Abstract
Aim
To assess the mechanical and the geometric accuracy of two different clinically used image guidance systems in radiotherapy for a period of 6 months.
Background
With the image guidance procedures being routine in the clinical radiotherapy department, the quality assurance tests for these systems become essential. The mechanical and geometric accuracy of these systems are crucial since it directly affects patient treatment set-up and delivery.
Materials and methods
We have assessed the mechanical and the geometric accuracy of two different image guidance systems (MV and kV based), being used clinically for a period of 6 months. The quality assurance tests such as imager positioning/repositioning, imaging and treatment beam isocentre coincidence, imager mechanical alignment, image scaling, geometric accuracy of cone beam computed tomography system, automatic image registration and offset calculation accuracy were assessed in this period.
Results
It was found that both systems were mechanically and geometrically accurate within ±2 mm in this period.
Conclusion
The quality assurance tests for MV based image guidance system were simple compared to kV based systems. We recommend performing periodic quality assurance tests to verify the integrity of both image guidance systems.
Keywords: Image guidance, Mechanical and geometric accuracy, Quality assurance
1. Background and aim
Image guidance in radiotherapy has become essential with sophisticated three dimensional treatment techniques, such as intensity modulated radiotherapy, stereo-tactic radiotherapy, volumetric modulated arc therapy, etc. It is being used routinely in radiotherapy centres to evaluate and correct inter-fractional patient setup errors and internal organ motion.1, 2, 3, 4, 5 In the past, orthogonal mega voltage (MV) portal images were acquired to verify patient positioning with respect to the treatment beam. Using portal images limits the visualisation only to bony structures. In recent times, the cone beam computed tomography (CBCT) has been used to provide a volumetric image of the patient that is acquired just prior to treatment delivery on the treatment table.6, 7, 8 Several CBCT systems are available commercially and are being used clinically in radiotherapy centres: MV CBCT, such as MVision®, kilo voltage (kV) CBCT, such as OBI®, XVI®. A good imaging system needs to be safe, mechanically and geometrically accurate providing good image quality with reasonable imaging dose. With the image guidance system capable of performing CBCT, it can be used for adaptive radiotherapy9, 10 to make decisions to change the treatment plan in the course of treatment to account for changes in patient anatomy due to tumour shrinkage or weight loss. With suitable CT to material density conversion curve, the CBCT can also be used for dose calculation in treatment planning.11, 12 Even though an image guidance system has several clinical applications, its primary use is to verify the patient position with respect to the treatment beam, which makes the mechanical and geometric accuracy of the system more important. Several studies have compared and reported MV and kV based imaging systems’ image quality and imaging dose.13, 14, 15, 16 These imaging systems differ in their geometry, acquisition and reconstruction methods. These systems need to be assessed for their alignment with respect to treatment beam and imaging accuracy in order to be used in clinics. We present a study on the mechanical and geometric accuracy assessment of two different clinically used image guidance systems in radiotherapy for a period of 6 months.
2. Materials and methods
2.1. System 1
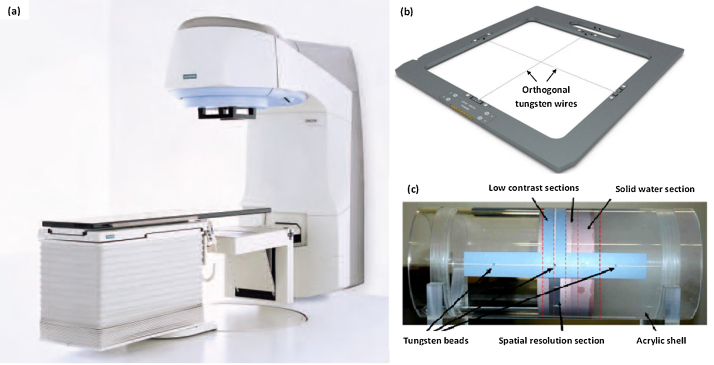
The Siemens Oncor Expression™ (Siemens Medical solutions Inc., Concord, CA) linear accelerator is capable of delivering high energy photons and electrons. It is equipped with the MV imaging guidance system (OPTIVUE 1000ST™) able to acquire MV planar and CBCT imaging and is attached to the gantry at the counter-part of the head of the linear accelerator, as shown in Fig. 1(a). The image guidance system consists of flat panel detectors which have the sensors of amorphous silicon (a-Si) photo diodes that are deposited on a glass substrate with a scintillator coating. The pixels have a pitch of 400 μm and there are 1024 × 1024 pixels covering a 40 × 40 cm2 area. The X-ray reticule (named ‘X-RETIC’) consists of two orthogonal radio-opaque tungsten wires, shown in Fig. 1(b), that intersect at the collimator rotation axis and can be inserted in the accessory slot in the gantry head and are used to represent the treatment co-ordinate axis in the acquired MV image. The image quality phantom (Siemens Medical Solutions, Concord, CA), as shown in Fig, 1(c), is a cylindrical acrylic shell of diameter 20 cm with four sections for image quality checks, positioned axially within the shell. It also has tungsten beads at three axial planes such that four tungsten beads are arranged at 12, 3, 6 and 9 o’ clock at each axial plane to determine the geometric positional accuracy of CBCT image.
Fig. 1.
(a) Siemens Oncor Expression linear accelerator system equipped with MV imager; (b) X-ray reticule orthogonal tungsten wires, to represent treatment co-ordinate axis; (c) image quality phantom consisting of four sections to check the image uniformity, noise, low contrast and spatial resolution. The tungsten beads at 3 o’ clock position in three axial planes are also shown.
2.2. System 2
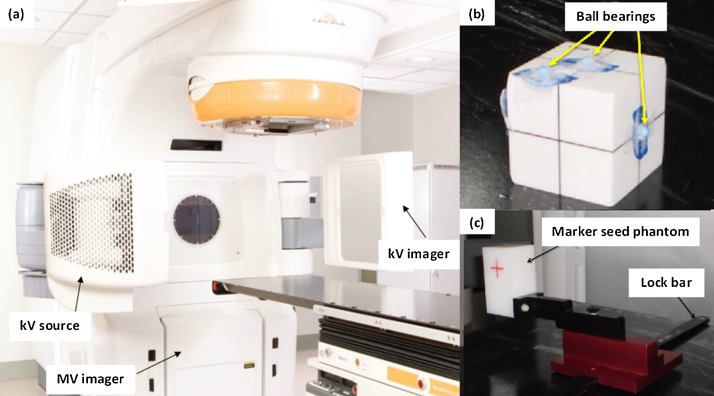
The NovalisSTx™ linear accelerator (Varian, Palo Alto, CA and BrainLAB, Heimstetten, Germany), as shown in Fig. 2(a), is capable of delivering high energy photons for conventional and stereotactic treatment delivery and high energy electrons. The MV and kV imaging systems are integrated with the linear accelerator. The MV electronic portal imager consists of a-Si based detector with 1024 × 768 pixel matrix covering an area of 40 × 30 cm2, mounted in the gantry at the counter-part of the head of the linear accelerator. The kV on-board imager (OBI) system, mounted orthogonal to the MV beam axis consists of a kV source and a kV a-Si detector-based imager covering an area of 40 × 30 cm2. The detailed description of the system can be found elsewhere.17 The MV imager can acquire planar images and the kV OBI can acquire both planar and CBCT volumetric images. The isocentre cube phantom and the OBI geometric phantom (Varian, Palo Alto, CA), as shown in Fig. 2(b) and (c), respectively, are used to perform geometric accuracy tests of the imaging system. The isocentre cube phantom consists of a 2 mm spherical radio-opaque ball bearing (bb) in the centre of the cube and several other bbs at the surface. The OBI geometric phantom also known as the Marker seed phantom has five radio-opaque markers inbuilt within the block and can be fixed on the couch using a lock bar.
Fig. 2.
(a) Varian NovalisSTx linear accelerator system equipped with MV imager, kV source and imager; (b) isocentre cube phantom with radio-opaque ball bearings; (c) OBI geometric phantom consisting of marker seed phantom with 5 inbuilt radio-opaque markers, which can be fixed on the treatment table using lock bar.
2.3. Mechanical and geometric accuracy tests
Periodic quality assurance tests were previously developed to assess and evaluate the performance of the imaging system.18 In this study, the mechanical and geometric accuracy for two different imaging systems were assessed for a period of 6 months. Table 1 lists the QA tests, their frequencies and tolerances. The tolerance values for the tests were set based on recommendations from the American Association of Physicists in Medicine (AAPM) task group (TG) reports19, 20 and manufactures’ specifications.
Table 1.
Mechanical and geometric accuracy QA tests for image guidance system.
| Frequency | QA test | Tolerance |
|---|---|---|
| Daily | Imager positioning/repositioning | ±1 mm |
| Imaging and treatment beam coincidence (single gantry angle) | ±1 mm | |
| Monthly | Imaging and treatment beam coincidence (4 cardinal gantry angles) | ±1 mm |
| Image scaling | ±2 mm | |
| Mechanical alignment – full range of travel | ±2 mm | |
| Geometric accuracy for CBCT | ±2 mm | |
| Automatic image registration and offset calculation accuracy | ±2 mm |
2.4. Imager positioning/repositioning
The imaging device attached to the machine can be extended during imaging and retracted when not in use for easy access for radiographers to set up the patient. This mechanical movement of the imager requires performing imager positioning accuracy and reproducibility tests. The test involves acquiring planar image of a phantom with inbuilt radio opaque markers and verifying its position with respect to image centre with every imager positioning and repositioning. System 1: a slab phantom with 5 radio opaque markers at known distances was used for testing the MV flat panel imager. System 2: the test includes testing the positioning and repositioning accuracy of the MV imager, kV source and detector arms. A cube phantom with inbuilt radio-opaque markers was used for this system. The positioning accuracy of the imager was measured with the variation between the expected and measured distances of each marker from the centre. The repositioning accuracy was measured with the variations in the markers’ positions between the two images acquired before and after repositioning.
2.5. Imaging and treatment beam isocentre coincidence
The imager co-ordinate axis has to be coincident with the treatment beam coordinate axis in order to have correspondence between the treatment verification images of patient position with respect to the treatment beam. This alignment was checked daily at gantry angle of 0° and monthly in the other cardinal gantry angles, i.e. 90°, 180° and 270°. For system 1, the MV beam and the MV imager alignment was checked by acquiring planar MV image of the XRETIC, which represents the treatment co-ordinate axis and its projection on the planer image was verified against the imager coordinate axis which can be visualised with the graphic grid display option. The misalignment between the two axes was measured using a ruler or angle measuring tool option. For system 2, the MV beam and MV imager alignment was checked to verify the MV isocentre position, then KV beam and KV imager alignment with reference to the MV isocentre was checked. This test was performed using the isocentre cube phantom.
2.6. Mechanical movement of the imaging system
The accuracy of mechanical movement of the imager was checked throughout its range of travel in the lateral, longitudinal and vertical directions using a graph sheet and a measuring tape. For system 1, the MV imager can be moved only vertically along the beam axis. The lateral and longitudinal alignment was checked at various source to imager distances (SIDs). For system 2, the MV imager has 5 stored vertical positions and can have a range of movement in the lateral and longitudinal directions. The kV source and detector position from isocentre was also measured. The alignment was checked by moving the imager to various positions and comparing against expected values.
2.7. Scaling
For both the systems, the planar and cone beam image scaling was checked by imaging an object of known dimension and measuring its dimension on their respective images.
2.8. Geometric accuracy for CBCT
The tungsten beads at the centre, head and foot of the image quality phantom, were used to verify geometric positional accuracy of CBCT imaging system 1. For system 1, MV CBCT images were acquired with the phantom aligned at the machine isocentre. In the system software, the reference point cursor was placed at the centre of each bead in the CBCT image to read the three dimensional co-ordinates of each bead and compared with the actual known position.
For system 2, the isocentre cube phantom was scanned on a CT simulator and used as the planning CT dataset. Then the cube was placed on the treatment table and aligned with the room lasers such that the central bb lies at the machine isocentre. CBCT images of the cube were acquired and the dataset was aligned with the planning CT dataset such that the central bb on both datasets coincides with each other. The table co-ordinates in the lateral, longitudinal and vertical directions calculated to align the two datasets gives the geometric positioning accuracy of CBCT.
2.9. Automatic image registration and table offset calculation accuracy
The automatic image registration tool in the image guidance system software registers the planning CT dataset with the CBCT dataset acquired before treatment delivery to verify the patient positioning. The registration is based on mutual information algorithm. Once the registration is checked and accepted by the user, the software displays the table offset for set-up correction. The automatic image registration and table offset calculation accuracy of the system were checked by introducing a known off-set between the two datasets. The treatment table scales accuracy was checked monthly to be within ±1 mm. Image quality phantom and OBI geometric phantom were used for this test for system 1 and system 2, respectively. The Phantoms were positioned and scanned on the CT similar to that of a patient. A simple plan was made in treatment planning system (TPS) with planning isocentre coinciding with the centre of the phantom. The phantom was set-up on the treatment machine with the planned isocentre coinciding with the machine mechanical isocentre. CBCT images were acquired and the system started automatic registration of acquired CBCT dataset and the planning CT dataset. The registration was visually verified in the axial, coronal and sagittal views and, when accepted, the system calculated and displayed the table offset in the lateral, longitudinal and vertical directions. Any residual offset in the phantom positioning from the isocentre were noted. The CBCT scans were repeated for different known shifts introduced to the CBCT images by shifting the table in the lateral, longitudinal and vertical directions. Each time the system computed offset from automatic registration was noted and compared with the applied table offset.
3. Results
3.1. Imager positioning/repositioning
The tests were performed daily for a period of 6 months and the mean and standard deviation in the marker position during this period for both systems are shown in Table 2. The positioning and repositioning accuracy of both systems were within ±1 mm in this 6-month period.
Table 2.
Results showing the positioning/repositioning accuracy of the two imaging systems.
| System | Device | Mean variation ± standard deviation (mm) |
|
|---|---|---|---|
| Positioning accuracy | Repositioning accuracy | ||
| 1 | MV Imager | 0.2 ± 0.4 | 0.0 ± 0.1 |
| 2 | MV Imager | 0.3 ± 0.3 | 0.1 ± 0.1 |
| kV source & Detector | 0.1 ± 0.3 | 0.0 ± 0.1 | |
3.2. Imaging and treatment beam isocentre coincidence
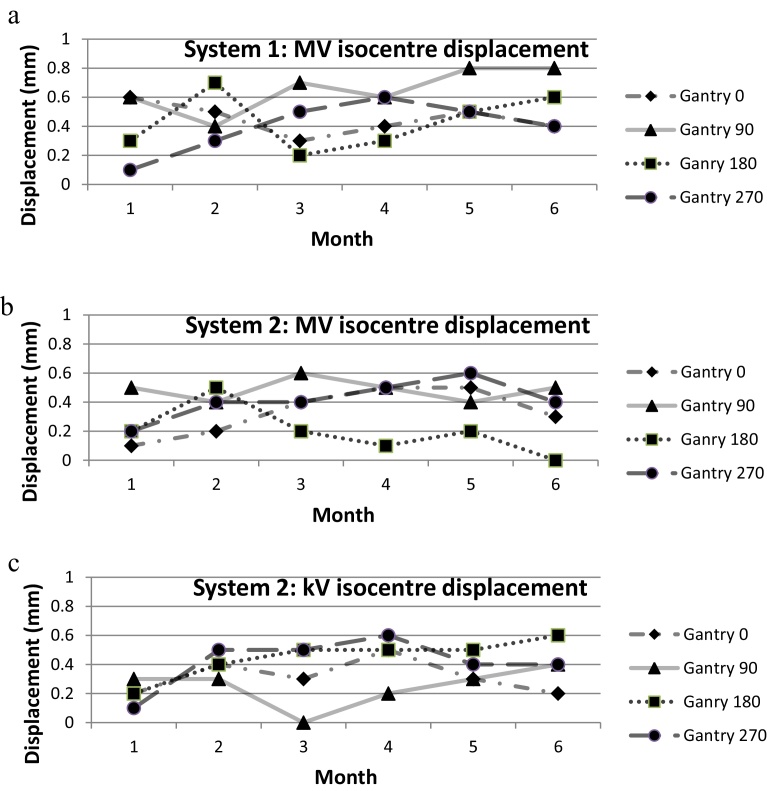
Fig. 3(a) shows the accuracy of alignment of MV imager with respect to the treatment beam for system 1. Fig. 3(b) and (c) show the accuracy of MV isocentre and kV beam isocentre for system 2. In the period of 6 months, the misalignment was within ±1 mm at any gantry angle for both systems.
Fig. 3.
(a) System 1: accuracy of alignment of MV imager to the MV treatment beam isocentre for a period of 6 months; (b) system 2: accuracy of alignment of MV imager to the MV treatment beam isocentre for a period of 6 months; (c) system 2: accuracy of alignment of kV imager to the kV beam isocentre with reference to the MV beam isocentre for a period of 6 months.
3.3. Mechanical movement of the imaging system
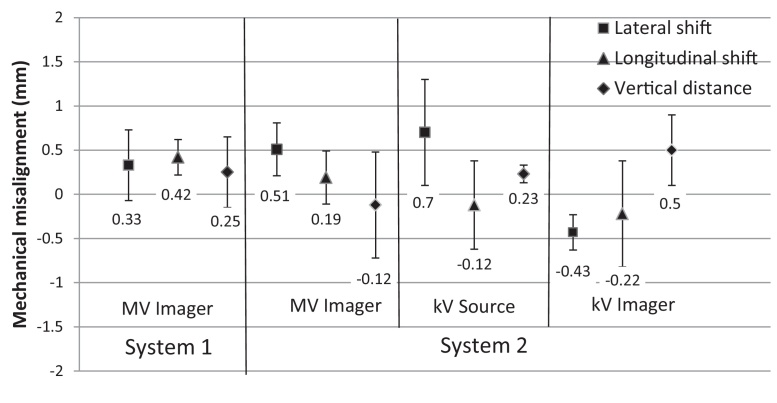
Fig. 4 shows the mean mechanical misalignment of both imaging systems along the lateral, longitudinal and vertical directions for a period of 6 months. For both systems the misalignment was within ±1 mm in any direction.
Fig. 4.
Mean mechanical misalignment of the two imaging systems with error bars representing one standard deviation in lateral, longitudinal and vertical directions for a period of 6 months.
3.4. Scaling
The image scaling accuracy was assessed monthly for both systems and the results were analysed for a period of 6 months. For system 1 MV planar and CBCT imaging, the mean variation between the measured and actual dimension were 0.2 ± 0.2 mm and 0.1 ± 0.2 mm, respectively. For system 2 MV planar, kV planar and CBCT, the mean ± standard deviations were 0.3 ± 0.2 mm, 0.2 ± 0.1 mm and 0.4 ± 0.1 mm, respectively.
3.5. Geometric accuracy for CBCT
For system 1, the mean variation in the geometric position of all beads was averaged for 6 months and shown in Table 3. For system 2, the average geometric positioning accuracy of the cube bb was tabulated. It was found that the accuracy of both systems was within ±2 mm during this period.
Table 3.
Geometric positioning accuracy of CBCT systems.
| Direction | Mean variation ± standard deviation (mm) |
|
|---|---|---|
| System 1 | System 2 | |
| Lateral (x) | −0.8 ± 0.8 | 0.3 ± 0.6 |
| Longitudinal (y) | 0.0 ± 0.3 | −0.3 ± 0.1 |
| Vertical (z) | −0.5 ± 0.8 | 0.6 ± 0.9 |
3.6. Automatic image registration and table offset calculation accuracy
The automatic registration and offset calculation accuracy test results were within ±2 mm for both systems and the results were averaged for a period of 6 months. The mean ± standard deviation of the test results of system 1 and 2 were 0.6 ± 0.5 mm and 0.5 ± 0.8 mm, respectively.
4. Discussion and conclusion
The mechanical and geometric accuracy were assessed for a period of 6 months for two image guidance systems used clinically. For both systems, the results were well within the tolerance.
For system 1, the system geometry is simple and robust and requires less manpower and time to perform the QA tests compared to system 2. The treatment and imaging co-ordinate coincidence tests were not implicit with system 2; it requires the MV mechanical isocentre, MV radiation isocentre, kV mechanical isocentre and kV radiation isocentre to be within ±1 mm in any direction, which requires considerable time daily. For both systems, manufacturer provided equipment was sufficient to perform these tests.
The geometric accuracy test is very crucial, since any inaccuracies may directly affect the patient set-up and treatment delivery. For the CBCT systems, geometric calibrations were performed frequently as recommended by the manufacturer. These calibrations involve defining the three dimensional imaging co-ordinates of the system. The geometric calibrations of these systems were mentioned elsewhere.21, 22, 23
Even though a MV-based imaging system has simple geometry, it lags behind in terms of image quality and imaging dose to the patient. Even if the imaging dose can be accounted in the treatment planning, the volume of tissue irradiated with the low imaging dose may be significant. This restricts frequent treatment verification imaging. However, the imaging protocols can be optimised to have daily MV imaging with 3 times 2D imaging and 2 times 3D imaging per week. kV-based imaging systems require several additional tests and calibrations with respect to the MV system, such as the kV X-ray output test, kV beam quality tests, mAs linearity and kV blade calibration which requires additional time and maintenance costs for the system.
Our assessment shows that both systems were suitable to perform image guided radiotherapy. These systems require periodic assessment of mechanical and geometric accuracy as any geometrical flex in the mechanical movement of the gantry and/or the imaging system may affect the imaging accuracy and quality.
In conclusion, the mechanical and geometric tests for the MV-based image guidance system are simple and straight forward compared with kV-based system. Both systems require periodic quality assurance tests to assure their integrity with the treatment machine.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Kapanen M., Laaksomaa M., Tulijoki T. Effects of remedies made in patient setup process on residual setup errors and margins in head and neck cancer radiotherapy based on 2D image guidance. Rep Pract Oncol Radiother. 2015;20(4):292–298. doi: 10.1016/j.rpor.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semaniak A., Kukolowicz P. Set-up uncertainty during post mastectomy radiotherapy with segmented photon beams technique. Rep Pract Oncol Radiother. 2015;20(3):181–187. doi: 10.1016/j.rpor.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cendales R., Torres F., Arbelaez J. Displacements of fiducial markers in patients with prostate cancer treated with image guided radiotherapy: a single-institution descriptive study. Rep Pract Oncol Radiother. 2015;20(1):38–42. doi: 10.1016/j.rpor.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laaksomaa M., Kapanen M., Skyttä T. Estimation of optimal matching position for orthogonal kV setup images and minimal setup margins in radiotherapy of whole breast and lymph node areas. Rep Pract Oncol Radiother. 2014;19(6):9–375. doi: 10.1016/j.rpor.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piotrowski T., Yartsev S., Rodrigues G. Comparative analysis of image guidance in two institutions for prostate cancer patients. Rep Pract Oncol Radiother. 2014;19(3):206–213. doi: 10.1016/j.rpor.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma C.M., Paskalev K. In-room CT techniques for image-guided radiation therapy. Med Dosim. 2006;31(1):30–39. doi: 10.1016/j.meddos.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Xing L., Thorndyke B., Schreibmann E. Overview of image-guided radiation therapy. Med Dosim. 2006;31:91–112. doi: 10.1016/j.meddos.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Goyal S., Kataria T. Image guidance in radiation therapy: techniques and applications. Radiol Res Pract. 2014 doi: 10.1155/2014/705604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghilezan M., Yan D., Martinez A. Adaptive radiation therapy for prostate cancer. Semin Radiat Oncol. 2010;20(April (2)):130–137. doi: 10.1016/j.semradonc.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanderup K., Georg D., Pötter R. Adaptive management of cervical cancer radiotherapy. Semin Radiat Oncol. 2010;20(2):121–129. doi: 10.1016/j.semradonc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Schreibmann E., Li T. Evaluation of on-board kV cone beam CT CBCT-based dose calculation. Phys Med Biol. 2007;52(3):685–705. doi: 10.1088/0031-9155/52/3/011. [DOI] [PubMed] [Google Scholar]
- 12.Morin O., Chen J., Aubin M. Dose calculation using megavoltage cone-beam CT. Int J Radiat Oncol Biol Phys. 2007;67:1201–1210. doi: 10.1016/j.ijrobp.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 13.Stützel J., Oelfke U., Nill S. A quantitative image quality comparison of four different image guided radiotherapy devices. Radiother Oncol. 2008;86:20–24. doi: 10.1016/j.radonc.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Kamath S., Song W., Chvetsov A. An image quality comparison study between XVI and OBI CBCT systems. J Appl Clin Med Phys. 2011;12(2):376–390. doi: 10.1120/jacmp.v12i2.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song W.Y., Kamath S., Ozawa S. A dose comparison study between XVI® and OBI® CBCT systems. Med Phys. 2008;35:480–486. doi: 10.1118/1.2825619. [DOI] [PubMed] [Google Scholar]
- 16.Murphy M.J., Balter J., Balter S. The management of imaging dose during image-guided radiotherapy: report of the AAPM Task Group 75. Med Phys. 2007;34(10):4041–4063. doi: 10.1118/1.2775667. [DOI] [PubMed] [Google Scholar]
- 17.Yoo S., Kim G.Y., Hammoud R. A quality assurance program for the on-board imagers. Med Phys. 2006;33(11):4431–4447. doi: 10.1118/1.2362872. [DOI] [PubMed] [Google Scholar]
- 18.Kanakavelu N., Samuel E.J.J. Assessment and evaluation of MV image guidance system performance in radiotherapy. Rep Pract Oncol Radiother. 2015;20(3):188–197. doi: 10.1016/j.rpor.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein E.E., Hanley J., Bayouth J. Task group 142 report: quality assurance of medical accelerators. Med Phys. 2009:4197–4212. doi: 10.1118/1.3190392. [DOI] [PubMed] [Google Scholar]
- 20.Bissonnette J.P., Balter P.A., Dong L. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179. Med Phys. 2012;39:1946–1963. doi: 10.1118/1.3690466. [DOI] [PubMed] [Google Scholar]
- 21.Matsinos E., Kaissl W. Varian Medical Systems Imaging; Switzerland: 2006. The Geometric Calibration of Cone-Beam Imaging and Delivery Systems in Radiation Therapy. [Google Scholar]
- 22.Gayou O., Miften M. Commissioning and clinical implementation of a mega-voltage cone beam CT system for treatment localization. Med Phys. 2007;34:3183–3192. doi: 10.1118/1.2752374. [DOI] [PubMed] [Google Scholar]
- 23.Abou-Elenein H.S., Attalla E.M., Ammar H. Megavoltage cone beam computed tomography: commissioning and evaluation of patient dose. J Med Phys. 2011:205–212. doi: 10.4103/0971-6203.89969. [DOI] [PMC free article] [PubMed] [Google Scholar]