Abstract
Background
Adenosine is a purine nucleoside implicated in the regulation of the innate and adaptive immune systems, acting through its interaction with four cell surface receptors: A1, A2A, A2B, and A3. There is intense interest in understanding how adenosine functions in health and during disease, but surprisingly little is known about the actual role of adenosine-mediated mechanisms in systemic lupus erythematosus (SLE). With this background, the aim of the present study was to test the hypothesis that dysregulation of A1, A2A, A2B, and A3 adenosine receptors (ARs) in lymphocytes of patients with SLE may be involved in the pathogenesis of the disease and to examine the correlations between the status of the ARs and the clinical parameters of SLE.
Methods
ARs were analyzed by performing saturation-binding assays, as well as messenger RNA and Western blot analysis, with lymphocytes of patients with SLE in comparison with healthy subjects. We tested the effect of A2AAR agonists in the nuclear factor kB (NF-kB) pathway and on the release of interferon (IFN)-α; tumor necrosis factor (TNF)-α; and interleukin (IL)-2, IL-6, IL-1β, and IL-10.
Results
In lymphocytes obtained from 80 patients with SLE, A2AARs were upregulated compared with those of 80 age-matched healthy control subjects, while A1, A2B, and A3 ARs were unchanged. A2AAR density was inversely correlated with Systemic Lupus Erythematosus Disease Activity Index 2000 score disease activity through time evaluated according to disease course patterns, serositis, hypocomplementemia, and anti-double-stranded DNA positivity. A2AAR activation inhibited the NF-kB activation pathway and diminished inflammatory cytokines (IFN-α, TNF-α, IL-2, IL-6, IL-1β), but it potentiated the release of anti-inflammatory IL-10.
Conclusions
These data suggest the involvement of A2AARs in the complex pathogenetic network of SLE, acting as a modulator of the inflammatory process. It could represent a compensatory pathway to better counteract disease activity. A2AAR activation significantly reduced the release of proinflammatory cytokines while enhancing those with anti-inflammatory activity, suggesting a potential translational use of A2AAR agonists in SLE pharmacological treatment.
Keywords: Systemic lupus erythematosus, A2A adenosine receptor, Disease activity
Background
Systemic lupus erythematosus (SLE) is the prototypic multisystem autoimmune disorder with a broad spectrum of clinical presentations encompassing almost all organs and tissues [1]. The irreversible break in immunological tolerance is manifested by immune responses against endogenous nuclear antigens and the subsequent formation of autoantibodies and immune complexes (ICs). SLE has classically been considered an autoimmune disease with a predominant adaptive immune system component because T and B cells have been considered the most important pathogenetic players [2].
During the early inflammatory phase, plasmacytoid dendritic cells (DCs) are able to internalize nucleic acids containing interferogenic ICs that reach the endosomes and stimulate Toll-like receptor 7 or 9, leading to interferon (IFN)-α gene transcription [3–5]. IFN-α contributes to the maturation of myeloid DCs that can activate autoreactive T cells through antigen presentation and costimulation. This favors the development of T helper 1 cells responsible for the high-level production of proinflammatory cytokines [6–8] and enhances B-cell maturation and differentiation, antibody production, and IC formation. In SLE, the IC- and IFN-α-secreting monocytes modulate interleukin (IL)-10 function [8]. The capability of IL-10 to suppress production of inflammatory cytokines such as tumor necrosis factor (TNF)-α and IL-6, implicated in promoting autoimmunity and tissue inflammation in SLE, is attenuated [8].
Growing evidence emphasizes that the purine nucleoside adenosine plays an active role as a local regulator of inflammation in different pathologies. Adenosine is a ubiquitous nucleoside involved in various physiological and pathological functions by stimulating the G protein-coupled A1, A2A, A2B, and A3 adenosine receptors (ARs) [9–12]. The role of ARs is well known in physiological conditions and in a variety of pathologies, including inflammatory damage, neurodegenerative disorders, and cancer [13–15]. In particular, A2AAR stimulation mediates inhibition of TNF-α, IL-1β, IL-2, IL-6, and IFN-α [16–18] and increases the production of the anti-inflammatory cytokine IL-10 [19]. With this background, the aim of the present study was to explore the arrangement and functionality of ARs in SLE and to evaluate their relationship with clinical phenotype and disease activity.
Methods
Patients and study design
Patients with SLE regularly attending our lupus clinic and satisfying the 1997 revised American College of Rheumatology criteria [20] were consecutively recruited from the Rheumatology Unit, Sant’Anna Hospital, University of Ferrara, Ferrara, Italy. We recorded clinical, demographic, and serological data, as well as data regarding therapy, including corticosteroids (measured as prednisone equivalent), antimalarials, and immunosuppressants.
Disease activity routinely assessed using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2 K) [21] and cumulative damage assessed with the Systemic Lupus International Collaborating Clinics Index were extracted by retrieving information from clinical records and a dedicated database. Moreover, disease activity and progress through time were considered according to four different patterns defined using the SLEDAI-2 K, excluding serological descriptors (hypocomplementemia and anti-double-stranded DNA [anti-dsDNA] antibodies) to focus on clinical activity: chronic active disease (CAD), relapsing-remitting disease, clinical quiescent disease (CQD), and minimal disease activity [22].
Seroimmunologic tests included complement components C3 and C4 dosages, antinuclear antibody (ANA), anti-dsDNA, anti-Sjögren’s-syndrome-related antigen A (anti-SSA), anti-Sjögren’s-syndrome-related antigen B (anti-SSB), anti-Smith (anti-Sm), antiribonucleoprotein (anti-RNP), anticardiolipin (aCL), anti-β2-glycoprotein I (anti-β2-GPI), and lupus anticoagulant (LA). C3 and C4 (in grams per liter) were measured by nephelometry, and hypocomplementemia was defined by local laboratory reference values (e.g., C3 < 0.8 g/L and C4 < 0.11 g/L detected on at least two separate occasions). ANA were detected by indirect immunofluorescence using HEp-2 cells as a substrate; positivity was defined as a titer ≥1:160. Anti-dsDNA were detected by indirect immunofluorescence using Crithidia luciliae with a cutoff titer of 1:40; positivity was certified if confirmed in two separate measurements. Anti-SSA, anti-SSB, anti-Sm, and anti-RNP were detected by using an immunoblotting technique. aCL and anti-β2-GPI were measured by enzyme-linked immunosorbent assay (ELISA) [23]. LA was measured in accordance with the recommendation of the Scientific and Standardization Committee of the International Society of Thrombosis and Hemostasis. Positivity for antiphospholipid antibodies (aPL) and LA was defined if confirmed in two separate measurements performed 12 weeks apart [24]. Healthy volunteers (n = 80) matched for age and sex ratio from the Ferrara University Hospital Blood Bank served as a control group.
Sample collection and human lymphocyte preparation
Lymphocytes were isolated and prepared as previously described from the peripheral blood of control subjects and patients with SLE [25–27]. Leukocytes were separated from erythrocytes with a solution of 6 % dextran T500 (Sigma-Aldrich, St. Louis, MO, USA), suspended in Krebs-Ringer phosphate buffer, and layered onto 10 ml of Ficoll-Hypaque density gradient (GE Healthcare Life Sciences, Little Chalfont, UK).
After centrifugation, mononuclear cells were washed in 0.02 M phosphate-buffered saline (PBS) at pH 7.2 and containing 5 mM MgCl2 and 0.15 mM CaCl2. They were then decanted into a culture flask and placed in a humidified incubator (5 % CO2) for 2 h at 37 °C. This procedure, aimed at removing monocytes that adhered to the culture flasks, resulted in a purified lymphocyte preparation containing at least 99 % small lymphocytes identified by morphological criteria.
To obtain membrane suspensions, cell fractions were centrifuged in a hypotonic buffer at 20,000 × g for 10 minutes. The resulting pellet was incubated in Tris-HCl 50 mM buffer, pH 7.4, with 2 IU/ml adenosine deaminase (Sigma-Aldrich) for 30 minutes at 37 °C. After centrifugation at 40,000 × g for 10 minutes, the final pellet was used for radioligand binding assays. The protein concentration was determined by using a Bio-Rad Laboratories (Hercules, CA, USA) method with bovine serum albumin as the reference standard [25].
Real-time reverse transcriptase-polymerase chain reaction experiments
Total cytoplasmic RNA was obtained from human lymphocytes by using the acid guanidinium thiocyanate phenol method. Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay [25–28] of A1, A2A, A2B, and A3 ARs messenger RNAs (mRNAs) was performed using gene-specific fluorescently labeled TaqMan MGB Probe (minor groove binder) in an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Real-time RT-PCR for A1, A2A, A2B, and A3 ARs was carried out with the Assays-on-Demand TM Gene expression Products NM_000674, NM_000675, NM_000676, and NM_000677 (Applied Biosystems), respectively. For the real-time RT-PCR of the reference gene, the endogenous control human β-actin was used, and the probe was fluorescently labeled with VIC™ dye (Applied Biosystems).
Western blot analysis
Human lymphocytes were washed with ice-cold PBS and lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich) containing protease inhibitors and 1 mM sodium orthovanadate. Proteins were eluted in Laemmli buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene fluoride membranes. Next, the membranes were incubated with specific antibodies for ARs (Alpha Diagnostic International, San Antonio, TX, USA), followed by washing and incubation with HRP-conjugated secondary antibodies. After a stripping step, the blots were reprobed with anti-β-actin antibody (clone EPR1123Y; EMD Millipore, Billerica, MA, USA).
Saturation binding to ARs
Because AR mRNA and protein expression experiments in patients with SLE have shown an upregulation of A2AARs compared with control subjects, we carried out experiments to examine saturation binding to this receptor subtype. For these assays, different concentrations of 3H-ZM 241385 (0.01–30 nM) as a radioligand, and cell membranes (60 μg of protein per assay) were incubated for 60 minutes at 4 °C [27]. The radioligand 3H-4-(2-(7-amino-2-(2-furyl)-[1, 2, 4]triazolo[2,3-a] [1, 3, 5] triazin-5-ylamino)ethyl)phenol (specific activity 27 Ci/mmol) was purchased from BIOTREND Chemikalien (Cologne, Germany). Nonspecific binding was determined in the presence of 1 μM 3H-ZM 241385. Bound and free radioactivity were separated by filtering the assay mixture through Whatman GF/B glass fiber filters (GE Healthcare Life Sciences) by using a Brandel cell harvester (Brandel, Gaithersburg, MD, USA) [28]. The filter-bound radioactivity was counted in a 2810TR liquid scintillation counter (PerkinElmer, Waltham, MA, USA).
Pro- and anti-inflammatory cytokines release in cultured lymphocytes
Isolated lymphocytes from healthy subjects or patients with SLE were suspended at a density of 106 cells/ml in RPMI 1640 medium supplemented with 2 % fetal bovine serum (EuroClone, Pero, Italy) and seeded into 24-well plates. Lymphocytes were incubated for 24 h in the absence or in the presence of an A2AAR agonist, CGS-21680 (2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine; 100 nM and 1 μM). In some experiments, cells were treated with a selective A2AAR antagonist, SCH 442416 (2-(2-furanyl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo[4,3-e] [1, 2, 4] triazolo[1,5-c]pyrimidin-5-amine; 1 μM), 15 minutes before the agonist CGS-21680 to verify the specific involvement of these receptors in cytokine release. CGS-21680 was obtained from Sigma-Aldrich and SCH 442416 was purchased from Tocris Bioscience (Bristol, UK). At the end of incubation, the cell suspension was collected and centrifuged at 1000 × g for 10 minutes at 4 °C. IFN-α, TNF-α, IL-2, IL-6, IL-1β, and IL-10 levels were determined using a specific quantitative sandwich ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions [25].
Nuclear factor kB activation in human cultured lymphocytes
Nuclear extracts from human cultured lymphocytes of the examined patients were obtained by using a nuclear extract kit (Active Motif, Carlsbad, CA, USA) following the manufacturer’s instructions. Nuclear factor (NF)-kB subunit p65 activation was evaluated in lymphocyte nuclear extracts by using the TransAM NF-kB kit (Active Motif). The primary antibody against NF-kB recognized an epitope in the subunits that is accessible only when it is activated and bound to its DNA target. The reaction was developed with streptavidin-HRP, and optical density was read by spectrophotometry at 450-nm wavelength [27].
Data and statistical analysis
Using dissociation equilibrium constants for saturation binding, affinity or Kd values, and the maximum densities of specific binding sites, Bmax values were calculated for a system of one- or two-binding-site populations by nonlinear curve fitting [28]. All experimental data are reported as mean ± SEM of independent experiments as indicated in the figure legends. Statistical analysis of the data was performed by using Student’s t test or one-way analysis of variance (ANOVA) followed by Dunnett’s test. The analysis was carried out using the GraphPad Prism 5.0 statistical software package (GraphPad Software, La Jolla, CA, USA), and differences were considered statistically significant with a p value less than 0.01.
Results
Clinical characteristics
A total of 80 patients with SLE (71 women and 9 men) with a mean ± SD age of 44 ± 11.9 years, disease duration of 139 ± 100 months, and SLEDAI-2 K score of 4 ± 4.3 were studied. In addition, 80 healthy subjects matched for age and sex ratio were enrolled. Demographic, clinical, and pharmacological treatments of the study subjects are reported in Table 1.
Table 1.
Clinical and demographic features of the study subjects, as well as pharmacological treatments in patients with systemic lupus erythematosus
| Patients with SLE (n = 80), n (%) | |
|---|---|
| Clinical parameters | |
| Female/male | 71/9 |
| Age, years, mean ± SD | 44 ± 11.9 |
| Disease duration, monthsa | 139 ± 100 |
| SLEDAI-2 K score, mean ± SD | 4 ± 4.3 |
| SDI, mean ± SD | 0.8 ± 1.2 |
| Disease activity patterns | |
| CQD | 46 (57.5 %) |
| CAD | 18 (22.5 %) |
| MDA | 14 (17.5 %) |
| RRD | 2 (2.5 %) |
| Serological parameters | |
| aPL (aCL, β2-GPI, and/or LA) | 39 (48.75 %) |
| ENA | 48 (60 %) |
| Hypocomplementemia | 53 (62.2 %) |
| Anti-dsDNA antibody, ongoing/previous | 41 (51.2 %)/17 (21.1 %) |
| Treatments | |
| Corticosteroids, 2.5 up to 12.5 mg/day | 67 (83.7 %) |
| Hydroxychloroquine, 200 mg/day | 48 (60 %) |
| Ongoing immunosuppressant therapy | 25 (31.2 %) |
| Mycophenolate mofetil | 11 (13.7 %) |
| Cyclosporine A | 4 (5 %) |
| Azathioprine | 5 (6.2 %) |
| Methotrexate, 10–15 mg/week | 3 (3.7 %) |
| Thalidomide | 1 (1.2 %) |
| IVIg | 1 (1.2 %) |
| PEX | 1 (1.2 %) |
| Anticoagulants | 11 (13.7) |
| Antiaggregant | 33 (41.25) |
Abbreviations: SLEDAI-2 K Systemic Lupus Erythematosus Disease Activity Index 2000, SDI Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index, CQD clinical quiescent disease, MDA minimal disease activity, CAD chronic active disease, RRD relapsing-remitting disease, aPL antiphospholipid antibodies, aCL anticardiolipin antibodies, LA lupus anticoagulant, β 2 -GPI β2-glycoprotein I antibodies, ENA extractable nuclear antigen antibodies, PEX plasma exchange, IVIg intravenous immunoglobulin, anti-dsDNA anti-double-stranded DNA
aDisease duration at the time of sample collection
A2AAR mRNA and protein expression are upregulated in lymphocytes of patients with SLE
AR mRNA and protein expression were evaluated in lymphocytes of patients with SLE in comparison with those of healthy subjects by means of quantitative RT-PCR assay and Western blot analysis, respectively. Figure 1a reports the relative A1, A2A, A2B, and A3 AR mRNA levels determined by RT-PCR in human lymphocytes of healthy subjects and patients with SLE. Among these receptors, only A2AAR mRNA expression was significantly increased in patients with SLE respect to control subjects. Western blot and densitometric analysis indicated a significant increase in A2AAR protein expression in lymphocytes of patients with SLE compared with those of healthy subjects, while no differences were found in A1, A2B, or A3 ARs (Fig. 1b, c).
Fig. 1.
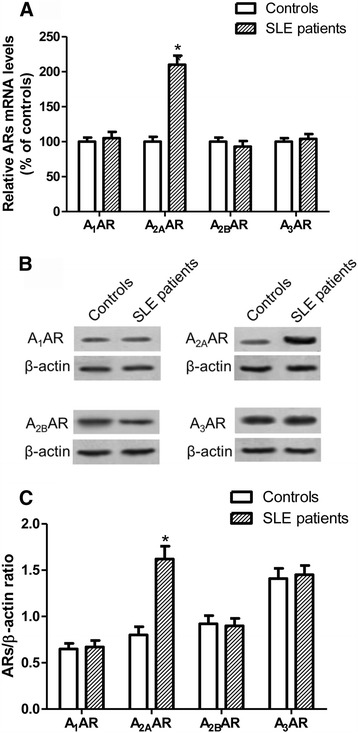
Messenger RNA (mRNA) and protein expression of adenosine receptors (ARs) in human lymphocytes from patients with systemic lupus erythematosus (SLE) and healthy subjects. a Relative AR mRNA levels were determined by real-time reverse transcriptase-polymerase chain reaction. Experiments were performed in duplicate with lymphocytes obtained from individual patients with SLE (n = 80) and healthy subjects (n = 80), and data are shown as mean ± SEM. b Western blot analysis showing immunoblot signals of ARs in one patient with SLE and one healthy subject, representative of blots obtained with lymphocytes from 80 patients with SLE and 80 healthy control subjects. β-actin was used as a loading control. c Densitometric analysis of AR expression in human lymphocytes from patients with SLE (n = 80) and healthy subjects (n = 80) indicated as a ratio of β-actin (loading control). Data are expressed as the mean ± SEM of densitometric analysis results obtained from the indicated number of subjects. * p < 0.01 versus control group by one-way analysis of variance with Dunnett’s test
Alteration of A2AAR affinity and density in lymphocytes of patients with SLE
Figure 2a and b show the saturation curves and Scatchard plots of [3H]-ZM 241385 in human lymphocytes, confirming the upregulation of A2AARs in patients with SLE compared with healthy subjects. The affinity of the radioligand [3H]-ZM 241385 for A2AAR (expressed as Kd, nanomoles) was decreased in lymphocytes from patients with SLE compared with that of the control group. Interestingly, the A2AAR density, expressed as a Bmax value, significantly increased in patients with SLE compared with healthy subjects, reaching a 2.3-fold increment (Fig. 2a, b).
Fig. 2.
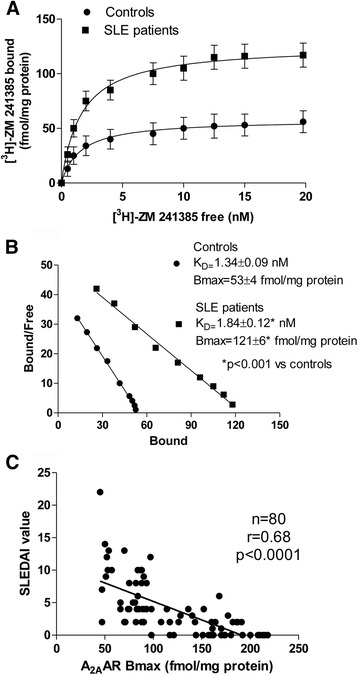
Adenosine 2A receptors (A2AARs) are upregulated in lymphocytes from patients with systemic lupus erythematosus (SLE). a Saturation curves and b Scatchard plot showing the binding of 3H-ZM 241385 to A2AARs in lymphocyte membranes derived from 80 healthy control subjects (solid circles) and 80 patients with SLE (solid squares) are also shown. Saturation binding experiments were performed as described in the Methods section. Data in the saturation curves are expressed as the mean ± SEM of results pooled from one experiment performed in duplicate for the indicated number of subjects. c Linear regression analysis between Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2 K) score and maximum number of A2AARs (Bmax) in lymphocytes (n = 80, r = 0.68, p < 0.0001 by using Pearson’s or Spearman’s correlation (Pearson’s r = −0.68, Spearman’s r = −0.75)
Clinical correlations
An inverse correlation was found between A2AAR density (expressed as Bmax values in femtomoles per milligram of protein), SLEDAI-2 K measured at the time of blood sampling (Fig. 2c), and disease activity through time evaluated according to the course patterns (CQD versus CAD; p < 0.0001). In addition, A2AAR density inversely correlated with serositis (p = 0.0043), hypocomplementemia (p = 0.0005), and anti-dsDNA positivity (Table 2). Regarding treatments, modulation of A2AAR density was found among corticosteroid users (p = 0.0078). With regard to A2AAR affinity, only one significant correlation was found with anti-dsDNA-positive patients (p = 0.008) (Table 2).
Table 2.
Clinical, serological, and pharmacological treatments in patients with systemic lupus erythematosus: correlation with adenosine A2A receptor affinity and density
| Patients (n) | K d (nM) | p Value | Bmax (fmol/mg of protein) | p Value | |
|---|---|---|---|---|---|
| Disease activity patterns | |||||
| CQD/CAD | 46/18 | 1.96 ± 1.19/1.77 ± 0.89 | NS | 141.6 ± 48.9/76 ± 28.6 | < 0.0001 |
| Organ involvement | |||||
| Renal, yes/no | 13/67 | 1.33 ± 0.59/1.94 ± 1.15 | 0.06 | 114 ± 45.05/120.8 ± 52.3 | NS |
| Neuropsychiatric, yes/no | 20/60 | 1.73 ± 0.81/1.88 ± 1.18 | NS | 103.85 ± 48.2/125 ± 51.2 | NS |
| Articular, yes/no | 46/34 | 1.79 ± 1.22/1.9 ± 0.9 | NS | 110.85 ± 49.3/131.8 ± 51.5 | 0.06 |
| Cutaneous, yes/no | 25/55 | 1.46 ± 0.71/1.89 ± 1.2 | NS | 105.60 ± 43.9/123.8 ± 53.7 | NS |
| Hematological, yes/no | 41/39 | 1.70 ± 0.89/1.98 ± 1.28 | NS | 116.85 ± 47.8/123.6 ± 54.5 | NS |
| Serositis, yes/no | 21/59 | 1.60 ± 1.3/1.9 ± 0.9 | NS | 92.86 ± 38.7/129.2 ± 51.6 | 0.0043 |
| Serological parameters | |||||
| aCL, yes/no | 22/58 | 1.91 ± 1.01/1.81 ± 1.14 | NS | 134.04 ± 52.55/114.27 ± 47.3 | NS |
| Anti-β2-GPI, yes/no | 9/71 | 1.59 ± 0.46/1.87 ± 1.15 | NS | 116.11 ± 53.15/120.2 ± 51.1 | NS |
| LA, yes/no | 28/52 | 1.92 ± 1.20/1.8 ± 1.05 | NS | 109.03 ± 51.60/125.5 ± 50.2 | NS |
| ENA, yes/no | 58/22 | 1.92 ± 1.15/1.77 ± 1.07 | NS | 114,5 ± 47.34/123.1 ± 53.52 | NS |
| Hypocomplementemia, yes/no | 53/27 | 1.57 ± 0.78/2.15 ± 1.32 | NS | 97.88 ± 39.25/145.08 ± 51.78 | < 0.0001 |
| Anti-dsDNA, yes/no | 41/39 | 1.65 ± 1.01/2.31 ± 1.18 | 0.008 | 106.26 ± 48.42/153.04 ± 41.76 | < 0.0001 |
| Treatments | |||||
| Corticosteroids, 2.5 up to 12.5 mg/day, yes/no | 67/13 | 1.83 ± 1.17/1.88 ± 0.63 | NS | 113.12 ± 50.30/153.69 ± 41.47 | 0.0078 |
| Hydroxychloroquine, 200 mg/day, yes/no | 48/32 | 1.93 ± 1.07/1.69 ± 1.13 | NS | 126.89 ± 51.09/108.93 ± 49.72 | NS |
| Immunosuppressants or induction therapy, yes/no | 25/55 | 1.98 ± 1.19/1.54 ± 0.8 | NS | 124.31 ± 48.04/110.15 ± 56.46 | NS |
| Anticoagulants, yes/no | 11/69 | 2.01 ± 1.47/1.81 ± 1.04 | NS | 128.91 ± 64.01/128.91 ± 64 | NS |
| Antiaggregants, yes/no | 33/47 | 2.03 ± 1.24/1.7 ± 0.97 | NS | 123.39 ± 50.61117.13 ± 51.66 | NS |
Abbreviations: aCL anticardiolipin antibodies, anti-β 2 -GPI anti-β2-glycoprotein I, CAD chronic active disease, CQD clinical quiescent disease, dsDNA double-stranded DNA, ENA extractable nuclear antigen antibodies, LA lupus anticoagulant, NS not significant
Analysis was carried out using unpaired t tests.
A2AAR activation reduces proinflammatory cytokine production from lymphocytes
To investigate the potential anti-inflammatory role of A2AAR stimulation in SLE, we evaluated the effect of CGS-21680 on the release of some of the most relevant proinflammatory cytokines involved in the pathogenesis of SLE, such as IFN-α, TNF-α, IL-6, IL-1β, and IL-2. In cultured lymphocytes of patients with SLE, a marked release of IFN-α was observed following the incubation of the cells with 0.1 mg/ml lipopolysaccharide (LPS) for 24 h (Fig. 3a). Interestingly, CGS-21680 at concentrations of 100 nM and 1 μM was able to inhibit the LPS-induced IFN-α release in lymphocytes of both patients with SLE and healthy subjects. However, the effect of CGS-21680 was significantly greater in lymphocytes obtained from patients with SLE than in those of healthy subjects (p < 0.0001), most likely due to the upregulation of A2AARs (see Table 3). The inhibitory effect of the A2AAR agonist was counteracted by the selective antagonist SCH 442416 (1 μM), demonstrating the specific A2AAR-mediated response (Fig. 3a). Similar results were obtained when we evaluated the capability of CGS-21680 to inhibit the release of TNF-α induced by LPS (Fig. 3b). Again, the inhibitory effect of the A2AAR agonist was more evident in lymphocytes of patients with SLE than in those of healthy subjects (p < 0.0001) (see Table 3). The anti-inflammatory effect of A2AAR activation induced by CGS-21680 was also confirmed when we analyzed the production of other proinflammatory ILs, such as IL-6 (Fig. 3c), IL-1β (Fig. 3d), and IL-2 (Fig. 4a). As reported in Table 3, a greater inhibitory effect was obtained in lymphocytes of patients with SLE. Moreover, the use of the selective A2AAR antagonist SCH 442416 (1 μM) demonstrated that the effect was mediated by A2AARs.
Fig. 3.
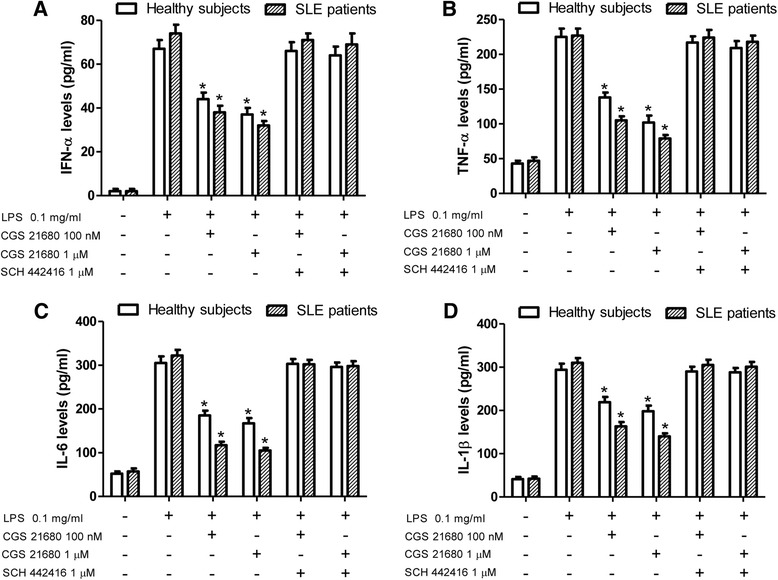
Adenosine 2A receptor (A2AAR) stimulation inhibits proinflammatory cytokine release. The effect of a well-known A2AAR agonist (CGS-21680; 100 nM and 1 μM) and antagonist (SCH 442416; 1 μM) on (a) interferon (IFN)-α, (b) tumor necrosis factor (TNF)-α, (c) interleukin (IL)-6, and (d) IL-1β release in cultured lymphocytes of patients with systemic lupus erythematosus (SLE) (n = 20) and healthy subjects (n = 20) that were stimulated by 0.1 mg/ml lipopolysaccharide (LPS) as determined by enzyme-linked immunosorbent assay. Data are expressed as the mean ± SEM of three independent experiments performed in triplicate * p < 0.01 versus LPS-treated cells by one-way analysis of variance with Dunnett’s test
Table 3.
Effect of CGS 21680 in lymphocytes from healthy subjects (n = 20) or SLE patients (n = 20) on different inflammatory mediators
| Healthy subjects | SLE patients | |||||
|---|---|---|---|---|---|---|
| Cellular stimulation | CGS 21680 (1μM) stimulation | % of reduction/fold of increase | Cellular stimulation | CGS 21680 (1μM) stimulation | % of reduction/fold of increase | |
| IFN-α | 67 ± 4a | 37 ± 3a | 44.39 ± 1.21d | 74 ± 4a | 32 ± 2a | 56.89 ± 1.52d,* |
| TNF-α | 225 ± 12a | 102 ± 10a | 54.66 ± 1.14d | 227 ± 10a | 79 ± 5a | 64.95 ± 2.33d,* |
| IL-6 | 305 ± 15a | 167 ± 12a | 45.38 ± 1.18d | 322 ± 13a | 105 ± 6a | 67.71 ± 1.25d,* |
| IL-1β | 294 ± 14a | 198 ± 13a | 32.64 ± 1.02d | 310 ± 11a | 140 ± 7a | 54.20 ± 2.35d,* |
| IL-2 | 36 ± 3b | 21 ± 2b | 41.59 ± 1.08d | 42 ± 3b | 19 ± 2b | 54.59 ± 1.76d,* |
| IL-10 | 2832 ± 112a | 4802 ± 189a | 1.70 ± 0.02e | 2973 ± 104a | 5329 ± 199a | 1.79 ± 0.02e,** |
| NF-kB | 245 ± 14c | 161 ± 13c | 34.07 ± 1.08d | 266 ± 10c | 150 ± 6c | 43.69 ± 1.47d,* |
The data are expressed as mean ± SEM; *p < 0.0001 vs healthy subjects; ** p < 0.01 vs healthy subjects
a Cellular stimulation with LPS; values in pg/ml
b Cellular stimulation with PMA + ionomycin; values in pg/ml
c Cellular stimulation with LPS; values in % of controls
d Percentage of reduction
e Fold of increase
Fig. 4.

CGS-21680 effect on interleukin (IL)-2 and IL-10 release and on nuclear factor (NF)-kB activation. Effect of CGS-21680 (100 nM and 1 μM) and SCH 442416 (1 μM) in cultured lymphocytes of patients with systemic lupus erythematosus (SLE) (n = 20) and healthy subjects (n = 20) stimulated by lipopolysaccharide (LPS) (0.1 mg/ml) or phorbol 12-myristate 13-acetate (PMA) (2 ng/ml) and ionomycin (0.2 μM) on (a) IL-2 release, (b) IL-10 release, and (c) NF-kB activation. Data are expressed as the mean ± SEM of three independent experiments performed in triplicate * p < 0.01 versus LPS-treated cells by one-way analysis of variance with Dunnett’s test
CGS-21680 increases production of anti-inflammatory cytokine IL-10 in lymphocytes
The incubation of lymphocytes with the A2AAR agonist CGS-21680 (1 μM) augmented basal IL-10 release in lymphocytes from healthy subjects and from patients with SLE (Fig. 4b). A more pronounced effect of CGS-21680 was obtained when cells were stimulated with LPS (0.1 mg/ml), although LPS alone did not alter IL-10 production. In the presence of LPS, the effect of CGS-21680 was significantly greater (p < 0.01) in lymphocytes of patients with SLE than in those of healthy subjects (see Table 3).
A2AAR activation inhibits LPS-induced NF-kB activation in lymphocytes
Many of the anti-inflammatory effects of A2AAR stimulation are mediated by the inhibition of NF-kB activation [25]. To verify if CGS-21680 was able to inhibit NF-kB in lymphocytes from patients with SLE in comparison with those of healthy subjects, the activation of NF-kB p65 subunits following LPS treatment was investigated. As shown in Fig. 4c, the A2AAR agonist determined a marked reduction of LPS-stimulated NF-kB p65 subunit activation in nuclear extract from lymphocytes obtained from patients with SLE and healthy subjects, with a significantly greater effect in the former (see Table 3). The inhibitory effect of CGS-21680 was completely counteracted by the selective A2AAR antagonist SCH 442416 (1 μM).
Discussion
The primary aim of this study was to investigate the role of ARs in SLE pathogenesis and to assess potential relationships between these receptors and clinical data. Within the complexity of the pathogenic mechanisms of lupus, innate immune responses play a significant role contributing either to tissue injury via release of inflammatory cytokines or to the aberrant hyperactivation of T and B cells, qualified as the most important players leading to autoreactive autoantibody production and resultant end-organ injury [3, 5, 29–31]. The role of the adenosinergic system is attractive for its multifunctionality in this wide spectrum of inflammation-related processes [10, 13, 14] and for its potential engagement in SLE.
AR mRNA and protein analysis supported higher A2AAR expression in lymphocytes from patients with SLE with than in those of control subjects, while no changes in A1, A2B, or A3 ARs were found, suggesting a specific A2AAR alteration. Moreover, saturation binding experiments confirmed the upregulation of A2AARs in lymphocytes of patients with SLE.
Notably, the highest levels of A2AAR density were tightly correlated with the lowest levels of clinical—namely, clinimetric—indexes and serological parameters (anti-DNA, C3 and C4) of disease activity, suggesting that the endogenous activation of these receptors could lead to mitigation of the disease. This aspect is further supported by the finding of an inverse correlation between CAD progression of the disease and receptor density, suggesting that the mutual modulation of A2AAR expression identifies well a persistent and stable regulation of the inflammatory status.
The hypothesis that A2AAR upregulation could represent a compensatory mechanism to better counteract the inflammatory background in SLE is supported by a preclinical study in an MRL/lpr mouse model of lupus nephritis [32] in which the A2AAR mRNA expression in the kidneys of MRL/lpr mice was significantly increased compared with that in control mice. In this study, the treatment with an A2AAR agonist ameliorated the severity of nephritis and renal vasculitis and reduced leukocytic infiltration.
Because IFN-α plays a central role in SLE pathogenesis [5], we investigated the anti-inflammatory effect of A2AAR activation on this cytokine in cultured lymphocytes. The effects of the IFN signature on lupus lymphocytes have been studied mainly in the regulatory T-cell subpopulation, where the action of IFN-α diminished their activity [33], while in B cells it stimulated antibody production [34]. We demonstrated that the A2AAR agonist CGS-21680 inhibited IFN-α release in cultured lymphocytes with a greater effect in patients with SLE than in healthy subjects. This observation further supports the competence of A2AAR signaling, as suggested by a previous study [35], to promote peripheral tolerance by generation of regulatory T cells. The reduction of inflammatory response by A2AAR activation was also confirmed when we studied the release of typical proinflammatory cytokines such as TNF-α, IL-6, IL-1β, and IL-2. Furthermore, we found that CGS-21680 mediated a significant increase of anti-inflammatory IL-10, which is an important immunoregulator that supports T-cell differentiation and suppresses proinflammatory cytokines [36].
It is well known that activation of NF-kB pathways leads to enhanced B-cell survival and T-cell activation and maturation [37]. Moreover, NF-kB positively regulates gene-encoding cytokines and other inflammatory factors, suggesting that this transcription factor could be one of the master regulators of inflammatory responses. Thus, the inhibition of NF-kB by CGS-21680 could explain the reduction of LPS-stimulated proinflammatory cytokines in cultured lymphocytes of patients with SLE.
Conclusions
Taken together, our data demonstrate the presence of A2AAR upregulation in patients with SLE and a significant inverse correlation of A2AAR density with SLEDAI-2 K score and CAD. The anti-inflammatory response of A2AARs opens up a new perspective on the translational role of the A2AAR agonists in the pharmacological treatment of SLE, highlighting their therapeutic potential in the management of this disorder.
Acknowledgements
The authors are grateful to the study participants.
Funding
Internal funding.
Availability of data and materials
For access to study data, please contact the corresponding author.
Authors’ contributions
AB designed and performed the study, analyzed and interpreted data, and drafted the manuscript. KV, MG, and PAB performed the study, interpreted data, and revised the manuscript. MP interpreted and discussed the data and critically revised the manuscript. FV and AR analyzed and interpreted data, and critically revised the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
The authors received informed consent from the participants for data publication in an anonymized form.
Ethics approval and consent to participate
The study was approved by the local ethics committee of the University Hospital of Ferrara (Italy), and written informed consent was obtained from each participant in accordance with the principles outlined in the Declaration of Helsinki.
Abbreviations
- aCL
Anticardiolipin antibodies
- ANA
Antinuclear antibody
- ANOVA
Analysis of variance
- anti-β2-GPI
Anti-β2-glycoprotein I
- anti-RNP
Antiribonucloprotein
- anti-SSA
Anti-Sjögren’s-syndrome-related antigen A
- anti-SSB
Anti-Sjögren’s-syndrome-related antigen B
- anti-Sm
anti-Smith
- aPL
Antiphospholipid antibodies
- AR
Adenosine receptor
- C3 and C4
Complement components C3 and C4
- CAD
Chronic active disease
- CQD
Clinical quiescent disease
- DC
Dendritic cell
- dsDNA
Double-stranded DNA
- ELISA
Enzyme-linked immunosorbent assay
- ENA
Extractable nuclear antigen antibodies
- IC
Immune complex
- IFN-α
Interferon-α
- IL
Interleukin
- IVIg
Intravenous immunoglobulin
- LA
Lupus anticoagulant
- LPS
Lipopolysaccharide
- MDA
Minimal disease activity
- mRNA
Messenger RNA
- NF-kB
Nuclear factor kB
- NS
not significant
- PBS
Phosphate-buffered saline
- PEX
Plasma exchange
- PMA
phorbol 12-myristate 13-acetate
- RRD
Relapsing-remitting disease
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- SDI
Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index
- SLE
Systemic lupus erythematosus
- SLEDAI-2 K
Systemic Lupus Erythematosus Disease Activity Index 2000
- TNF-α
tumor necrosis factor-α
Contributor Information
Alessandra Bortoluzzi, Phone: +390532239651, Email: brtlsn1@unife.it.
Fabrizio Vincenzi, Email: vncfrz@unife.it.
Marcello Govoni, Email: gvl@unife.it.
Melissa Padovan, Email: pdvmss@unife.it.
Annalisa Ravani, Email: rvnnls@unife.it.
Pier Andrea Borea, Email: andrea.borea@unife.it.
Katia Varani, Email: vrk@unife.it.
References
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2010;10:3–7. doi: 10.1016/j.autrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Celhar T, Magalhães R, Fairhurst AM. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res. 2012;53:58–77. doi: 10.1007/s12026-012-8270-1. [DOI] [PubMed] [Google Scholar]
- 4.Batteux F, Palmer P, Daëron M, Weill B, Lebon P. FCγRII (CD32)-dependent induction of interferon-α by serum from patients with lupus erythematosus. Eur Cytokine Netw. 1999;10:509–14. [PubMed] [Google Scholar]
- 5.Obermoser G, Pascual V. The interferon-α signature of systemic lupus erythematosus. Lupus. 2010;19:1012–9. doi: 10.1177/0961203310371161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwata Y, Furuichi K, Kaneko S, Wada T. The role of cytokine in the lupus nephritis. J Biomed Biotechnol. 2011;2011:594809. doi: 10.1155/2011/594809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 8.Yuan W, DiMartino SJ, Redecha PB, Ivashkiv LB, Salmon JE. Systemic lupus erythematosus monocytes are less responsive to interleukin-10 in the presence of immune complexes. Arthritis Rheum. 2011;63:212–8. doi: 10.1002/art.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. Introductory overview of purinergic signalling. Front Biosci Elite Ed. 2011;3:896–900. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- 10.Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol. 2007;83:263–76. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–83. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gessi S, Merighi S, Fazzi D, Stefanelli A, Varani K, Borea PA. Adenosine receptor targeting in health and disease. Expert Opin Investig Drugs. 2011;20:1591–609. doi: 10.1517/13543784.2011.627853. [DOI] [PubMed] [Google Scholar]
- 14.Antonioli L, Csóka B, Fornai M, Colucci R, Kókai E, Blandizzi C, et al. Adenosine and inflammation: what’s new on the horizon? Drug Discov Today. 2014;19:1051–68. doi: 10.1016/j.drudis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Di Virgilio F, Vuerich M. Purinergic signaling in the immune system. Auton Neurosci Basic Clin. 2015;191:117–23. doi: 10.1016/j.autneu.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Haskó G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, et al. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–74. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 17.Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, et al. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood. 2004;103:1391–7. doi: 10.1182/blood-2003-06-1959. [DOI] [PubMed] [Google Scholar]
- 18.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J Immunol. 2005;174:1073–80. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 19.Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM. An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC Infect Dis. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Gladman DD, Ibañez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 22.Zen M, Bassi N, Nalotto L, Canova M, Bettio S, Gatto M, et al. Disease activity patterns in a monocentric cohort of SLE patients: a seven-year follow-up study. Clin Exp Rheumatol. 2012;30:856–63. [PubMed] [Google Scholar]
- 23.Harris EN, Gharavi AE, Patel SP, Hughes GR. Evaluation of the anti-cardiolipin antibody test: report of an international workshop held 4 April 1986. Clin Exp Immunol. 1987;68:215–22. [PMC free article] [PubMed] [Google Scholar]
- 24.Horbach DA, van Oort E, Donders RC, Derksen RH, de Groot PG. Lupus anticoagulant is the strongest risk factor for both venous and arterial thrombosis in patients with systemic lupus erythematosus: comparison between different assays for the detection of antiphospholipid antibodies. Thromb Haemost. 1996;76:916–24. [PubMed] [Google Scholar]
- 25.Varani K, Padovan M, Vincenzi F, Targa M, Trotta F, Govoni M, et al. A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res Ther. 2011;13:R197. doi: 10.1186/ar3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varani K, Caramori G, Vincenzi F, Adcock I, Casolari P, Leung E, et al. Alteration of adenosine receptors in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:398–406. doi: 10.1164/rccm.200506-869OC. [DOI] [PubMed] [Google Scholar]
- 27.Varani K, Massara A, Vincenzi F, Tosi A, Padovan M, Trotta F, et al. Normalization of A2A and A3 adenosine receptor up-regulation in rheumatoid arthritis patients by treatment with anti-tumor necrosis factor α but not methotrexate. Arthritis Rheum. 2009;60:2880–91. doi: 10.1002/art.24794. [DOI] [PubMed] [Google Scholar]
- 28.Varani K, Vincenzi F, Tosi A, Gessi S, Casetta I, Granieri G, et al. A2A adenosine receptor overexpression and functionality, as well as TNF-α levels, correlate with motor symptoms in Parkinson’s disease. FASEB J. 2010;24:587–98. doi: 10.1096/fj.09-141044. [DOI] [PubMed] [Google Scholar]
- 29.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–56. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 30.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–53. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 31.Robson MG, Walport MJ. Pathogenesis of systemic lupus erythematosus (SLE) Clin Exp Allergy. 2001;31:678–85. doi: 10.1046/j.1365-2222.2001.01147.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Yang N, Wang S, Huang B, Li F, Tan H, et al. Adenosine 2A receptor is protective against renal injury in MRL/lpr mice. Lupus. 2011;20:667–77. doi: 10.1177/0961203310393262. [DOI] [PubMed] [Google Scholar]
- 33.Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+, CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-α-producing antigen-presenting cells. Arthritis Rheum. 2008;58:801–12. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- 34.Oka H, Hirohata S. Regulation of human B cell responsiveness by interferon-α: interferon-α-mediated suppression of B cell function is reversed through direct interactions between monocytes and B cells. Cell Immunol. 1993;146:238–48. doi: 10.1006/cimm.1993.1023. [DOI] [PubMed] [Google Scholar]
- 35.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–9. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz DA, Gray JD, Behrendsen SC, Kubin M, Rengaraju M, Ohtsuka K, et al. Decreased production of interleukin-12 and other Th1-type cytokines in patients with recent-onset systemic lupus erythematosus. Arthritis Rheum. 1998;41:838–44. doi: 10.1002/1529-0131(199805)41:5<838::AID-ART10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Chuang HC, Lan JL, Chen DY, Yang CY, Chen YM, Li JP, et al. The kinase GLK controls autoimmunity and NF-kB signaling by activating the kinase PKC-θ in T cells. Nat Immunol. 2011;12:1113–8. doi: 10.1038/ni.2121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For access to study data, please contact the corresponding author.


