Abstract
Aim
To determine the influence of IGRT in terms of toxicities compared to non-IGRT patients undergoing definitive RT.
Background
Image-guided radiotherapy (IGRT) enables immediate correction of target movement by online imaging. For prostate cancer patients undergoing radiation therapy (RT), a geographical miss of the prostate may result in increased dose–volume effects in the rectum and bladder.
Methods
A total of 198 prostate cancer patients treated between 2003 and 2013 were recruited randomly for this evaluation. The rates of genitourinary (GU) and gastrointestinal (GI) toxicity for 96 non-IGRT patients (total dose: 72/73.8 Gy) were compared to those for 102 IGRT patients (total dose: 77.4 Gy) according to the Common Toxicity Criteria Version 3.0 (CTCAEv3.0). Follow-up information included treatment-related symptoms and PSA relapse.
Results
After a median follow-up of 55.4 months, a statistically significant difference was noted for acute GI toxicities ≥1 in favour of IGRT. Significantly more patients treated by IGRT were free of acute GI symptoms (43% vs. 19%, p = 0.0012). In the non-IGRT group, more patients experienced acute GU side effects (89% vs. 80%, p = 0.07). Late toxicity scores were comparable for both cohorts.
Conclusions
Based on the data, we demonstrated that despite dose escalation, IGRT enabled us to reduce the GI side effects of radiation. IGRT can therefore be considered to be the standard of care for dose-escalated RT of localized prostate cancer.
Keywords: Acute toxicity, Image guidance, Late toxicity, Prostate cancer, Radiotherapy
1. Background
The efficacy of curative high-dose radiotherapy for localized prostate cancer has been proven in several randomized trials.1, 2, 3 It has been shown that improvements of radiation treatment techniques could enhance biochemical relapse-free survival rates, but this has not been translated into better survival. Acute and chronic toxicities experienced by patients are dependent on the total radiation doses and the technique used for the treatment of prostate cancer. Pollack et al.3 demonstrated that the biochemical control rate was improved by increasing the total dose (70–78 Gy), but this approach also increased toxicities to the organs at risk.
In the literature, acute (grade 2/3) genitourinary (GU) and gastrointestinal (GI) side effects have been reported in 30–50% of cases, and severe side effects, including chronic rectal bleeding ≥ grade 2, have been reported in 6–20% of patients.4, 5
Several studies analyzed the inter-and intrafractional displacements of the prostate, which ranged from 0.2 to 21 mm6 depending on rectal and bladder filling. Our prospective analysis of 66 prostate cancer patients demonstrated that standardized prostate immobilization using an endorectal balloon significantly reduced organ motion.7 Furthermore, organ motion of the prostate can be reduced significantly by standardized bladder-filling.8 Image-guided radiotherapy enables an immediate correction of target movement by online imaging.6, 9 A geographical miss of the prostate may result in increased dose–volume effects on the organs at risk. Higher mean doses to the rectum and bladder are more likely to increase treatment-related side effects, such as proctitis or cystitis. In addition, missing the target volume might result in higher rates of local failure.7, 9, 10 In 2009, image guidance for dose-escalated irradiation of prostate cancer was implemented in our clinic as the standard of care.
2. Aim
In this retrospective analysis, the influence of IGRT versus non-IGRT in terms of treatment-related side-effects was determined for patients undergoing definitive radiotherapy with validated rectal and bladder filling protocols. For quality assurance, all patients underwent the same toxicity assessment and were treated uniformly based on standardized in-house protocols with the same planning constraints. Biochemical relapse rates for all patients were analyzed to demonstrate treatment efficacy.
3. Material and methods
A total of 207 prostate cancer patients treated in our department between 2003 and 2013 were randomly recruited for this retrospective evaluation. To exclude any selection bias we randomly chose a two years period before and a two years period after commencing the IGRT program for our study, during which we evaluated all patients with newly diagnosed prostate cancer that had undergone definitive radiotherapy.
Follow-up data were not complete for 9 patients and 198 patients were evaluable. In 60% of the patients androgen suppression was performed. 52 (25%) of the patients were diagnosed at stage T1, 111 (62.2%) were diagnosed at stage T2, 33 (17.9%) were diagnosed at stage T3, including 3 patients with lymph node metastasis, and 2 (1%) were diagnosed as stage T4, including 1 patient with lymph node metastasis.
85% of patients had either intermediate- or high-risk features defined by Gleason Score, PSA and T stage. Low-risk patients had a PSA ≤ 10 ng/ml combined with Gleason score 2–6 and stage cT1-2a. Intermediate risk was defined as PSA ≥ 10.0 ≤ 20.0 and/or Gleason = 7 and/or stage cT2b. High risk was defined as PSA ≥ 20.0 and/or Gleason 8–10 and/or cT2c-T3.11
Prior to the implementation of IGRT, 96 patients were irradiated with a total dose of 72.0 Gy (15 patients)/73.8 Gy (81 patients). After commencing the IGRT program, 102 patients were irradiated with a total dose of 77.4 Gy, 1.8 Gy fractions, 5 days/week.
All patients followed a standardized bladder-filling protocol. In addition, immobilization of the prostate was improved by using an endorectal balloon (filled with 40 ml of air), as reported previously.7
All patients underwent 3D CT virtual simulation (GE ADVSIM®) with knee and footstocks fitted onto an immobilization board, and CT was conducted at 2.5 mm spacing and 2.5 mm thickness. If possible, diagnostic MRI images and CT data sets were coregistered using the AW Server Fusion 2.0 (GE Healthcare). The prostate and seminal vesicles were contoured and the gtv, ctv and ptv were generated using the ADVSIM® system. For low-risk patients, the ctv included the prostate and the base of the seminal vesicles; for intermediate- and high-risk patients, the ctv encompassed the prostate and the entire seminal vesicles. For high-risk patients, the regional pelvic lymph nodes were involved. Margins for expansion were 10 mm in all directions, except posteriorly, which was set at 6 mm for the ptv1 (50.4 Gy). For boost contouring, the ctv2 included the prostate gland and any gross tumours observed outside of the prostate gland, and the expansion margins were 5 mm in all directions to generate the ptv2.
The bladder and femoral heads/bone were delineated consecutively, and the rectum was defined as an organ at risk beginning from 1 cm above the ptv1 to 1 cm below the ptv1. The small bowel was drawn from the inferior small bowel loop as a continuous structure to 1 cm above the planning target volume.
For treatment planning, the dose–volume constraints for the rectum were as follows: V50 Gy ≤ 50%, V60 Gy ≤ 40%, and V70 Gy ≤ 25%. The dose constraints for the bladder were V65 < 50% and a mean dose of <60 Gy. The dose constraint for the small bowel was a maximum dose of <50 Gy and V45 ≤ 25 cm3. 3D conformal radiotherapy (3D-CRT) and IMRT/RapidArc® (Varian Medical Systems) planning were performed using the Eclipse™ Treatment Planning System.
All IGRT patients underwent daily online matching. Furthermore, for 46 IGRT patients, fiducial markers were used. Three gold markers were implanted under ultrasound (US) guidance into the prostate before CT planning. Before each treatment, MV/KV images were obtained daily to align the markers to the planning data set. For 56 IGRT patients, the prostate position was detected by cone beam-CT (CBCT) scan before treatment. The patients were shifted for discrepancies in setup, and additional images were obtained to verify treatment accuracy. The alignment was performed with the contours based on soft tissue 3D images. According to published recommendations, a standardized patient setup verification protocol based upon a daily acquisition of portal images and cone-beam CTs were used to eliminate systematic deviations in patient setup.12, 13
Treatment-related side effects were recorded weekly during the course of radiotherapy. All acute and late GI and GU symptoms were scored according to the Common Toxicity Criteria Version 3.0 (CTCAEv3.0).14 Follow-up information included history, PSA values and physical examinations during the interval from the completion of irradiation to the last follow-up contact. Late toxicity was defined as side effects occurring later than 6 months after the completion of treatment. In addition, radiation-dependent cutaneous toxicities and fatigue symptoms were documented. The highest acute and late toxicity scores for each type of toxicity were evaluated for each patient.
The data were evaluated by descriptive statistical methods using the SPSS software package (Windows) and the Real Statistics Data Analysis Tool™/Excel/Microsoft Word. The different variables were evaluated by contingency tables. Associations between the different variables were calculated using the chi-square test and Fisher's exact test (two-tailed). P < 0.05 was considered to be statistically significant. Biochemical relapse rates for each treatment group (±IGRT) were compared. Biochemical failure was defined using the Phoenix definition.15
Approval of the study (no. 2191-2014) was given by the ethics committee of the Hannover Medical School.
4. Results
A total of 207 patients were randomly included in this analysis. Follow-up data were not complete for 9 patients. In the non-IGRT cohort, 96 patients were evaluable, and 102 evaluable patients underwent image-guided radiotherapy. The patient characteristics are presented in Table 1. The baseline data for the patients in each treatment group were comparable except for the risk score which was graded as high-risk in 40% of the non-IGRT patients and 61% of the IGRT patients (p < 0.05). Anti-hormonal therapy was applied in 74% (non-IGRT) and 75% (IGRT) of the high-risk patients. In addition, comorbidities (diabetes and hypertension) were reported for 36% and 34% of the patients in the non-IGRT and IGRT groups, respectively. The median follow-up for all the patients was 55.4 months (range 13–101 months), and the median follow-up periods by treatment group were 7.3 years (range 76–101 months, non-IGRT,) and 1.9 years (range 13–37 months, IGRT). Dose–volume analysis was performed for each patient during treatment planning. The mean dose to the bladder was 47.8 Gy, and the mean dose received by the rectum was 48.2 Gy. There was no significant difference between the treatment groups concerning the dose–volume relationships of the organs at risk.
Table 1.
Patients baseline of the IGRT and non-IGRT patient cohort (NA: not available).
| Non-IGRT n (%) | IGRT n (%) | |
|---|---|---|
| T stage | ||
| 1 | 29 (30) | 23 (23) |
| 2 | 53 (55) | 58 (57) |
| 3 | 13 (14) | 20 (20) |
| 4 | 1 (1) | 1 (1) |
| Gleason score | ||
| ≤6 | 51 (52) | 32 (31) |
| 7 | 33 (34) | 47 (46) |
| 8 | 8 (8) | 15 (15) |
| 9 | 2 (2) | 6 (6) |
| 10 | 0 (0) | 2 (2) |
| NA | 2 (2) | 0 (0) |
| Risk score | ||
| Low | 23 (24) | 7 (7) |
| Intermediate | 35 (36) | 33 (32) |
| High | 38 (40) | 62 (61) |
| Histopathological grade | ||
| G1 | 11 (1) | 1 (1) |
| G2 | 74 (77) | 65 (64) |
| G3 | 10 (10) | 22 (22) |
| NA | 1 (1) | 14 (14) |
| Age (years) | ||
| Mean (range) | 72.3 (41–86) | 73.6 (47–82) |
| Androgen suppression | ||
| Yes | 57 (59) | 61(60) |
| No | 39 (40) | 41 (37) |
| Pretreatment PSA (ng/ml) | ||
| <10 | 68 (71) | 74 (73) |
| 10 to <20 | 14 (14) | 20 (19) |
| ≥20 | 14 (14) | 8 (8) |
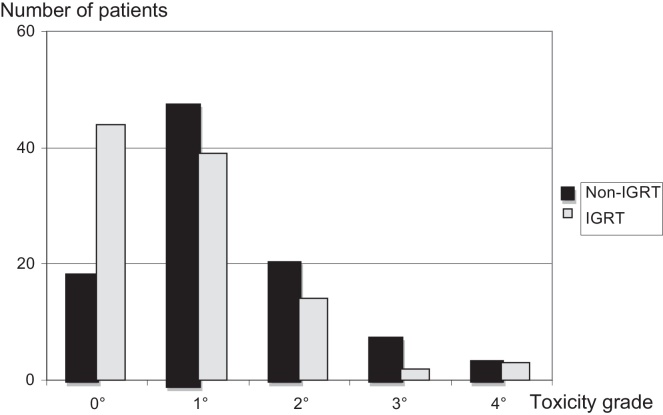
Acute GI and GU toxicities based on treatment type are documented in Table 2. Acute GI toxicities, according to the CTCAE score, were noted in 44% (grade 1), 17% (grade 2) and 5% (grade 3) of the cases, respectively. Severe acute side effects (grade 4) were observed in 3% of each treatment group. 3 IGRT patients and 2 non-IGRT patients suffered from haemodynamic collapses due to severe diarrhoea and 1 non-IGRT patient suffered from significant spontaneous rectal bleeding. Acute GU side effects were observed in 50% (grade 1), 27% (grade 2) and 7% (grade 3) of the patients. No patient had acute grade 4 GU toxicities. The comparisons of acute toxicities ≥1, ≥2, and ≥3 are shown in Table 3a, Table 3b. A statistically significant difference was noted for acute GI toxicities ≥1 in favour of IGRT treatment. Significantly more patients treated by definitive IGRT were free of acute GI symptoms (43% vs. 19%, p = 0.0012, Fig. 1, Table 3a). There were no statistically significant differences in acute GU toxicities between the groups, but in the non-IGRT group, more patients experienced treatment-related symptoms (89% vs. 80%, p = 0.07, Table 3b). Proportionally, there were more patients with grade 1 and grade 3 GU reactions in this group.
Table 2.
Maximum toxicity score during radiotherapy according to CTCAEv3.0a (GI: gastrointestinal, GU: genitourinary, n: number of patients).
| Grade | Acute GI toxicity |
Acute GU toxicity |
||
|---|---|---|---|---|
| Non-IGRT | IGRT | Non-IGRT | IGRT | |
| n (%) | n (%) | n (%) | n (%) | |
| 0 | 18(19%) | 44 (43) | 11 (11) | 20 (20) |
| 1 | 48 (50) | 39 (38) | 51 (53) | 48 (47) |
| 2 | 20 (21) | 14 (14) | 25 (26) | 29 (28) |
| 3 | 7 (7) | 2 (2) | 9 (9 | 5 (5) |
| 4 | 3 (3) | 3 (3) | 0(0) | 0(0) |
Common Toxicity Criteria Version 3.0.13
Table 3a.
Comparison of acute gastrointestinal toxicities (GI), maximum toxicity score during radiotherapy according to CTCAEv3.0a (n: number of patients).
| Acute GI toxicity grade | Non-IGRT | IGRT | p-value |
|---|---|---|---|
| in % | in % | ||
| ≥1 | 81 | 57 | 0.0012 |
| ≥2 | 31 | 19 | 0.15 |
| ≥3 | 10 | 5 | 0.18 |
Common Toxicity Criteria Version 3.0.13
Table 3b.
Comparison of acute genitourinary toxicities (GU), maximum toxicity score during radiotherapy according to CTCAEv3.0a (n: number of patients).
| Acute GU toxicity grade | Non-IGRT | IGRT | p-value |
|---|---|---|---|
| in % | in % | ||
| ≥1 | 89 | 80 | 0.07 |
| ≥2 | 34 | 34 | 1 |
| ≥3 | 9 | 5 | 0.41 |
Common Toxicity Criteria Version 3.0.13
Fig. 1.
Comparison of acute gastrointestinal toxicity in IGRT vs. non-IGRT patients, p < 0.05.
Late toxicity scores were comparable for both irradiation cohorts, indicating that most patients recovered quickly within 6 weeks after treatment. Chronic grade 1 GI toxicities were observed in 34% of the cases. 13.5% of the patients suffered from late grade 2 GI toxicities. Only one non-IGRT patient developed chronic rectal bleeding (grade 3). Complete chronic GI and GU toxicities depending on treatment modality are listed in Table 4, Table 5. Although IGRT patients had a higher rate of grade 2 GU late effects (17% vs. 9%), this group experienced relatively less grade 1 toxicity (10% vs. 34% in non-IGRT patients).
Table 4.
Late toxicity score after radiotherapy according to CTCAEv3.0a (GI: gastrointestinal, GU: genitourinary, n: number of patients).
| Grade | late GI toxicity |
late GU toxicity |
||
|---|---|---|---|---|
| Non-IGRT | IGRT | Non-IGRT | IGRT | |
| n (%) | n (%) | n (%) | n (%) | |
| 0 | 51 (54%) | 51 (50) | 77 (80) | 50 (49) |
| 1 | 34 (35) | 34 (33) | 10 (10) | 35 (34) |
| 2 | 10 (10) | 17 (17) | 9 (9) | 17 (17) |
| 3 | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Common Toxicity Criteria Version 3.0.13
Table 5.
Comparison of late toxicities ≥ grade 2 according to CTCAEv3.0.a
| Toxicity ≥ grade 2 | Non-IGRT (%) | IGRT (%) | p-value |
|---|---|---|---|
| Diarrhoea | 10 | 17 | 0.15 |
| Rectal bleeding | 1 | 0 | 0.32 |
| Urgency, dysuria | 9 | 17 | 0.09 |
| Fatigue | 4 | 5 | 0.73 |
Common Toxicity Criteria Version 3.0.13
Chronic grade 2 and 3 GI toxicity was mostly caused by diarrhoea and rectal bleeding, and GU toxicity mainly developed from urgency and dysuria. We did not observe any cases of urethral stricture during the follow-up period. Overall, fatigue-related symptoms were reported by 4.5% of the patients without a treatment-related difference.
All non-IGRT patients and 80.4% of the 102 patients treated with IGRT received 3D-CRT, whereas 19.6% of the IGRT patients were irradiated using the IMRT/RapidArc technique. The median follow-up time for the patients treated with IMRT/RapidArc was 1.4 years. A subset analysis did not reveal any significant differences compared to the entire IGRT group.
Kaplan–Meier analysis was performed to determine survival rates. The probabilities of PSA relapse-free survival were 100%, 95%, 86% and 84% at 6, 10, 12 and 18 months, respectively, for all the patients.
Considering the short median follow-up for the IGRT patients the comparison of PSA relapse free survival rates between the two treatment groups was not reliable to detect any differences.
5. Discussion
The incidence of acute and late side effects after definitive irradiation varies among published studies, but grade 2/3 toxicities have been reported in up to 40% of cases.2, 4, 16, 17, 18, 19 Until now, acute and chronic gastrointestinal and genitourinary toxicities are the main sources of morbidity for prostate cancer patients. Severe treatment-related side effects may reduce patient compliance and thereby impede the completion of therapy protocols as well as the effective treatment of the cancer. Furthermore, such side effects may adversely affect the patient's quality of life.20
The toxicity experienced by patients is mainly influenced by the irradiation technique employed. Modern high-dose treatment techniques have been shown to improve biochemical control rates and clinical results,1, 2, 3 but toxicity is a serious concern for treatments administering dose-escalated irradiation up to 78–84 Gy.21 Therefore, many efforts are made to spare the organs at risk by using internal and external immobilization devices.7, 8, 17, 22 The application of an endorectal balloon with 40-ml air filling spares the rectal and anal wall during 3D-CRT7, 22, 23 leading to reduced acute and late gastrointestinal toxicity rates. According to these data, our patients followed a validated bladder and rectal filling protocol.
In our study, we evaluated the toxicity in patients before and after the implementation of image guidance and dose-escalated irradiation up to a total dose of 77.4 Gy. Despite the limitations of a retrospective analysis, the baseline characteristics of each a study group were comparable (Table 1) without significant differences. In addition, patients were treated uniformly on standardized immobilization and setup protocols, and we were able to directly analyze the influence of image guidance and dose escalation.
In our trial, significantly more patients treated by definitive IGRT were free of acute GI symptoms. The proportion of benefit was 24% less for grade ≥1 reactions, despite escalation of the total dose from 73.8 to 77.4 Gy. We could not prove any significant effects concerning GU side effects, but more patients experienced dysuria symptoms in the non-IGRT group.
As shown by Haaren et al.,24 a large dose spread by organ movement can be reduced by including daily setup corrections. More precise targeting reduces unnecessary radiation of the rectum. In addition, our subset analysis did not observe any significant differences between IMRT patients and all IGRT patients, as we used the same dose constraints for 3D-CRT and IMRT planning; we observed grade 2 toxicities in 14% of the IGRT patients.
Other studies of IGRT have reported acute grade 2 GI toxicities in between 8% and 35%.3, 9, 19, 25, 26 These data are limited in terms of the quantifiable benefits of IGRT due to differences in the number of patients, the protocols and the dose constraints. Contradictory to our results, the incidence of grade 2 GI effects was 30%, compared to 35% in the non-IGRT group, in the study by Gill et al.9 after delivering a total dose of 78 Gy. Lips et al.26 evaluated 330 patients treated by IMRT/IGRT with a total dose of 76 Gy. Acute grade 2 GU and GI toxicities occurred in 47% and 30% of the cases, respectively, but the data were not compared to those for non-IGRT cases. Comparable to our data, a study that analyzed 238 IGRT-treated prostate cancer patients reported an incidence of grade 2 side effects of 19%.27 Acute grade 3 and 4 toxicities were described in 0–8% of the patients. Pollack et al.28 evaluated 100 prostate cancer patients irradiated by IGRT with a total dose of 76 Gy, and only 2% of the patients suffered from severe toxicities. In our trial, severe GI and GU toxicities were reduced from 10% and 9%, respectively, to 5% by implementing IGRT without any grade 4 GU reactions. In comparison to other studies, the incidence of acute GI toxicity was lower, potentially because of the validated application of an endorectal balloon to effectively stabilize prostate motion.7 Early grade ≥2 GU toxicity occurred in 34% of our patients. Notably, 20% of the IGRT patients did not complain of any GU symptoms, compared to 11% of the non-IGRT patients (p = 0.07). As reported by Ghadjar et al.,4 acute urinary symptoms occur in up to 56% of cases. Severe GU side effects were rare, occurring in 9% of the non-IGRT group and in 5% of the IGRT group, without any grade 4 reactions. These results corresponded with published data.4, 9, 25, 26
The incidence of late grade ≥2 GI toxicity was 17% (IGRT) vs. 11% (non-IGRT), and only one patient suffered from rectal bleeding 12 months after treatment. There were no significant differences between treatment groups in regard to late grade ≥2 GU toxicity.
The limitation of our retrospective analysis was the shorter follow-up period of only 1.9 years for IGRT-treated patients compared to the 55.4-month mean follow-up for all patients. According to published data, the majority of later rectal side effects manifest during a follow-up period of 36 months.26, 29 Nevertheless, these data compare well to those from the randomized trial published by Zietman et al.,29 which reported late grade 2 GI side effects in 17% of the patients and grade 3 reactions in 1% of the patients (total dose of 79.2 Gy). In addition, acute toxicity is predictive of late rectal and GU side effects.30 A meta-analysis21 of randomized trials of dose-escalated irradiation reported late grade ≥2 GI and GU toxicity rates of 17–43% and 21–56%, respectively. Although there were more cases of late grade ≥2 GI toxicity after dose escalation, no significant increase in RTOG grade 3 late urinary or rectal morbidity was identified (high-dose RT vs. conventional-dose RT).21 Therefore, most studies have applied IGRT/IMRT with smaller ptv margins. It is noteworthy that we demonstrated the advantage of image guidance without margin reduction.
Approximately 5% of the patients reported late grade ≥2 fatigue symptoms, but there was no difference between the treatment groups. Fatigue is a systemic symptom, but organ toxicity, such as severe diarrhoea, has been shown to be a significant determinant. We did not find any associations with severe reactions due to the small number of fatigue cases. A quality of life study of prostate cancer patients observed a deterioration in global health status, fatigue and pain at occasional time points.31
As proven in randomized trials,3, 29 men with clinically localized prostate cancer have a lower risk of biochemical failure if they receive high-dose, rather than conventional-dose, conformal radiation. Due to the median follow-up time of only 1.9 years after dose-escalation from 73.8 to 77.4 Gy our analysis was not able to show any significant improvement in PSA relapse rate or biochemical relapse-free survival. The advantage of dose escalation might be evident with a longer follow-up period.
6. Conclusion
Even though this study was retrospective, we consider our conclusions to be of significant informative value. Notably, all of the patients included in the study were treated at the same institution with identical positioning aids and internal immobilization, including rectal balloons and a drinking protocol to standardize the bladder content. Furthermore, all of the patients were treated given the availability of the same technical means and conditions and were evaluated for toxicities at the same centre. Based on the data generated from a large patient cohort, we showed that despite dose escalation, IGRT enabled us to reduce the GI side effects of radiation. Therefore, IGRT can be considered the standard of care for dose-escalated radiation therapy of localized prostate cancer.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Zietman A.L., DeSilvio M.L., Slater J.D. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. J Am Med Assoc. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 2.Cahlon O., Zelefsky M.J., Shippy A. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Pollack A., Zagars G.K., Smith L.G. Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. J Clin Oncol. 2000;18:3904–3911. doi: 10.1200/JCO.2000.18.23.3904. [DOI] [PubMed] [Google Scholar]
- 4.Ghadjar P., Vock J., Vetterli D. Acute and late toxicity in prostate cancer patients treated by dose escalated intensity modulated radiation therapy and organ tracking. Radiat Oncol. 2008;3:35. doi: 10.1186/1748-717X-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalski J.M., Yan Y., Watkins-Bruner D. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87(5):932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanakavelu N., Samuel E.J. Assessment and evaluation of MV image guidance system performance in radiotherapy. Rep Pract Oncol Radiother. 2015;20(3):188–197. doi: 10.1016/j.rpor.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiebe M., Pinkert U., Hennig I., Förg T., Hoffmann W. Konformale Strahlentherapie des Prostatakarzinoms–Einfluss der internen Immobilisation durch Rektumballonkatheter auf die Risikoorganbelastung. Strahlenther Onkol. 2001;177(1):25. [Google Scholar]
- 8.Mullaney L.M., O'Shea E., Dunne M.T. A randomized trial comparing bladder volume consistency during fractionated prostate radiation therapy. Pract Radiat Oncol. 2014;4(5):e203–e212. doi: 10.1016/j.prro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Gill S., Thomas J., Fox C. Acute toxicity in prostate cancer patients treated with and without image-guided radiotherapy. Radiat Oncol. 2011;6:145–152. doi: 10.1186/1748-717X-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Crevoisier R., Tucker S., Dong L. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:965–973. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico A.V., Whittington R., Schultz D., Malkowicz S.B., Tomaszewski J.E., Wein A. Outcome based staging for clinically localized adenocarcinoma of the prostate. J Urol. 1997;158(4):1422–1426. [PubMed] [Google Scholar]
- 12.De Boer H.C., Heijmen B.J. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys. 2001;50(5):1350–1365. doi: 10.1016/s0360-3016(01)01624-8. [DOI] [PubMed] [Google Scholar]
- 13.Korreman S., Rasch C., McNair H. The European Society of Therapeutic Radiology and Oncology-European Institute of Radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: a practical and technical review and guide. Radiother Oncol. 2010;94(2):129–144. doi: 10.1016/j.radonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Trotti A., Colevas A.D., Setser A. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 15.Roach M., III, Hanks G., Thames H., Jr. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974. doi: 10.1016/j.ijrobp.2006.04.029. http://www.ncbi.nlm.nih.gov/pubmed/16798415. [DOI] [PubMed] [Google Scholar]
- 16.Koper P.C., Jansen P., van Putten W. Gastro-intestinal and genito-urinary morbidity after 3D conformal radiotherapy of prostate cancer: observations of a randomized trial. Radiother Oncol. 2004;73:1–9. doi: 10.1016/j.radonc.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Peeters S.T., Hoogeman M.S., Heemsbergen W.D. Volume and hormonal effects for acute side effects of rectum and bladder during conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1142–1152. doi: 10.1016/j.ijrobp.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 18.Schultheiss T.E., Lee W.R., Hunt M.A. Late GI and GU complications in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:3–11. doi: 10.1016/s0360-3016(96)00468-3. [DOI] [PubMed] [Google Scholar]
- 19.Pervez N., Small C., MacKenzie M. Acute toxicity in high risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:57–64. doi: 10.1016/j.ijrobp.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Van Tol-Geerdink J.J., Leer J.W., van Oort I.M. Quality of life after prostate cancer treatments in patients comparable at baseline. Br J Cancer. 2013;108(9):1784–1789. doi: 10.1038/bjc.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viani G.A., Stefano E.J., Afonso S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74(5):1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 22.Wang K.K., Vapiwala N., Bui V. The impact of stool and gas volume on intrafraction prostate motion in patients undergoing radiotherapy with daily endorectal balloon. Radiother Oncol. 2014;112(1):89–94. doi: 10.1016/j.radonc.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Smeenk R.J., Teh B.S., Butler E.B. Is there a role for endorectal balloons in prostate radiotherapy? A systematic review. Radiother Oncol. 2010;95(3):277–282. doi: 10.1016/j.radonc.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Van Haaren P.M., Bel A., Hofman P. Influence of daily setup measurements and corrections on the estimated delivered dose during IMRT treatment of prostate cancer patients. Radiother Oncol. 2009;90(3):291–298. doi: 10.1016/j.radonc.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Fonteyne V., Villeirs G., Speleers B. Intensity-modulated radiotherapy as primary therapy for prostate cancer: report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int J Radiat Oncol Biol Phys. 2008;72(3):799–807. doi: 10.1016/j.ijrobp.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Lips I.M., Dehnad H., van Gils C.H. High-dose intensity-modulated radiotherapy for prostate cancer using daily fiducial marker-based position verification: acute and late toxicity in 331 patients. Radiat Oncol. 2008;3:15. doi: 10.1186/1748-717X-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soete G., Verellen D., Michielsen D., Rappe B., Keuppen F., Storm G. Image guided conformation arc therapy for prostate cancer. Early side effects. Int J Radiat Oncol Biol Phys. 2006;66:S141–S144. [Google Scholar]
- 28.Pollack A., Hanlon L., Horwitz M. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zietman A.L., DeSilvio M.L., Slater J.D. Comparison of conventional-dose vs highdose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. J Am Med Assoc. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien P., Franklin C., Poulsen M. Acute symptoms, not rectally administered sucralfate, predict for late radiation proctitis: longer term follow-up of a phase III trial-TransTasman Radiation Oncology group. Int J Radiat Oncol Biol Phys. 2002;54 doi: 10.1016/s0360-3016(02)02931-0. 442-229. [DOI] [PubMed] [Google Scholar]
- 31.Choo R., Pearce A., Danjoux C. Prospective evaluation of quality of life in prostate cancer patients receiving combined treatment of postoperative radiotherapy plus androgen suppression for PT3 or positive resection margin after radical prostatectomy. Eur Urol. 2007;52(6):1645–1650. doi: 10.1016/j.eururo.2006.11.018. [DOI] [PubMed] [Google Scholar]