Abstract
Marie Sklodowska-Curie, an extraordinary woman, a Polish scientist who lived and worked in France, led to the development of nuclear energy and the treatment of cancer. She was the laureate of two Nobel Prizes, the first woman in Europe who obtained the degree of Doctor of Science and opened the way for women to enter fields which had been previously reserved for men only. As a result of her determination and her love of freedom, she has become an icon for many female scientists active in radiation sciences. They are successors of Maria Curie and without the results of their work, improvement in radiation oncology will not be possible. Many of them shared some elements of Maria Curie's biography, like high ethical and moral standards, passionate dedication to work, strong family values, and scientific collaboration with their husbands. The significance of Tikvah Alper, Alma Howard, Shirley Hornsey, Juliana Denekamp, Helen Evans, Eleanor Blakely, Elizabeth L. Travis, Fiona Stewart, Andree Dutreix, Catharine West, Peggy Olive, Ingela Turesson, Penny Jeggo, Irena Szumiel, Eleonor Blakely, Sara Rockwell and Carmel Mothersill contribution to radiation oncology is presented. All the above mentioned ladies made significant contribution to the development of radiotherapy (RT) and more efficient cancer treatment. Due to their studies, new schedules of RT and new types of ionizing radiation have been applied, lowering the incidence of normal tissue toxicity. Their achievements herald a future of personalized medicine.
Keywords: Marie Curie, Radiobiology, Ionizing radiation, Tumor radiobiology
“Therapy should be permanently backed up by scientific research without which no progress is possible. Moreover, the search for pure knowledge is one of the important needs of mankind…”.
Marie Curie (From Marie Curie's speech made at the opening of the Radium Institute in Warsaw, May 1932)
In 1911, Marie Sklodowska-Curie was awarded her second Nobel Prize for the discovery of radium. Her pioneering work demonstrated that radiation is a powerful tool with a wide range of potential applications, which include a range of diagnostic and therapeutic medical procedures. Marie Curie has left a great deal to the world. Her work led to the development of nuclear energy and radiotherapy (RT) for the treatment of cancer. She also improved the image of science. Marie Curie, the first woman in Europe to obtain the degree of Doctor of Science, the first female professor at Sorbonne, opened the way for women to enter fields which had been previously reserved for men. As a result of her determination and her love of freedom, she has become a role model for 20th century female radiobiologists presented here and who continue Marie Curie's legacy development of radiotherapy. It is therefore worthwhile to highlight her many achievements and her personality, which make her a role model for scientists today.
Marie Sklodowska-Curie was born in Warsaw, Poland, on 7 November 1867, and because women were not allowed to study at Polish universities she left the country to continue her education in France. She won her licenciate in physics at the Faculty des Science at Sorbonne already in 1893, and in mathematics a year later.
In 1894 she was introduced to Pierre Curie (1859–1906), a French physicist. Soon after, Pierre realized he had found a matching soul and wrote to Marie: “How wonderful would it be if we could spend our lives together, following our ideas. Your patriotic ideas, and our common ideas of humanity and scientific research”. Their marriage (25 July 1895) marked the beginning of a remarkable partnership. They worked in an old wooden shed, with a skylight roof, a former prosectorium of a medical school. Pierre concentrated on physics, Marie on chemistry, she was mainly occupied with isolating uranium salts from tones of ores. Marie published her first research paper on magnetism of tempered steel.1 She presented the results of her solo work on Becquerel's rays and suggested the existence of a new element. Pierre Curie joined her research work in March 1898. The Curies’ investigation of the radiation in uranium ore led, in 1898, to the discovery of two highly radioactive new chemical elements, polonium (named by Marie in honor of Poland) and radium.2, 3
Marie struggled to obtain pure radium in the metallic state. When this was achieved, the Curies idealistically resigned from the right to patent the radium isolation method. They published all the results of their studies along with the detailed manufacturing processes and offered free information on the subject. Marie Curie wrote: “Radium is a chemical element, a property of all humans”.
In 1903, Marie Sklodowska-Curie received both her doctoral degree for her studies on radioactive substances and, together with her husband and Henri Becquerel, the Nobel Prize in physics. But the prize nearly remained in the male company. Only Pierre Curie and H. Becquerel were initially nominated as candidates, while Marie's contribution was ignored. Marie's role was finally acknowledged after Pierre Curie wrote to the nominating committee saying that either he and his wife were honored together or he would resign from his candidacy. This is the example of the Curies’ supportive relationship.
After Pierre Curie's sudden death in an accident on 19 April 1906, Marie was appointed to succeed him as head of the physics department at the Sorbonne.
In 1911, she received the Nobel Prize in chemistry for her work on the isolation of metallic radium. In the same year she took part in the Solway Conference, where she was the only woman amongst men-scientists that included Niels Bohr, Max Planck and Albert Einstein.
Due to Marie Sklodowska-Curie's efforts, the Radium Intitute in Paris (Pierre's dream) was founded in 1912. In 1914, Marie became head of the Curies Pavilion and worked there until her death. The social impact of Marie Curie's work can be seen during the World War I, when she organized a mobile radiographic unit and took it to the battlefront where she frequently operated it herself examining the wounded and training radiographic assistants. At a rough estimate, as a result of Marie Curie's involvement more than 150 radiologists were trained at her courses; over 200 X-ray laboratories (including 20 mobile units) were organized for military hospitals, and more than a million soldiers were helped by radiography before surgery.
In 1921 and 1929, Marie Curie made two triumphant journeys to the United States. During the first trip, the President of the United States, Warren Harding, presented her with a gram of radium that had been bought by American women. She donated it to the Radium Institute in Paris. From the second trip to the United States (1929) she brought money to buy radium for the Radium Institute in Warsaw. Both decisions were examples of her engagement in charity work.
In 1922 she was appointed member of the International Commission on Intellectual Co-operation by the Council of the League of Nations. Without regretting her early attitude to resist patenting her own ideas, she became an advocate of scientists’ right to patent their discoveries and inventions. She also campaigned for free access to international scientific literature, for internationally recognized scientific symbols and standards and for free international exchange of scientists.
In spite of her commitment to France, Marie Curie always maintained close contact with her native country. Due to her generosity and initiative, the Radium Institute in Warsaw was founded in 1932, with its foremost task of utilizing the healing properties of radium to protect health and save human lives. Marie Sklodowska-Curie died on 4 July 1934 of leukemia caused by prolonged exposure to radioactive substances.
Under Maria Sklodowska-Curie's guidance, about 483 scientific publications were published, and 34 doctorates were awarded. The Institute of Radium provided treatment with radium to about 8000 patients. She was widely acclaimed for her scientific achievements and granted numerous awards and distinctions, honorary degrees from many universities and honorary memberships of learned societies. Apart from scientific achievements, she was a happy mother of two daughters; Irene and Eve.
In Marie Curie's time, first cancers treated using radium were easily accessible surface and body cavity tumors. Of the latter, cancer of the cervix was the most frequently treated. In the mid-1930s, cancers in many sites were considered to be incurable. But with time the situation changed, owing to, among others, many female scientists faithful to Marie Curie's idea of fight against cancer. Their achievements increased the understanding of cell-killing mechanisms, normal tissue toxicity, effect of dose fractionation and tumor biology and influenced progress in RT. Many of them shared some elements of Marie's biography, including, emigration to a foreign land, high ethical and moral standards, passionate dedication to work, strong family values, and scientific collaboration with their husbands. The best examples could be the cooperation of Tikvah Alper with Michael Sterne, Juliana Denekamp with Bo Littbrand, Fiona Stewart with Adrian Begg, Peggy Olive with Ralph Durand or Carmel Mothersill with Colin Seymour.
Because of space limit, I will concentrate on their main achievements, omitting their honorable awards and positions, distinctions, books and hundreds of publications.
This subjective review starts with Alma Howard (1913–1984), a pioneer of cell cycle studies, who worked in the early 1950s at Hammersmith Hospital in London. She (with Stephen Pelc) was the first to explore and describe the cell cycle in eukaryotic cells.4 Using radioactive tracers (the technique which revolutionized the field of cell biology), they found that, unlike bacteria, eucariotic cells synthesized DNA only in S-phase rather than continually through interphase. The definition of cell phases: G1, S, G2 and mitosis formed the basis for the modern studies of growth control in molecular biology and biomedicine.
One of the pioneers of British radiobiology was Tikvah Alper (1909–1995) who had a great influence on several generations of radiobiologists, not only in Britain but internationally. In the early 1950s, she worked on target theory at the Experimental Radiopathology Research Unit at Hammersmith Hospital in London. Radiobiology then became heavily concentrated on cell survival curves, based on target theory, which were described by mean lethal dose (Do) and hit number. If was found that hit numbers for different cell lines in vitro spread from 1.0 to many hundreds, Tikvah Alper changed their name from ‘hit number’ to extrapolation number.5, 6 She was interested also in oxygen effect, Relative Biological Effectiveness (RBE) of fast neutrons and transmissible (neither a virus nor a bacterium) agent in scrapie and BSE (bovine spongiform encephalopathy).7 She was the first to recognize, by UV absorption studies, that the infectious agents (prions), which include scrapie and BSE, also known as “mad cow disease” in cattle (and Creutzfeldt-Jakob disease in humans), are not replicated or transmitted as nucleic acid nor proteins (epidemic first diagnosed in 1986 in Great Britain). Prions are infectious agents composed of protein in a misfolded form. This is in contrast to all other known infectious agents (virus/bacteria/fungus/parasite) which must contain nucleic acids (either DNA, RNA, or both). The word prion is derived from the words protein and infection.
In the 1950s, Tikvah Alper suggested that membranes may constitute another (apart from DNA) important radiation target in cells. This has led to attempts at explaining the involvement of membrane lesions in cell killing (apoptosis) and in promoting radiation resistance.8
She was born in South Africa and married to a bacteriologist (Michael Sterne). At that time, it was impossible for a married woman to obtain a good scientific post in South Africa so she devoted herself to her family. Nevertheless, she also worked together with her husband, in a small private laboratory in the garden of their home. Later, she became employed as head of the Biophysics Section of National Physics Laboratory but in 1951 when she lost her position, she came to Great Britain and joined the group of scientists working at Hammersmith Hospital with Hal Gray.9 In 1962, she was appointed director of the unit. Tikvah Alper was an outstanding radiobiologist who had to overcome many obstacles in her private and professional life. Alper was a lifelong feminist. She was happily married for 62 years but never changed her name and never wore a wedding ring.9 She was a skillful educator of deaf children (her oldest son was born deaf). She was an important role model for many women, showing them that they could succeed in a very demanding career without having to sacrifice family values and that the gender of scientists was irrelevant. She opposed the common social practice of giving little public recognition to women.
Under Tikvah Alper's direction, the Experimental Radiopathology Research Unit at Hammersmith became one of the world's leading radiobiology centers. After her retirement in 1973, she worked as a senior advisor at the Gray Laboratory from 1974 until 1977 (Fig. 1). Tikvah Alper cooperated with Shirley Hornsey who was a very productive scientist working on radiation injury in mouse lung,10 spinal cord,11 jejunum,12 RBE of fast neutrons,13 and hypoxia. Her most remarkable achievement was obtained in cooperation with Hal Gray. She was involved in studies showing the relationship between oxygen tension and radiosensitivity, using mouse and chicken cells. She studied the effect of oxygen on tumor response to irradiation. Hornsey is the co-author of the widely known paper by Hal Gray14 reporting that oxygen might sensitize a tumor to X-rays more than normal tissues. The authors of the study were the first to suggest the use of oxygen to improve the therapeutic index of radiotherapy. Hal Gray later moved from the Hammersmith Hospital to the Mount Vernon Hospital and the Gray Laboratory.
Fig. 1.

Tikvah Alper (first from right) with Juliana Denekamp and Jack Fowler at the Gray Laboratory (about 1970), (Jack Fowler courtesy).
In 1968, Juliana Denekamp (1943–2001) was employed in the same center. She quickly became a distinguished English radiobiologist. Between 1988 and 1994, Juliana Denekamp was director of the Gray Laboratory, UK. Thereafter, she was appointed Professor of Translational Research at the Umea University, Sweden, where she moved to join her husband, radiation oncologist Bo Littbrand. They worked in close cooperation. She was very active there, both as a researcher and teacher, until her early death in June 2001. Juliana Denekamp was a leading international scientist in radiation biology applied to radiotherapy and well known and admired for her habit of asking penetrating questions at meetings.
Juliana Denekamp was recognized for significant contributions to dozens of topics in radiobiology applied to radiation oncology. She authored about 300 publications,15 therefore, only some of which are highlighted.
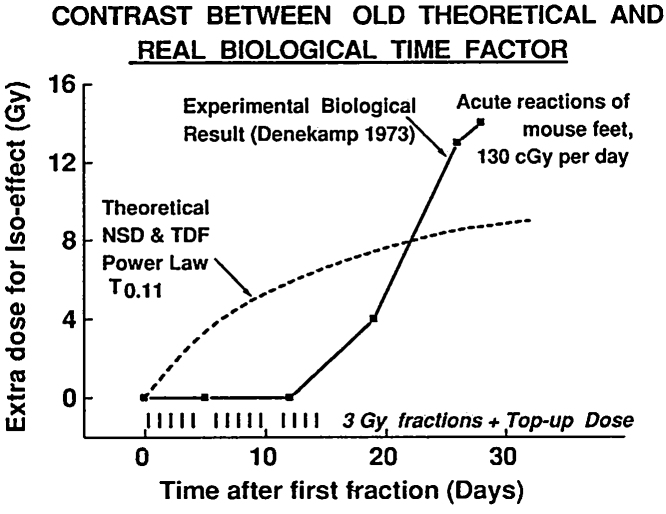
In 1973 Denekamp showed, for the first time experimentally, that the time factor for acute reactions in mouse skin consisted of a delay of one or two population turnover times followed by a rapid increase in the dose required to keep the biological effect constant. This increase was, as she showed, associated with higher proliferation rate in the basal layer of the skin.16 The time-course was exactly the opposite of the then accepted model of Nominal Standard Dose (NSD) and Time-Dose Factor (TDF), which provided for the biological effect to be large at first and then decreasing (Fig. 2). The difference between biological data measured experimentally and NSD and TDF theoretical curve was shown, for the first time, for acute reactions in mouse skin.16, 17
Fig. 2.
Graph presenting the difference between biological data (full curve) measured experimentally for the first time by J. Denekamp in mouse skin16 and Nominal Standard Dose and Time-Dose Factor theoretical curve (dashed) (Jack Fowler courtesy).
Denekamp examined cell kinetics in mouse tumor and the relationship between reoxygenation and cell loss rate. She pointed out greater cell loss factors (faster shrinkage following irradiation) in carcinomas than in sarcomas.18
She studied radioprotectors, like WR2721 (Amifostine) and showed radioprotection of WR-2721 in all the tumors tested, as well as in normal tissues.19, 20 Denekamp showed also much faster proliferation of endothelial cells in tumors than in normal tissues (except in wound-healing and fetal development).21
Denekamp made major contributions in designing radiotherapy schedules: CHART (Continuous hyperfractionated accelerated radiotherapy) and ARCON (Accelerated Radiotherapy with Carbogen and Nicotinamide). The CHART schedule, delivering a dose exceeding 50 Gy, with 3 F a day of 1.5 Gy (36 F) in 12 treatment days – without a weekend gap – was developed with Stanley Dische, Michele Saunders and Jack Fowler at the Mount Vernon Cancer Centre.22 ARCON was studied in several trials and has recently been shown to improve regional control in laryngeal tumors.
Julie Denekamp was involved in research methodology. She designed the “top-up” technique for investigating effects of many small fractions of X-rays and neutrons.
She led a major program of development in the Gray Lab of new techniques to investigate late complications in mice, with functional tests that did not require the sacrifice of the animal. Results could be obtained for a sequence of times after irradiation in the same animals. Techniques were developed to irradiate small, well-defined, regions of the mouse and to measure progressive functional damage in the lung (breathing rate and lethal pneumonitis),23 bladder (urinary frequency, bladder contraction),24, 25 and rectum (short feces).26, 27 The results clarified and underpinned the clear difference in α/β ratios between early and late-responding tissues described by Thames et al.,28 in the same period.
The American radiobiologist Elizabeth L. Travis from MD Anderson Cancer Center spent 3 years at the Gray Lab in the late 1970s. Having special pathology knowledge about radiation damage to the lungs, she made a great input to research on radiation induced normal tissue damage. Late effects that develop in normal tissues adjacent to the tumor site in months to years after therapy can reduce the quality of life in cancer survivors. Working in the Gray Laboratory between 1976 and 1979, together with Juliana Denekamp, she introduced to experimental radiobiology non-harmful assays performed on living animals (Fig. 3). The end-point was breathing rate which was measured simply by the frequency of pressure changes in a mouse spirometer, for each mouse on each experimental day after irradiation.23, 29, 30 Later, she looked at molecular susceptibility to radiation induced pulmonary fibrosis.31, 32 The now well known contrasts between the early and late types of radiation injury were then identified and investigated, and found indeed to mirror the early pneumonitis and later fibrosis occurring in human lungs. The studies are so important, because late effects that develop in normal tissues adjacent to the tumor site in months to years after radiotherapy can reduce the quality of life in cancer survivors.
Fig. 3.

Juliana Denekamp and Elizabeth Travis in Los Angeles at their leisure time (Jack Fowler courtesy).
Elizabeth Travis stays faithful to the ideas of Marie Curie. She is a strong advocate for the advancement of women in academic medicine, both at MD. Anderson and nationally.
Over her career as a professor, she was the Vice President of Women Faculty Programs, the Provost's Office, the University of Texas MD Anderson Cancer Center, Houston, TX. Elizabeth Travis established and secured an endowment for the Margaret L. Kripke Legend Award for promotion of women in cancer medicine and cancer science. In 2009 she obtained an AAMC Women in Medicine Leadership Development Award (Association of American Medical Colleges).
Fiona Stewart, professor of radiobiology in the Division of Experimental Therapy, the Netherlands Cancer Institute, Amsterdam, developed a reputation for her studies on normal tissue damage and the radiobiology of both acute and late complications.24, 25 Whilst working in the Gray Laboratory with Juliana Denekamp (till 1984) she showed, using labeled cell autoradiography, that the natural pre-irradiation turnover time (Tpotential doubling time) of a normal tissue determines whether radiation damage in an organ occurs early or late.33 Late responding normal tissues were shown to behave differently from tumors and acutely responding normal tissues to fractionated radiotherapy, which had profound clinical implications (Fig. 4). She also studied hypoxic-cell radiosensitizers, and capillary-damaging agents in tumors (including interactions between radio-protecting compounds and light-sensitization compounds for therapy and other interactions of chemotherapy and radiotherapy).
Fig. 4.

Fiona Stewart (first from right) during discussion with Anamaria Rojas and Elizabeth Travis at one of the conferences (Jack Fowler courtesy).
After moving to Amsterdam (1984), she continued to investigate mechanisms of late radiation injury, particularly the long term recovery potential of tissues and their tolerance to re-irradiation.34, 35 She studied the link between functional damage and vascular mediated inflammatory and thrombotic changes.36 One of her big interests is photodynamic therapy in oncology. During the 1990s, Fiona Stewart set up a pre-clinical program to investigate the potential of photodynamic therapy (PDT) for the treatment of small superficial tumors. Her work demonstrated that vascular mediated damage is an essential component to curative PDT and this knowledge had a major influence on the design of optimal clinical schedules.
Fiona Stewart received the 2012 Weiss Medal (Association for Radiation Research), the 2014 ESTRO Lifetime Achievement Award, the 2014 Bacq & Alexander Award (European Radiation Research Society), and the 2015 Gray Medal (ICRU).
Andree Dutreix from the Institute of Gustave-Roussy, Villejuive, France, is a medical physicist. She developed standards for quality assurance in brachytherapy and tele-radiotherapy.37, 38 A. Dutreix was involved in development of the European Quality Assurance Network for external RT based on thermoluminescent dosimetry.39 In the early 1980s she worked on the RBE of neutrons, helium ions and Californium-251 (Fig. 5).
Fig. 5.

Andree Dutreix (second from left) with Joseph Rotblat (Nobel Peace Prize Laureate in 1995), Juliana Denekamp and the author during barbecue at Marie Curie Symposium in Umea (Sweden, June 1998), (Author's archive).
Since the early 1980s, clinical studies have shown that patients with the same tumor types and treated according to the same RT schedule have different tumor responses and different degrees of normal tissue damage. It became obvious that the variation in RT responses may depend on biological factors. Knowledge of these parameters could provide clinicians with relevant information needed to select appropriate treatments, and this has led to the search for predictive assays.
There are three important radiobiological factors involved in determining tumor response to radiotherapy: proliferation, hypoxia and cellular radiosensitivity. In terms of radiosensitivity, there is general agreement that not only tumors, but also normal individuals differ in their intrinsic radiosensitivity. Pretreatment assessment of the parameter has potential for use by clinicians to allow dose or treatment modifications.
The inherent radiosensitivity of a cell has been defined by many different factors. In vitro studies suggest that three factors produce most of the variations seen in tumor cells: DNA repair, cell growth characteristics, and genome instability.
The largest study looking at tumor cellular radiosensitivity in relation to RT outcome was carried out by Catharine West from the Institute of Cancer Sciences, Christie Hospital NHS Trust in Manchester. She used a clonogenic assay (considered the gold standard method) to examine radiosensitivity of cells derived from primary tumor biopsies. Radiosensitivity was measured as a surviving fraction after 2 Gy in vitro irradiation (SF2). The test was suggested as a predictive assay.40
Her work showed that in vitro measurement of tumor cell radiosensitivity is a highly significant prognostic factor for carcinoma of the cervix treated with radiotherapy41 (Fig. 6). She examined also other methods (chromosomal aberrations) and other biological parameters (hypoxia) in human tumors.42 West established the Translational Radiobiology Group with the aim to characterize molecular patient profiles that reflect relevant biological phenotypes and predict tumor and normal tissue response to radiation. She uses assays carried out in various clinical samples at the DNA (genome), RNA (transcriptome) or protein (proteome) level.43 At present, she is interested in the genome-wide association study for identification genes of late RT toxicity.44 This is an important problem as late toxicity in RT can limit treatment intensity, thus preventing increase in dosage and lowering the probability of tumor control.
Fig. 6.
Catharine West, the author and Peggy Olive at the 15th L.H. Gray Conference in Canterbury in 1989 (Author's archive).
Peggy Olive from the British Columbia Cancer Research Centre in Canada developed a method to measure DNA damage in individual cells based on the technique of microelectrophoresis.45 Cells embedded in agarose are lysed, subjected briefly to an electric field, stained with a fluorescent DNA-binding stain, and viewed using a fluorescence microscope. Broken DNA migrates farther in the electric field and the cell then resembles a “comet” with a brightly fluorescent head and a tail region which increases as damage grows.
This method became popular after its usefulness had been proved in some clinical studies. This method has been used for measuring DNA damage, hypoxia and apoptosis.45, 46 Peggy Olive used also H2AX foci as the method for indication of lethal DNA damage.47, 48 She also studied the expression of endogenous hypoxia markers (HIF-1, CAIX) and application of pimonidazole and Eppendorf electrode in human tumors.
Ingela Turesson, Professor of Radiation Oncology from the Department of Radiology, Oncology and Radiation Science, Uppsala, Sweden, has significant achievements in assessing intrinsic radiosensitivity in a clinical setting. She looked for correlation between in vitro cellular radiosensitivity with in vivo normal-tissue responses. She also performed prospective clinical fractionation studies on acute and late reactions in the skin of breast cancer patients.
Turesson studied the phenomenon of HRS/IRR and indicated high sensitivity in the radiation survival response of mammalian cells at doses below <0.5 Gy. This phenomenon, which has been termed hyperradiosensitivity (HRS), precedes the occurrence of a relative resistance (per unit dose) to cell killing by radiation over the dose range 0.5–1 Gy. The latter phenomenon has been named increased radioresistance (IRR).49 In the late 1980s, Ingela Turesson & Howard Thames from the MD Anderson Cancer Center examined the repair capacity and kinetics of human skin during fractionated radiotherapy. Endpoints were: erythema, desquamation, and telangiectasia after 3 and 5 years’ follow up.50 The authors found 2 values of repair half time (T1/2) in human skin: there was a fast repair component of 0.3–0.4 h and slower components of 1.1–1.3 for acute effects and 3.5 h for late effects. Since then, 6 h or longer intervals between fractions are recommended in radiotherapy schedules.51 Recently, she became involved in studies of proton therapy.52
Irena Szumiel from the Institute of Nuclear Chemistry and Technology in Warsaw is interested in the monitoring and signaling of radiation-induced damage in mammalian cells. Her main interests cover factors determining mammalian cell sensitivity to ionizing radiation in a model of mouse leukemia L5178Y (LY)-consisting of 2 sublines differing in susceptibility to cytotoxic agents and DNA repair.53, 54 She studies DNA repair, cell signaling, and radiosensitivity markers. Irena Szumiel has authored a number of reviews on the subject.55, 56, 57
Penny Jeggo from the University of Sussex, Brighton, England, studies mechanisms of DNA double-strand breaks (DSBs) repair in mammalian cells.58, 59 She is especially interested in DNA non-homologous end-joining (NHEJ), a major DSB repair pathway in mammalian cells.60 She works on the identification of genes involved in this process and looks for proteins interacting with the well characterized components of the DSB repair machinery.61 Her recent work concerns differences in repair of DSBs localized in eu- and heterochromatin, which has highlighted how chromatin complexity influences not only the factors required for DSB repair but also the pathway choice. Understanding the consequences of defects in DSB repair mechanisms for the cellular response to ionizing radiation may contribute to improvements of radiotherapy.62
High-LET irradiation is recommended for radioresistant tumors. For these new particles, heavy-ion radiobiology is needed. A representative of this field is Eleanor Blakely, a biophysicist from Ernest Orlando Lawrence Berkeley National Laboratory, California. She has studied the mechanisms underlying the increased biological effectiveness of densely ionizing radiations, including alpha particles, neutrons and highly energetic heavy charged particles.63 She examined DSBs, their repair, induction of chromosomal aberrations in mammalian cells after irradiation with heavy-ion beams. She showed that the increased biological effectiveness of densely ionizing radiations may be a result of clustered DNA damages that lead to a greater proportion of nonreparable DNA strand breaks, compared to sparsely ionizing radiation.64, 65 Recently, (July 2014) in CERN, she presented a seminar on “60 years of particle therapy” showing the efforts of CERN and the Lawrence Berkely National Laboratory in developing treatment for cancers with protons and carbon. Eleonor Blakeley serves also as consultant in support of clinical radiotherapy trials, and of issues pertinent to radiation protection.
In the USA, Maria Curie's ideas were developed by Helen Evans (1924–2007), Professor of Radiation Oncology, University of Wisconsin. Her work was focused on molecular and cellular consequences of exposure to ionizing radiation. She was a pioneer in elucidating the mechanisms of ionizing radiation mutagenesis in mammalian cells and the induction of genomic instability (some have even referred to her as the Mother of Mutagenesis).66, 67 She worked with a wide range of biological systems (viruses, bacteria, slime molds, rodents).
Definition of mutagenesis given by Evans: genomic instability can be initiated by complex, poorly repaired DNA damage induced by low doses of ionizing radiation that leads to a mutator phenotype.68 She was also interested in photodynamic therapy (PDT). The technique is used clinically to treat malignant tumors and is considered as both minimally invasive and minimally toxic. Most modern PDT involves three key components, a photosensitizer, a light source and tissue oxygen.69 Hellen Evans was active in many professional societies and her favorite was the Radiation Research Society of which she was the President between 1994 and 1995.
At present, we know that microenvironment plays a significant role in tumor development and growth and is considered a target in tumor response to radiotherapy. Great achievements in this field and understanding of the biology of solid tumors and improvement in cancer treatment were obtained by Sara Rockwell, professor of Therapeutic Radiology and Pharmacology at Yale School of Medicine in Boston. Her work is focused on studying the unphysiological microenvironments of cells within solid tumors, its influence on the biology of tumor cells and of the stromal elements within solid tumors.70 She showed that the microenvironmental inadequacies within solid tumors alter the proliferation patterns of the cells, the metabolic pathways used by the cells, the ability of the cells to tolerate stress, DNA damage, and other injuries, and the response of the cells to radiation and antineoplastic drugs.71, 72 Rockwell studies their implication on the development and progression of solid tumors. She is involved in developing therapeutic strategies to improve cancer therapy. Sara Rockwell is also the author of a review on the evolution of experimental radiotherapy over the past century.73
The old way of thinking about the pathophysiology of normal tissue effects has changed in the last years because of our better understanding of radiation injury mechanisms at the molecular level. At present, it is believed that normal tissue response to radiation is an integrated response involving cell death (target cell theory) and the production of cytokines, reactive oxygen species and alterations in gene expression of many cells. Apart of direct, cytocidal effects, radiation can produce indirect and functional effects. Examples of indirect effects are the bystander effect and the secretion of pro-inflammatory cytokines that can induce an inflammatory response.
Carmel Mothersill, an Irish radiobiologist (McMaster University, Ontario, Canada), is famous for pivotal studies on radiation-induced bystander effect (RIBE). This is the phenomenon in which unirradiated cells exhibit irradiation effects as a result of signals received from nearby irradiated cells. This phenomenon contradicts the old radiobiological dogma that the damage effects of radiation are the results of direct ionization of cell structures, particularly DNA. Prof. Carmel Mothersill showed that the RIBE is induced by agents and signals emitted by directly irradiated cells and manifests as lowering of survival, cytogenetic damage, apoptosis and biochemical changes in neighboring non-irradiated cells.74, 75 The bystander effect can be induced by radiation doses as low as 2 mGy; however, ‘low dose’ in RT means generally less than 0.5 Gy. Carmel Mothersill has shown that low dose effects are complex and induce, among others, apart from RIBE, genomic instability (commonly described as a non-targeted effect).76 Recently, Carmel Mothersill's research has moved from in vitro to in vivo studies using fish species and mammals and provides results for the general discussion on radiation protection.77
In the times of Marie Sklodowska-Curie, she was virtually the only woman recognized as a great scientist among many men. The ladies presented in the article also functioned among numerous male scientists involved in radiobiology. Although they constituted less than 40% of the community, they made significant contribution to the development of radiotherapy and more efficient cancer treatment. Due to their studies, new schedules of RT and new types of ionizing radiation have been applied, lowering the incidence of normal tissue toxicity. Their achievements herald the future of personalized RT.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgements
The author would like to thank Prof. Jack Fowler for all the information, photographs and for his being so helpful, encouraging and enthusiastic towards all women scientists who cooperated with him.
Footnotes
The paper was delivered on the Scholars in Training on pre 14th ICRR Conference Workshop in Warsaw, 27th August 2011.
References
- 1.Sklodowska-Curie M. Rayon semis par les composes de l’Uranium et du Thorium. Compt Rend Acad Sci Paris. 1898;126:1101. [Google Scholar]
- 2.Curie P., Skłodowska-Cuire M. Sur une subsatnce nouvelle radio-active contenue dans la pechblende. Compt Rend Acad Sci Paris. 1898;127:175. [Google Scholar]
- 3.Curie P., Bemont G. Sur une nouvelle substance fortemont radioactive contenue dans la pitchblende. Compt Rend Acad Sci Paris. 1898;127:1215. [Google Scholar]
- 4.Howard A., Pelc S.R. Synthesis of DNA in normal and irradiated cells and its relation to chromosome breakage. Heredity. 1953;(Suppl. 6):261–273. [Google Scholar]
- 5.Alper T., Gillies N.E., Elkind M.M. The sigmoid survival curve in radiobiology. Nature. 1960;186:1062–1063. doi: 10.1038/1861062a0. [DOI] [PubMed] [Google Scholar]
- 6.Alper T. Cambridge University Press; 1979. Cellular radiobiology. [Google Scholar]
- 7.Alper T. The scrapie enigma: insights from radiation experiments. Radiat Res. 1993;135:283–292. [PubMed] [Google Scholar]
- 8.Alper T. The role of membrane damage in radiation-induced cell death. In: Miller M.V., Shramou A.E., editors. Membrane toxicity. Plenum; New York: 1977. pp. 139–165. [DOI] [PubMed] [Google Scholar]
- 9.Fowler J. In memoriam Tikvah Alper 1909–1995. Radiat Res. 1995;142:110–112. [PubMed] [Google Scholar]
- 10.Hornsey S., Kutsutani Y., Field S.B. Damage to mouse lung with fractionated neutrons and X-rays. Radiology. 1975;116:171–174. doi: 10.1148/116.1.171. [DOI] [PubMed] [Google Scholar]
- 11.White A., Hornsey S. Radiation damage to the rat spinal cord: the effect of single and fractionated doses of X rays. Br J Radiol. 1978:515–523. doi: 10.1259/0007-1285-51-607-515. [DOI] [PubMed] [Google Scholar]
- 12.Griffin C.S., Hornsey S. The effect of neutron dose rate on jejunal crypt survival. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:589–595. doi: 10.1080/09553008514552831. [DOI] [PubMed] [Google Scholar]
- 13.Hornsey S. RBE for lung and cord. Int J Radiat Oncol Biol Phys. 1982:2099–2102. doi: 10.1016/0360-3016(82)90552-1. [DOI] [PubMed] [Google Scholar]
- 14.Gray H., Conger A., Ebert M., Hornsey S., Scott O. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 15.Fowler J.F. The Denekamp Diadems. A review of Julie's achievements. Acta Oncol. 2001;40:903–912. doi: 10.1080/02841860152708161. [DOI] [PubMed] [Google Scholar]
- 16.Denekamp J. Changes in the rate of repopulation during multifraction irradiation of mouse skin. Br J Radiol. 1973;46:381–387. doi: 10.1259/0007-1285-46-545-381. [DOI] [PubMed] [Google Scholar]
- 17.Denekamp J., Fowler J.F. Further investigations of the response of irradiated mouse skin. Int J Radiat Biol. 1966;10:435–441. doi: 10.1080/09553006614550541. [DOI] [PubMed] [Google Scholar]
- 18.Denekamp J. CC Thomas; Springfield, IL: 1982. Cell kinetics and cancer therapy; p. 162. [Google Scholar]
- 19.Rojas A., Stewart F.A., Denekamp J. Experimental radiotherapy with WR-2721 and misonidazole. Int J Radiat Oncol Biol Phys. 1982;8:527–530. doi: 10.1016/0360-3016(82)90676-9. [DOI] [PubMed] [Google Scholar]
- 20.Denekamp J., Stewart F.A., Rojas A. Rebuttal: is the outlook grey for WR-2721 as a clinical radioprotector? Int J Radiat Oncol Biol Phys. 1983;9:1247–1249. doi: 10.1016/0360-3016(83)90194-3. [DOI] [PubMed] [Google Scholar]
- 21.Denekamp J., Hill S.A., Stewart F.A. Vascular occlusion and tumor cell death. Eur J Cancer. 1983;19:271–275. doi: 10.1016/0277-5379(83)90426-1. [DOI] [PubMed] [Google Scholar]
- 22.Saunders M.I., Dische S., Fowler J.F. Radiotherapy employing three fractions of each twelve consecutive days. Acta Oncol. 1988;27:163–167. doi: 10.3109/02841868809090336. [DOI] [PubMed] [Google Scholar]
- 23.Travis E.L., Vojnovic B., Davies E.E., Hirst D.G. A plethysmographic method for measuring function in locally irradiated mouse lung. Br J Radiol. 1977;52:67–74. doi: 10.1259/0007-1285-52-613-67. [DOI] [PubMed] [Google Scholar]
- 24.Stewart F.A., Denekamp J., Michael B.D. Late radiation damage in the mouse bladder as measured by increased urination frequency. Radiat Res. 1978;78:649–659. [PubMed] [Google Scholar]
- 25.Stewart F.A., Radhawa V.S., Michael B.D. Multifraction irradiation of mouse bladders. Radiother Oncol. 1984;2:131–140. doi: 10.1016/s0167-8140(84)80049-3. [DOI] [PubMed] [Google Scholar]
- 26.Terry N.H.A., Denekamp J. RBE values end repair characteristics for colorectal injury after Caesium137 gamma-ray and neutron irradiation. II. Fractionation up to ten doses. Br J Radiol. 1984;57:617–629. doi: 10.1259/0007-1285-57-679-617. [DOI] [PubMed] [Google Scholar]
- 27.Gasinska A., Dubray B., Hill S.A., Denekamp J., Thames H.D., Fowler J.F. Early and late injuries in mouse rectum after fractionated X-ray and neutron irradiation. Radiother Oncol. 1993;26:244–253. doi: 10.1016/0167-8140(93)90266-b. [DOI] [PubMed] [Google Scholar]
- 28.Thames H.D., Withers H.R., Peters L.J., Fletcher G.H. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 29.Travis E.L., Down J.D., Holmes S.J., Hobson B. Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiat Res. 1980;84:133–143. [PubMed] [Google Scholar]
- 30.Travis E.L., Down J.D. Repair in mouse lung after split doses of X rays. Radiat Res. 1981;87:166–174. [PubMed] [Google Scholar]
- 31.Rosen I.I., Fischer T.A., Antolak J.A. Correlation between lung fibrosis and radiation therapy dose after concurrent radiation therapy and chemotherapy for limited small cell lung cancer. Radiology. 2001;221:614–622. doi: 10.1148/radiol.2213992043. [DOI] [PubMed] [Google Scholar]
- 32.Haston C.K., Zhou X., Gumbiner-Russo L. Universal and radiation-specific loci influence murine susceptibility to radiation-induced pulmonary fibrosis. Cancer Res. 2002;62:3782–3788. [PubMed] [Google Scholar]
- 33.Stewart F.A., Soranson J.A., Alpen E.L., Williams M.V., Denekamp J. Radiation-induced renal damage: the effects of hyperfractionation. Radiat Res. 1984;98:407–420. [PubMed] [Google Scholar]
- 34.Stewart F.A., Van der Kogel A.J. Retreatment tolerance of normal tissues. Semin Radiat Oncol. 1994;4:103–111. doi: 10.1053/SRAO00400103. [DOI] [PubMed] [Google Scholar]
- 35.Stewart F.A. Re-treatment after full-course radiotherapy: is it a viable option? Acta Oncol. 1999;38:855–862. doi: 10.1080/028418699432545. [DOI] [PubMed] [Google Scholar]
- 36.Stewart F.A., Te Poele J.A., Van der Val A.F. Radiation nephropathy: the link between functional damage and vascular mediated inflammatory and thrombotic changes. Acta Oncol. 2001;40:952–957. doi: 10.1080/02841860152708233. [DOI] [PubMed] [Google Scholar]
- 37.Dutreix A. When and how can we improve precision in radiotherapy? Radiother Oncol. 1984;2:275–292. doi: 10.1016/s0167-8140(84)80070-5. [DOI] [PubMed] [Google Scholar]
- 38.Bridier A., Dutreix A. A method for dose calculation for high energy photon beams based on measurements performed at reference depth. Acta Oncol. 1993;32:425–433. doi: 10.3109/02841869309093621. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira I.H., Dutreix A., Bridier A., Chavaudra J., Svensson H. The ESTRO-QUALity assurance network (EQUAL) Radiother Oncol. 2000;55:273–284. doi: 10.1016/s0167-8140(99)00101-2. [DOI] [PubMed] [Google Scholar]
- 40.West C.M.L. Predictive assays in radiation therapy. Adv Radiat Biol. 1994;18:149–180. [Google Scholar]
- 41.West C.M.L., Davidson S.E., Roberts S.A., Hunter R.D. The independence of intrinsic radiosensitivity as a prognostic factor for patients response to radiotherapy of carcinoma of the cervix. Br J Cancer. 1997;76:1184–1190. doi: 10.1038/bjc.1997.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nordsmark M., Loncaster J., Aquino-Parsons C.h. The prognostic value of pimonidazole and tumor pO2 in human cervix carcinomas after radiation therapy: a prospective international multi-center study. Radiother Oncol. 2006;80:123–131. doi: 10.1016/j.radonc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 43.West C.M., Elliott R.M., Burnet N.G. The genomics revolution and radiotherapy. Clin Oncol. 2007;19:470–480. doi: 10.1016/j.clon.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Fachal L., Gomez-Caamano A., Barnett G.C. A three-stage genome-wide association study identifies a susceptibility locus for late radiotherapy toxicity at 2q24.1. Nat Genet. 2014;46:891–894. doi: 10.1038/ng.3020. [DOI] [PubMed] [Google Scholar]
- 45.Olive P.L., Banath J.P., Durand R.E. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat Res. 1990;122:86–94. [PubMed] [Google Scholar]
- 46.Olive P.L., Durand R.E., Jackson S.M. The comet assay in clinical practice. Acta Oncol. 1999;38:839–844. doi: 10.1080/028418699432527. [DOI] [PubMed] [Google Scholar]
- 47.Yu T., MacPhail S.H., Banath J.P., Klokov D., Olive P.L. Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA-double strand breaks and genomic instability. DNA Repair (Amst) 2006;5:935–946. doi: 10.1016/j.dnarep.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 48.Olive P.L. Retention of gamma H2AX foci as an indication of lethal DNA damage. Radiother Oncol. 2011;101:18–23. doi: 10.1016/j.radonc.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 49.Turesson I., Joiner M.C. Clinical evidence of hypersensitivity to low doses in radiotherapy. Radiother Oncol. 1986;40:1–3. doi: 10.1016/0167-8140(96)01765-3. [DOI] [PubMed] [Google Scholar]
- 50.Turesson T., Thames H.D. Repair capacity and kinetics of human skin during fractionated radiotherapy: erythema, desquamation, and telangiectasia after 3 and 5 year's follow-up. Radiother Oncol. 1989;15:169–188. doi: 10.1016/0167-8140(89)90131-x. [DOI] [PubMed] [Google Scholar]
- 51.Nyman J., Turesson I. Does the interval between fractions matter in the range of 4–8 h in radiotherapy? A study of acute and late human skin reactions. Radiother Oncol. 1995;34:171–178. doi: 10.1016/0167-8140(95)01525-l. [DOI] [PubMed] [Google Scholar]
- 52.Isacsson U., Nilsson K., Asplund S. A method to separate rectum from the prostate during proton beam radiotherapy of prostate cancer patients. Acta Oncol. 2010;49:500–505. doi: 10.3109/02841861003745535. [DOI] [PubMed] [Google Scholar]
- 53.Szumiel I. Response of 2 strains of L5178Y cells to cis-dichlorobis(cyclopentylamine) platinum(II) I. Cross-sensitivity to cis-pad and UV-light. Chem Biol Interact. 1979;24:51–72. doi: 10.1016/0009-2797(79)90102-9. [DOI] [PubMed] [Google Scholar]
- 54.Szumiel I. L5178Y sublines: a look back from 40 years. Part I: General characteristics. Int J Radiat Biol. 2005;81:339–352. doi: 10.1080/09553000500143518. [DOI] [PubMed] [Google Scholar]
- 55.Szumiel I. Intrinsic radiosensitivity of proliferating mammalian cells. Adv Radiat Biol. 1981;9:281–320. [Google Scholar]
- 56.Szumiel I. Review. Ionizing radiation-induced cell death. Int J Radiat Biol. 1994;66:329–341. doi: 10.1080/09553009414551271. [DOI] [PubMed] [Google Scholar]
- 57.Szumiel I. Monitoring and signaling of radiation-induced damage in mammalian cells. Radiat Res. 1998;(Suppl. 150):92–101. [PubMed] [Google Scholar]
- 58.Jeggo P.A., Crr A.M., Lehmann A.R. Splitting the ATM: distinct repair and checkpoint defects in ataxia-teleangiectasia. Trends Genet. 1998;14:312–316. doi: 10.1016/s0168-9525(98)01511-x. [DOI] [PubMed] [Google Scholar]
- 59.Jeggo P.A. Identification of genes involved in repair of DNA double-strand breaks in mammalian cells. Radiat Res. 1998;(Suppl. 150):S80–S91. [PubMed] [Google Scholar]
- 60.Girard P.M., Foray N., Stumm M., Jeggo P. Radiosensitivity in Nijmegen Breakage Syndrome cells is attributable to a repair defect and not cell cycle checkpoint defects. Cancer Res. 2000;60:4881–4888. [PubMed] [Google Scholar]
- 61.Jeggo P.A. The fidelity of repair radiation damage. Radiat Prot Dosimetry. 2002;99:1–4. doi: 10.1093/oxfordjournals.rpd.a006740. [DOI] [PubMed] [Google Scholar]
- 62.Jeggo P.A. The role of the DNA damage response mechanisms after low-dose radiation exposure and a consideration of potentially sensitive individuals. Radiat Res. 2010;174:825–832. doi: 10.1667/RR1844.1. [DOI] [PubMed] [Google Scholar]
- 63.Blakely E.A., Kronenberg A. Heavy-ion radiobiology: new approaches to delineate mechanisms underlying enhanced biological effectiveness. Radiat Res. 1998;(Suppl. 150):126–145. [PubMed] [Google Scholar]
- 64.Tobias C.A., Blakley E.A., Alpen E.L. Molecular and cellular radiobiology of heavy ions. Int J Radiat Oncol Biol Phys. 1982;8:2109–2120. doi: 10.1016/0360-3016(82)90554-5. [DOI] [PubMed] [Google Scholar]
- 65.Chang P.Y., Bjornstad K.A., Rosen C.J. Effects of iron ions, protons and X-rays on human lens cell differentiation. Radiat Res. 2004;162:326–331. doi: 10.1667/rr3368.1. [DOI] [PubMed] [Google Scholar]
- 66.Evans H.H., Wilkins L., Horng M.F. Mutagenesis and transformation of C3H 10T 1/2 cells treated with ethyl methanesulfonate. Mutat Res. 1981;84:203–219. doi: 10.1016/0027-5107(81)90063-4. [DOI] [PubMed] [Google Scholar]
- 67.Beer J.Z., Jacobson E.D., Evans H.H., Szumiel I. X-ray and UV mutagenesis in two L5178Y cell strains differing in tumorigenicity, radiosensitivity, and DNA repair. Br J Cancer Suppl. 1984;6:107–111. [PMC free article] [PubMed] [Google Scholar]
- 68.Evans H.H. Failla Memorial Lecture. The prevalence of multilocus lesions in radiation-induced mutants. Radiat Res. 1994;137:131–144. [PubMed] [Google Scholar]
- 69.Oleinick N.L., Evans H.H. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150:146–156. [PubMed] [Google Scholar]
- 70.Rockwell S., Dobrucki I.T., Kim E.Y. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9:442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lally B.E., Rockwell S., Fischer D.B., Collingridge D.R., Piepmeier J.M., Knisely J.P. The interaction of polarographic measurements of oxygen and histological grade in human glioma. Cancer J. 2006;12:461–466. doi: 10.1097/00130404-200611000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Rockwell S., Tumor cell survival . In: Tumor models in cancer research. 2nd ed. Teicher B.A., editor. Humana Press; 2010. pp. 607–623. [Google Scholar]
- 73.Rockwell S. Experimental radiotherapy: a brief history. Radiat Res. 1998;(Suppl. 150):157–169. [PubMed] [Google Scholar]
- 74.Mothersill C., Seymour C.B. Medium from irradiated human epithelial cells but not from fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 75.Mothersill C., Seymour C.B. Radiation-induced bystander effects: past history and future directions. Radiat Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 76.Mothersill C., Seymour C. Uncomfortable issues in radiation protection posed by low-dose radiobiology. Radiat Environ Biophys. 2013;52:293–298. doi: 10.1007/s00411-013-0472-y. [DOI] [PubMed] [Google Scholar]
- 77.Mothersill C., Fernandez-Palomo C., Fazzari J. Transmission of signals from rats receiving high doses of microbeam radiation to cage mates: an inter-mammal bystander effect. Dose-Response. 2014;12:72–92. doi: 10.2203/dose-response.13-011.Mothersill. [DOI] [PMC free article] [PubMed] [Google Scholar]