Abstract
Some low molecular weight biomolecules, i.e., NAD(P)H, are unstable at high temperatures. The use of these biomolecules by thermophilic microorganisms has been scarcely analyzed. Herein, NADH stability has been studied at different temperatures and viscosities. NADH decay increased at increasing temperatures. At increasing viscosities, NADH decay rates decreased. Thus, maintaining relatively high cellular viscosity in cells could result in increased stability of low molecular weight biomolecules (i.e., NADH) at high temperatures, unlike what was previously deduced from studies in diluted water solutions. Cellular viscosity was determined using a fluorescent molecular rotor in various prokaryotes covering the range from 10 to 100°C. Some mesophiles showed the capability of changing cellular viscosity depending on growth temperature. Thermophiles and extreme thermophiles presented a relatively high cellular viscosity, suggesting this strategy as a reasonable mechanism to thrive under these high temperatures. Results substantiate the capability of thermophiles and extreme thermophiles (growth range 50–80°C) to stabilize and use generally considered unstable, universal low molecular weight biomolecules. In addition, this study represents a first report, to our knowledge, on cellular viscosity measurements in prokaryotes and it shows the dependency of prokaryotic cellular viscosity on species and growth temperature.
Introduction
The upper temperature limit at which microorganisms can grow has been widely discussed (1, 2). Currently, there is evidence of cell proliferation at 122°C by Methanopyrus kandleri strain 116 isolated from deep-sea vents (3). It is known that these microorganisms use essentially the same biomolecules and similar metabolic machinery as all other living beings (1, 2). However, some of the biomolecules required for cell functioning and growth (i.e., nicotinamide dinucleotides, adenosine triphosphate, and phosphoribosyl phosphate, among others) are unstable at high temperature (4). Therefore, there must be mechanisms to provide stability to essential biomolecules for life to be feasible in hot environments (2, 5).
Numerous studies have attempted to explain the stability at high temperatures of essential macrobiomolecules, such as DNA and proteins (6, 7). The stability of certain proteins under high temperature conditions is explained both by intrinsic mechanisms (modifications in sequence and structure) and by extrinsic mechanisms (interactions depending on solution composition) (7, 8, 9). Research on protein thermostability at high temperatures has explained relevant aspects of their functionality under extreme conditions (7).
However, living cells also depend on other small, low molecular weight biomolecules, like redox and energy cofactors, required for cell functioning. A number of these molecules show high instability at high temperatures (2, 10), suggesting that there must be some mechanisms for thermophiles to overcome this limitation under high temperature (4). For instance, although some factors like pH and certain ions have influence on NADH stability (11), there are no known mechanisms that explain NADH stability under high temperature (2, 4). Understanding the thermostability of small biomolecules is crucial to explain how thermophilic microorganisms are able to thrive under elevated temperatures (1).
It has been well described that cells accumulate some specific compatible solutes under osmotic stress (12, 13). Several studies have shown that some compatible solutes are also accumulated in the cytoplasm of hyperthermophiles under conditions of heat stress (14, 15, 16). The stabilization of some enzymes at high temperatures has also been shown in vitro in the presence of some compatible solutes like betaine, choline, 1-glyceryl-1-myo-inosityl phosphate, glycine, ectoine, or trehalose, among others (14, 16, 17, 18). Despite these results, the mechanisms conferring thermal stability by these solutes remain in most cases to be deciphered.
The cytoplasm of cells can be considered as a highly concentrated solution of multitude of interacting molecules, as the model of molecular crowding suggests (19, 20, 21). Consequently, its consistency is expected to be more like a viscous gel than an aqueous solution (20, 22), which can influence several cellular properties (23). For instance, viscosity has been shown to play an important role in maintaining cell structure and function (22, 24, 25). Viscosity decreases with temperature (26). A viscous solution greatly reduces the physical impacts among biomolecules, and, so, it should result in higher stability (27, 28). High temperature will induce a decrease in cell viscosity (26) and so, molecular and cell stability could be jeopardized.
Intracellular viscosity strongly influences diffusion and interaction between biomolecules (20, 22, 28). However, estimating intracellular viscosity is a difficult task. Traditional methods (i.e., mechanical viscometers) cannot be used to determine viscosity inside living cells. To solve this problem, fluorescent ratiometric methods have been introduced (29). More recently, the use of fluorescent molecular rotors has been proposed to accurately determine intracellular viscosity (30). These molecular rotors typically comprise a conjugated domain that can freely rotate in low-viscosity solutions, but viscous environments limit that action. This rotation is reflected in fluorescence intensity. Peng et al. (30) reported a new generation fluorescent molecular rotor, RY3, which provides dual emission peaks (456 nm, blue; 650 nm, red) showing minimum fluorescence in nonviscous environments. Red fluorescence increases with viscosity while blue fluorescence remains insensitive, providing the basis for ratiometric measurements using a small molecule fluorescence sensor with cell-membrane permeability to investigate on intracellular viscosity (30). Results of cellular viscosity have been carried out only on eukaryotic cells. Viscosity reported for the cytoplasm of cells from tissue cultures presented values only slightly above those expected for water or dilute solutions (29).
The aim of this study is to infer whether viscosity could represent a potential factor to partially maintain the thermostability of small biomolecules (i.e., NADH) at increasing temperatures. NADH decay rates as a function of temperature and viscosity were evaluated. Also determined was the cellular viscosity for prokaryotes over a broad temperature range, to evaluate the relevance of the proposed strategy. Understanding how labile, low molecular weight biomolecules can be maintained stable at high temperatures contributes to explaining how thermophiles are able to thrive under extreme temperatures using mainly the same biomolecules as mesophiles.
Materials and Methods
Nicotinamide adenine dinucleotide (NADH) was the selected low molecular weight biomolecule due to the simplicity of use and lower cost (31). Differences in stability of NADH were tested as a function of temperature and viscosity. Dynamic viscosity (herein viscosity as mPa·s) was considered in this study. Viscosity is dependent on temperature (26). Increased viscosity was obtained by supplementing assay solutions with either ethylene glycol or ectoine. Many other solutions were tested, but most showed interference with the NADH assay. Viscosity was determined using a VisioLab AMVn Micro-Viscometer (Anton-Paar, Graz, Austria) following the manufacturer’s recommendations.
A first type of experiment aimed to determine the effect of viscosity on the stability of NADH over a range of temperatures (20–90°C). These experiments were carried out in solution of phosphate buffer (pH 7.7) at concentrations of 0.05 and 0.1 M for ethylene glycol (50% vol/vol) or ectoine (0.45 mg mL−1) solutions, respectively. These buffering conditions resulted in a maximum stability for NADH (11). A buffered-water control lacking these compounds (ethylene glycol and ectoine) was also carried out. NADH was added to these assay solutions at 1 mM final concentration. Independent triplicate NADH solutions were assayed. Incubation was carried out over the temperature range from 20 to 90°C. Decomposition of NADH was followed over time collecting aliquots at specific time periods up to a maximum of 30–50 h or until NADH decreased down to undetectable levels. NADH was quantified spectrophotometrically at 340 nm (32) using a Nanodrop 2000c (Thermo Fisher Scientific). As previously studied in Hofmann et al. (33), the biologically oxidized form (NAD+) is not the main intermediate. The major products of NADH thermal degradation resulted from the hydrolysis and oxidative ring opening of the reduced nicotinamide (33). Preliminary results showed that NADH decay followed first-order kinetics and the NADH decay rate was estimated by linear regression (34) plotting the natural logarithm of NADH concentration versus time. NADH decay rates are expressed as absolute values so that the highest absolute value corresponds to the fastest decay and lowest stability. Analysis of variance and comparison of linear regression coefficients were used to evaluate the significance of differences between estimates, following Sokal and Rohlf (34).
A second type of experiment aimed to determine the direct effect of viscosity on NADH decay at a constant temperature. In these experiments, a series of solutions with different viscosities were prepared. These viscosities were achieved by using the following concentrations of ethylene glycol: 0 (lacking ethylene glycol), 10, 20, 30, 40, 50, and 60% vol/vol. Each one of these solutions was assayed at 20, 50, 70, and 90°C. Assay conditions and estimates were carried out as described above. Unlike the first type of experiment (previous paragraph), this second type of experiment allowed us to monitor the effect of viscosity at a given temperature.
Intracellular viscosity was estimated using a fluorescent molecular rotor, RY3 (30). RY3 was synthesized as previously described in Peng et al. (30). For these experiments, different prokaryotic strains were used. The following bacteria were employed: Escherichia coli K12 (CECT 433; 20, 30, and 40°C), Pseudomonas aeruginosa PAO1 (CECT 4122; 20 and 40°C), Lactococcus lactis spp. lactis (CECT 4433; 10, 15, 20, 30, and 40°C), Geobacillus thermoglucosidasius (CECT 4038; 50, 60, and 70°C), and Fervidobacterium FC2004 (JCM 18757; 60, 70, and 80°C), and the archaeon Pyrococcus furiosus (DSM 3638; 70, 80, 90, and 99°C). The growth temperatures for those species covered the temperature range from 10 to 100°C. Each strain was grown in the recommended culture medium under the required temperature condition. Cultures were harvested by centrifugation, then washed with saline solution (0.9% NaCl) or a salt solution (recommended culture medium lacking organic components) for marine species. Cell suspensions were adjusted to an OD 1. RY3 was added to cell suspensions at 5 μM final concentration (30) and incubated at the temperature of growth for 1 h. Ratiometric fluorescence measurements were carried out as described by Peng et al. (30) at the excitation (400 nm) and emission (456 and 650 nm) peaks. The intensity (I) of blue emission at 456 nm was insensitive to changes in viscosity and the red fluorescence intensity (650 nm) increased with viscosity. Ratiometric changes with viscosity followed a linear relationship between log(I650/I456) and log(viscosity) that fitted the Föster-Hoffmann model (35). This calibration was achieved with different solutions of ethylene glycol (from 10 to 60%). Viscosity versus growth temperature was analyzed by nonlinear curve fitting using the Log-Normal (three parameters) function available in SigmaPlot software (Systat Software, London, UK). To estimate the potential interference of RY3 dye and biomolecules in the tested cells, fluorescence lifetime measurements were performed using a time-correlated single photon counting module (Fluorohub; Horiba Jobin Yvon, Edison, NJ). Samples were optically excited with 900-ps-long pulses from a tunable laser source (SC400; Fianium, Southampton, UK) operating at λ = 633 nm and fluorescence was collected at λ = 715 nm. The fluorescence decay curves were best fitted (1.1 < χ2 <1.3) to a biexponential function:
and an intensity-weighted average decay time was extracted from
Results
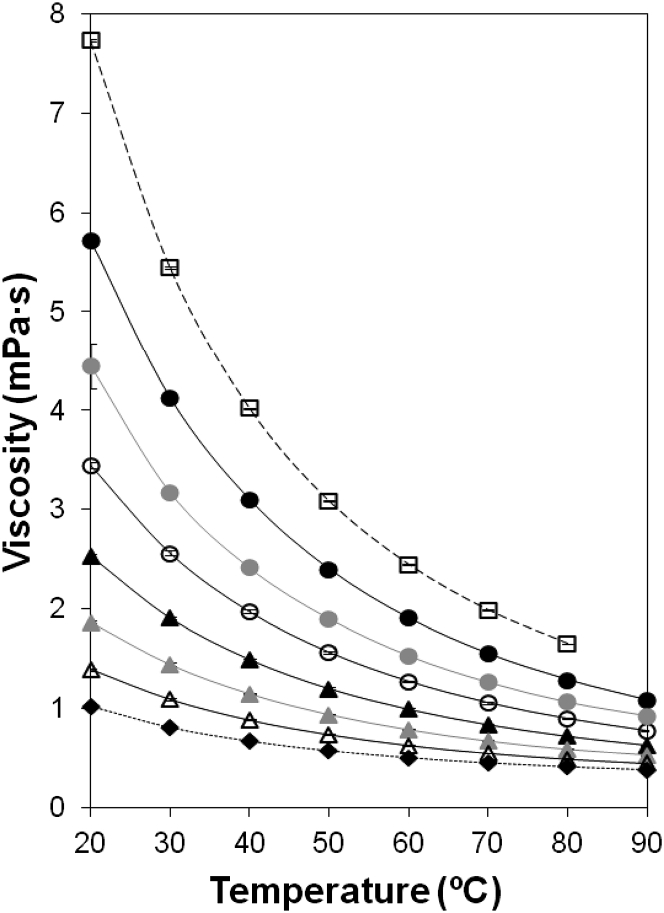
The persistence of NADH in solutions with different viscosities was analyzed within a wide temperature range (20–90°C). Fig. 1 shows the relationships between temperature and viscosity for the solutions used in this study. Water viscosity decreases threefold from 20 to 80°C. Solutions of ethylene glycol and ectoine were assayed to obtain higher viscosities than water.
Figure 1.
Effect of temperature on viscosity of the different solutions used in this study: buffered-water (dotted line with solid diamonds), ectoine (0.45 mg mL−1; dashed line with open squares), and ethylene glycol at 10% (open triangles), 20% (shaded triangles), 30% (solid triangles), 40% (open circles), 50% (shaded circles), and 60% (solid circles). Error bars are shown except when covered by solid symbols.
Effects of temperature and viscosity on NADH decay
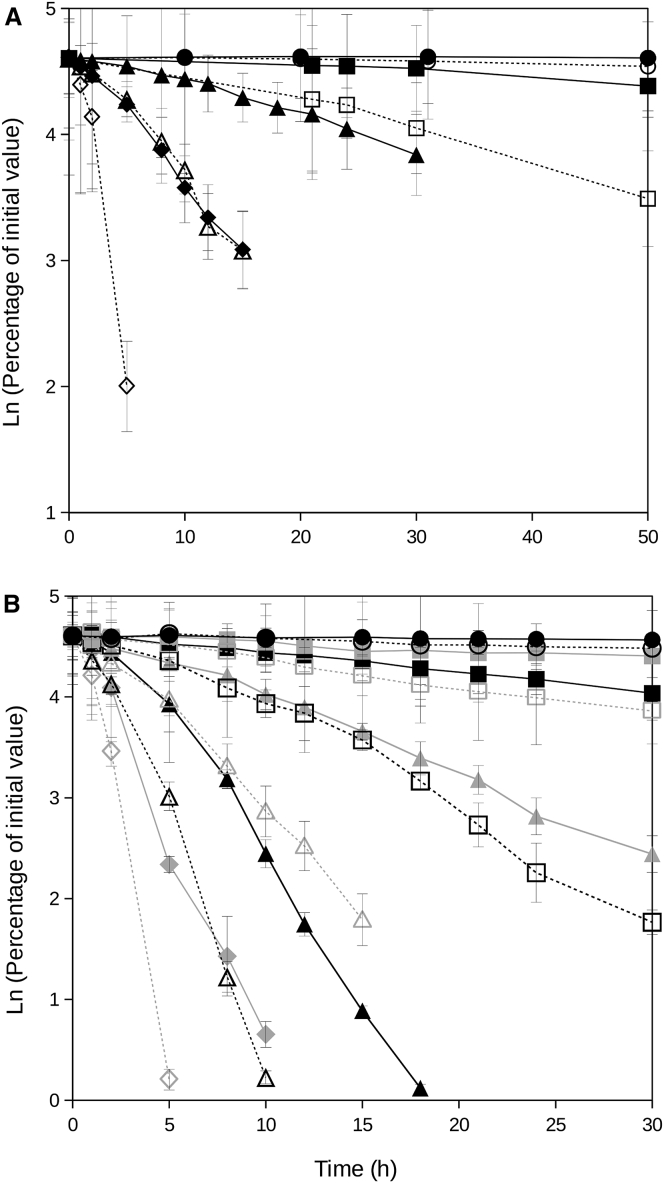
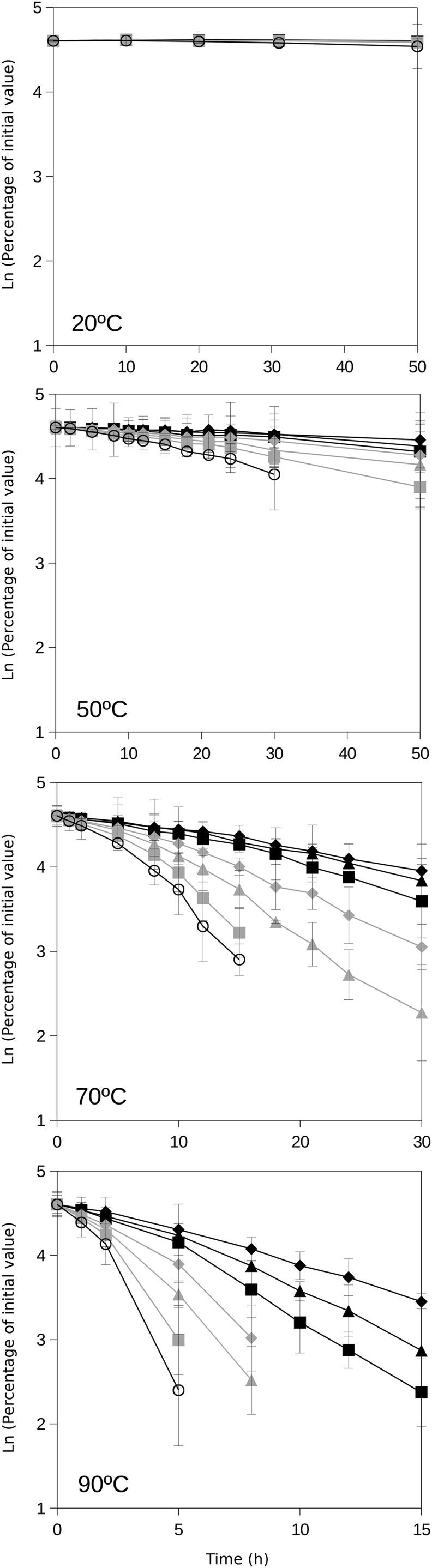
Stability of NADH in solutions at different viscosities and temperatures was assayed over time in ethylene glycol (50%, final concentration) or ectoine (0.45 mg mL−1). A similar pattern was observed in both solutions (Fig. 2). At the lowest temperature tested (20°C), NADH remained stable over the time period assayed in this study both in buffered-water and the ethylene glycol solution (Fig. 2 A). Increasing temperatures resulted in a sharp increase of NADH decay. At temperatures of 50°C and above (up to 90°C), NADH decay rates at high viscosity (i.e., ethylene glycol solution) were significantly lower (as absolute values) than in control solutions at low viscosity (i.e., buffered-water) at the same temperature (P < 0.001) (Fig. 2 A).
Figure 2.
NADH persistence in buffered-water (dotted lines with open symbols) and ethylene glycol (A) or ectoine (B) containing solutions with high viscosities (filled symbols) at different temperatures: 20°C (solid circles), 40°C (shaded squares), 50°C (solid squares), 60°C (shaded triangles), 70°C (solid triangles), 80°C (shaded diamonds), and 90°C (solid diamonds). Error bars are shown except when covered by solid symbols.
NADH decay in ectoine-containing solutions (i.e., high viscosity) showed a lower decrease rate (P < 0.001) than in the ectoine-lacking solutions (i.e., low viscosity control solution) over a range of temperatures (40–80°C; (Fig. 2 B). At 20°C, NADH remained approximately constant over the length of the experiment. Increasing temperatures resulted in increasing NADH decay rates (Fig. 2 B).
Stabilization of NADH by viscosity
These experiments to evaluate the stabilization of NADH by viscosity were performed at four different temperatures (20, 50, 70, and 90°C). Results are shown in Fig. 3. At 90°C, increasing viscosities resulted in decreasing NADH decays for a range of viscosities from 0.4 to 1.1 mPa·s. At 70°C and 50°C, an increase of viscosity also resulted in decreases of the rates of NADH decay with viscosities 0.5–1.5 mPa·s and 0.6–2.4 mPa·s, respectively. At a given temperature (at and above 50°C), increasing viscosity resulted in higher NADH stability (Fig. 3). At 20°C, NADH showed practically no decay during these experiments at a range of viscosities from ∼1 to >5 mPa·s. The differences of NADH decay rates between the lowest and highest viscosities were comparatively decreasing as temperature decreased (Fig. 4). At low viscosity (i.e., ∼0.5 mPa·s), the effect of temperature was dramatic, leading to increasing NADH decay rates with temperature up to 16 h−1 at 90°C. When comparing these effects at high viscosities (i.e., ∼1.5 mPa·s), NADH remained much more stable over the full temperature range tested (20–90°C) showing null decay (at 20°C) and decays below 3 h−1 (at higher temperatures).
Figure 3.
NADH persistence at different viscosities. The tested range of viscosities was prepared by supplementing the solutions with ethylene glycol at 0% (unsupplemented, ethylene glycol lacking solution; open symbols), 10% (shaded squares), 20% (shaded triangles), 30% (shaded diamonds), 40% (solid squares), 50% (solid triangles), and 60% (solid diamonds) final concentrations. The experiments were performed at 20, 50, 70, and 90°C. Error bars are shown except when covered by solid symbols.
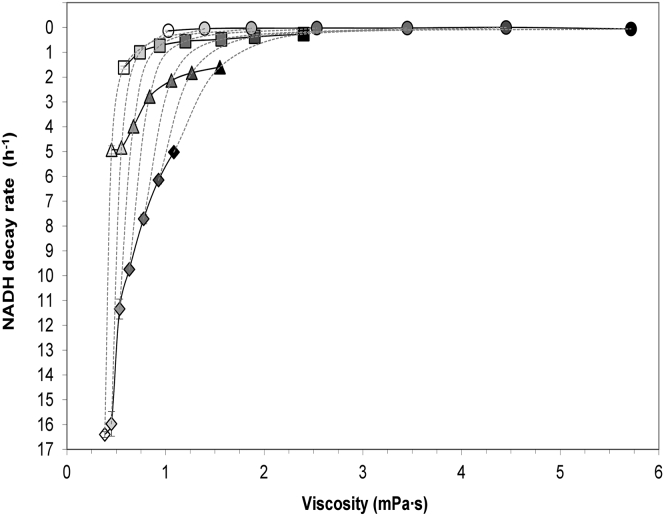
Figure 4.
Relationships between NADH decay rates and viscosity for experiments performed at 20°C (circles), 50°C (squares), 70°C (triangles), and 90°C (diamonds). Different viscosities at a single temperature were obtained by using different concentrations of ethylene glycol (0, 10, 20, 30, 40, 50, and 60%, final concentrations). Open symbols represent control solutions without ethylene glycol. Solid symbols of increasing darkness represent increasing ethylene glycol concentrations. The solutions with different concentrations of ethylene glycol assayed at the same temperature are linked by a continuous solid line and they show the apparent trend of NADH stabilization at increasing viscosity. Solutions of equal composition are linked by a dotted line showing the effect of temperature on viscosity and NADH decay rate. Error bars are shown except when covered by solid symbols.
Curves of NADH decay rate versus viscosity (Fig. 4) showed a reduction of NADH decay at increasing viscosity. The curves at the tested temperatures (Fig. 4) pointed toward minimum decays at increasingly high viscosity values and drastic increases of NADH decay rates at low viscosities and high temperatures.
Cellular viscosity
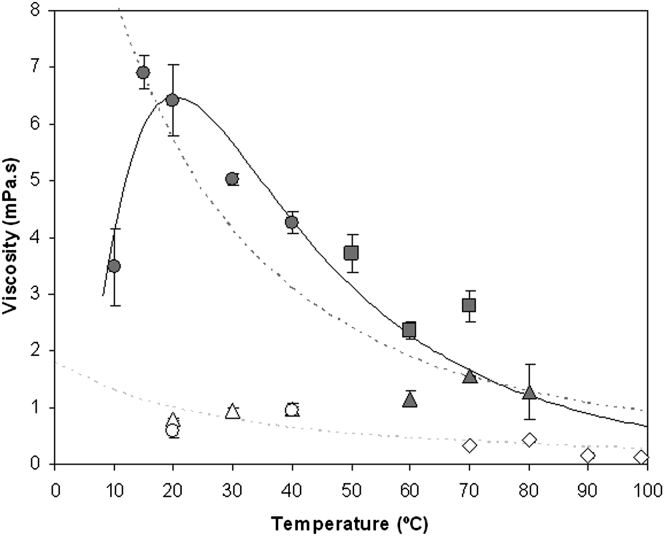
Different prokaryotic strains showed different cellular viscosity. Fig. 5 presents the viscosity estimates as a function of growth temperature for different prokaryotes. Escherichia and Pseudomonas growing in the temperature range from 20 to 40°C presented very similar values and low cellular viscosity around the viscosity of water. The Lactococcus strain resulted in a large variation of viscosity at different temperatures. Lactococcus cells grown at the lowest studied temperature (10°C) showed much lower cellular viscosity than these cells growing at 15–20°C and above. Lactococcus cells presented a peak of maximum viscosity at 15–20°C, decreasing at higher and lower temperatures. The decrease of cellular viscosity in Lactococcus cells at 10°C might be explained by a physiological adaptation to the limit imposed by exponentially increased viscosity at lowering temperatures. Geobacillus cells are thermophiles, growing from 50 to 70°C; they showed lower cellular viscosity than Lactococcus cells but higher than Fervidobacterium cells grown from 60 to 80°C. Pyrococcus cells grown from 70 to 99°C presented the lowest viscosity values determined during this study and around the range of viscosity expected for water in its growth temperature range. For comparison purposes, Fig. 5 presents the lines corresponding to the relationships of viscosity versus temperature for water and a solution of 60% ethylene glycol, which are indicative of water and high viscosity conditions, respectively, used during the NADH decay experiments described above. The predicted line resulting from nonlinear curve-fitting analysis including the data points from Lactococcus, Geobacillus, and Fervidobacterium (P < 0.0001; r = 0.95; n = 11) is also plotted in Fig. 5. Estimated cellular viscosity of Escherichia, Pseudomonas, and Pyrococcus was in the range of viscosity expected for water at their growth temperatures. Lactococcus, Geobacillus, and Fervidobacterium presented values of cellular viscosity much higher than water at their growth temperatures but in the range of viscosity corresponding to a 60% ethylene glycol solution.
Figure 5.
Relationship between cellular viscosity and growth temperature for several prokaryotes. Escherichia coli (open triangles), Pseudomonas aeruginosa (open circles), Lactococcus lactis (solid circles), Geobacillus thermoglucosidasius (solid squares), Fervidobacterium sp. (solid triangles), and Pyrococcus furiosus (open diamonds). Error bars are shown except when covered by solid symbols. A predictive nonlinear curve (solid continuous line) fitting the data points from species with solid symbols (Lactococcus, Geobacillus, Fervidobacterium) is presented. For comparison, the viscosity to temperature relationships of buffered-water (light-shaded dashed line) and 60% ethylene glycol solution (dark-shaded dashed line) are shown.
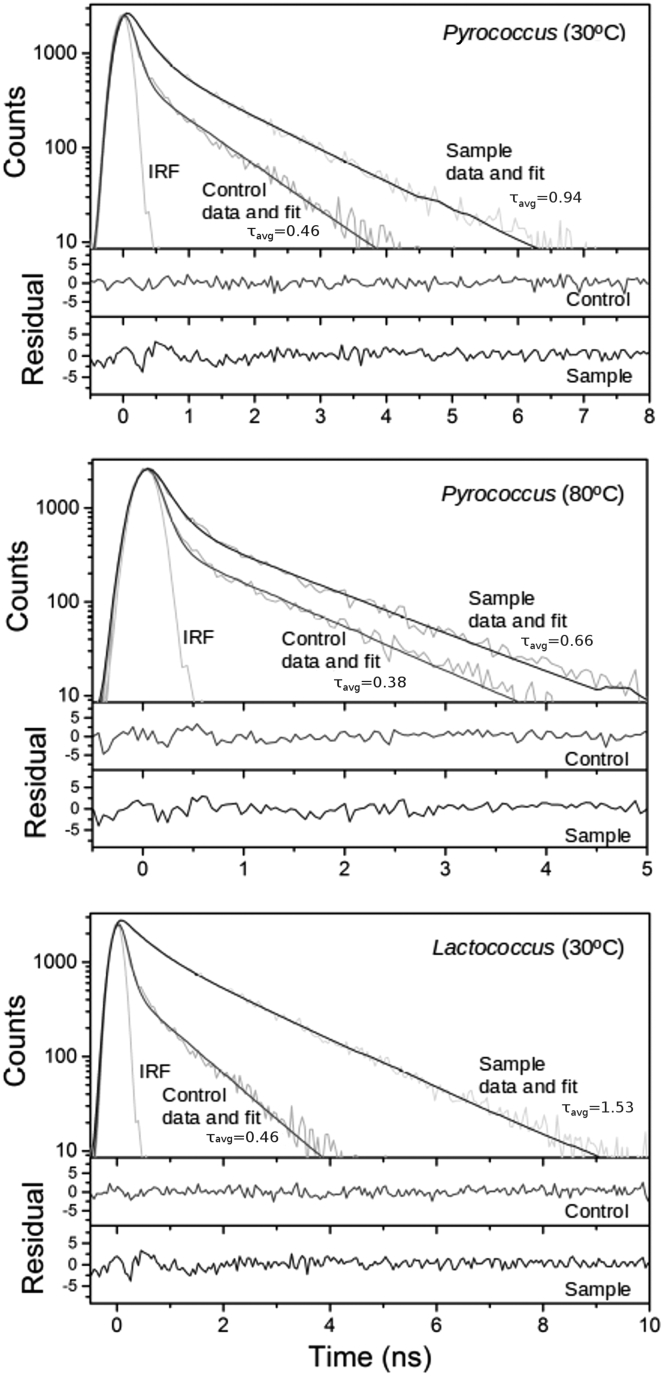
Fluorescence decay curves (Fig. 6) showed no interference between RY3 dye and biomolecules in cells (where a faster decay rate would be expected as a consequence of potential fluorescence quenching). These curves presented similar patterns under the tested conditions (with and without cells, and for different cell types, Pyrococcus and Lactococcus). Estimates of τavg values were lower at high temperature (Fig. 6). The τavg value for Pyrococcus cells was higher than the control lacking cells. The high τavg value obtained for Lactococcus confirmed the much higher cellular viscosity than can be reached in this bacterium.
Figure 6.
Fluorescence decay curves of RY3 for Pyrococcus furiosus cells grown at 80°C (measured at 30°C, upper figure, and at 80°C, center figure) and Lactococcus lactis cells grown at 30°C (measured at 30°C, lower figure). Experimental data, fitted curves, residuals, and estimated τavg values (ns) are shown for controls and cells.
Discussion
A number of biomolecules required for cell functioning are known to show in vitro instability at high temperatures (2, 4). All living cells, including thermophiles, use basically the same types of biomolecules. Several studies have attempted to clarify this issue focusing mainly on protein structure (7, 8, 9). Besides proteins, cell functioning requires numerous low molecular weight biomolecules. For instance, NADH has been reported to be unstable at elevated temperatures (4). NADH is essential for cells by participating in numerous central metabolic processes involving electron transfers. This study shows evidence of the influence of cellular viscosity on the stability of low molecular weight biomolecules at elevated temperatures and centers on the model provided by NADH.
The reported thermal instability of NADH (2, 4, 11) has been confirmed in this study, showing increased decay at increasing temperatures. At temperatures of 50°C and above, NADH showed clear decline over time that is in agreement with previous observations (33). Increasing viscosity resulted in increasing stability of NADH at various temperatures. Higher-than-water viscous solutions were obtained by different concentrations of ethylene glycol or supplementing with ectoine. For example, ectoine has been reported in thermophilic cells as a compatible solute that could be either synthesized by the cells or taken up from the medium contributing to cell survival at increasing temperatures (36, 37). Previous studies have proposed other strategies to overcome the instability of biomolecules at high temperature, but the actual mechanisms remain to be deciphered (1, 4). High viscosity has been reported as associated with cells adapted to heat stress in fungal spores (24), representing a preliminary example that maintaining viscosity could facilitate long-term preservation and the thermal stability of biomolecules. As a consequence, one could propose that the accumulation in thermophilic cells of a variety of solutes could be a possible mechanism to maintain cytoplasm viscosity and compensate, at least partially, for the expected decrease of viscosity in the cell interior at increasing temperatures.
Viscosity greatly decreases with temperature, and life at high temperatures is expected to be adapted to this phenomenon. Results have shown that viscous solutions contributed to significantly enhancing the stability of NADH at high temperatures. Thus, maintaining viscosity in the cytoplasm of thermophiles could represent a potential mechanism to increase the stability of low molecular weight biomolecules under elevated temperatures.
To evaluate the relevance of cellular viscosity to stabilize low molecular weight biomolecules at high temperature, cellular viscosity was estimated for different prokaryotes using a novel fluorescent molecular rotor. This study is the first report, to our knowledge, comparing cellular viscosity among prokaryotes from a wide temperature range (from 10 to 100°C). These results indicated that cellular viscosity is species-dependent, and that it can depend on growth temperature.
In this study we have observed two basic strategies of cellular viscosity in prokaryotes: 1) those species that maintain relatively high cellular viscosity (around the values of a 60% ethylene glycol solution); and 2) those that present relatively low cellular viscosity (in the viscosity range of water and diluted solutions). Prokaryotes following the first strategy should be potentially able to overcome, at least partially, the instability of low molecular weight biomolecules at high temperatures by maintaining relatively high viscosities. This strategy fits with the observation that thermophilic cells are able to accumulate organic solutes (14, 15, 16) that could regulate cellular viscosity. This group includes thermophiles and extreme thermophiles and taxonomic phyla, such as Firmicutes and Thermotogae among the tested species, with growing temperatures in the range of 50–80°C. An interesting example from this study is Lactococcus, which presented a clear response of its cellular viscosity as a function of growth temperature. Lactococcus maximum cellular viscosity was observed for cells growing at 15–20°C. Lactococcus showed the ability to drastically modify its cellular viscosity as a response to growth at low (10°C) and moderately high (30–40°C) temperature conditions. This is a first report, to our knowledge, showing the ability of bacterial cells to regulate its cellular viscosity depending on growing temperature.
The γ-Proteobacteria examined in this study correspond to typical mesophilic, Gram-negative, bacteria and followed the second strategy. These species presented a cellular viscosity at the level of water within the 20–40°C temperature range. These levels of viscosity present no compromise for biomolecule instability at that temperature range. However, a hyperthermophilic archaeon, Pyrococcus, also showed cellular viscosities around the expected values for water despite growing at the highest temperatures herein tested. Fluorescence decay curves indicated a lack of interference between RY3 dye and biomolecules in cells over a wide temperature range. The τavg obtained for Pyrococcus cells at 80°C showed that these cells present cellular viscosity slightly above the water value; this can only partially contribute to stabilizing small biomolecules. The decrease of viscosity with temperature suggests the existence of distinct or complementary physiological mechanisms to circumvent the thermal instability of key low molecular weight biomolecules in hyperthermophiles (i.e., >80°C). Some potential mechanisms have been proposed, including bearable rapid turnover rates, metabolite channeling, and other strategies of local stabilization (4).
Conclusions
Cellular viscosity in prokaryotes is species-dependent and it can vary as a result of growth temperature within a species. Increasing cellular viscosity enhances NADH stability at high temperature. Cellular viscosity observed for thermophiles and extreme thermophiles (growing optimally between 50 and 80°C) corresponds to values of viscosity able to provide effective stability for NADH and likely other low molecular weight biomolecules. Some mesophiles, such as Lactococcus, are capable of modifying cellular viscosity depending on growing temperature, likely as an adaptive mechanism to low/high temperature stress. On the other hand, some other cells (γ-Proteobacteria, Pyrococcus) present relatively low cellular viscosity slightly above the level of water values. This study significantly contributes to explain the stability of low molecular weight biomolecules in thermophiles and extreme thermophiles, and to determine the cellular viscosity in prokaryotes and its variability.
Author Contributions
All authors contributed to improve the final drafts of the article.
A.C. performed most experiments with the help of J.C.; X.P. contributed with RY3 and participated in the cellular viscosity measurements; J.M.G. designed the experiments and wrote most of the article; and J.F.G.-L. performed and analyzed the fluorescence decay curves.
Acknowledgments
The authors are thankful to M. C. Portillo for her inputs and comments.
The authors acknowledge funding from projects No. CSD2009-0006 and No. CGL2014-58762-P from the Spanish Ministry of Economy and Competitiveness and grants No. BIO-288 and No. RNM2529 from the Andalusian Government. Federal funds cofinanced these projects. Funding from the mobility program No. 003-ABEL-CM-2013 (NILS Science and Sustainability program, EEA grants) is also acknowledged.
Editor: Paul Wiseman.
References
- 1.Cowen D.A. The upper temperature of life—where do we draw the line? Trends Microbiol. 2004;12:58–60. doi: 10.1016/j.tim.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Stetter K.O. Extremophiles and their adaptation to hot environments. FEBS Lett. 1999;452:22–25. doi: 10.1016/s0014-5793(99)00663-8. [DOI] [PubMed] [Google Scholar]
- 3.Takai K., Nakamura K., Horikoshi K. Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc. Natl. Acad. Sci. USA. 2008;105:10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel R.M., Cowan D.A. Biomolecular stability and life at high temperatures. Cell. Mol. Life Sci. 2000;57:250–264. doi: 10.1007/PL00000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White R.H. Hydrolytic stability of biomolecules at high temperatures and its implication for life at 250°C. Nature. 1984;310:430–432. doi: 10.1038/310430a0. [DOI] [PubMed] [Google Scholar]
- 6.Grogan D.W. Hyperthermophiles and the problem of DNA instability. Mol. Microbiol. 1998;28:1043–1049. doi: 10.1046/j.1365-2958.1998.00853.x. [DOI] [PubMed] [Google Scholar]
- 7.Vieille C., Zeikus G.J. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 2001;65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berezovsky I.N., Shakhnovich E.I. Physics and evolution of thermophilic adaptation. Proc. Natl. Acad. Sci. USA. 2005;102:12742–12747. doi: 10.1073/pnas.0503890102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M.K., González J.M., Robb F.T. Extremely thermostable glutamate dehydrogenase (GDH) from the freshwater archaeon Thermococcus waiotapuensis: cloning and comparison with two marine hyperthermophilic GDHs. Extremophiles. 2002;6:151–159. doi: 10.1007/s007920100238. [DOI] [PubMed] [Google Scholar]
- 10.Hudson R.C., Ruttersmith L.D., Daniel R.M. Glutamate dehydrogenase from the extremely thermophilic archaebacterial isolate AN1. Biochim. Biophys. Acta. 1993;1202:244–250. doi: 10.1016/0167-4838(93)90011-f. [DOI] [PubMed] [Google Scholar]
- 11.Rover Júnior L., Fernandes J.C., Serrano S.H. Study of NADH stability using ultraviolet-visible spectrophotometric analysis and factorial design. Anal. Biochem. 1998;260:50–55. doi: 10.1006/abio.1998.2656. [DOI] [PubMed] [Google Scholar]
- 12.da Costa M.S., Santos H., Galinski E.A. An overview of the role and diversity of compatible solutes in bacteria and archaea. Adv. Biochem. Eng. Biotechnol. 1998;61:117–153. doi: 10.1007/BFb0102291. [DOI] [PubMed] [Google Scholar]
- 13.Calderón M.I., Vargas C., Nieto J.J. Complex regulation of the synthesis of the compatible solute ectoine in the halophilic bacterium Chromohalobacter salexigens DSM 3043T. Microbiology. 2004;150:3051–3063. doi: 10.1099/mic.0.27122-0. [DOI] [PubMed] [Google Scholar]
- 14.Empadinhas N., da Costa M.S. Diversity and biosynthesis of compatible solutes in hyper/thermophiles. Int. Microbiol. 2006;9:199–206. [PubMed] [Google Scholar]
- 15.Martins L.O., Huber R., Santos H. Organic solutes in hyperthermophilic archaea. Appl. Environ. Microbiol. 1997;63:896–902. doi: 10.1128/aem.63.3.896-902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos H., da Costa M.S. Organic solutes from thermophiles and hyperthermophiles. Methods Enzymol. 2001;334:302–315. doi: 10.1016/s0076-6879(01)34478-6. [DOI] [PubMed] [Google Scholar]
- 17.Caldas T., Demont-Caulet N., Richarme G. Thermoprotection by glycine betaine and choline. Microbiology. 1999;145:2543–2548. doi: 10.1099/00221287-145-9-2543. [DOI] [PubMed] [Google Scholar]
- 18.Knapp S., Ladenstein R., Galinski E.A. Extrinsic protein stabilization by the naturally occurring osmolytes β-hydroxyectoine and betaine. Extremophiles. 1999;3:191–198. doi: 10.1007/s007920050116. [DOI] [PubMed] [Google Scholar]
- 19.Goodsell D.S. Springer; New York: 1993. The Machinery of Life. [Google Scholar]
- 20.Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer J. From water and ions to crowded biomacromolecules: in vivo structuring of a prokaryotic cell. Microbiol. Mol. Biol. Rev. 2011;75:491–506. doi: 10.1128/MMBR.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack G.H. Ebner and Sons; Seattle, WA: 2001. Cells, Gels and the Engines of Life: a New, Unifying Approach to Cell Function. [Google Scholar]
- 23.Zimmerman S.B., Minton A.P. Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 1993;22:27–65. doi: 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- 24.Dijksterhuis J., Nijsse J., Golovina E.A. High viscosity and anisotropy characterize the cytoplasm of fungal dormant stress-resistant spores. Eukaryot. Cell. 2007;6:157–170. doi: 10.1128/EC.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun W.Q. Dielectric relaxation of water and water-plasticized biomolecules in relation to cellular water organization, cytoplasmic viscosity, and desiccation tolerance in recalcitrant seed tissues. Plant Physiol. 2000;124:1203–1216. doi: 10.1104/pp.124.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogelson R.L., Likhachev E.R. Temperature dependence of viscosity. Tech. Phys. 2001;46:1056–1059. [Google Scholar]
- 27.Finkelstein I.J., Massari A.M., Fayer M.D. Viscosity-dependent protein dynamics. Biophys. J. 2007;92:3652–3662. doi: 10.1529/biophysj.106.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauscher A.Á., Simon Z., Malnasi-Csizmadia A. Temperature dependence of internal friction in enzyme reactions. FASEB J. 2011;25:2804–2813. doi: 10.1096/fj.11-180794. [DOI] [PubMed] [Google Scholar]
- 29.Luby-Phelps K., Mujumdar S., Waggoner A.S. A novel fluorescence ratiometric method confirms the low solvent viscosity of the cytoplasm. Biophys. J. 1993;65:236–242. doi: 10.1016/S0006-3495(93)81075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng X., Yang Z., Yan M. Fluorescence ratiometry and fluorescence lifetime imaging: using a single molecular sensor for dual mode imaging of cellular viscosity. J. Am. Chem. Soc. 2011;133:6626–6635. doi: 10.1021/ja1104014. [DOI] [PubMed] [Google Scholar]
- 31.Chenault H.K., Whitesides G.M. Regeneration of nicotinamide cofactors for use in organic synthesis. Appl. Biochem. Biotechnol. 1987;14:147–197. doi: 10.1007/BF02798431. [DOI] [PubMed] [Google Scholar]
- 32.Wu J.T., Wu L.H., Knight J.A. Stability of NADPH: effect of various factors on the kinetics of degradation. Clin. Chem. 1986;32:314–319. [PubMed] [Google Scholar]
- 33.Hofmann D., Wirtz A., Pohl M. Structure elucidation of the thermal degradation products of the nucleotide cofactors NADH and NADPH by nano-ESI-FTICR-MS and HPLC-MS. Anal. Bioanal. Chem. 2010;398:2803–2811. doi: 10.1007/s00216-010-4111-z. [DOI] [PubMed] [Google Scholar]
- 34.Sokal R.R., Rohlf F.J. 3rd Ed. W.H. Freeman; New York: 1995. Biometry: the Principles and Practice of Statistics in Biological Research. [Google Scholar]
- 35.Förster T., Hoffmann G. Die viskositätsabhängigkeit der fluoreszenzquantenausbeuten einiger farbstoffsysteme. Z Phys Chem (Munich) 1971;75:63–76. [Google Scholar]
- 36.Bursy J., Kuhlmann A.U., Bremer E. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl. Environ. Microbiol. 2008;74:7286–7296. doi: 10.1128/AEM.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Estepa R., Argandoña M., Vargas C. The ectD gene, which is involved in the synthesis of the compatible solute hydroxyectoine, is essential for thermoprotection of the halophilic bacterium Chromohalobacter salexigens. J. Bacteriol. 2006;188:3774–3784. doi: 10.1128/JB.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]