Abstract
Previous research has shown that podocytes unable to assemble heparan sulfate on cell surface proteoglycan core proteins have compromised cell-matrix interactions. This report further explores the role of N-sulfation of intact heparan chains in podocyte-matrix interactions. For the purposes of this study, a murine model in which the enzyme N-deacetylase/N-sulfotransferase 1 (NDST1) was specifically deleted in podocytes and immortalized podocyte cell lines lacking NDST1 were developed and used to explore the effects of such a mutation on podocyte behavior in vitro. NDST1 is a bifunctional enzyme, ultimately responsible for N-sulfation of heparan glycosaminoglycans produced by cells. Immunostaining of glomeruli from mice whose podocytes were null for Ndst1 (Ndst1−/−) showed a disrupted pattern of localization for the cell surface proteoglycan, syndecan-4, and for α-actinin-4 compared with controls. The pattern of immunostaining for synaptopodin and nephrin did not show as significant alterations. In vitro studies showed that Ndst1−/− podocytes attached, spread, and migrated less efficiently than Ndst1+/+ podocytes. Immunostaining in vitro for several markers for molecules involved in cell-matrix interactions showed that Ndst1−/− cells had decreased clustering of syndecan-4 and decreased recruitment of protein kinase-Cα, α-actinin-4, vinculin, and phospho-focal adhesion kinase to focal adhesions. Total intracellular phospho-focal adhesion kinase was decreased in Ndst1−/− compared with Ndst1+/+ cells. A significant decrease in the abundance of activated integrin α5β1 on the cell surface of Ndst1−/− cells compared with Ndst1+/+ cells was observed. These results serve to highlight the critical role of heparan sulfate N-sulfation in facilitating normal podocyte-matrix interactions.
Keywords: heparan sulfate, glycosaminoglycans, proteoglycans, syndecan, podocyte
podocyte cell-matrix interactions are well known to play key roles in glomerular homeostasis [for a review see (37)]. Animal models that have mutations in genes encoding proteins that are either directly or peripherally associated with cellular subsystems involved in podocyte-matrix interaction have often led to aggressive, catastrophic failure of podocyte function.
Although the importance of heparan sulfate (HS) in glomerular biology has been investigated by numerous research groups over the past 30+ yr, the role of HS in mediating podocyte-matrix interactions still remains relatively underexplored [for a review see (31)].
At the cell surface, the HS carbohydrate structures (known as glycosaminoglycans or GAGs) are normally found covalently attached to one or more species of cell surface proteoglycans (PGs); in particular, members of the syndecan 1–4 (Sdc 1–4) and glypican 1–6 families of cell surface PGs. Although each family of PG core proteins has specific yet diverse bioactivities inherent in part to amino acid motifs within the core protein, the members of each family are capable of presenting/extending one or more HS chains from the cell membrane out into the pericellular milieu. With HS, the “reach” of the GAG structure extending from the PG core protein can exceed 80 nm (48), thus the combined distance (core protein + GAG) has the potential of superseding the extended length of integrin receptors (50 nm).
The role that cell surface HS proteoglycans (HSPGs) and their respective GAGs play in mediating in podocyte-matrix interactions in vivo and in vitro was highlighted in the results of two earlier studies carried out by laboratory (8, 9). In those reports, we showed that podocytes, incapable of assembling HS on all secreted PGs (both basement membrane and cell surface) developed foot process effacement early in life. In vitro studies using immortalized HS+ and HS− podocytes in adhesion and migration assays showed that the HS− podocytes were less efficient in adhering to fibronectin than their wild-type counterparts. Syndecan-4 (Sdc-4) immunostaining has demonstrated that the localization of Sdc-4 to focal adhesions was compromised in the HS− podocytes in vitro (8). Immunostaining of tissue sections from HS+ and HS− mice using antibodies directed against Sdc-4 showed a disruption of the podocyte-associated pattern of Sdc-4 and synaptopodin immunostaining in the HS− mutant animals compared with HS+ animals (8, 31).
To refine the specificity of the former approach, we developed a mouse model having a podocyte-specific deletion of NDST1 (Ndst1−/− mouse), a key enzyme involved in GAG N-sulfation (42). During HS assembly, NDST1 (1) catalyzes the removal of the acetyl group from N-acetyl glucosamine (2) and subsequently adds a sulfate group in its place (28). In addition, N-sulfation is a prerequisite for subsequent modifications to HS (e.g., 2-0, 3-0, 6-0 sulfation and C5 epimerization) (17). Thus the loss of NDST1 activity in podocytes may affect the pattern/amount of HS sulfation via effects on downstream modifications of the HS chain. The patterns of sulfated residues in HS chains, known as NS (N-sulfated) domains, are important in HS-ligand interactions [see (20, 27, 39) for a review]. In light of our previous studies (8, 9) we postulated that the loss of NDST1 activity in podocytes might hinder the ability of podocytes to adhere and properly organize on the underlying glomerular basement membrane (GBM), despite the HS chains still being present on the PGs. Our initial in vivo studies in the podocyte-specific Ndst1−/− mouse model (42) provided evidence to this effect, showing that loss of NDST1 and thus N-sulfation led to the development of glomerular hypertrophy and foot process effacement, and the onset of a modest age-associated microalbuminuria. In this report, we further explored changes in podocyte-matrix interactions in the mutant mouse models and using immortalized podocytes having the deletion of Ndst1. The results show that the loss of N-sulfation also leads to the disruption of the pattern of distribution of Sdc-4 within the glomeruli of Ndst1−/− mutant mice. The change in glomerular Sdc-4 distribution correlated with a change in the pattern of distribution of α-actinin-4. In vitro studies further explored the effects of Ndst1−/− deletion at the cellular level, and showed that Ndst1−/− podocytes do not efficiently adhere and migrate on extracellular matrices compared with wild-type cells, the deficiency being mediated in part by the inefficient clustering of Sdc-4 in focal adhesions and a net change in the cell surface activation of at least one member of the integrin family, α5β1 integrin. In turn, changes in some of these interactions are manifested in vivo by disruption of Sdc-4/α-actinin-4 organization in glomerular podocytes.
MATERIALS AND METHODS
Animals and animal care.
Immortomice (H2Kb-tsA58) were purchased from Charles River Laboratories (Wilmington, MA). Ndst1fl/fl mice were a gift from Dr. Jeffery Esko (University of California, San Diego). All animals were housed under controlled climate conditions and given food and water ad libitum in the Animal Resource Facility at Louisiana State University (LSU) Health Sciences Center-Shreveport. Experiments performed during the course of the study were conducted under an approved protocol of the LSU Health Sciences Center-Shreveport Animal Care and Use Committee. Tissue sections used during the course of this study were from archival tissues from animals used in an earlier study (42).
Antibodies.
Antibodies used in this study and their concentrations were as follows: anti-HS (1:1 HS4C3; TVK), which recognizes a carbohydrate epitope present on all heparan chains (44); mouse monoclonal antibody 10E4, which recognizes N-sulfated residues on HS (47) (1:50, 05604; Seikagaku America); rat anti-mouse Sdc-4 monoclonal antibody, which recognizes a 71-amino acid region near the amino terminal of the ectodomain of Sdc-4 (53) (1:21, clone KY/8.2; BD Pharmigen, San Jose CA); rabbit polyclonal anti-α-actinin-4 (4 μg/ml, 42–1400; Zymed, San Francisco, CA); rabbit polyclonal anti-vinculin (1:50, V4139; Sigma Chemical, St. Louis MO); rabbit polyclonal phosphorylated focal adhesion kinase (pFAK) (1:50, 3283; Cell Signaling Technology, Beverly MA); rabbit polyclonal anti-PKCα (1:50, 2056; Cell Signaling Technology); rabbit anti-synaptopodin (1:750, 163002; Synaptic Systems, Goettingen, Germany); guinea pig polyclonal anti-nephrin (1:500, BP5030; Acris, Rockville, MD); and rabbit monoclonal anti-WT1 (1:20, 52933; AbCam, Cambridge MA). A mouse monoclonal antibody against glutathione S-transferase (GST) (1:1000, sc-138; Santa Cruz Biotechnology) and rabbit anti-GAPDH (1:8,000, 2118; Cell Signaling Technology) were used for Western blot immunoassays. Species-specific secondary antibodies (either fluorochrome-conjugated or horseradish peroxidase conjugates) were purchased from Jackson Immunoresearch (Malverne, PA).
Development of immortalized Ndst1−/− podocytes.
Immortalized podocytes having floxed Ndst1 alleles were developed by breeding the Ndst1fl/fl mouse with the Immortomouse, which expresses a transgene containing the temperature-sensitive SV-40 large T-antigen. The genomic integration site for the large SV40 transgene in the Immortomouse has been recently mapped (22), the insert localized to an area that has no predicted gene at this particular location. Verification of the correct genotype was carried out as previously described (8) for the Immortomouse PCR; PCR primers for the detection of the floxed Ndst1 allele were 5′-CCAGGGCGTCAGGGCCTCCTG-3′ (forward) and 5′-TCCCACATGGCGAGACTGAGGTTC-3′ (reverse); and the cycling parameters were 95°C for 15 min (denature); 35 cycles of 95°C for 55 s, 57°C for 55 s, and 72°C for 1.5 min. The resultant double-mutant mouse, Immortomouse/Ndst1fl/fl, was used as a source for primary podocyte cultures in a similar fashion to what has been previously reported by our laboratory (8). Briefly, glomeruli from these mice were isolated using magnetic separation, and primary podocytes were isolated from glomerular explant cultures using limiting dilution methods (43). The podocytes were expanded in culture by growing them under permissive conditions (33°C in the presence of IFN-γ). Using this approach, eight cell lines bearing the floxed allele were initially developed. The cell type of the resulting monoclonal cell lines was verified by immunolabeling of podocyte-specific cell markers WT-1 and synaptopodin (data not shown). The Ndst1fl/fl gene was excised in vitro by using an adenoviral-mediated delivery of a green fluorescent protein (GFP)-Cre recombinase construct (Vector Biolabs, Philadelphia, PA), or alternatively, an adenoviral construct expressing only GFP was used in control infections. Virally transduced cells were sorted by fluorescence-activated cell sorting, resulting in paired monoclonal cell lines of podocytes either possessing (Ndst1+/+) or lacking (Ndst1−/−) Ndst1. For the purposes of our studies, only podocytes grown at the restrictive conditions (37°C in the absence of IFN-γ) for a minimum of 14 days prior were used in the cell-based assays.
Immunostaining and microscopy.
To confirm a loss of N-sulfation in NDST1-deficient podocytes, subconfluent monolayers of Ndst1+/+ and Ndst1−/− cells were double-label immunostained with the monoclonal antibody 10E4, which recognizes N-sulfated HS and the phage display anti-HS GAG antibody, HS4C3. Confluent Ndst1+/+ and Ndst1−/− podocyte cultures were also immunostained to explore the sulfation/glycosylation status of the matrix secreted/assembled by those cells. To investigate focal adhesion organization and stress fiber formation, fully differentiated podocytes were treated with cycloheximide to inhibit de novo matrix synthesis; seeded on fibronectin-coated (100 μg/ml) slides; and allowed to adhere for 1, 2, and 4 h. Subsequently, the cells were fixed with 3.5% formaldehyde in PBS and permeabilized using 0.1% Tween 20. Cells were then double-label immunostained with anti-Sdc-4 and anti-α-actinin-4, or anti-vinculin, anti-PKCα, or anti-pFAK. In initial tissue microscopy studies and for cell culture studies, images were digitized using a Hamamatsu Orca digital camera interfaced with an Olympus IX-70 microscope equipped for epifluorescent illumination. The camera signal was ported to an Apple Macintosh computer hosting the imaging software I Vision-MAC (Biovision, Exton, PA). In subsequent microscopy studies tissue sections were imaged using a Zeiss LSM 510 laser scanning confocal microscope, the aperture on the microscope set to give a 0.9-μm focal plane section.
α5 integrin activation assay.
The GST-FNIII9–11 peptide-based integrin activity assay was carried out as previously described (35). Briefly, cells were seeded at 80% confluence on a fibronectin-coated (l μg/ml) 6-well plate in serum-free medium for 4 h. After cells were adhered to the substratum, the medium was removed and PBS containing 1 mM MgCl2 and the α5β1 integrin peptide, GST-FNIII (20 μg/ml), was added to the wells. The peptide was incubated with the cells for 30 min at 37°C. Following incubation, the unbound peptide was removed with two rinses of ×1 PBS containing MgCl2. The cultures were subsequently lysed and the amount of cell surface-associated GST-FNIII9–11 peptide was measured by Western blot immunoassay. The interwell variability between assays was normalized using GAPDH as a loading control.
Adhesion and migration assays.
Podocyte cell adhesion, spreading, and migration assays were carried out as previously described (8). Podocytes in culture express α5β1 integrin, an integrin that has been shown to undergo affinity modulation. The rationale for using fibronectin in our studies was that it has RGD motifs and heparin-binding domains, allowing fibronectin to engage both integrin and syndecans in a consistent fashion. Although fibronectin is not considered to be an integral matrix component of the GBM, it has been immunolocalized to the lamina rarae of the GBM, and is thus available to the basal surface of the podocyte (14, 15).
Ndst1+ and Ndst1− podocytes were preincubated with cycloheximide (250 μg/ml) to inhibit endogenous matrix protein synthesis in serum-free medium (RPMI 1640 medium with 5 mmol/l glucose) for 2 h prior to adhesion and spreading assays. For cell-adhesion assays, Cell Tracker green (CMFDA, Invitrogen) was added to the cells at a concentration of 2.5 μM and allowed to incorporate into the cells for 30 min. The cells were then rinsed with serum-free medium (SFM) and allowed to recover for 30 min. Cells were then trypsinized and plated on a fibronectin-coated (100 μg/ml) 24-well plate and allowed to adhere for 2 and 4 h. At each time point, the nonadherent cells were removed and the wells were rinsed twice with PBS. The adherent cells were lysed with 100 mM NaOH. The nonadherent cells and the adherent cell lysates were transferred to a 96-well plate and analyzed for fluorescence on a plate reader.
For measurement of differences in spread cell area, the cells were pretreated with cycloheximide as described above, subsequently trypsinized, and plated on a fibronectin-coated (100 μg/ml) 24-well plate and allowed to interact with the substratum for 2 h. The unattached cells were removed by rinsing with PBS. The cells were fixed and permeabilized with 3.5% formaldehyde and 0.1% Tween 20. The actin cytoskeleton was stained with Alexa 488 phalloidin (Life Sciences), and cell nuclei were counterstained using Hoechst 33342. The spread areas of cells were measured on digitized images using a planimetry subroutine in the image analysis software Ivision-MAC (Biovision).
A scratch assay was used to measure cell migration as previously described (8). Briefly, cells were grown to confluence on a fibronectin-coated (100 μg/ml) 24-well plate and allowed to fully differentiate at the restrictive temperature (37°C). The resulting monolayer of cells was subsequently wounded in a cruciform pattern. Registration marks were etched into the bottom of the wells to allow for imaging the same field of view at each time point. For measurement purposes, photomicrographs were taken (×40 final magnification) of the entire cruciform area at each time period and assembled into a larger montage. The total open area of the residual scratch was measured at 0, 24, and 48 h to quantify the migration of the leading cell boundary into the inflicted wound.
Radiolabeling and column chromatography.
Cells were seeded at a subconfluent density in serum-containing complete medium for 24 h. Subsequently, the medium was decanted and cells were then labeled with 50 μCi/ml 35S and 25 μCi/ml 3H glucosamine in serum-free media, and the incubation continued for 48 h at 37°C. Afterward, the media was decanted and cleared by centrifugation. The supernatants were loaded onto a 1-cm DEAE Trisacryl M ion-exchange column equilibrated in 4 M urea, 1 mM dithioethrietol (DTT), 10 mM EDTA, 0.2 M NaCl, 0.1% Triton X-100, and 50 mM Tris, pH 8.0. The column was then rinsed sequentially with 10 column volumes of the former buffer, followed by 10 column volumes of 4 M urea, 1 mM DTT, 10 mM EDTA, 0.2 M NaCl, 0.1% Triton X-100, and 50 mM sodium acetate, pH 4.0. The PGs were eluted from the column in a buffer containing 4 M urea, 1 mM DTT, 10 mM EDTA, 1.5 M NaCl, 0.1% Tween 20, and 50 mM sodium acetate, pH 4.0. Fractions (1 ml) were collected and analyzed for radioactivity using liquid scintillation counting. The peak fractions were pooled, dialyzed against water, and lyophilized. The resulting samples were treated with alkaline borohydride (0.3 M NaBH4) (36) followed by nitrous acid degradation using 0.5 M nitrous acid, pH 1.5 (41) and then neutralized to pH 8.5 with Na2CO3. The nitrous acid-treated samples were loaded onto a G25 superfine column (0.06 × 40 cm) and run at a flow rate of 0.09 ml/min and collected at 30-s intervals. These fractions were analyzed for radioactivity using liquid scintillation counting, and the elution profile was plotted as radioactivity vs. eluted volume.
Flow cytometry.
Flow cytometry of podocytes immunostained for α5 integrin (see Antibodies) was carried out as previously described (8).
RESULTS
Podocyte-glomerular basement membrane interactions and cytoskeletal organization are disrupted in the glomeruli of the Ndst1−/− mutant mouse model.
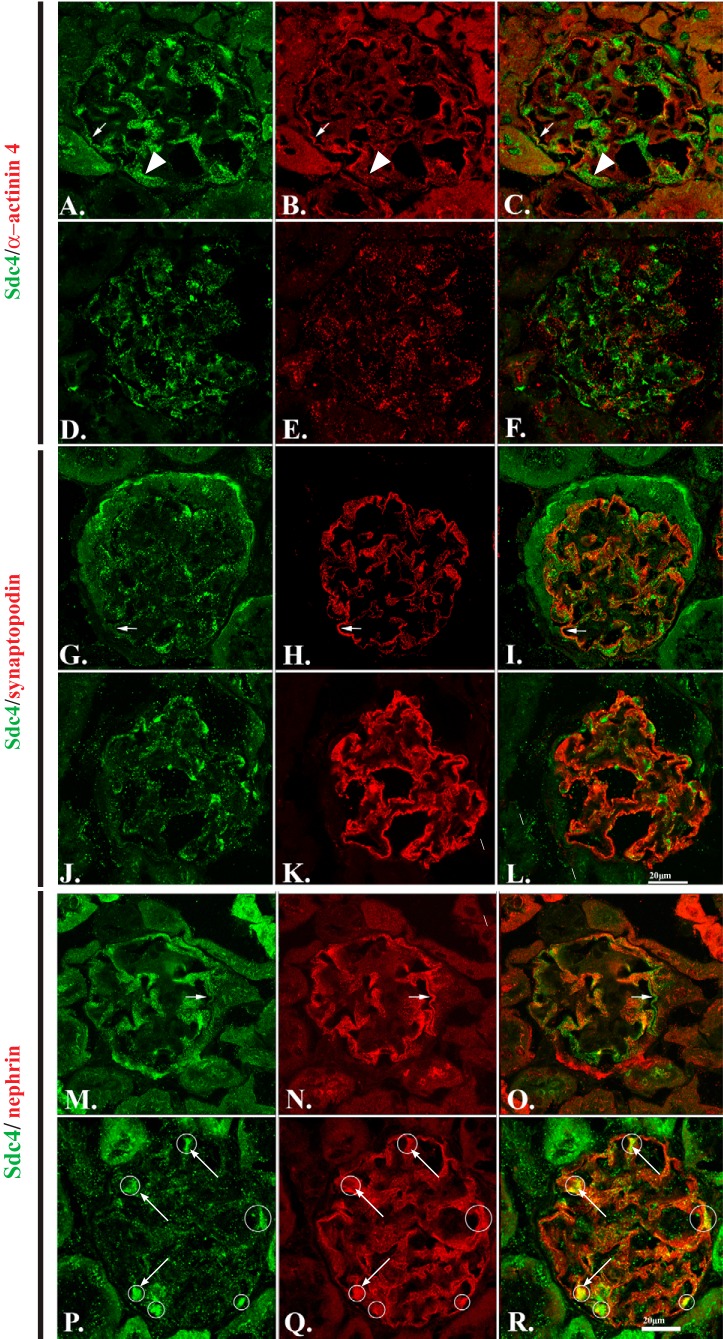
Our earlier study of the Ndst1−/− mouse model (42) showed that podocytes lacking NDST1 developed foot process effacement, a morphologic indication that podocyte cell-matrix interactions were disrupted. To further understand the nature of this disruption, we immunostained tissue sections from Ndst1+/+ (Fig. 1, A, B, G, H, M, and N) and Ndst1−/− (Fig. 1, D, E, J, K, P, and Q) mice (control and mutant) with a monoclonal antibody against Sdc-4 (Fig. 1, A, D, G, J, M, and P) and with antibodies against α-actinin-4 (Fig. 1, B and E), synaptopodin (Fig. 1, H and K), and nephrin (Fig. 1, N and Q). The age of 15 mo was chosen because this age was just before the development of an increase in urinary albumin excretion in this model (42). In Ndst1+/+ control animals (Fig. 1, A, G, and M) the pattern of Sdc-4 staining within the glomerulus has a variable appearance that is dependent on plane of the cut section. In areas where the glomerular capillary basement membrane is cut in transverse section, Sdc-4 staining appears as a linear pattern of punctate dots (Fig. 1A, arrow) that follow along the length of the capillary wall. In regions of the glomerulus where the plane of section in tangential to the capillary GBM (Fig. 1A, arrowhead), Sdc-4 staining appears as an orderly array of punctate dots. In the Ndst1+/+ animals, Sdc-4 and α-actinin-4 are codistributed (Fig. 1, A–C, arrowheads) in a linear pattern along the length of the GBM. Similar to what was observed in the PEXTKO model (8), whose podocytes cannot synthesize HS chains, the pattern of localization of Sdc-4 was disrupted in the glomeruli of the Ndst1−/− animals (Fig. 1, D, J, and P) compared with Ndst1+/+ controls (Fig. 1, A, G, and M). In the Ndst1−/− glomeruli, in some areas codistribution was observed, but the overall pattern of Sdc-4 and α-actinin-4 was disrupted compared with that of control animals. Sdc-4 also codistributed with synaptopodin (Fig. 1, G–I, arrows) and nephrin (Fig. 1, M–O, arrows) in the glomeruli from Ndst1+/+ animals. In the glomeruli from Ndst1−/− mutants the codistribution of Sdc-4/synaptopodin and Sdc-4/nephrin was also changed in both staining pairs; this was ostensibly due the change in the distribution of Sdc-4 rather than the other staining partners in the double-labeled specimens. The pattern of synaptopodin staining in the Ndst1−/− glomeruli (Fig. 1K) did have a relatively denser/more intense staining along the length of the GBM compared with that of controls (Fig. 1H). In the tissue sections that had been double-labeled with Sdc-4/nephrin, although the linear GBM distribution pattern for nephrin was not ostensibly altered, there were consistent areas of colocalization in the form of Sdc-4/nephrin aggregates (Fig. 1, P–R, circles surrounding the yellow fluorescence). Similar colocalization patterns were not found in tissue sections that had been doubled-stained for Sdc-4/synaptopodin or Sdc-4/α-actinin-4.
Fig. 1.
Podocytes from Ndst1−/− mice show disruption in syndecan-4 (Sdc-4) distribution and alterations in cytoskeletal organization in vivo. A–C, G–I, and M–O: laser scanning confocal micrographs of kidneys from Ndst1+/+ animals at 15 mo of age. D–F, J–L, and P–R: micrographs of kidneys from Ndst1−/− mice of the same ages. This age range was selected because microalbuminuria is evident at this age in the Ndst1−/− mouse model [see (42)]. The sections were stained for Sdc-4 (A, D, G, J, M, and P) and α-actinin-4 (B and E), synaptopodin (H and K), and nephrin (N and Q). C, F, I, L, O, and R: overlay images of the panels within the same row of images. The data show that the glomerular podocytes in Ndst1−/− mice show significant changes in the organization/localization of Sdc-4 (D, J, and P). Of the three molecules commonly associated with glomerular podocytes, the localization of α-actinin-4 (E) is most disrupted in Ndst1−/− podocytes compared with that of controls (B). The pattern of synaptopodin staining is also changed (H and K), albeit not to the same extent observed with α-actinin-4 (B and E). The pattern of nephrin staining (N and Q) appeared relatively the same in the glomeruli from both strains of mice, with the only difference being the colocalization/coclustering of Sdc-4/nephrin in aggregates (R, arrows) in the glomeruli of Ndst1−/− mice. Neither synaptopodin nor α-actinin-4 show a similar pattern of colocalization in the glomeruli of Ndst1−/− mice (final magnification ×400, bar = 20 μm).
Development and characterization of Ndst1+/+ and Ndst1−/− immortalized podocyte cell lines.
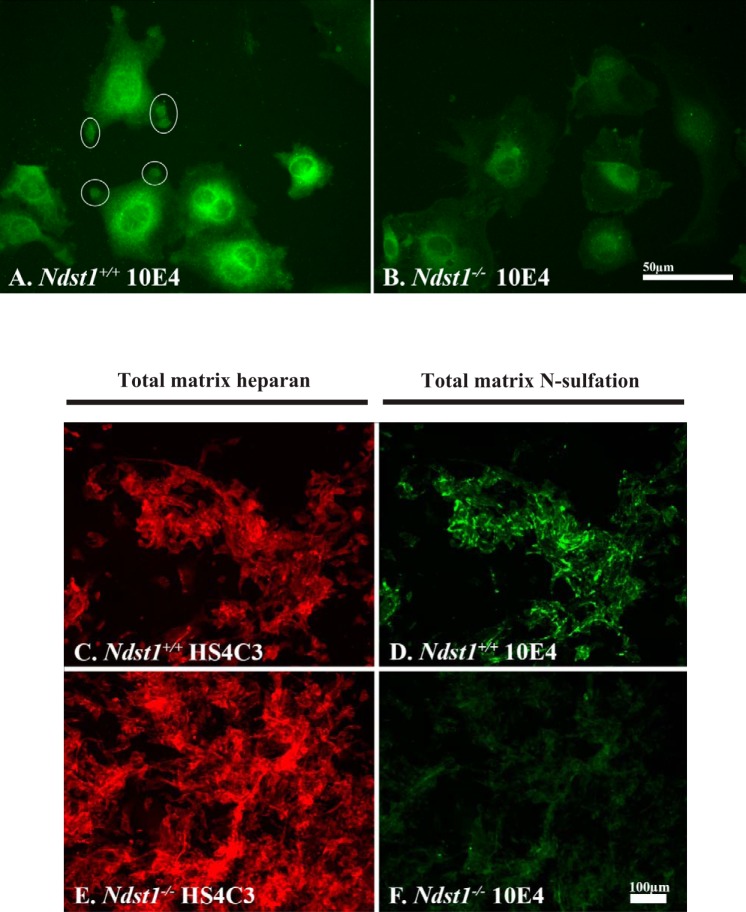
Immortalized podocyte cell lines expressing (Ndst1+/+) or lacking (Ndst1−/−) Ndst1 were developed to further explore potential changes in cell-matrix interactions (see materials and methods). To confirm the loss of HS N-sulfation in NDST1-deficient podocytes, Ndst1+/+ and Ndst1−/− podocytes (Fig. 2, A and B) or their extracellular matrices (Fig. 2, C–F) were immunostained with the monoclonal antibody 10E4 (Fig. 2, A, B, D, and F), which recognizes N-sulfated carbohydrate epitopes on HSPGs (45). 10E4 antibody staining of Ndst1+/+ cells was localized to large, circular aggregates (Fig. 2A, circles) of positive staining present at the periphery of Ndst1+/+ cells. These aggregates indicate regions of podocyte focal adhesion with the extracellular matrix, similar to the pattern of aggregation for Sdc-4 described in our earlier report (8). In contrast, Ndst1−/− cells did not show the same pattern of 10E4 staining, indicative that the HS present on cell surface PGs was not N-sulfated (Fig. 2B), and that the efficiency of focal adhesion formation was dependent on the presence of N-sulfated GAGs. To examine potential changes in the extent of N-sulfation of matrix PGs, cells were grown in culture for 5 days then double-label immunostained with 10E4 (Fig. 2, D and F) and HS4C3 antibodies (Fig. 2, C and E). The HS4C3 antibody, which recognizes a carbohydrate epitope present on all HS chains, stained matrices assembled by Ndst1+/+ (Fig. 2C) and Ndst1−/− (Fig. 2E) cells, whereas the 10E4 antibody stained only the matrices assembled by Ndst1+/+ cells (Fig. 2B). The latter data serve to show that Ndst1−/− cells are incapable of modifying newly made matrix-associated HS via N-sulfation (Fig. 2F) but are still capable of assembling HS and organizing an extracellular matrix (Fig. 2E). The absence of 10E4 staining is also indicative that possible compensatory activity of one of the other members the NDST1 enzyme family (NDST2-4) did not occur. The loss of HS N-sulfation was further confirmed by measuring the change in the elution profile of chemically fragmented HS isolated from the conditioned medium of Ndst1+/+ and Ndst1−/− cells (Fig. 3).
Fig. 2.
Ndst1−/− podocytes are unable to N-sulfate heparan sulfate (HS) glycosaminoglycans. A and B: Ndst1+/+ and Ndst1−/− podocytes grown in short-term cell culture, and the cells are immunostained with the 10E4 antibody to demonstrate N-sulfated residues. On the periphery of the Ndst1+/+ cells, “pods” of 10E4-positive immunoreactivity (white circles) can be seen around the periphery of the cells, whereas in Ndst1−/− cells (B) there is little to no 10E4 staining at the cell periphery. The perinuclear staining observed in Ndst1−/− cells for 10E4 is the result of nonspecific staining of the secondary antibody. For comparative purposes, both images were shot at the same exposure and subjected to the same normalization with regard to gray-level intensity. C–F: Ndst1+/+ and Ndst1−/− podocytes grown in culture for 5 days, and cultures were subsequently immunostained for the presence of heparin sulfate (HS) (C: E-HS4C3 antibody) or the presence of N-sulfated residues (D: F-10E4 antibody). For comparative purposes, the exposures in C and E were identical as were exposures in D and F. Each image set was subjected to the same normalization with regard to gray-level intensity. C and D: Ndst1+/+ podocytes are able to assemble a pericellular matrix containing N-sulfated (D) HS (C); the Ndst1−/− podocytes are able to assemble a pericullar matrix containing HS (E) but with little or no N-sulfation (F). Final magnification: A and B, ×400, bar = 50 μm; C–F, ×100, bar = 100 μm.
Fig. 3.
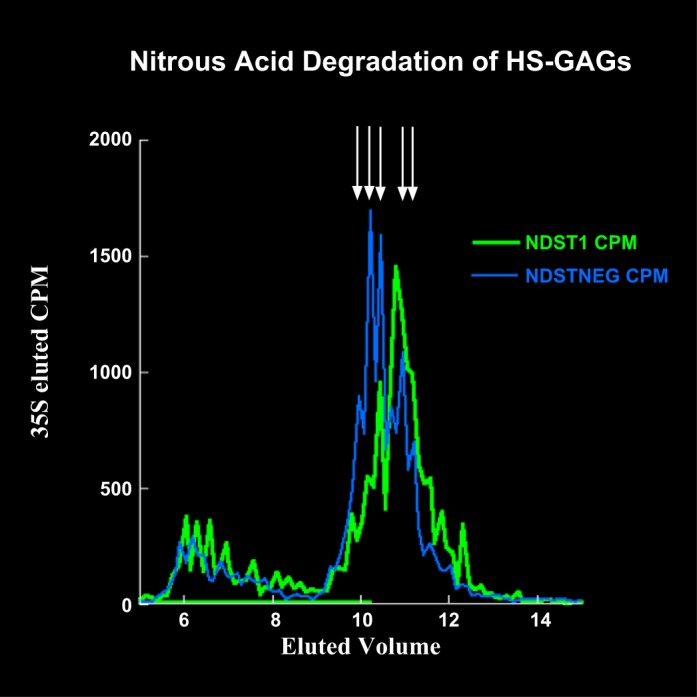
3H/35S radiolabeling studies further confirm that Ndst1−/− podocytes are unable to N-sulfate HS glycosaminoglycans (GAGs). To further confirm the reduction in N-sulfation, Ndst1+/+ and Ndst1−/− cells were radiolabeled with 35S and 3H-glucosamine, and the radiolabeled secreted proteoglycans (PGs) were subsequently isolated from conditioned medium using DEAE ion-exchange chromatography. The radiolabeled PGs were treated with alkaline borohydride (36), which releases intact GAG chains from the PG core protein. Radiolabeled HS was subsequently treated with nitrous acid (41), the reaction of which cleaves HS chains specifically at N-sulfated GlcN residues, the resultant reaction products were analyzed by gel filtration chromatography. The graph shows the 35S labeled HS peaks from Ndst1−/− cell-conditioned medium eluted earlier in the gel filtration chromatography profile (blue trace) compared with the HS isolated from Ndst1+/+ cell-conditioned medium (green trace), indicating the generation of larger HS fragments (arrows) from the HS made by Ndst1−/− cells. This would be indicative of a net decrease in the available number of cleavable N-sulfated sites, thus providing additional evidence that the mutant cells have a deficiency in the ability to N-sulfate heparan.
Ndst1−/− podocytes show a decreased ability to adhere, spread, and migrate on extracellular matrix.
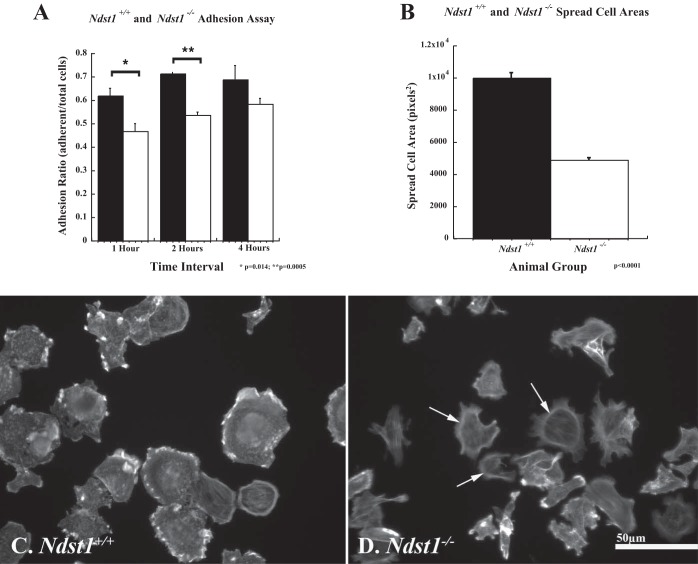
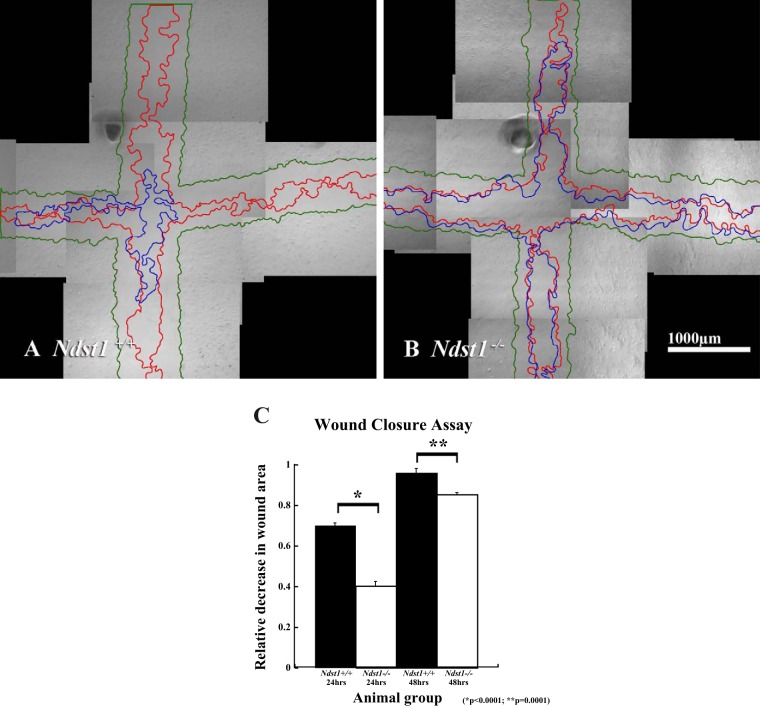
To understand how the loss of N-sulfation might alter podocyte cell-matrix interactions, cell adhesion and migration assays were performed as previously described (8). The assays used plasma fibronectin as a substratum to engage both integrins and cell surface HS. The rationale for using fibronectin as a substratum was severalfold: 1) fibronectin is present in the GBM (33) and thus accessible for interactions with podocyte integrins; 2) fibronectin engages both the α5β1 integrins and cell surface HS (see discussion); and 3) the bipartite cell-matrix interaction (α5β1 integrin/Sdc-4) is known to promote the formation of focal adhesion in culture. The data (Fig. 4) show that at 1 and 2 h after plating the Ndst1−/− cells were less efficient in cell-matrix adhesion (P = 0.014, P = 0.005, 1 and 2 h, respectively) compared with Ndst1+/+ cells. At 4 h after plating a difference in adhesion between Ndst1+/+ and Ndst1−/− cells (Fig. 4A) was observed, but the difference was not significant. Measurement of cell areas showed that at 2 h the Ndst1−/− cells covered significantly less surface area (P = 0.001) on the substratum than Ndst1+/+ cells (Fig. 4B). At 2 h after attachment the Ndst1+/+ cells labeled with phalloidin showed robust development of actin-rich cell-matrix contacts (Fig. 4C). Although the Ndst1−/− cells were adherent and some had developed stress fibers, many of the cells still possessed a circumferential (cortical) actin pattern (Fig. 4D), indicative that the attachment process lagged behind in the Ndst1−/− cells compared with Ndst1+/+ cells. To determine whether differences in cell-matrix adhesion extended into in-cell migration, a scratch assay of confluent Ndst1+/+ and Ndst1−/− podocyte monolayers was used as previously described (8). The Ndst1−/− podocytes showed a significant (P = 0.001) decrease in wound closure at 24 and 48 h after injury, compared with the ability of the Ndst1+/+ cells (Fig. 5, A–C). Taken together, the above results indicate that lack of N-sulfation affects the ability of podocytes to efficiently interact with a matrix substratum that concomitantly engages both Sdc-4 and integrins.
Fig. 4.
Ndst1−/− podocytes do not attach and spread as efficiently on fibronectin compared with Ndst1+/+ podocytes. The graphs show the results of the cell adhesion assay (A) and spread cell area measurements (B) for Ndst1+/+ and Ndst1−/− podocytes in culture. A: differences in adhesion of Ndst1−/− podocytes on fibronectin at 1, 2, and 4 h. A significant difference was observed in Ndst1−/− podocyte adhesion (white bars) to fibronectin at 1 (P = 0.014) and 2 (P = 0.0005) h after seeding compared with Ndst1+/+ podocytes. A similar but not significant difference in adhesion was observed at 4 h after seeding between Ndst1+/+ and Ndst1−/− podocytes. B: results of spread cell area measures at T = 2 h after seeding between Ndst1+/+ (white bars) and Ndst1−/− (black bars) podocytes, the Ndst1−/− cells showing a twofold difference (P = 0.0001) in area compared with Ndst1+/+ cells. C and D: Ndst1+/+ and Ndst1−/− podocytes stained with Alexa 488 phalloidin to demonstrate differences in actin organization at 2 h after seeding. Prominent clusters of actin can be seen around the periphery of the Ndst1+/+ cells (C) and some cells show the beginning of stress fiber formation. The Ndst1−/− cells (D) show less spreading and fewer clusters of actin around their periphery, some still retaining a pattern of cortical actin staining. Final magnification in C and D: ×400, bar = 50 μm.
Fig. 5.
Ndst1−/− podocytes migrate less efficiently compared with Ndst1+/+ podocytes. The micrographs show representative fields of view of wounded monolayers of Ndst1+/+ (A) and Ndst1−/− (B) podocytes. In each micrograph, the monolayer boundaries at a given postwounding time interval are demarcated by green lines (T = 0), red lines (T = 24 h), and blue lines (T = 48 h). The white asterisk in each micrograph denotes one of several marks that were used as registration points for image alignment. C: differences in respective areas (A) for 24 h (A0-A24) and 48 h (A0-A48). The data show that at each time interval the amount of wound closure is significantly greater (P = 0.001) in the Ndst1+/+ cells compared with the Ndst1−/− cells. Final magnification in A and B = ×40, bar = 1,000 μm.
Lack of N-sulfation affects focal adhesion assembly and downstream signaling.
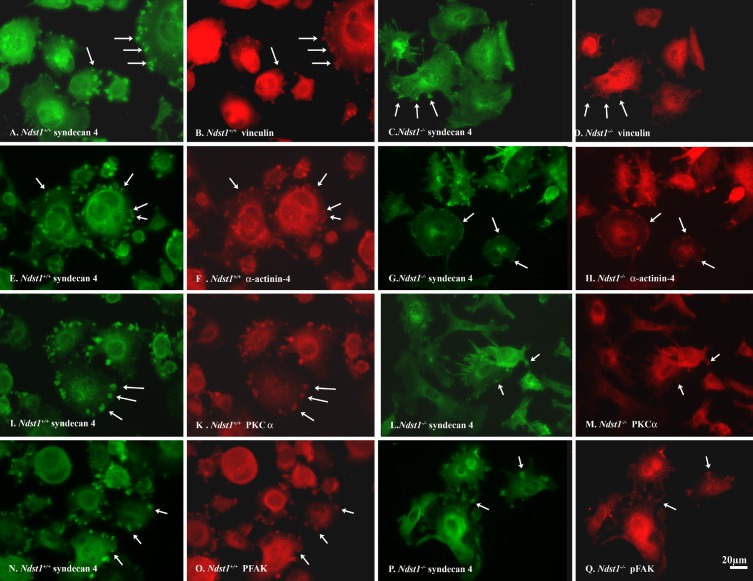
We speculated, as in the PEXTKO model, that the compromise with regard to cell matrix adhesion, spreading, and migration observed in the Ndst1−/− podocytes was based in the inability to properly engage the HS that was covalently attached to Sdc-4. From this perspective, we examined potential differences in the focal adhesion morphology and organization via immunostaining for well-known focal adhesion components such as vinculin (Fig. 6, B and D), α-actinin-4 (Fig. 6, F and H), PKCα (Fig. 6, J and L), and pFAK (Fig. 6, N and P). Immunostaining of Ndst1+/+ and Ndst1−/− podocytes showed a pronounced difference in the size of Sdc-4 aggregates between the two cell types, the aggregates being smaller in size in the Ndst1−/− (Fig. 6, C, G, L, and P) podocytes compared with those in the Ndst1+/+ podocytes (Fig. 6, A, E, I, and N). In turn, the size differential of the Sdc-4 aggregates is mirrored in the colocalization of the above focal adhesion components (Fig. 6), indicating that the lack of N-sulfation has a negative effect on the lateral aggregation of Sdc-4, which would subsequently translate into the downstream organization of focal adhesion signaling complexes in Ndst1−/− podocytes.
Fig. 6.
Ndst1−/− podocytes do not readily form adhesion complexes on fibronectin compared with Ndst1+/+ podocytes. A, B, E, F, I, K, N, and O: micrographs of Ndst1+/+ podocytes; C, D, G, H, L, M, P, and Q: micrographs of Ndst1−/− podocytes immunostained for Sdc-4 (A, C, E, G, I, L, N, and P), vinculin (B and D), α-actinin-4 (F and H), PKCα (K and M), or pFAK (O and Q). In Ndst1+/+ cells, Sdc-4 staining appears as large aggregates (“pods”) at the periphery of the cells (arrows in A, E, I, and N). In Ndst1−/− cells (C, G, L, and P) the aggregates are smaller in size. In accord, in Ndst1+/+ cells, the relative size of vinculin (B), α-actinin-4 (F), PKCα (K), and pFAK (O) clusters are larger than those observed in Ndst1−/− cells (D, H, M, and Q, respectively). Final magnification ×400, bar = 20 μm.
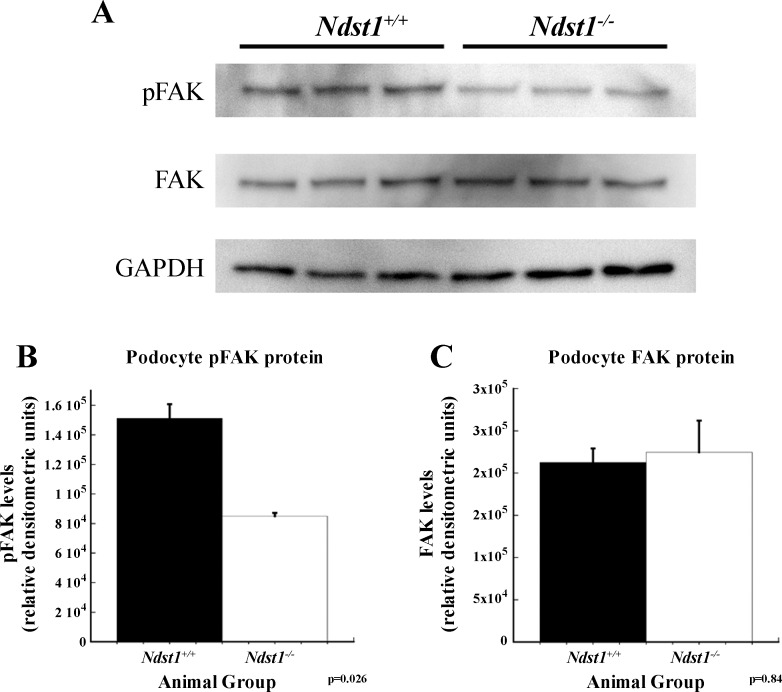
To investigate potential differences in signaling downstream from the focal adhesion complex between Ndst1+/+ and Ndst1−/− podocytes, cell lysates were analyzed by Western blot immunoassays for intracellular focal adhesion kinase (FAK) and pFAK. Western blot immunoassays showed that the levels of pFAK were significantly decreased (P = 0.026) in Ndst1−/− cells compared with control Ndst1+/+ podocytes, whereas the total levels of FAK were unchanged (Fig. 7).
Fig. 7.
Signaling from focal adhesions is diminished in Ndst1−/− podocytes compared with Ndst1+/+ podocytes. A: Western immunoblot for phosphorylated focal adhesion kinase (pFAK) (Tyr397) and focal adhesion kinase (FAK; total FAK). GAPDH immunoblotting serves as a loading control for band normalization. B and C: graphs of densitometry measurements of the blots in (A) showing significantly less pFAK in Ndst1−/− cells compared with Ndst1+/+ cells (B). No significant difference between the two groups was observed for total intracellular levels of FAK (C).
Lack of N-sulfation on HS affects the activation status and cell surface levels of integrin.
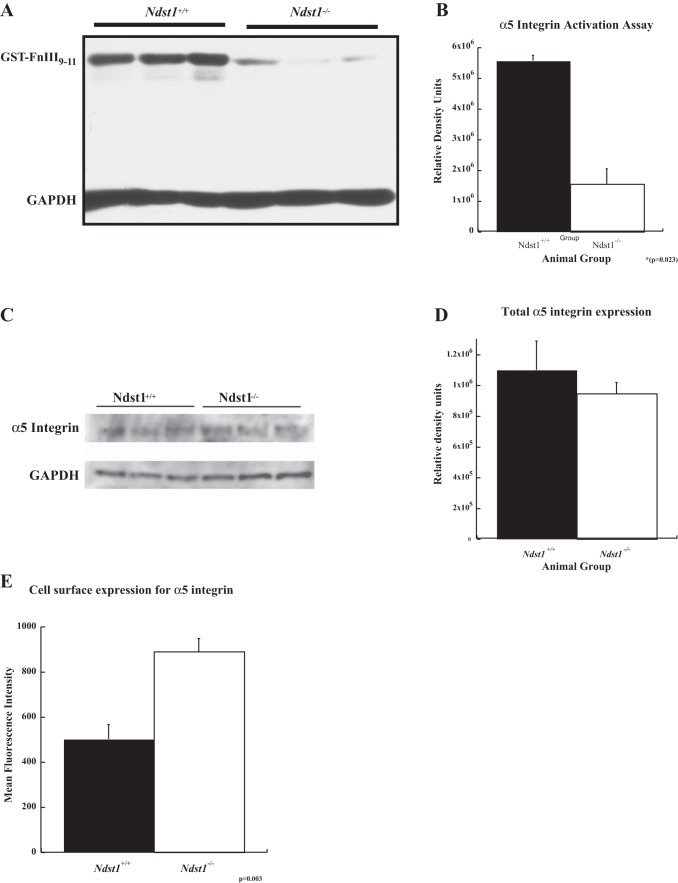
It is well known that syndecans work in tandem with integrins to mediate cell-matrix adhesion processes (13, 50, 51). Furthermore, recent studies have shown that the ectodomains of several syndecan core proteins are capable of affecting integrin affinity-modulation (i.e., inside-out signaling leading to integrin activation) (1, 7, 38). With the latter concept in mind, the activation status of α5β1 integrin was examined via the use of a GST-FNIII9–11 probe that specifically binds only the active configuration of α5β1 integrin (35). The binding of the GST-FNIII9–11 probe to the surface of Ndst1−/− cells was significantly less (P = 0.023) than that on Ndst1+/+ cells (Fig. 8, A and B), indicating that there was less active α5β1 integrin on the surface of Ndst1−/− cells. Western blot immunoassays were performed for the α5 portion of the integrin heterodimer of total cell lysates from Ndst1+/+ and Ndst1−/− podocytes to determine whether the decrease in active α5β1 integrin was related to a net decrease in the cellular abundance of α5β1 integrin. The assays showed that there was no difference in the total amount of α5 protein between the Ndst1+/+ and Ndst1−/− cells, further suggesting that the observed decrease in the binding of the GST-FNIII9–11 probe was a result of a net decrease in the activation status of α5β1 integrin. Alternatively, Sdc-4 ligand binding has been shown in other systems to have an effect on the levels of integrin on the cell surface (32). To test the latter possibility, immunostaining for α5 protein of nonpermeabilized Ndst1+/+ and Ndst1−/− cells in suspension was performed followed by flow cytometry to measure the relative difference in surface levels of the protein. The flow cytometry studies showed that the Ndst1−/− cells had significantly greater (×1.6 compared with control, P = 0.03) levels of α5 integrin on their surface of relative to that observed on the Ndst1+/+ cells.
Fig. 8.
The presence of α5β1 integrin in the active state at the cell surface in Ndst1−/− podocytes is diminished compared with that observed on the surface of Ndst1+/+ podocytes. A: Western immunoblot for GST-tagged FNIII9–11 peptide that had been bound to the activated α5β1 integrin on the surfaces of Ndst1+/+ and Ndst1−/− podocytes. B: graph of the densitometry of the respective bands of the gel shown in (A), the measures normalized to the GAPDH loading control. The amount of GST-FNIII9–11 peptide bound to activated integrin is significantly less (P = 0.023) in the Ndst1−/− cells compared with the Ndst1+/+ cells. C: Western blot of total cellular α5 integrin expression of Ndst1+/+ and Ndst1−/− podocytes. D: densitometry measures of the blot in (C) normalized to the GAPDH loading control. The data show that there was not a significant difference in the levels of total α5β1 integrin expression between of Ndst1+/+ and Ndst1−/− podocytes. E: results of surface immunostaining/flow cytometry measurements of Ndst1+/+ and Ndst1−/− podocytes for the presence of α5 integrin subunit at the cell surface. The data show Ndst1−/− podocytes that have more α5 integrin on their surfaces than Ndst1+/+ cells. Taken together, the data indicate that Ndst1−/− podocytes have more α5 integrin on their surfaces but is held in the low-affinity state for ligand binding.
DISCUSSION
The above data show that the loss of N-sulfation affects several key aspects of podocyte cell-matrix interactions such as attachment, spreading, focal adhesion formation, and migration. The majority of the results of the present study are similar to those previously reported for HS-null podocytes (8). However, the data serve to highlight the fact that although HS chains are present on PGs secreted in the Ndst1−/− podocytes (Fig. 2C), the presence of N-sulfate groups along the length of the HS chain are key and critical in mediating efficient podocyte-matrix interactions.
Extended NS domains are rapidly becoming known to be essential in mediating the interactions between HS and its protein ligands. Unlike integrins, which engage their ligands in a 1:1 receptor-ligand interaction/molecule, the presence of multiple NS domains along the length of HS and the presence of several HS carbohydrate chains per cell surface PG molecule impart polyvalency to GAG-protein interactions. Each PG molecule, via HS, has the potential to engage ligands at multiple sites within each ligand or, alternatively, to engage multiple species of ligands simultaneously (e.g., laminins and type IV collagens) per interaction. HS-protein interactions occur via Cardin-Weintraub (CW) motifs, which are embedded within the primary structure of the protein (6). The sequences of these motifs are [-X-B-B-X-B-X-] or [-X-B-B-B-B-X-X-B-X-], where X is a hydropathic residue and B is a basic residue (6). Plasma fibronectin, the matrix molecule used in our adhesion assays, contains at least four CW motifs (6), one encompassing the heparin-binding motif that has been shown to work in synergy with the RGD integrin-binding site to promote focal adhesion formation in other cell types (52). The data from the cell adhesion/spreading studies indicate that N-sulfation is important in driving the fibronectin-HS interactions that occur in the early stages of cell-matrix adhesion. Like the HS-null podocytes in our previous report (8), the Ndst1−/− podocytes at 2 h of cell-matrix interaction showed persistence of cortical actin organization (Fig. 4D, arrows) whereas the cytoskeletal organization observed in the Ndst1+/+ podocytes (Fig. 4C) had already moved toward forming stress fibers. A similar finding was reported in CHO 745 cells, which are also unable to make HS (16). Over time, the Ndst1−/− podocytes eventually reorganize their actin cytoskeleton into stress fibers (data not shown). The latter transition of the cortical actin organization toward the development of stress fibers may be the result of the Sdc-4 core protein itself, albeit with less efficiency, to directly engage matrix molecules to promote cell-matrix interactions (16). Because our 35S radiolabeling data (Fig. 3) also show that some degree of sulfation is maintained in the Ndst1−/− podocytes, it would also suggest that HS N-sulfation is important for efficiently mediating initial cell-matrix interactions, but may not be an absolute requirement for cell-matrix adhesion because other sulfate residues still present on HS could possibly engage the CW motifs over a longer course of time.
Because HS is always associated with PG core proteins, it was logical to explore how loss of N-sulfation might have effects on the organization of cell surface PGs in podocytes as they begin to engage the extracellular matrix. With regard to podocyte-matrix interactions, the data suggest that as in the PEXTKO mouse/HS-null podocytes (8, 9), some of the effects observed in the Ndst1−/− model are mediated via altering the ability of Sdc-4 to function properly during cell-matrix interactions. Sdc-4 plays a role in adhesion and cytoskeletal rearrangement through its intracellular associations with PKCα (23), FAK (49), and α-actinin (19). It is known that lateral aggregation and oligomerization of Sdc-4 plays a key role in mediating signaling through Sdc-4 (34) via several key pathways. Moreover, changes in oligomerization of Sdc-4 leads to a decrease in the β1 integrin-dependent recruitment of α-actinin to focal adhesion complexes (10). The VAR domain in the cytoplasmic tail of Sdc-4 is known to engage α-actinin; the lack of Sdc-4 to oligomerize efficiently leads to a decrease in α-actinin in focal adhesions (10). Our in vivo data (Fig. 1, B cf. E) and in vitro data (Fig. 6, F cf. H) reflect these same findings. An additional consequence of a decreased ability of Sdc-4 to multimerize is that PKCα is not activated as efficiently (34), and thus downstream signaling from Sdc-4 via PKCα is decreased. Abrogation of Sdc-4 signaling, in turn, has also been shown to have a negative effect on FAK phosphorylation (Tyr397) (49). The results in this study are consistent with previous reports showing that pFAK localization to sites of cell-matrix contact was decreased in Ndst1−/− cells (Fig. 6Q) compared with control cells (Fig. 6O). The overall abundance of intracellular pFAK (Fig. 7, A and B) in Ndst1−/− cells also was significantly decreased (P = 0.026) compared with controls in Western immunoblot assays. As mentioned above, our previous studies with HS-null podocytes showed similar results with regard to aggregation of Sdc-4 at the cell surface (8). Yang et al. (54) showed that the presence of HS on Sdc-1 core proteins was essential for targeting Sdc-1 to Triton X-100-resistant aggregates on the cell surfaces of B-lymphocytes. Sdc-4 has also been shown to be present in detergent-resistant lipid rafts (46) as aggregates of Sdc-4. Our results suggest that by changing the N-sulfate pattern within cell surface HS, Ndst1−/− cells were not able to efficiently form large Sdc-4 multimeric aggregates at the stages of early adhesion that would facilitate the efficient assembly of nascent signaling complexes associated with lipid rafts.
Another level of complexity of the Ndst1−/− podocyte model is the unexpected change in the baseline affinity of α5β1 integrin in Ndst1−/− podocytes for the GST-FNIII9–11 peptide, as suggested by the significant decrease (P = 0.023) in abundance of the GST-FNIII9–11 peptide (35) bound to the Ndst1−/− cell surfaces compared with controls. The decrease in binding of the GST-FNIII9–11 peptide (35), which was used to demonstrate the level of active, nonligated α5β1 integrin on the cell surface, is mirrored by the decrease in FAK activation (Fig. 7). At first glance the decrease in GST-FNIII9–11 peptide probe binding could have been explained by a net decrease in the cell surface levels of α5β1 integrin. This observation would have been consistent with what has been previously described in the literature for fibroblasts, in which cell surface levels of α5β1 integrin are dynamically regulated via endocytotic recycling of unengaged α5β1 receptors upon Sdc-4 adhesion (4). However, the flow cytometry data in our study would suggest that α5β1 may not follow that recycling pathway (4) in Ndst1−/− podocytes because the data show the net surface abundance for α5 integrin increased in those cells compared with controls (P = 0.003). Affinity modulation of integrins for their respective ligands has been well described in the literature [see (3, 18, 29, 40) for review] and, as mentioned previously, several recent studies show that integrin interactions with Sdc-1 (2, 7) and Sdc-4 (38) core proteins may be capable of evoking that effect.
From a historical perspective, the decreased sulfation of HS has been shown to be a consequence of diabetes mellitus in the kidney (5, 11, 12). The majority of these former studies used 35S sulfate radiolabeling as a means to detect the presence of undersulfated GAG. Given that N-sulfation of HS is a key step in the sulfation of HS, it would be logical to assume that the undersulfation of HS could be the result of the diabetic milieu affecting the intracellular levels/activity of NDST1. Although studies in rodent models did demonstrate a significant decrease in NDST1 activity in diabetic animals (24, 25), other studies exploring the expression (55) or activity (26) of NDST1 using human skin fibroblasts isolated from control and diabetic patients led to equivocal results. The former study showed no difference in the level of expression of NDST1 mRNA, but a decrease in the levels of NDST2 mRNA in diabetic individuals compared with controls (55). The same study reported that levels of mesangial cell expression of NDST1 or NDST2 were not affected by hyperglycemia. The latter study showed no difference in the level of NDST1 activity between control and diabetic fibroblasts isolated from normal patients and those with diabetes (26). Exploring the Nephromine database (30) and polling the Ju Podocyte dataset (21) (healthy living donor vs. those with diabetic nephropathy) we found that the most significant decrease in glomerular mRNA expression occurred with NDST1 mRNA (2.228-fold decrease, P = 0.00018) >NDST3 (1.94-fold decrease, P = 0.006) >NDST4 (1.65-fold decrease, P = 0.013), NDST2 (1.14, unchanged, P = 0.987). Thus the discrepancies in the literature over the levels of expression of NDST1 may be more indicative of a cell/tissue-specific changes in the level of NDST1 mRNA expression/activity in diabetes mellitus.
We believe that the changes in podocyte organization and cell-matrix interactions observed in the Ndst1−/− models (42) may be very germane to explaining changes observed in the human condition because NDST1−/− expression is downregulated in diabetes mellitus (see the previous paragraph). Thus the power of this model is that it emulates changes in podocyte-matrix interactions that are often found in diabetes mellitus (and possibly other renal diseases) without having many of the confounding pathophysiological sequelae that occur in diabetes mellitus.
GRANTS
This work supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-077860 to K. J. McCarthy and a Predoctoral Research Award to T. Sugar from the Malcolm Feist Institute for Cardiovascular Diseases and Imaging.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.S., D.J.W.-M., A.W.O., T.H.v.K., and K.J.M. conception and design of research; T.S., D.J.W.-M., J.G., and K.J.M. performed experiments; T.S., D.J.W.-M., A.W.O., and K.J.M. analyzed data; T.S., D.J.W.-M., A.W.O., and K.J.M. interpreted results of experiments; T.S. and K.J.M. drafted manuscript; A.W.O. and K.J.M. edited and revised manuscript; K.J.M. prepared figures; K.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jeffery Esko from the Department of Cellular and Molecular Medicine at the School of Medicine, University of California-San Diego, for his generosity in sharing the Ndst1fl/fl mice for this study and for his helpful discussions. The research in this paper was carried out in partial fulfillment of a Ph.D. dissertation project by T. Sugar in the Department of Cell Biology and Anatomy at Louisiana State University Health Sciences Center-Shreveport.
REFERENCES
- 1.Altemeier WA, Schlesinger SY, Buell CA, Brauer R, Rapraeger AC, Parks WC, Chen P. Transmembrane and extracellular domains of syndecan-1 have distinct functions in regulating lung epithelial migration and adhesion. J Biol Chem 287: 34927–34935, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altemeier WA, Schlesinger SY, Buell CA, Parks WC, Chen P. Syndecan-1 controls cell migration by activating Rap1 to regulate focal adhesion disassembly. J Cell Sci 125: 5188–5195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol 19: 495–507, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass MD, Williamson RC, Nunan RD, Humphries JD, Byron A, Morgan MR, Martin P, Humphries MJ. A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis. Dev Cell 21: 681–693, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown DM, Klein DJ, Michael AF, Oegema TR. 35S-glycosaminoglycan and 35S-glycopeptide metabolism by diabetic glomeruli and aorta. Diabetes 31: 418–425, 1982. [DOI] [PubMed] [Google Scholar]
- 6.Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9: 21–32, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Abacherli LE, Nadler ST, Wang Y, Li Q, Parks WC. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PloS One 4: e6565, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Wassenhove-McCarthy D, Yamaguchi Y, Holzman L, van Kuppevelt TH, Orr AW, Funk S, Woods A, McCarthy K. Podocytes require the engagement of cell surface heparan sulfate proteoglycans for adhesion to extracellular matrices. Kidney Int 78: 1088–1099, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, Wijnhoven TJ, Woods AC, McCarthy KJ. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int 74: 289–299, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi Y, Kim S, Lee J, Ko SG, Lee W, Han IO, Woods A, Oh ES. The oligomeric status of syndecan-4 regulates syndecan-4 interaction with alpha-actinin. Eur J Cell Biol 87: 807–815, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MP, Klepser H, Wu VY. Undersulfation of glomerular basement membrane heparan sulfate in experimental diabetes and lack of correction with aldose reductase inhibition. Diabetes 37: 1324–1327, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MP, Surma ML. [35S]sulfate incorporation into glomerular basement membrane glycosaminoglycans is decreased in experimental diabetes. J Lab Clin Med 98: 715–722, 1981. [PubMed] [Google Scholar]
- 13.Couchman JR, Chen L, Woods A. Syndecans and cell adhesion. Int Rev Cytol 207: 113–150, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Courtoy PJ, Kanwar YS, Hynes RO, Farquhar MG. Fibronectin localization in the rat glomerulus. J Cell Biol 87: 691–696, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtoy PJ, Timpl R, Farquhar MG. Comparative distribution of laminin, type IV collagen, and fibronectin in the rat glomerulus. J Histochem Cytochem 30: 874–886, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Echtermeyer F, Baciu PC, Saoncella S, Ge Y, Goetinck PF. Syndecan-4 core protein is sufficient for the assembly of focal adhesions and actin stress fibers. J Cell Sci 112, Pt 20: 3433–3441, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71: 435–471, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol 17: 509–516, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Greene DK, Tumova S, Couchman JR. Syndecan-4 associates with alpha-actinin. J Biol Chem 278: 7617–7623, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Jastrebova N, Vanwildemeersch M, Lindahl U, Spillmann D. Heparan sulfate domain organization and sulfation modulate FGF-induced cell signaling. J Biol Chem 285: 26842–26851, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju W, Greene CS, Eichinger F, Nair V, Hodgin JB, Bitzer M, Lee YS, Zhu Q, Kehata M, Li M, Jiang S, Rastaldi MP, Cohen CD, Troyanskaya OG, Kretzler M. Defining cell-type specificity at the transcriptional level in human disease. Genome Res 23: 1862–1873, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kern G, Flucher BE. Localization of transgenes and genotyping of H-2kb-tsA58 transgenic mice. Biotechniques 38: 38, 40, 42, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Keum E, Kim Y, Kim J, Kwon S, Lim Y, Han I, Oh ES. Syndecan-4 regulates localization, activity and stability of protein kinase C-alpha. Biochem J 378: 1007–1014, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kofoed-Enevoldsen A. Inhibition of glomerular glucosaminyl N-deacetylase in diabetic rats. Kidney Int 41: 763–767, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Kofoed-Enevoldsen A, Noonan D, Deckert T. Diabetes mellitus induced inhibition of glucosaminyl N-deacetylase: effect of short-term blood glucose control in diabetic rats. Diabetologia 36: 310–315, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Kofoed-Enevoldsen A, Petersen JS, Deckert T. Glucosaminyl N-deacetylase in cultured fibroblasts; comparison of patients with and without diabetic nephropathy, and identification of a possible mechanism for diabetes-induced N-deacetylase inhibition. Diabetologia 36: 536–540, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Kreuger J, Spillmann D, Li J, Lindahl U. Interactions between heparan sulfate and proteins: the concept of specificity. J Cell Biol 174: 323–327, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindahl U, Kusche-Gullberg M, Kjellen L. Regulated diversity of heparan sulfate. J Biol Chem 273: 24979–24982, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 23: 607–614, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Martini S, Eichinger F, Nair V, Kretzler M. Defining human diabetic nephropathy on the molecular level: integration of transcriptomic profiles with biological knowledge. Rev Endocr Metab Disord 9: 267–274, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy KJ, Wassenhove-McCarthy DJ. The glomerular basement membrane as a model system to study the bioactivity of heparan sulfate glycosaminoglycans. Microsc Microanal 18: 3–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan MR, Hamidi H, Bass MD, Warwood S, Ballestrem C, Humphries MJ. Syndecan-4 phosphorylation is a control point for integrin recycling. Dev Cell 24: 472–485, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberley TD, Mosher DF, Mills MD. Localization of fibronectin within the renal glomerulus and its production by cultured glomerular cells. Am J Pathol 96: 651–662, 1979. [PMC free article] [PubMed] [Google Scholar]
- 34.Oh ES, Woods A, Couchman JR. Multimerization of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C. J Biol Chem 272: 11805–11811, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell 17: 4686–4697, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson HC, Hopwood JJ. The alkaline cleavage and borohydride reduction of cartilage proteoglycan. Biochem J 133: 457–470, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachs N, Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat Rev Nephrol 9: 200–210, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Saito Y, Imazeki H, Miura S, Yoshimura T, Okutsu H, Harada Y, Ohwaki T, Nagao O, Kamiya S, Hayashi R, Kodama H, Handa H, Yoshida T, Fukai F. A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4. J Biol Chem 282: 34929–34937, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 11: 288–300, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shively JE, Conrad HE. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry 15: 3932–3942, 1976. [DOI] [PubMed] [Google Scholar]
- 42.Sugar T, Wassenhove-McCarthy DJ, Esko JD, van Kuppevelt TH, Holzman L, McCarthy KJ. Podocyte-specific deletion of NDST1, a key enzyme in the sulfation of heparan sulfate glycosaminoglycans, leads to abnormalities in podocyte organization in vivo. Kidney Int 85: 307–318, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takemoto M, Asker N, Gerhardt H. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ten Dam GB, Kurup S, van de Westerlo EM. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J Biol Chem 281: 4654–4662, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Ten Dam GB, Kurup S, van de Westerlo EM, Versteeg EM, Lindahl U, Spillmann D, van Kuppevelt TH. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J Biol Chem 281: 4654–4662, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Tkachenko E, Simons M. Clustering induces redistribution of syndecan-4 core protein into raft membrane domains. J Biol Chem 277: 19946–19951, 2002. [DOI] [PubMed] [Google Scholar]
- 47.van den Born J, Salmivirta K, Henttinen T, Ostman N, Ishimaru T, Miyaura S, Yoshida K, Salmivirta M. Novel heparan sulfate structures revealed by monoclonal antibodies. J Biol Chem 280: 20516–20523, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9: 121–167, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Wilcox-Adelman S, Denhez F, Goetinck P. Syndecan-4 modulates focal adhesion kinase phosphorylation. J Biol Chem 277: 32970–32977, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Woods A. Syndecans: transmembrane modulators of adhesion and matrix assembly. J Clin Invest 107: 935–941, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woods A, Couchman JR. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell 5: 183–192, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woods A, McCarthy JB, Furcht LT, Couchman JR. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol Biol Cell 4: 605–613, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashita Y, Oritani K, Miyoshi EK, Wall R, Bernfield M, Kincade PW. Syndecan-4 is expressed by B lineage lymphocytes and can transmit a signal for formation of dendritic processes. J Immunol 162: 5940–5948, 1999. [PubMed] [Google Scholar]
- 54.Yang Y, Borset M, Langford JK, Sanderson RD. Heparan sulfate regulates targeting of syndecan-1 to a functional domain on the cell surface. J Biol Chem 278: 12888–12893, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Yard B, Feng Y, Keller H, Mall C, van Der Woude F. Influence of high glucose concentrations on the expression of glycosaminoglycans and N-deacetylase/N-sulphotransferase mRNA in cultured skin fibroblasts from diabetic patients with or without nephropathy. Nephrol Dial Transplant 17: 386–391, 2002. [DOI] [PubMed] [Google Scholar]