Abstract
Aims and background
To present survival and toxicity outcomes in patients with clinically localized, non-metastatic prostate cancer (PCa) treated with external beam radiotherapy (EBRT) combined with androgen deprivation therapy (ADT).
Materials and methods
Retrospective study of 849 PCa patients (pts) treated from 1996 to 2005. Until August 2000, all patients (281) were treated with conventional dose EBRT (<76 Gy); subsequent pts received ≥76 Gy (565 pts). Median age was 70 years (range, 39–82). Most pts were intermediate (353; 42.8%) or high-risk (344; 41.7%). Mean PSA was 10.1 ng/ml. Median dose to the prostate was 75 Gy. Complete ADT was administered to 525 pts (61.8%).
Results
Median follow-up was 109.6 months (range, 68.3–193.4). Overall survival (OS) was 92.5% and 81.1% at 5 and 10 years; by risk group (low, intermediate, high), 5- and 10-year OS rates were 94.3% and 85.9%, 92.3% and 79.2%, and 91.9% and 80.2% (p = 0.728). Five- and 10-year BRFS was 94.1% and 80.6% (low risk), 86.4% and 70.9% (intermediate), and 85.2% and 71.4% (high) (p = 0.0666). Toxicity included rectitis: grade 1 (G1) (277 pts; 32.6%), G2 (108; 12.7%), and G3 (20; 2.6%) and urethritis: G1 (294; 34.6%); G2 (223; 26.2%), and G3 (11; 1.3%). By dose rate (<76 Gy vs. ≥76 Gy), 5 and 10-year BRFS rates were 83.1% and 68.3% vs. 88.4% and 74.8% (p = 0.038).
Conclusions
Our results are comparable to other published series in terms of disease control and toxicity. These findings confirm the need for dose escalation to achieve better biochemical control and the benefits of ADT in high-risk PCa patients.
Keywords: Prostate cancer, High-dose, Toxicity, Radiation therapy, Survival rate
1. Background
External beam radiation therapy (EBRT) has long been used to treat localized prostate cancer (PCa). In the past, the prescribed radiation dose ranged from 64 to 70 Gy delivered in fractions of 1.8–2 Gy, although data from clinical trials1, 2 showed that such doses were insufficient to achieve better disease control. However, it was not possible to increase the maximum doses with conventional EBRT due to toxicity risk (primarily to the bladder and rectum). The advent of three-dimensional conformal radiotherapy (3D-CRT) in the 1990s increased the accuracy of dose delivery to the target, thus enabling the use of higher, more effective doses while keeping toxicity within acceptable levels. As a result, long-term outcomes have been improved and toxicity decreased.3
Dose escalation can be achieved with precise radiotherapy techniques such as 3D-CRT or intensity-modulated radiotherapy (IMRT), or by adding a boost delivered with high-dose rate brachytherapy (HDRB) or IMRT.4, 5, 6, 7 Overall survival in high risk patients can be further improved by combining EBRT with androgen deprivation therapy (ADT).8, 9
2. Aim
In the present retrospective study, we report long-term outcomes in a large patient cohort (849 patients) treated from 1996 to 2005 during a time period in which our treatment approach transitioned from conventional dose EBRT to high-dose 3D-CRT.
3. Materials and methods
3.1. Patient cohort
From 1996 to 2005, 849 patients (pts) underwent EBRT for clinically localized, non-metastatic PCa at our institution (Catalan Institute of Oncology; Barcelona, Spain). The median age was 70 years (range 39–82), with a median follow-up of 109.6 months (range, 68.3–193.4). Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics.
| Characteristic* | Value |
|---|---|
| Patients | 849 (100%) |
| Age (years) | |
| Median | 70 |
| Range | 39–82 |
| Follow-up (months) | |
| Median | 109.6 |
| Range | 68.3–193.4 |
| Gleason score | |
| ≤6 | 389 (45.8%) |
| =7 | 365 (43.0%) |
| >7 | 95 (11.2%) |
| Pretreatment PSA (ng/mL) | |
| Median | 10.1 |
| Range | 1.2–300.8 |
| T stage (DRE) (n = 819) | |
| T1 | 379 (46.3%) |
| T2 | 279 (34.1%) |
| T3 | 156 (19.0%) |
| T4 | 5 (0.6%) |
| T stage (MRI)) (n = 124) | |
| T1 | 14 (11.3) |
| T2 | 57 (46%) |
| T3 | 53 (42.7%) |
| T stage (US) (n = 477) | |
| T1 | 206 (43.2%) |
| T2 | 246 (51.6%) |
| T3 | 25 (5.2%) |
| Risk group (n = 824) | |
| Low | 127 (15.4%) |
| Intermediate | 353 (42.8%) |
| High | 344 (41.7%) |
MRI indicates magnetic resonance imaging; US, ultrasound; DRE, digital rectal examination.
Up to August 2000, all patients received conventional dose EBRT, at which time 3D-CRT was implemented. Thus, 281 of the 849 pts (33.1%) were treated with EBRT at doses <76 Gy. In the year 2000, our institution switched to high-dose radiotherapy based on findings from multiple studies10, 11, 12, 13, 14, 15 and updated international guidelines.16 Thus, all patients treated from August of that year (565 pts) were prescribed ≥76 Gy. Dose prescription data is not available for 3 cases.
The TNM-staging system of the American Joint Committee on Cancer17 was used to classify patients. The Table shows the patient characteristics at baseline. Staging and pre-treatment work-up for all patients consisted of a complete physical examination including digital rectal examination, complete blood count with PSA determination, chest X-ray, and transrectal ultrasound. CT scan, bone scintigraphy, and pelvic MRI were performed when needed.
The risk group classification system developed by D’Amico and colleagues,18 which includes blood PSA levels, Gleason score (GS) and tumour (T) stage, was used to assign patients to one of three risk groups: low, intermediate or high risk. Of the 849 pts in the study, data on risk group classification was available for 824 pts, as follows: 127 (15.4%) were considered low risk, 353 (42.8%) intermediate risk, and 344 (41.2%) high risk (Table 2). Mean pre-treatment prostate-specific antigen PSA was 10.1 ng/ml and Gleason score (GS) was ≤6, 7 and >7 in 389 pts (45.8%), 365 pts (43.0%), and 95 pts (11.2%), respectively. Perineural invasion was positive in 156 pts (18.4%).
Table 2.
Patient characteristics by risk group.
| Characteristic | Value |
||
|---|---|---|---|
| Low | Intermediate | High | |
| Patients | 127 (15.4%) | 353 (42.8%) | 344 (41.7%) |
| Age (y) | n = 124 | n = 353 | n = 340 |
| Median | 71 | 71 | 69 |
| Range | 39–78 | 50–80 | 41–82 |
| Gleason score | n = 127 | n = 353 | n = 344 |
| ≤6 | 127 (100%) | 121 (34.3%) | 123 (35.8%) |
| 7 | – | 232 (65.7%) | 129 (37.5%) |
| >7 | – | – | 92 (26.7%) |
| Pretreatment PSA (ng/mL) | n = 127 | n = 353 | n = 344 |
| Median | 6.9 | 9.3 | 20.9 |
| Range | 1.2–9.9 | 3.2–20 | 2.3–261 |
| ADT | n = 127 | n = 353 | n = 344 |
| Yes* | 31 (24.4%) | 164 (46.5%) | 310 (90.1%) |
ADT indicates androgen deprivation therapy (ADT).
In all cases, EBRT was performed with the patient in a supine position with legs and feet immobilized. Data from a CT scan performed with the patient in the treatment position were entered into the 3D treatment planning system to outline the prostate, vesicles, bladder and rectum. Regional lymph nodes were also contoured if the risk of the involvement was ≥15% (Partin tables).19 Patients with confirmed pelvic node involvement were excluded from the study.
The EBRT treatment was delivered in daily fractions of 2 Gy, 5 days per week. All patients received EBRT alone without boost. The median dose to the prostate was 75 Gy (range, 73.9–76). Most patients (565; 66.8%) were prescribed 76 Gy with the remaining (281; 33.2%) receiving <76 Gy. Data from 3 pts are missing.
Regional lymph nodes were treated with a conventional four-field box. The median dose in the pelvis was 50 Gy (42–50) in 138 pts. For treatment of the prostate and seminal vesicles, a six-field technique with opposed lateral and four oblique conformal field boost was implemented. The median dose in the prostate was 75 Gy (range, 60–78) and the median dose to the seminal vesicles was 70 Gy (46–76) in 760 patients. In the remaining pts, the seminal vesicles were not irradiated.
Applied margins (5 mm in the posterior direction and 10 mm in all other directions) were used to obtain the PTV, which included prostate, vesicles and margins. Custom blocking with multileaf collimators was designed using a beam's eye view and additional margins were adjusted to provide a minimum dose of 95% to the prostate PTV. Risk organ constraints included femoral heads (mean dose ≤45 Gy, V50 < 50%) and bladder/rectum (V75 < 15%, V70 preferably <25%, V60 preferably <40% maximum 60%, V40 < 60% maximum 80%).20
Complete ADT was administered to 525 pts (61.8%) for a mean of 21 months in accordance with clinical guidelines.9 Table 2 shows the number and percentage of patients who underwent ADT by risk category. As noted previously, data on risk group classification were not available for 25 patients (20 of whom received ADT), and these patients are, therefore, not shown in Table 2, which shows only the 505 patients for whom risk group data was available. All patients received bicalutamide 50 mg/d two weeks before and after administration of the first luteinizing hormone-releasing hormone (LHRH) analogues (Leuprorelin, Triptorelin, or Goserelin). The duration of ADT administration ranged from 6 months to 3 years depending on the risk group (intermediate vs. high risk, respectively).
Acute and late radiation toxicity data were obtained from follow-up notes in patient records and scored according to the Radiation Therapy Oncology Group (RTOG) morbidity scoring criteria. Biochemical failure was based on 3 rises in PSA per the American Society for Therapeutic Radiology and Oncology consensus guidelines.21
4. Statistical analysis
Time to events was measured from the start of treatment to failure, death, or to the most recent follow-up visit. Treatment outcomes were measured in terms of overall survival (OS), cause-specific survival (CSS), disease-free survival (DFS), and biochemical-free relapse (BRFS). These estimated rates were calculated using actuarial and Kaplan–Meier methods. Differences between groups were assessed using the log-rank test. Multivariate Cox analysis was performed to calculate hazard rate ratios (HRR) with 95% confidence intervals, and analyze hormonal therapy exposure as a time dependent covariate.
5. Results
5.1. Biochemical control and overall survival
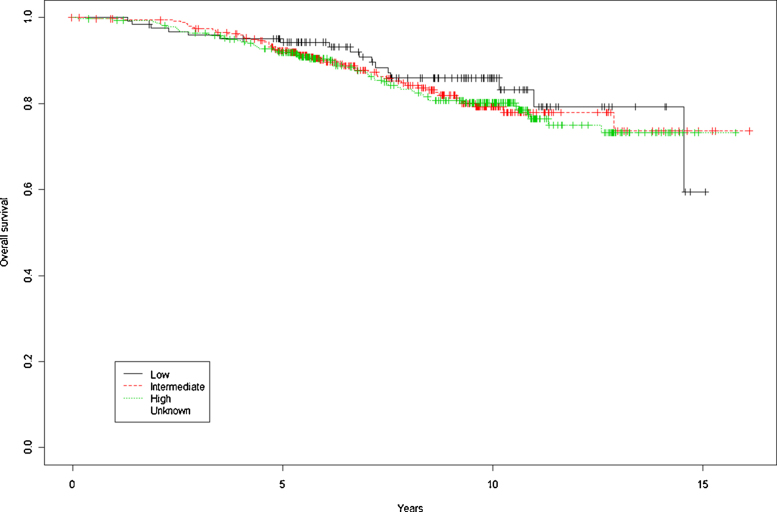
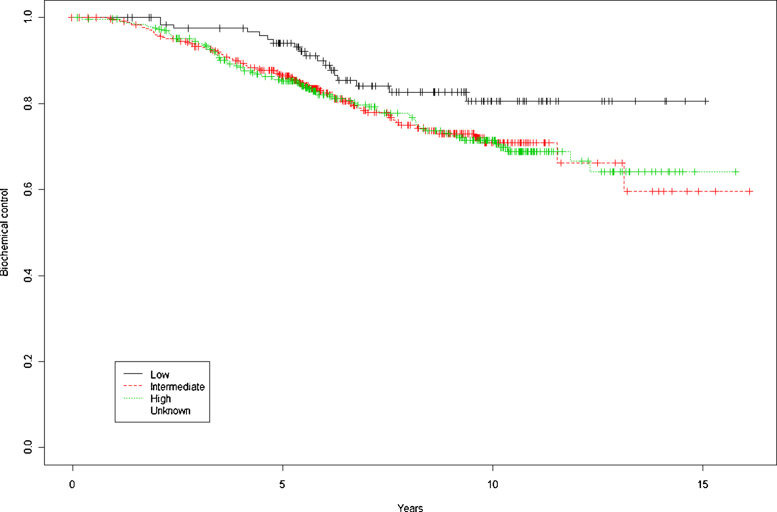
Overall survival rates for the entire cohort at 5 and 10 years were, respectively, 92.5% and 81.1% (Fig. 1). By risk group (low, intermediate, and high), OS at 5 and 10 years was, respectively, 94.3% and 85.9%, 92.3% and 79.2%, and 91.9% and 80.2% (p = 0.728). BRFS by risk group (low, intermediate, and high) at 5 and 10 years were, respectively, 94.1% and 80.6%, 86.4% and 70.9%, and 85.2% and 71.4% (p = 0.0666) (Fig. 2). DFS at 5 and 10 years was 86.1% and 70.8%, respectively. By risk group, (low, intermediate, and high), 5 and 10 year DFS rates were, respectively, 94.1% and 80.6%, 86.4% and 70.2%, and 84.1% and 69% (p = 0.00468).
Fig. 1.
Overall survival at 5 and 10 years by risk group.
Fig. 2.
Biochemical control at 5 and 10 years by risk group.
5.2. BRFS by dose <76 Gy vs. ≥76 Gy
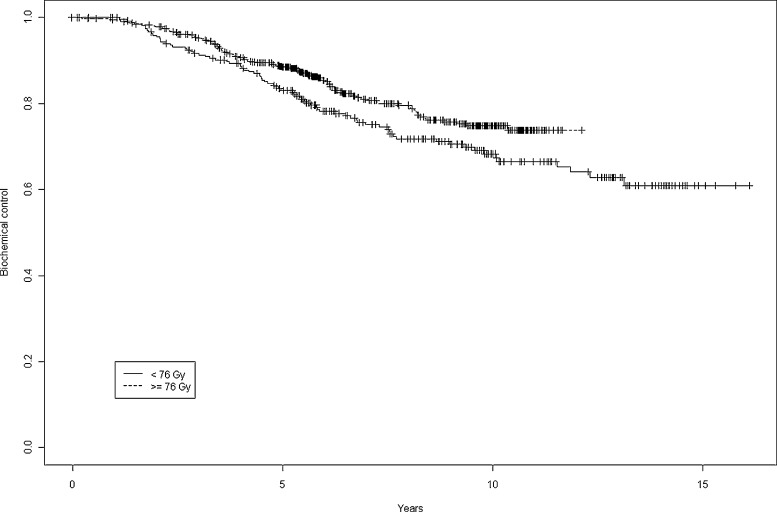
BRFS at 5 and 10 years was 83.1% and 68.3% when the dose was <76 Gy vs. 88.4% and 74.8% when the dose was ≥76 Gy, a significant between-group difference (p = 0.038) (Fig. 3). Based on the HRR analysis, dose >76 Gy is a protective factor for BRFS: HRR = 0.65 (0.4–0.9).
Fig. 3.
Biochemical control at 5 years and 10 years by dose <76 Gy vs. ≥76 Gy.
5.3. Comparative results by risk group with and without ADT
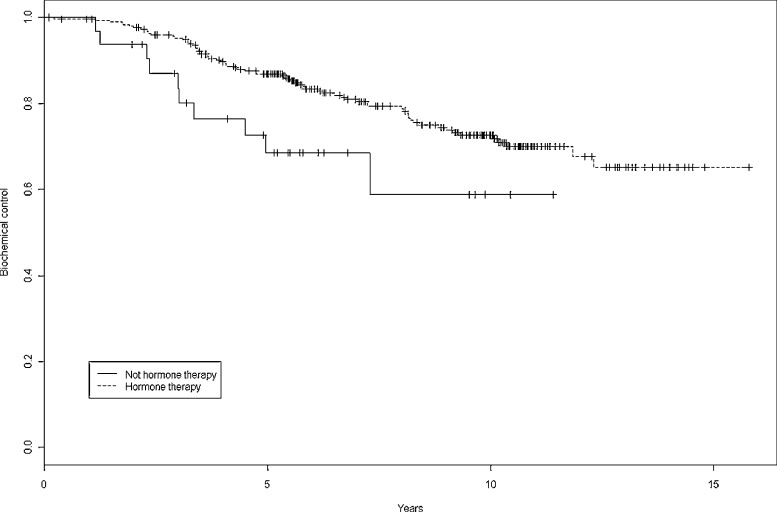
Fig. 4 shows biochemical control rates by group (ADT vs. no ADT). A total of 525 pts (61.8%) received ADT for a mean of 21 months. BRFS in intermediate risk pts at 5 and 10 years was, respectively, 82.9% and 65.8% in patients without ADT (183 pts) vs. 89.9% and 74.5% in pts who received ADT (163 pts) (p = 0.0937). In low risk pts, 5 and 10 year BRFS rates were, respectively, 94.4% and 85.8% without ADT (95 pts) vs 93.4% and 68.5% in pts with ADT (31 pts) (p = 0.0691). Finally, in high risk pts, the corresponding BRFS at 5 and 10 years, respectively was 68.6% and 58.8% without ADT (33 pts) vs. 86.9% and 72.7% with ADT (303 pts) (p = 0.0319).
Fig. 4.
Biochemical control at 5 years and 10 years with and without androgen deprivation therapy.
Administration of ADT did not result in any significant differences in DFS in low and intermediate risk patients. By contrast, significant differences were seen between high-risk patients who received ADT and those who did not: 5 and 10 year DFS rates without ADT were, respectively, 66% and 56.5% vs. 85.9% and 70.3% with ADT (p = 0.0232).
5.4. Survival according to the use of whole pelvic radiotherapy (WPRT)
A total of 195 pts underwent WPRT, with a median dose of 48.4 Gy (range, 48.1–48.6). No significant differences were observed in OS at 5 and 10 years, respectively: 92.1% and 81.2% without WPRT vs. 94.6% and 80.4% with WPRT (p = 0.606). In contrast, significant differences were observed in BRFS and DFS: at 5 and 10 years, BRFS was 88.2% and 73.9% without WPRT vs. 78.6 and 64.6% with WPRT (p = 0.00443). Similarly, 5 and 10 year DFS without WPRT was 87.6% and 72.7% vs. 78.1% and 62% with WPRT (p = 0.00194).
5.5. Toxicity
Most pts experienced some type of toxicity, including rectitis and urethritis. In most cases, rectitis was grade 1 (G1) (277 pts; 32.6%) or G2 (108 pts; 12.7%). Only 20 pts (2.6%) presented late G3 rectitis. Urethritis was as follows: G1 (294 pts; 34.6%); G2 (223; 26.2%), and G3 (11; 1.3%).
5.6. Multivariate analysis
A Cox proportional hazard model was performed to identify the factors associated with OS and biochemical failure. On the univariate analysis, the variables most closely associated with OS were PSA, pelvic irradiation, and intermediate and high risk classification (D’Amico). On the multivariate analysis, the only variable identified was patient age. The best model includes the following variables: Gleason, age, and radiation dose to prostate dose ≥76 Gy, with R2 = 0.016.
On the univariate analysis to determine significant predictors of biochemical failure, patient age was the only significant variable. In contrast, the multivariate analysis identified the following predictors: PSA, intermediate and high risk disease, and dose ≥76 Gy (dose <76 Gy increases risk of biochemical failure).
6. Discussion
This study presents results from a very large cohort of PCa patients treated with exclusive EBRT during the time in which our centre switched from conventional dose EBRT to high-dose 3D-CRT. As might be expected, our data confirm that higher doses of radiotherapy (≥76 Gy) had a significant positive impact on BRFS. Overall survival rates for the entire cohort at 5 and 10 years were, respectively, 92.5% and 81.1%, and are similar to other published studies that administered similar doses with long follow up.2, 3 ADT conferred a survival benefit only in high-risk patients but not in intermediate-risk patients. The long-follow up and excellent outcomes in our study confirm the effectiveness of EBRT, and further support the use of high-dose techniques.
In the present study, we present data from patients treated from 1996 to 2005. This time period covers a transitional period during which multiple publications reported the benefits of dose escalation in prostate cancer, thus leading to a shift in treatment approach. As a result, the patient cohort described here was a mixed group, with one-third of patients receiving conventional dose radiotherapy and the other two-thirds receiving high-dose radiotherapy. Since that time, multiple randomized-controlled trials (RCTs)1, 2 have been carried out, confirming the benefits of increased doses. In 2010, Zietman et al.3 reported long-term results of a RCT which demonstrated superior long-term cancer control for high-dose radiation without any increase in G3 or higher late urinary or rectal toxicity.
6.1. Survival
Survival rates in our study are very similar to other published studies for clinically localized PCa. Dearnaley et al.2 reported a 5-year progression-free survival rate of 90% in patients who received dose escalations, which is similar to our results in intermediate risk patients. At 10 years, Zietman et al.3 reported a DFS of 68% in the intermediate risk group vs. 70% in our study.
6.2. Use of androgen deprivation therapy
A large percentage (61.8%) of our patients received ADT, a fact that may partially explain the good results in our series. Confirming what other studies have shown, we found that ADT was most effective in high risk patients. We found that the use of ADT significantly improved BRFS and DFS in high-risk patients but not in intermediate-risk patients, a finding that confirms previously published reports about the benefits of ADT after EBRT in patients at a high risk of developing metastasis.9 Zapatero et al. recently reported that 2 years of ADT combined with high-dose radiotherapy improves biochemical control and overall survival with no increase in late radiation toxicity.22 Notably, those authors confirmed that long-term ADT was particularly beneficial for high-risk disease. The influence of ADT in intermediate-risk patients is still not clear, particularly when dose-escalated radiotherapy is administered8 and ADT has not been shown to provide any benefits in low-risk disease.
6.2.1. Whole pelvic radiotherapy
Although it might, at first, seem counterintuitive that patients in our series who underwent whole pelvic radiotherapy actually had worse outcomes (BRFS and DFS, but not OS) versus patients who did not undergo pelvic RT, this difference can be explained by the fact that patients prescribed WPRT had a worse initial prognosis. Nevertheless, it should be noted that the use of WPRT did not affect overall survival.
The use of WPRT is a hotly debated topic, but some studies have reported improved outcomes. Mantini et al.23 reported a significant improvement in BRFS for the patients who had WPRT and, more importantly, WPRT did not significantly increase rates of acute and late toxicity. In the RTOG 9413 trial, Roach et al.24 demonstrated better progression-free survival with WPRT versus prostate-only radiotherapy in patients whose risk of nodal involvement was >15%. In our series, we included regional lymph nodes if the risk of the involvement was ≥15%.
6.2.2. Benefits of dose escalation
We found significant differences in BRFS between patients who received <76 Gy vs. those who received ≥76 Gy EBRT. As we showed in this study, based on the HRR analysis, a dose >76 Gy is a protective factor for BRFS. The benefits of higher doses have been well-documented in numerous studies, including a meta-analysis.25 Moderate dose escalation (78 Gy) decreases biochemical and clinical failure as well as PCa death in patients with pretreatment PSA >10 ng/mL or high-risk disease.26 A multicenter randomized trial published in 2006 confirmed these findings, showing significantly lower rates of biochemical and clinical failure in PCa patients treated with a higher dose of radiotherapy.1
Despite the recognized benefits of dose escalation, the risks of increased toxicity must be considered. In our series, most pts experienced some type of toxicity, primarily rectitis and/or urethritis. However, in most cases, toxicity was either G1 or G2 toxicity. Rates of G3 toxicity were quite low, with only 20 pts (2.6%) presenting late G3 rectitis and 11 with G3 urethritis. Other series comparable to ours in which patients were treated with exclusive EBRT have reported higher toxicity in high dose groups. Pollack et al.27 reported G3 rectitis in 1% of pts in the 70 Gy arm vs. 10% in the 78 Gy arm. Similarly, Dearnaley et al.2 reported rectitis >G3 in 4% in the standard arm vs. 6% in the 74 Gy arm. In contrast, Zietman et al.3 reported no increase in G3 toxicity in the high-dose group. Zapatero and colleagues found that ADT and high-dose radiotherapy improved outcomes without causing additional toxicity.22 We believe that the low rates in our sample are, at least partially, due to the fact that one-third of the sample was treated with conventional rather than high-dose radiotherapy.
7. Conclusions
Our results are comparable to other published series in terms of disease control and side effects. These findings, obtained in a large cohort over a long time period, further support the benefits of dose escalation to achieve better disease control in the long-term. In our series, the benefit of adding ADT was clear only in high-risk patients. For this reason, more studies are needed to better identify the subset of patients that will most benefit from ADT, particularly in the light of the adverse effects of this treatment.
Conflict of interest
The authors declare no conflicts of interest related to this study.
Financial disclosure
None declared.
Acknowledgements
The authors wish to thank Bradley Londres for editing the manuscript, and Montse Ventura for her assistance with the statistical analysis.
References
- 1.Peeters S.T.H., Heemsbergen W.D., Koper P.C.M. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(May (13)):1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 2.Dearnaley D.P., Sydes M.R., Graham J.D. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(June (6)):475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 3.Zietman A.L., Bae K., Slater J.D. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95–09. J Clin Oncol. 2010;28(March (7)):1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoskin P.J., Rojas A.M., Bownes P.J., Lowe G.J., Ostler P.J., Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103(May (2)):217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Pistis F., Guedea F., Pera J. External beam radiotherapy plus high-dose-rate brachytherapy for treatment of locally advanced prostate cancer: the initial experience of the Catalan Institute of Oncology. Brachytherapy. 2010;9(March (1)):15–22. doi: 10.1016/j.brachy.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Boladeras A., Santorsa L., Gutierrez C. External beam radiotherapy plus single-fraction high dose rate brachytherapy in the treatment of locally advanced prostate cancer. Radiother Oncol. 2014;112(August (2)):227–232. doi: 10.1016/j.radonc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 7.López E., Lazo A., Gutiérrez A., Arregui G., Núñez I., Sacchetti A. Influence of 11C-choline PET/CT on radiotherapy planning in prostate cancer. Rep Pract Oncol Radiother. 2015;20(March (2)):104–112. doi: 10.1016/j.rpor.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dal Pra A., Cury F.L., Souhami L. Combining radiation therapy and androgen deprivation for localized prostate cancer – a critical review. Curr Oncol. 2010;17(October (5)):28–38. doi: 10.3747/co.v17i5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolla M., Collette L., Blank L. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(July (9327)):103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 10.Hanks G.E., Hanlon A.L., Schultheiss T.E. Dose escalation with 3D conformal treatment: five year outcomes, treatment optimization, and future directions. Int J Radiat Oncol Biol Phys. 1998;41(June (3)):501–510. doi: 10.1016/s0360-3016(98)00089-3. [DOI] [PubMed] [Google Scholar]
- 11.Pollack A., Smith L.G., von Eschenbach A.C. External beam radiotherapy dose response characteristics of 1127 men with prostate cancer treated in the PSA era. Int J Radiat Oncol Biol Phys. 2000;48(September (2)):507–512. doi: 10.1016/s0360-3016(00)00620-9. [DOI] [PubMed] [Google Scholar]
- 12.Lyons J.A., Kupelian P.A., Mohan D.S., Reddy C.A., Klein E.A. Importance of high radiation doses (72 Gy or greater) in the treatment of stage T1–T3 adenocarcinoma of the prostate. Urology. 2000;55(January (1)):85–90. doi: 10.1016/s0090-4295(99)00380-5. [DOI] [PubMed] [Google Scholar]
- 13.Pollack A., Zagars G.K., Smith L.G. Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. J Clin Oncol. 2000;18(December (23)):3904–3911. doi: 10.1200/JCO.2000.18.23.3904. [DOI] [PubMed] [Google Scholar]
- 14.Hanks G.E., Hanlon A.L., Pinover W.H., Horwitz E.M., Schultheiss T.E. Survival advantage for prostate cancer patients treated with high-dose three-dimensional conformal radiotherapy. Cancer J Sci Am. 1999;5(June (3)):152–158. [PubMed] [Google Scholar]
- 15.Bey P., Carrie C., Beckendorf V. Dose escalation with 3D-CRT in prostate cancer: French study of dose escalation with conformal 3D radiotherapy in prostate cancer-preliminary results. Int J Radiat Oncol Biol Phys. 2000;48(September (2)):513–517. doi: 10.1016/s0360-3016(00)00691-x. [DOI] [PubMed] [Google Scholar]
- 16.Bahnson R.R., Hanks G.E., Huben R.P. NCCN Practice Guidelines for Prostate Cancer. Oncology (Williston Park) 2000;14(November (11A)):111–119. [PubMed] [Google Scholar]
- 17.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(June (6)):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.D’Amico A., Altschuler M., Whittington R., Kao G., Malkowicz S.B., Wein A. The use of clinical parameters in an interactive statistical package to predict pathological features associated with local failure after radical prostatectomy for prostate cancer. Clin Perform Qual Health Care. 1993;1(December (4)):219–222. [PubMed] [Google Scholar]
- 19.Eifler J.B., Feng Z., Lin B.M. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2013;111(January (1)):22–29. doi: 10.1111/j.1464-410X.2012.11324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorino C., Cozzarini C., Vavassori V. Relationships between DVHs and late rectal bleeding after radiotherapy for prostate cancer: analysis of a large group of patients pooled from three institutions. Radiother Oncol. 2002;64(July (1)):1–12. doi: 10.1016/s0167-8140(02)00147-0. [DOI] [PubMed] [Google Scholar]
- 21.Cox J.D., Gallagher M.J., Hammond E.H. Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. J Clin Oncol. 1999;17(April (4)):1155. doi: 10.1200/JCO.1999.17.4.1155. [DOI] [PubMed] [Google Scholar]
- 22.Zapatero A., Guerrero A., Maldonado X. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(March (3)):320–327. doi: 10.1016/S1470-2045(15)70045-8. [DOI] [PubMed] [Google Scholar]
- 23.Mantini G., Tagliaferri L., Mattiucci G.C. Effect of whole pelvic radiotherapy for patients with locally advanced prostate cancer treated with radiotherapy and long-term androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2011;81(December (5)):e721–e726. doi: 10.1016/j.ijrobp.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Roach M., DeSilvio M., Valicenti R. Whole-pelvis, “mini-pelvis” or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Radiat Oncol Biol Phys. 2006;66(November (3)):647–653. doi: 10.1016/j.ijrobp.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 25.Zaorsky N.G., Hurwitz M.D., Keith S.W., Dicker A.P., Den R.B. Evaluation of biologically equivalent dose escalation, clinical outcome, and toxicity in prostate cancer radiation therapy: a meta-analysis of 12,000 patients from 40 institutions. Int J Radiat Oncol. 2013;87(October (2, Suppl.)):S24. [Google Scholar]
- 26.Kuban D.A., Levy L.B., Cheung M.R. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79(April (5)):1310–1317. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Pollack A., Zagars G.K., Starkschall G. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(August (5)):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]