Conductance of single alkanedithiols: conduction mechanism and effect of molecule-electrode contacts. J Am Chem Soc. 2006;128:2135–2141. doi: 10.1021/ja057316x. [DOI] [PubMed] [Google Scholar]
- 29.Li ZH, Pobelov I, Han B, Wandlowski T, Blaszczyk A, Mayor M. Conductance of redox-active single molecular junctions: an electrochemical approach. Nanotechnology. 2007;18:044018. doi: 10.1088/0957-4484/18/4/044018. [DOI] [Google Scholar]
- 30.Lennartz MC, Atodiresei N, Müller-Meskamp L, Karthäuser S, Waser R, Blügel S. Cu-adatom-mediated bonding in close-packed benzoate/Cu(110)-systems. Langmuir. 2009;25:856–864. doi: 10.1021/la801822e. [DOI] [PubMed] [Google Scholar]
- 31.Barbosa LAMM, Sautet P. Stability of chiral domains produced by adsorption of tartaric acid isomers on the Cu(110) surface: a periodic density functional theory study. J Am Chem Soc. 2001;123:6639–6648. doi: 10.1021/ja004336k. [DOI] [PubMed] [Google Scholar]


