Abstract
Background
Trematode infections of livestock are of global veterinary and public health importance causing serious economic losses. Majority of data on burden of trematode infections in Nigeria are based on abattoir surveys and there are very few data on herd level risk factors. The present study investigated the prevalence of, and herd level risk factors for, fasciolosis and other trematode infections in cattle in Edu Local Government Area (LGA).
Methods
A cross-sectional survey used two-stage study design to investigate cattle belonging to 65 households. Two questionnaires were administered for household-level and individual cattle-level data. Faecal and blood samples were obtained from the cattle. Logistic regression analyses were performed to determine risk factors for infections.
Results
Of 686 faecal samples analysed, 74.9 %, 16.1 %, 7.3 % and 1.2 % were positive for infections with Fasciola gigantica, paramphistomes, Dicrocoelium hospes and Schistosoma bovis respectively. Fasciola gigantica had higher prevalence in adult cattle (77.3 %) than weaners (62.5 %). Majority of co-infections was a combination of F. gigantica with paramphistomes 84/130 (64.6 %). Most (58.9 %) of the cattle belonged to FAMACHA© score 2. The mean packed cell volume (PCV) was 34.4 %. The sensitivity and specificity of FAMACHA© for anaemia (PCV < 24 %) were 18.2 and 96.9 %, respectively. Positive correlation was obtained between faecal egg counts for F. gigantica and paramphistomes (R = 0.15, P = 0.0001). Adult cattle were more likely to be infected with F. gigantica (odds ratio, OR: 1.94; Confidence Interval, CI: 1.19–3.16) than weaners. Cattle belonging to household heads aged between 40–59 years were more likely infected with paramphistomes (OR: 1.95; CI: 1.02–3.74) than those belonging to other age groups. Cattles from herds with size ≥ 100 were more likely infected with D. hospes than those from smaller herds (OR: 6.98; CI: 2.94–16.6).
Conclusion
This study revealed high prevalence of infection with F. gigantica in Kwara State. The co-infections by F. gigantica and paramphistomes with a positive correlation should be considered during anthelmintic therapy. There is a need to optimise and validate the FAMACHA© for use in cattle based on breeds and variation in colour of ocular mucous membrane. Risk factors identified could assist in tailoring control strategies for various trematode infections to particular groups of farmers and cattle.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1737-5) contains supplementary material, which is available to authorized users.
Keywords: Trematodes, Fasciola, Paramphistomes, Dicrocoelium, Schistosoma, Nigeria, Kwara, Prevalence, Risk factors, FAMACHA©
Background
Trematode infections cause serious economic losses to livestock globally. Many are zoonotic and thus a public health concern [1, 2]. Some of the trematode infections of cattle include species of Fasciola, Dicrocoelium, Schistosoma and paramphistomes. Fasciolosis due to F. gigantica has been reported in several parts of Africa [3–6] and Nigeria [7–10]. Other trematode infections in ruminants reported in Nigeria include species of Dicrocoelium [11], Schistosoma [12] and paramphistomes [7]. Trematode infections are known to cause clinical signs ranging from weight loss, sudden death [13] and anaemia in cattle [14, 15]. Tropical fasciolosis alone has been predicted to cause losses of about US$840 M per annum in the Africa’s 200 million cattle population [16] and this cost is likely to have increased significantly in the last sixteen years. Economic losses from fasciolosis may result directly from increased liver condemnation or indirectly from decreased livestock productivity [17]. Also about 165 million cattle are likely to be infected with Schistosoma spp. worldwide [18]. The cattle population in Nigeria is about 16 million [19] made up of predominantly humped zebu breeds (including the White Fulani, Sokoto Gudali and Red Bororo) and a limited number of hump less breeds including Keteku, Muturu and Kuri in the southwestern, southern and the northeastern parts, respectively [20]. They play a very important role in the Nigerian economy, contributing about 12.7 % of total agriculture gross domestic product (GDP) [21]. In the tropics, cattle are generally reared under the transhumance husbandry system with little supplementary feeding resulting in low productivity and high pre-weaning mortality [8]. Similarly, acute shortage of feeds during the dry season remains a common occurrence, compelling these animals to graze around water bodies that often contain large number of potential intermediate hosts of trematodes [8].
The majority of data on the burden of fasciolosis in Nigeria are based on abattoir surveys. However, there are very few data on the trematode prevalence in live cattle or on the herd level risk factors that may influence disease occurrence in Nigeria. Moreover, there are few recent data on infection of cattle with the other trematode species in Nigeria, and more recent information would be useful in formulating effective control strategies for this important group of parasites [7, 8, 22]. The present study investigated the prevalence of, and herd level risk factors for, fasciolosis and other trematode infections in cattle in the Edu Local Government Area (LGA).
Methods
Study location
A cross-sectional study was conducted from May to August 2013 to determine the prevalence of trematode infections and herd level risk factors in cattle from 11 villages of Edu LGA, Kwara State, North-central Nigeria. Kwara State lies between 8°05′ and 10°15′N; and 2°73′ and 6°13′E (Fig. 1). It has a total area of about 34,500 square kilometres comprising rainforest in the south and wooded savannah in the larger part of the state. It has 16 local government areas. Rainfall has an annual range of 1,000–1,500 mm and average maximum temperature between 30 and 35 °C [23]. Edu LGA was selected as the study location because it has very large pastoralist settlements and is one of the largest area for cattle production in Kwara State. Rice, sugarcane and melon are the major crops planted. Because Edu LGA is bounded by the River Niger in the north, the area is often inundated with flood leading to devastating losses of livestock and farmland. The pastoralists therefore migrate uphill away from flood plains (starting in July) to neighbouring states once the rains begin. They do not return until the end of the year when the rains cease. A local informant identified 11 cattle producing villages in Edu LGA and these formed the sample population of this study.
Fig. 1.
Map of Kwara State showing the location of Edu Local Government Area (study location). The inset map shows Kwara State within Nigeria
Study design and sampling
A two-stage sampling design was carried out. The first stage determined the number of households to be selected while the second stage determined the number of cattle to be sampled in each household. The number of households to be visited was calculated based on the formula: 1.962 * Pexp (1-Pexp)/ d2 [24] with an expected prevalence (Pexp) of 50 % (no previous data on herd level prevalence in the area), 10 % desired precision and 95 % confidence interval (1.96). The second sampling stage to determine the number of cattle to be sampled per household and was applied to the two-stage sampling protocol of Cameron & Baldock [25] developed to determine presence of disease. The protocol was implemented by using the FreeCalc software version 2 [(c) Copyright 2001-Angus Cameron AusVet Animal Health Services]. The sensitivity (92.7 %) and specificity (94.9 %) of the Flukefinder ® [26], 40 % minimum expected prevalence and cattle population interval of 1–250 animals were included as parameters. A maximum of 13 animals are required to be sampled in each household. Only cattle ≥ 12 month-old were sampled to ensure they had experienced at least one complete grazing season.
A total of 686 cattle in 65 households were sampled for trematode infections. While the sample size calculations indicated that 96 households were required, there were only 65 accessible households present with cattle during the survey (others had either migrated uphill away from floodplains or were inaccessible due to the flood). All study locations were georeferenced using Garmin® global positioning system (GPS). Both faecal and blood samples were collected from the animals.
Data collection
Two questionnaires were administered. The first focused on household level data such as farmer socio-demographic characteristics, management system, health practices, herd size, economic activities as well as knowledge and practice to control liver fluke disease. The second questionnaire was for individual cattle data and the information recorded included age, sex and breed. Both questionnaires were included in the analysis of herd level risk factors for trematode infections.
Coprological analysis
Faecal samples were obtained directly from the rectum of each cow into sterile plastic gloves or from the ground if seen being produced. The glove were turned inside out, carefully tied, labelled and transported under cool conditions to the laboratory for analysis. The commercially available kit FlukeFinder® Richard Dixon ID, USA (http://www.flukefinder.com/) was used to isolate the trematode eggs by differential sieving and sedimentation according to manufacturer’s instructions.
The sensitivity and specificity of the Flukefinder® has been previously compared to other sedimentation methods [26]; this device has also been used previously to isolate trematode eggs [6, 27]. Two grams of individual cattle faecal sample were used and analysed according to the manufacturers’ instructions. The Flukefinder® is made up of two 2-in. wide sieves; these were washed thoroughly in between samples to prevent cross-contamination. The material was poured into a 50 mm Petri dish, three drops of methylene blue was added for contrast and then examined under a stereomicroscope at a magnification of 40×. Eggs were identified using standard keys [28]. Although the eggs of F. gigantica and paramphistomes are similar (both oval and operculated), the eggs of F. gigantica possess a distinct yellowish-brown colour and measure 156–197 μm in length and 90–104 μm in width compared with those of paramphistomes, which are clear in colour and measure 114–176 by 73–100 μm. Eggs of D. hospes are small (36–45 × 22–30 μm), oval, dark brown and operculate, with two characteristic dark “eye-spots” [28] and eggs of S. bovis are spindle-shaped with characteristic terminal spines on both sides of the non-operculate eggs [28].
Haematological analysis
Blood samples for haematological analysis were collected from the jugular vein of the first three to four cattle sampled using EDTA anticoagulant (due to laboratory costs, blood samples were not obtained from all animals sampled in each household). The level of anaemia was also checked using the FAMACHA© anaemia chart to score the ocular mucous membrane [29]. The FAMCHA© chart is a low cost tool in determining anaemia status of ruminants [30]. The colour of ocular mucous membrane for each animal classified into five categories based on the FAMACHA© chart (ranging from bright red to pale) and recorded for individual animals. The packed cell volume (PCV) was also determined using the microhaematocrit method [31].
Statistical analysis
Descriptive analyses were carried out on cattle data. Cattle that had at least one trematode egg count was considered positive. The overall prevalence (in %) at the animal level was the total number of cattle positive divided by the total number of cattle sampled in the study. Chi-square or Fisher’s exact tests were used to explore the relationships between the trematode prevalence, household and cattle data. Descriptive statistics for trematode co-infections are also presented.
The sensitivity and specificity of the FAMACHA© score compared to PCV were determined. Animals with FS scores of 1–3 were classified as non-anaemic while those with FS scores of 4–5 were grouped as anaemic. Reference value of 24–46 % for PCV in cattle was used [32]. The PCV values of ≤24 % were classified as anaemic and those above 25 % were considered non anaemic.
Pearson’s correlation coefficient was used to explore the relationships between trematode infections. In order to carry out correlation analyses, trematode egg counts were log-transformed [log(egg count +1)] to stabilise variances. The relationship between log-transformed trematode faecal egg counts and PCV was also determined.
Trematode infections and herd level risk factors were investigated by using predictor variables such as the household level factors, individual cattle data and haematological indices. The outcome variable was cattle trematode infection status expressed as a binary variable (0 = negative; 1 = positive). Variables with values for P < 0.20 from the Chi-square or Fisher’s exact test obtained from the coprological analysis were included in the binary logistic regression analysis. The risk factors were explored by binary logistic regression using the forward stepwise variable selection method and the 95 % confidence interval of odds ratio was calculated for the predictors [33].
Statistical analyses were carried out using Microsoft Excel® and IBM SPSS® statistics version 21.0 software (IBM Corp. 2012, Armonk, NY, USA). Distribution of trematode infections were also represented using the QGIS® spatial software version 2.2.
Results
Household and cattle data
A total of 65 households was surveyed (Additional file 1: Table S1). The respondents were all male with a mean age of 42.75 (SD ± 11.24, range 23–70) years. They were mostly married (96.9 %) and are of Fulani (83 %) ethnic origin. The majority had no formal education (93.5 %). They kept mainly livestock (67.7 %) comprising primarily cattle (90.5 %). The mean cattle herd size was 62.31 ± 46.26; cattle were kept mainly by pastoral/nomadic grazing system (78.5 %).
A total of 686 cattle was investigated using questionnaire surveys, comprising 318 (46.8 %) males and 362 (53.2 %) females. The mean age of cattle was 3.57 (SD ± 1.38) years with minimum and maximum ages of one and ten years, respectively. The breed composition was predominantly humped zebu 655 (95.9 %) with a very small minority made up of Jersey 27 (4.0 %) and a single Friesian (0.1 %).
Coprological data
A total 686 faecal samples was analysed from cattle in 11 villages from which 536 (78.1 %) were positive for at least one of the parasites studied: 514 (74.9 %; 95 % CI: 72.0–79.0 %) for infections with F. gigantica, 110 (16.0 %; 95 % CI: 13.0–19.0 %) with paramphistomes, 50 (7.3 %; 95 % CI: 5.0–9.0 %) with Dicrocoelium hospes and 8 (1.2 %; 95 % CI: 0–2.0 %) with Schistosoma bovis. Of these 686 cattle sampled, 406 (75.7 %) had single species trematode infections of which 385/686 (56.1 %) were F. gigantica, 9/686 (1.3 %) paramphistome, 9/686 (1.3 %) D. hospes and 3/686 (0.4 %) S. bovis. One hundred and thirty cattle 130/386 (19.0 %) had trematode co-infections and 150 cattle were uninfected (Fig. 2). Of the 130 cattle with co-infections, 84/130 (64.6 %), 25/130 (19.2 %) and 4 (3.1 %) of the animals had co-infection of F. gigantica with paramphistomes, D. hospes and S. bovis, respectively. Fifteen cattle had co-infection with three species of trematode: 15/130 (11.5 %) with F. gigantica/ paramphistomes/ D. hospes and 1 (0.8 %) with F. gigantica/ paramphistomes/ S. bovis, but none were infected with all four species. Finally, one animal had a co-infection with paramphistomes and D. hospes 1 (0.8 %) but not with F. gigantica.
Fig. 2.
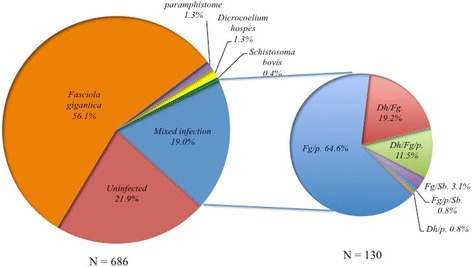
Proportions of single infections and co-infections by trematodes in cattle from the Edu Local Government Area of Kwara State, Nigeria
Infections with F. gigantica were found in cattle in all villages at prevalences ranging from 3.6 to 100 %, with seven out of the eleven villages having prevalence greater than 70 % (Table 1). The highest prevalence was recorded in Belle (100 %), Yelwa (91.3 %), Fedudangi (85.7 %) and Ndachewoye (85.2 %) whereas the lowest prevalence was recorded in Tshonga farm (3.6 %). Paramphistome infections were also found in all eleven villages, and D. hospes infections in all but one village (Mokwagi). The highest prevalences for paramphistomes and D. hospes were 58.3 % (Belle village) and 24.0 % (Ndabata), respectively. Three out of the eleven villages studied (Fanagun, Gonandogo and Ndachewoye) were positive for infection with S. bovis with prevalence rates of less than 2 %. Distribution maps of the prevalences of the various trematode species are shown in Fig. 3.
Table 1.
Households and cattle trematode infections from villages studied in Edu LGA, Kwara State, Nigeria
| Households | Cattle | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Village | Altitude (m) | F. gigantica | Paramphistomes | D. hospes | S. bovis | F. gigantica | Paramphistomes | D. hospes | S. bovis | ||||||
| N | n | N | N | N | N | n | (%) | n | (%) | n | (%) | n | (%) | ||
| Bacita | 106 | 2 | 2 | 2 | 2 | 0 | 22 | 7 | (31.8) | 6 | (27.3) | 3 | (13.6) | 0 | (0.0) |
| Belle | 76 | 5 | 5 | 5 | 1 | 0 | 48 | 48 | (100) | 28 | (58.3) | 6 | (12.5) | 0 | (0.0) |
| Bokungi | 221 | 3 | 3 | 3 | 2 | 0 | 23 | 12 | (52.2) | 2 | (8.7) | 2 | (8.7) | 0 | (0.0) |
| Fanagun | 82 | 14 | 14 | 7 | 5 | 3 | 158 | 122 | (77.2) | 12 | (7.6) | 9 | (5.7) | 3 | (1.9) |
| Fedudangi | 193 | 3 | 3 | 1 | 0 | 0 | 14 | 12 | (85.7) | 2 | (14.3) | 1 | (7.1) | 0 | (0.0) |
| Gonandogo | 84 | 10 | 10 | 4 | 4 | 2 | 136 | 100 | (73.5) | 2 | (1.5) | 3 | (2.2) | 2 | (1.5) |
| Mokwagi | 114 | 1 | 1 | 1 | 0 | 0 | 13 | 5 | (38.5) | 1 | (7.7) | 0 | (0.0) | 0 | (0.0) |
| Ndabata | 202 | 2 | 2 | 1 | 2 | 0 | 25 | 19 | (76.0) | 5 | (20.0) | 6 | (24.0) | 0 | (0.0) |
| Ndachewoye | 98 | 21 | 21 | 17 | 9 | 2 | 196 | 167 | (85.2) | 45 | (23.0) | 13 | (6.6) | 3 | (1.5) |
| Tshonga Farm | 175 | 2 | 1 | 1 | 2 | 0 | 28 | 1 | (3.6) | 1 | (3.6) | 3 | (10.7) | 0 | (0.0) |
| Yelwa | 77 | 2 | 2 | 2 | 2 | 0 | 23 | 21 | (91.3) | 6 | (26.1) | 4 | (17.4) | 0 | (0.0) |
| Total | 65 | 64 | 44 | 29 | 7 | 686 | 514 | (74.9) | 110 | (16.0) | 50 | (7.3) | 8 | (1.2) | |
Abbreviations: N number of samples, n number of infected, (%) prevalence
Fig. 3.
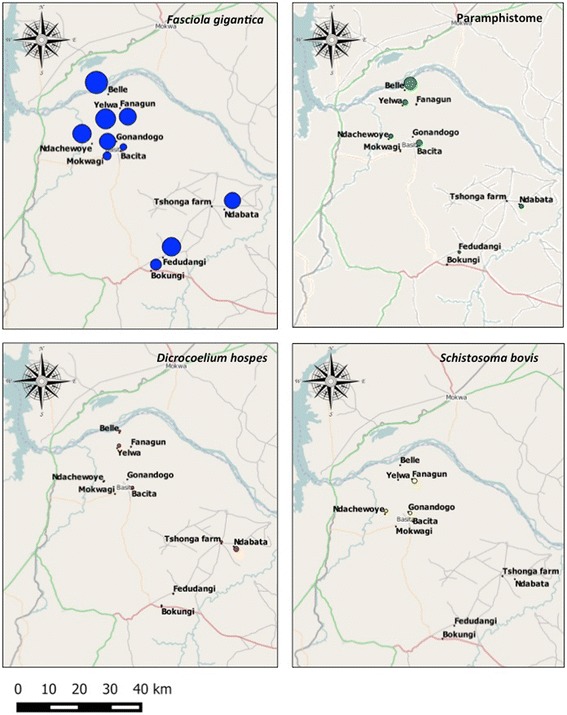
Distribution of trematode prevalence across villages sampled in Edu Local Government Area of Kwara State, North-central Nigeria. The size of the circles is proportional to prevalence: small circles (0 %); largest circle (100 %)
Faecal egg counts ranged from 0–73 (mean 5.92) eggs per gram (epg) for F. gigantica, 0–10 (mean 0.44) epg for paramphistomes, 0–7 (mean 0.15) epg for D. hospes and 0–4 epg for S. bovis. Fasciola gigantica had higher prevalence in adult cattle (77.3 %) than in those younger than two years (62.5 %) (χ2 = 0.002, P < 0.05). The differences in prevalence between age groups were statistically significant for F. gigantica (χ2 = 0.002, P < 0.05). The results of the univariate analyses are presented in Additional file 2: Table S2). The prevalence of S. bovis infection was too low to carry out further meaningful statistical analyses.
Correlations between trematode infections of cattle
A significant positive correlation was obtained between log-transformed data for faecal egg counts for F. gigantica and paramphistomes (R2 = 0.023, P = 0.0001) but there were no significant correlations between faecal egg counts for F. gigantica and D. hospes (R2 = 0.00003, P = 0.906) or between faecal egg counts for paramphistomes and D. hospes (R2 = 0.004, P = 0.088).
Haematological data
A total of 217 whole blood samples were analysed. The packed cell volume (PCV) varied within a range of 12–48 % with a mean value of 34.9 % (SD ± 7.25 %; normal range in cattle 24–46 %) [15]. Twenty-two (10.1 %) of these cattle had PCVs less than the lower normal limit of 24 %. The frequency distribution (Table 2) of FAMACHA® score revealed that the majority 58.9 % (128/217) of the cattle studied belonged to FS2 with the least being FS4. None of the cattle studied had a FS score 5.
Table 2.
Mean packed cell volume (PCV) of cattle in different categories of FAMACHA© score
| FAMACHA© score | Number of cattle | Mean PCV | Range PCV |
|---|---|---|---|
| N (% of total) | |||
| 1 | 56 (25.8) | 35.9 | 22–48 |
| 2 | 128 (58.9) | 34.9 | 20–48 |
| 3 | 23 (24.4) | 30.5 | 20–46 |
| 4 | 10 (4.6) | 27.7 | 12–41 |
Total number of cattle: 217
The sensitivity and specificity of the FAMACHA© ocular score in cattle were 18.2 % (4/22) and 96.9 % (189/195), respectively using PCV ≤ 24 % and FS of 4–5 as cut-off values for anaemia (Table 3).
Table 3.
Two-by-two contingency table of PCV and FAMACHA© score of cattle
| FAMACHA© Score | ||||
|---|---|---|---|---|
| FS 4–5 (Anaemic) | FS 1–3 (Non-anaemic) | Total | ||
| PCV | ≤ 24 % (Anaemic) | 4 | 18 | 22 |
| > 24 % (Non-anaemic) | 6 | 189 | 195 | |
| Total | 10 | 207 | 217 | |
Sensitivity: 18.2 %; Specificity: 96.9 %
Correlation between trematode infections and PCV in cattle
The correlation analysis between log-transformed trematode faecal egg counts and PCV revealed a weak negative but statistically non-significant correlation between PCV and F. gigantica (R = -0.050, R2 = 0.0023, P = 0.463) as well as between PCV and D. hospes (R = -0.070, R2 = 0.0049, P = 0.305).
Herd level risk factors of trematode infections
Cattle herd sizes greater than 100 were less likely to have F. gigantica infections than those of smaller herds (odds ratio OR: 0.28; 95 % confidence interval CI: 0.14–0.58). Adult cattle (≥ 2 years), were more likely to be infected (OR: 1.94; CI: 1.19–3.16) than younger cattle. Cattle belonging to heads of households aged between 40–59 years were more likely to be infected with paramphistomes (OR: 1.95; CI: 1.02–3.74) than respondents of other age groups (20–39 and > 60 years), while those belonging to the ‘Zabaruma’ ethnic group were less likely infected (OR: 0.05; CI: 0.01–0.22) than the Fulani or Zimbabwean farmers. Cattle herd size greater than 100 were more likely to be infected with D. hospes than those in smaller herds (OR: 6.98; CI: 2.94–16.6). Logistic regression could not be performed on S. bovis infection because there were too few infected animals (Table 4).
Table 4.
Binary logistic regression to investigate risk factors for cattle trematode infections in Kwara State, Nigeria
| Parasite | Risk factor | Odds ratio | 95 % CI | P |
|---|---|---|---|---|
| F. gigantica | Cattle herd size > 100 | 0.28 | 0.14–0.58 | 0.001 |
| Adult cattle (≥ 2 years) | 1.94 | 1.19–3.16 | 0.008 | |
| Paramphistomes | Ethnicity of head of respondents | 0.05 | 0.01–0.22 | 0.001 |
| Head of respondents (40–59 years) | 1.95 | 1.02–3.74 | 0.043 | |
| D. hospes | Cattle herd size > 100 | 6.98 | 2.94–16.6 | 0.001 |
Discussion
This is the first reported study to determine the burden of trematode infections in live cattle in Kwara state, North-central, Nigeria. Zebu cattle were the most predominant breeds because they are the predominant breed in Nigeria kept primarily for milk and beef [34]. The few exotic breeds sampled were from the government owned intensive farm in Tshonga district. There were more female (53.5 %) animals sampled than males (46.5 %) because more female animals, are usually kept by farmers for herd growth and milk production [35].
On the basis of coprological examination, trematode infections were common in cattle in Edu LGA, occurring in 78.1 % of animals investigated. The most frequent species of trematode identified was Fasciola gigantica in 74.9 % of cattle. This is considerably higher than the 22.5 % prevalence reported in an abattoir study in the state capital [36] but similar to a study carried out in Bauchi State of northern Nigeria reporting a prevalence of 76.9 % [37].
Egg counts for F. gigantica observed in our study (range 0–73 epg) were considerably lower than reported in previous studies in zebu cattle, for example, a range of 400–1,100 epg in faeces of cattle in Bangladesh [38], a mean of 81.2 egg per 2 g of faeces in Ethiopia [39] and range of 0–167 epg in Tanzania [6]. The values from the present study were however higher than those reported in Ghana [40] and in a previous abattoir study in Nigeria [41]. Further experimental studies are recommended to determine the egg output index of adult F. gigantica in Nigeria and Africa. Fasciolosis causes losses in livestock due to losses from mortality and reduced productivity and is one of the leading causes of liver condemnation in abattoir in Kwara state [36].
Fasciolosis is also an important zoonotic disease [42]. Ndachewoye houses a large abandoned dam that was used by the Bacita sugar factory. It serves as a watering point for both cattle and humans. It is an all year round dam that could favour the life cycle of F. gigantica and other trematode species. Transmission studies on Fasciola spp. revealed that some free-floating metacercariae might be suspended in water where they can be ingested by the definitive hosts [43]. Moreover, human fasciolosis has been reported to occur from eating uncooked watercress derived from endemic areas where infected cattle range freely and probably from contaminated water [44, 45].
The other trematodes prevalent in this study were D. hospes, S. bovis and paramphistomes. The prevalence of paramphistomes recorded here were lower than in previous reports from southern Ghana [40], Zambia [46], Tanzania [6] but higher than reported in Turkey [47]. Paramphistome species has been reported in Nigeria with up to 2,000 adult parasite in cattle [48].
Only a few studies are available on D. hospes infection in Nigeria the majority representing abattoir surveys [7, 8]. Although the clinical disease when present can lead to severe anaemia, oedema and emaciation, it is usually asymptomatic [49]. A prepatent period of up to 59 days post experimental infection with Dicrocoelium dendriticum has been reported in lambs, hence the low faecal egg count (range 0–7 epg) reported in this study may not fully reflect the level of infection [50]. The prevalence (7.3 %) reported in this study is considerably lower than the 38 % previously reported in cattle at slaughter in Zaria, Nigeria [8].
Infections with S. bovis was reported in only three of the villages studied (Gonandogo, Ndachewoye and Fanagun) with low prevalences (< 2 %) similar to those observed in a recent study in Tanzania [6]. However, another study in Zambia, reported up to 22 % prevalence for bovine schistosomiasis [51].
There was a significantly higher prevalence of F. gigantica infections in adult cattle (77.3 %) than weaners (62.5 %). This is in agreement with a study in Tanzania with similar findings [6] and may be due to longer exposure of adult animals to infection [52].
Although some past studies revealed higher trematode prevalence rates in female than in male animals [40, 53], no significant difference was found in this study. This finding agrees with a previous study in Uganda that reported no significant difference in F. gigantica prevalence between sexes [27].
Out of the 686 faecal samples analysed, 130 (19.0 %) were from cattle with trematode co-infections, the majority of which were F. gigantica co-infecting with paramphistomes (64.6 %). This may reflect the similarity of the life-cycles of these parasites, which require lymnaeid snails as intermediate hosts [54, 55]. The metacercariae of both trematodes could be found in the same places and might be ingested together by the cattle [56]. This finding was also supported by a significant positive correlation between F. gigantica and paramphistomes faecal egg counts. This positive relationship between the two parasites has been reported previously [46, 51, 57].
Although the haematological results obtained from this study indicated the mean PCV of 34.5 % was within normal range for cattle, which is 24–46 % [32], anaemia due to fasciolosis has been attributed to blood-sucking by adult parasites over long period of time in chronic infections [38, 58]. Failure to demonstrate any effect of trematode infection on PCV may have been due to improved nutritional status of the cattle during the rainy season when they were sampled. The nutritional status of cattle has been previously reported to influence the effect of liver fluke disease [59].
The FAMCHA© chart is a low cost tool for determining anaemia status in ruminants [60, 61] and can be used as part of an integrated worm control program [61]. Selective treatment of animals based on anaemic status is important in preventing anthelmintic resistance [29]. The usefulness of the FAMACHA© chart been evaluated previously in small ruminants in Nigeria [62, 63]. Using scores of 4 or 5 as being indicative of anaemia, the FAMACHA© chart gave a low sensitivity (18.2 %) compared with PCV of ≤ 24 % as a gold standard indicator for anaemia. The PCV cut-off value used to represent anaemia has a significant impact on sensitivity and specificity; values of 64.1 % and 91.3 %, respectively, have been reported in sheep using FAMACHA© score 4 or 5 and PCV ≤ 19 % [30], but there were too few cattle in our study with low enough PCVs for comparable analysis. The FAMACHA© was designed specifically for use in sheep with haemonchosis, optimising the chart for use in cattle based on breeds and variation in colour of ocular mucous membrane might be rewarding.
Analysis of risk factors for trematode infections indicated that cattle in large herds were significantly less likely to be infected with F. gigantica than those from smaller herds. This is because of the possibility of better herd management such as routine anthelmintic treatment. The risk of F. gigantica infection has been previously shown to be lower in medium and large sized non-dairy Danish cattle herds managed intensively [64]. This was however not the case with D. hospes infection where large cattle herds were seven times more likely to be infected than small herds. Other factors such as improper dosing of drugs, anthelmintic resistance or other management system factors may therefore predispose to D. hospes infection. The age and ethnicity of respondents proved to be important in predicting paramphistome infection, cattle belonging to respondents aged 40–59 years of age were 1.95 times more likely to have paramphistomes than cattle belonging to respondents of other age groups, and cattle of responents of Zabaruma ethnic groups were less likely to have paramphistomosis. These ethnic groups keep their cattle semi-intensively, and therefore are less likely to come in contact with infected pasture.
Conclusions
This study revealed the presence of infections with F. gigantica, D. hospes, S. bovis and paramphistomes in cattle sampled from Edu, Kwara State. There was a high variability in the prevalence of trematodes across villages and this may be important in successful control of the parasites. The high prevalence recorded for F. gigantica suggests that an anthelmintic resistance survey on the currently available drugs in the state would be advisable. The high positive correlation between F. gigantica and paramphistome infections and hence likelihood of co-infections should be considered when carrying out anthelmintic therapy. The drugs of choice should be effective against both parasites. There is also a need to optimise and validate the FAMACHA© chart for use in cattle based on breeds and variation in colour of ocular mucous membrane. This would improve its usefulness in identifying cattle with anaemia as a means of selective treatment and hence reduce anthelmintic use/resistance. Finally the risk factors identified in this survey such as herd size and cattle age could assist in tailoring control strategies for various trematode infections to particular group of farmers and cattle.
Abbreviations
EDTA, Ethylene diamine tetra acetic acid; epg, eggs per gram; FS, FAMACHA© score; GDP, Gross Domestic Product; GPS, Global positioning system; LGA, Local Government Area; PCV, Packed cell volume; QGIS, Quantum geographic information system; SD, Standard deviation; Sp, Specificity; Ss, Sensitivity; UIN, University investigation number
Acknowledgements
We are grateful to the staff of the Directorate of Veterinary services, Kwara State for their corporation in this study. Dr Gimba and Dr Deji Lawal are especially appreciated for their technical assistance. Also Mallam Idris for providing laboratory assistance. All the farmers that took part in the study are also acknowledged for their willingness to participate in the study. We also wish to thank the Universities of Ilorin, Kwara State, Nigeria and Bristol, UK for providing support for the PhD study.
Funding
PhD tuition was awarded to the corresponding author (NE) by Nigerian government (Tertiary education trust fund) but no specific funding was provided for the study.
Availability of data and materials
The data supporting the findings of this study are included within the article and its Additional files.
Authors’ contributions
NE carried the field sampling, analysis and writing of manuscript, AGA supervised the field sampling of cattle, GCC participated in the study design and manuscript correction, MCE participated in the study design, statistical analysis and writing of the manuscript. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was carried out with ethical approval from the Kwara State Ministry of Agriculture, Directorate of Veterinary Services. Consent was also sought from all of the cattle farmers in the study before sampling. The study design was approved by the University of Bristol ethical review group, with university investigation number UIN/13/020.
Additional files
General characterisitics of households surveyed in Edu LGA, Kwara State. (XLSX 10 kb)
Univariate analysis of trematode infections in cattle based on household and cattle data in Edu, Kwara State, Nigeria. (XLSX 16 kb)
Contributor Information
Nusirat Elelu, Email: nusyelelu@yahoo.com.
Abdulganiyu Ambali, Email: aambali076@yahoo.com.
Gerald C. Coles, Email: gerald.c.coles@bristol.ac.uk
Mark C. Eisler, Email: mark.eisler@bristol.ac.uk
References
- 1.Chen MG, Mott KE. Progress in assessment of morbidity due to Fasciola hepatica infection: a review of recent literature. Trop Dis Bull. 1990;87:1–38. [Google Scholar]
- 2.Wolfe MS. Spurious Infection with Dicrocoelium hospes in Ghana. Am J Trop Med Hyg. 1966;15(2):180–182. doi: 10.4269/ajtmh.1966.15.180. [DOI] [PubMed] [Google Scholar]
- 3.Phiri AM, Phiri IK, Sikasunge CS, Monrad J. Prevalence of fasciolosis in Zambian cattle observed at selected abattoirs with emphasis on age, sex and origin. J Vet Med B Infect Dis Vet Public Health. 2005;52(9):414–416. doi: 10.1111/j.1439-0450.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- 4.Abebe R, Abunna F, Berhane M, Mekuria S, Megersa B, Regassa A. Fasciolosis: Prevalence, financial losses due to liver condemnation and evaluation of a simple sedimentation diagnostic technique in cattle slaughtered at Hawassa Munincipal abattoir. Southern Ethiopia Ethiop Vet J. 2010;14(1):39–51. [Google Scholar]
- 5.Ngole IU, Ndamukong KJN, Mbuh JV. Internal parasites and haematological values in cattle slaughtered in Buea subdivision of Cameroon. Trop Anim Health Prod. 2003;35:409–413. doi: 10.1023/A:1025811428008. [DOI] [PubMed] [Google Scholar]
- 6.Nzalawahe J, Kassuku A, Stothard J, Coles G, Eisler M. Trematode infections in cattle in Arumeru District, Tanzania are associated with irrigation. Parasit Vectors. 2014;7(1):107. doi: 10.1186/1756-3305-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schillhorn van Veen TW, Folaranmi DOB, Usman S, Ishaya T. Incidence of liver fluke infections (Fasciola gigantica and Dicrocoelium hospes) in ruminants in northern Nigeria. Trop Anim Health Prod. 1980;12(2):97–104. doi: 10.1007/BF02242616. [DOI] [PubMed] [Google Scholar]
- 8.Ulayi BM, Umaru-Sule B, Adamu S. Prevalence of Dicrocoelium hospes and Fasciola gigantica Infections in cattle at Slaughter in Zaria. Nigeria J Anim Vet Adv. 2007;6(9):1112–1115. [Google Scholar]
- 9.Nwosu CO, Srivastava GC. Liver fluke infections in livestock in Borno State. Nigeria Vet Quart. 1993;15:182–183. doi: 10.1080/01652176.1993.9694403. [DOI] [PubMed] [Google Scholar]
- 10.Umar AG, Nwosu CO, Philip HR. Seasonal prevalence and economic importance of bovine fascioliasis in Jalingo Abattoir, Taraba State. Nigeria Nig Vet J. 2009;30:44–50. [Google Scholar]
- 11.Biu AA, Oluwafunmilayo A. Identification of some paramphistome infecting sheep in Maiduguri. Nigeria Pak Vet J. 2004;24(4):187–189. [Google Scholar]
- 12.Ndifon GT, Betterton C, Rollinson D. Schistosoma curassoni Brumpt, 1931 and S. bovis (Sonsino, 1876) in cattle in northern Nigeria. J Helminthol. 1988;62(01):33–34. doi: 10.1017/S0022149X00011160. [DOI] [PubMed] [Google Scholar]
- 13.Boray JC. Experimental Fasciolosis in Australia. Adv Parasitol. 1969;7:95–210. doi: 10.1016/S0065-308X(08)60435-2. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell G. Update on fasciolosis in cattle. In Pract. 2002;24:378–385. doi: 10.1136/inpract.24.7.378. [DOI] [Google Scholar]
- 15.Radostits OM, Clive C, Douglas CB, Kenneth WH. Textbook of diseases of cattle, sheep, goats, pigs and horses. 9th ed. Book Power formerly ELST with Saunders. 2000;1380-82.
- 16.Spithill T, Smooker P, Copeman D. Fasciola gigantica: epidemiology, immunology and molecular biology. In: Fasciolosis. Edited by Dalton JP: CABI Publishing. 1999;465–525.
- 17.Taylor EL. Fasciolasis and the liver fluke., vol. 64: Food and Agriculture Organization of the United Nations Agricultural Studies; 1964.
- 18.De Bont J, Vercruysse J, Southgate VR, Rollinson D, Kaukas A. Cattle schistosomiasis in Zambia. J Helminthol. 1994;68(04):295–299. doi: 10.1017/S0022149X00001516. [DOI] [PubMed] [Google Scholar]
- 19.FAOSTAT. Stock head/year: Nigeria [http://faostat.fao.org/site/573/DesktopDefault.aspx?PageID=573-ancor].
- 20.FAO . Country Forage/Forest Resource ProfileNigeria Edited by Aregheore EM. FAO Rome: Food and Agriculture Organization of United Nations; 2009. [Google Scholar]
- 21.CBN. Central Bank of Nigeria, Annual Report; 1999.
- 22.Bogatko W. Massive mixed Paramphistome cervi and F. gigantica infection in cattle herds in Northern Nigeria. Med Weter. 1975;31:469–470. [Google Scholar]
- 23.Kwara State Government N: Kwara State Diary; 2012.
- 24.Thrusfield MV. Veterinary Epidemiology. 3. Oxford: Blackwell; 2007. [Google Scholar]
- 25.Cameron AR, Baldock FC. Two-stage sampling in surveys to substantiate freedom from disease. Prev Vet Med. 1998;34(1):19–30. doi: 10.1016/S0167-5877(97)00073-1. [DOI] [PubMed] [Google Scholar]
- 26.Faria RN, Cury MC, Lima WS. Concordância entre duas técnicas coproparasitológicas para diagnóstico de Fasciola hepatica em bovinos. Arq Bras Med Vet Zootec. 2008;60:1023–1025. [Google Scholar]
- 27.Howell A, Mugisha L, Davies J, LaCourse EJ, Claridge J, Williams DJL, et al. Bovine fasciolosis at increasing altitudes: Parasitological and malacological sampling on the slopes of Mount Elgon. Uganda Parasit Vectors. 2012;5:196. [DOI] [PMC free article] [PubMed]
- 28.Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. London: Baillière Tindall; 1982. [Google Scholar]
- 29.Van Wyk JA, Bath GF. The FAMACHA© system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Vet Res. 2002;33(5):509–529. doi: 10.1051/vetres:2002036. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan RM, Burke JM, Terrill TH, Miller JE, Getz WR, Mobini S, et al. Validation of the FAMACHA© eye color chart for detecting clinical anemia in sheep and goats on farms in the southern United States. Vet Parasitol. 2004;123(1–2):105–20. [DOI] [PubMed]
- 31.McInroy RA. A micro-haematocrit for determining the packed cell volume and haemoglobin concentration on capillary blood. J Clin Pathol. 1954;7(1):32–36. doi: 10.1136/jcp.7.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radostits O, Clive C, Douglas C, Kenneth W. Textbook of diseases of cattle, sheep, goats, pigs and horses, 9th ed: Book Power formerly ELST with Saunders; 2000:1877.
- 33.Field A. Discovering statistics using SPSS: SAGE Publications; 2007:856.
- 34.Blench R. Traditional livestock breeds: Geographical distribution and dynamics in relation to the ecology of West Africa., vol. Working paper 122: Overseas development institute; 1999:1–66.
- 35.Majekodunmi A, Fajinmi A, Dongkum C, Shaw A, Welburn S. Pastoral livelihoods of the Fulani on the Jos Plateau of Nigeria. Pastoralism. 2014;4(1):20. doi: 10.1186/s13570-014-0020-7. [DOI] [Google Scholar]
- 36.Adewole SO. Prevalence and pathology of Fasciola species in slaughtered cattle. J Life Sci. 2010;4(4(Serial No. 29):28–31
- 37.Sugun SY, Ehizibolo DO, Ogo NI, Timothy SY, Ngulukun SS. Prevalence of bovine fasciolosis in Bauchi State, Nigeria. Sahel J Vet Sci. 2010;9(2):16-20.
- 38.Howlader MMR, Begum S, Islam K, Hai MA, Hossain MG. Further observations on the packed cell volume and haemoglobin concentration in cattle naturally infected with Fasciola gigantica. Bangladesh J Vet Med. 2004;2:No 2. [Google Scholar]
- 39.Yilma JM, Mesfin A. Dry season bovine fasciolosis in Northwestern part of Ethiopia. Rev Med Vet (Toulouse) 2000;151(6):493–500. [Google Scholar]
- 40.Squire SA, Amafu-Dey H, Beyuo J. Epidemiology of gastrointestinal parasites of cattle from selected locations in Southern Ghana. Livest Res for Rural Dev. 2013;25(117). www.lrrd.org/lrrd25/7/squi25117.htm.
- 41.Schilhorn van Veen TW. Ovine fascioliasis (Fasciola gigantica) on the Ahmadu Bello University farm. Trop Anim Health Prod. 1979;11(3):151–156. doi: 10.1007/BF02237791. [DOI] [PubMed] [Google Scholar]
- 42.Mas-Coma S, Bargues M, Valero M. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35(11–12):1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Dreyfuss G, Rondelaud D. Fasciola gigantica and F hepatica: a comparative study of some characteristics of Fasciola infection in Lymnaea truncatula infected by either of the two trematodes. Vet Res. 1997;28(2):123–130. [PubMed] [Google Scholar]
- 44.Stemmermann GN. Human infestation with Fasciola gigantica. Am J Pathol. 1953;29(4):731–759. [PMC free article] [PubMed] [Google Scholar]
- 45.Toledo R, Esteban J, Fried B. Current status of food-borne trematode infections. Eur J Clin Microbiol Infect Dis. 2012;31:1705–1718. doi: 10.1007/s10096-011-1515-4. [DOI] [PubMed] [Google Scholar]
- 46.Phiri AM, Phiri IK, Monrad J. Prevalence of amphistomiasis and its association with Fasciola gigantica infections in Zambian cattle from communal grazing areas. J Helminthol. 2006;80(01):65–68. doi: 10.1079/JOH2005313. [DOI] [PubMed] [Google Scholar]
- 47.Ozdal N, Gul A, Ilhan F, Deger S. Prevalence of Paramphistomum infection in cattle and sheep in Van Province, Turkey. Helminthologia. 2010;47(1):20–24. doi: 10.2478/s11687-010-0003-1. [DOI] [Google Scholar]
- 48.Dube SO, Obiamiwe BA, Aisein MSO. Descriptive studies of the Genus Paramphistomum Fischoeder, 1901 in some Nigerian cattle. Discov and Innov. 2005;17(3&4):186–192. [Google Scholar]
- 49.Otranto D, Traversa D. Dicrocoeliosis of ruminants: a little known fluke disease. Trends Parasitol. 2003;19(1):12–15. doi: 10.1016/S1471-4922(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 50.Campo R, Manga-González MY, González-Lanza C. Relationship between egg output and parasitic burden in lambs experimentally infected with different doses of Dicrocoelium dendriticum (Digenea) Vet Parasitol. 2000;87(2–3):139–149. doi: 10.1016/S0304-4017(99)00165-X. [DOI] [PubMed] [Google Scholar]
- 51.Yabe J, Phiri IK, Phiri AM, Chembensofu M, Dorny P, Vercruysse J. Concurrent infections of Fasciola, Schistosoma and Amphistomum spp. in cattle from Kafue and Zambezi river basins of Zambia. J Helminthol. 2008;82:373–376. doi: 10.1017/S0022149X08054904. [DOI] [PubMed] [Google Scholar]
- 52.Keyyu JD, Monrad J, Kyvsgaard NC, Kassuku AA. Epidemiology of Fasciola gigantica and amphistomes in cattle on traditional, small scale dairy and large scale dairy farms in the southern highlands of Tanzania. Trop Anim Health Prod. 2005;37:303–314. doi: 10.1007/s11250-005-5688-7. [DOI] [PubMed] [Google Scholar]
- 53.Adedokun OA, Ayinmode AB, Fagbemi BO. Seasonal prevalence of Fasciola gigantica infection among the sexes in Nigerian cattle. Vet Res. 2008;2(1):12–14. [Google Scholar]
- 54.FAO . The Epidemiology of helminth parasites. 2. 1993. pp. 1–30. [Google Scholar]
- 55.Keyyu JD, Kassuku AA, Msalilwa LP, Monrad J, Kyvsgaard NC. Cross-sectional prevalence of helminth infections in cattle on traditional, small-scale and large-scale dairy farms in Iringa district, Tanzania. Vet Res Commun. 2006;30(1):45-55. [DOI] [PubMed]
- 56.Szmidt-Adjidé V, Abrous M, Adjidé CC, Dreyfuss G, Lecompte A, Cabaret J, Rondelaud D. Prevalence of Paramphistomum daubneyi infection in cattle in central France. Vet Parasitol. 2000;87(2–3):133–138. doi: 10.1016/S0304-4017(99)00168-5. [DOI] [PubMed] [Google Scholar]
- 57.Nzalawahe J, Kassuku AA, Stothard JR, Coles GC, Eisler MC. Associations between trematode infections in cattle and freshwater snails in highland and lowland areas of Iringa Rural District, Tanzania. Parasitology. 2015;FirstView:1–10. doi: 10.1017/S0031182015000827. [DOI] [PubMed] [Google Scholar]
- 58.Sinclair KB. Observation on the clinical pathology of ovine fasciolosis. Br Vet J. 1962;118:37–53. [Google Scholar]
- 59.Hammond JA, Sewell MM. The pathogenic effect of experimental infections with Fasciola gigantica in cattle. Bri Vet J. 1974;130(5):453–465. doi: 10.1016/s0007-1935(17)35788-3. [DOI] [PubMed] [Google Scholar]
- 60.Grace D, Himstedt H, Sidibe I, Randolph T, Clausen PH. Comparing FAMACHA© eye color chart and Hemoglobin Color Scale tests for detecting anemia and improving treatment of bovine trypanosomosis in West Africa. Vet Parasitol. 2007;147(1–2):26–39. doi: 10.1016/j.vetpar.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 61.Reynecke DP, van Wyk JA, Gummow B, Dorny P, Boomker J. Validation of the FAMACHA© eye colour chart using sensitivity/specificity analysis on two South African sheep farms. Vet Parasitol. 2011;177(3–4):203–211. doi: 10.1016/j.vetpar.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 62.Glaji YA, Mani AU, Bukar MM, Igbokwe IO. Reliability of FAMACHA© chart for the evaluation of anaemia in goats in and around Maiduguri. Sokoto J Vet Sci. 2014;12(3):9–14. doi: 10.4314/sokjvs.v12i3.2. [DOI] [Google Scholar]
- 63.Idika IK, Iheagwam CN, Nwobi LG, Nwosu CO. Evaluation of anaemia in Nigerian goats using FAMACHA© eye colour chart: a preliminary study. Comp Clin Path. 2013;22(4):627–630. doi: 10.1007/s00580-012-1456-z. [DOI] [Google Scholar]
- 64.Olsen A, Frankena K, Bodker R, Toft N, Thamsborg S, Enemark H, Halasa T. Prevalence, risk factors and spatial analysis of liver fluke infections in Danish cattle herds. Parasit Vectors. 2015;8(1):160. doi: 10.1186/s13071-015-0773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are included within the article and its Additional files.


