Abstract
Androgens have profound effects on hippocampal structure and function, including induction of spines and spine synapses on the dendrites of CA1 pyramidal neurons, as well as alterations in long-term synaptic plasticity (LTP) and hippocampally dependent cognitive behaviors. How these effects occur remains largely unknown. Emerging evidence, however, suggests that one of the key elements in the response mechanism may be modulation of brain-derived neurotrophic factor (BDNF) in the mossy fiber (MF) system. In male rats, orchidectomy increases synaptic transmission and excitability in the MF pathway. Testosterone reverses these effects, suggesting that testosterone exerts tonic suppression on MF BDNF levels. These findings suggest that changes in hippocampal function resulting from declining androgen levels may reflect the outcome of responses mediated through normally balanced, but opposing, mechanisms: loss of androgen effects on the hippocampal circuitry may be compensated, at least in part, by an increase in BDNF-dependent MF plasticity.
Keywords: testosterone, hippocampus, BDNF, mossy fibers, CA3
Introduction
Over the past 15 years, a considerable body of information has emerged indicating that androgens modulate the structure and functions of the hippocampus. Effects on hippocampal-dependent behavior, long-term potentiation (LTP), dendritic spine and spine synapse density, patterns of dendritic arborization, as well as hippocampal neuronal survival have all been reported. In many respects, these effects resemble those of the principal ovarian estrogen, 17β-estradiol, in females (Harte-Hargrove and others 2013; MacLusky and others 2006b). However, although the majority of studies have shown that estradiol action in females is associated with increases in hippocampal excitability, as well as in behaviors that depend on hippocampal function, this is not true for androgens. Responses to androgens, in fact, appear to be mixed. For example, although testosterone appears to induce formation of excitatory spine synapses on the dendrites of hippocampal pyramidal neurons (MacLusky and others 2006b), the effects of testosterone on brain-derived neurotrophic factor (BDNF) and measures of hippocampal excitability, such as LTP in the mossy fiber system, appear to be inhibitory rather than stimulatory (Skucas and others 2013).
The goal of this brief review is to sort through this apparently conflicting set of data and suggest possible mechanisms to explain how androgens may regulate hippocampal function. Much of the data on the hippocampal effects of androgens has been reviewed elsewhere and this will not be repeated in extenso here. Our emphasis will be to highlight recent developments and, in particular, to focus on the possible role of BDNF as a mediator of androgen responses.
Evidence for Androgen Effects on Hippocampal Function
Evidence for androgen effects on hippocampal function comes from a number of different fields: from studies of the effects of changing androgen levels on cognitive behavior in people, from correlations between androgen levels and the incidence of neurodegenerative disorders, and finally from studies in experimental animals of the effects of androgen administration. All three lines of evidence point to the same overall conclusion: whereas androgen clearly affects hippocampal function, the responses are complex and include both positive and negative components.
A number of studies suggest that testosterone supplementation may improve cognitive function, in particular spatial memory, in men (Cherrier and others 2001; Driscoll and Resnick 2007; Janowsky 2006; Sherwin 2003). However, the magnitude of androgen effects remains controversial. Although androgen deprivation during hormone ablation therapy for treatment of prostate cancer has been reported by some authors to impair both verbal (Beer and others 2006) and spatial (Jenkins and others 2005) memory performance, other studies have reported mild to insignificant cognitive deficits in such patients (Alibhai and others 2010; Joly and others 2006; Matousek and Sherwin 2010; Nelson and others 2008).
Men exhibit a steady age-related decline in circulating testosterone levels, with a concomitant increase in sex hormone-binding globulin production (Feldman and others 2002; Morley and others 1997). A decline in the production of the major adrenal androgen, dehydroepiandrosterone (DHEA) is also observed with age (Labrie and others 1997). Reduced levels of free circulating testosterone have been postulated to contribute in men to diseases that increase with age, such as Alzheimer’s disease (Barron and Pike 2012; Drummond and others 2009; Fuller and others 2007), epilepsy (Harden and MacLusky 2004; Herzog 1991; Reddy 2004), and Parkinson’s disease (Johnson and others 2010). This hypothesis is teleologically attractive in view of the neurotrophic and neuroprotective effects of testosterone (Hammond and others 2001; Kurth and others 2014), as well as the effects of this steroid on other mechanisms that may indirectly contribute to neurodegenerative disease, including inflammatory responses (Butchart and others 2013) and cerebral blood flow (Moffat and Resnick 2007). However, studies suggest that the contribution of changes in androgen levels to Alzheimer’s disease may be relatively modest—particularly in women (Hogervorst and others 2005). With respect to cognitive deficits associated with Alzheimer’s disease, circulating testosterone levels do not appear to be predictive of neuropsychological outcome (Seidl and Massman 2014). Testosterone replacement therapy has also been reported to have no significant effect on motor and nonmotor symptoms of Parkinson’s disease (Okun and others 2006) although, as is the case for many clinical studies, interpretation of the results is limited by sample size.
A similar, inconclusive picture emerges from data obtained in animals. Androgens significantly influence working and spatial memory in male rodents, although the direction and magnitude of effect varies across studies. For instance, nonspatial working memory, as measured by spontaneous novel-object recognition, has been shown to be worse in gonadectomized (GDX) male rats than in either GDX males implanted with testosterone or in sham-GDX controls (Aubele and others 2008). Positive effects of androgens have also been detected using various spatial working memory tasks, including the Y-maze (Hawley and others 2013) and the Morris water maze (Khalil and others 2005). However, these studies contrast with others that report either impairment or no effect on learning (Goudsmit and others 1990) and memory (Naghdi and others 2001) in response to androgen treatment. A similar degree of variation is seen in studies that use nonspatial operant tests, such as the Sidman avoidance task to measure the impact of androgens on learning in rats (Milner 1976). Electrophysiological studies, likewise, suggest that the effects on the hippocampus of manipulating androgen levels are mixed. Testosterone application to hippocampal slices of adult male rats potentiates Schaffer collateral transmission in area CA1 (Smith and others 2002). However, testosterone depletion via prepubertal orchidectomy has been reported to facilitate LTP in the CA1 region of adult male rats, suggesting that androgens act to reduce CA1 plasticity (Harley and others 2000).
How Do Androgens Exert Their Effects? The Critical role of Local Steroid Metabolism
The complexity of androgen action at least in part reflects the fact that circulating androgens are converted to metabolites that have a wide range of biological activities, in different regions of the brain. This extensive metabolism provides mechanisms for both control, in terms of regulating the cellular responses that can be obtained via modulation of the activity of the relevant rate-limiting enzymes, as well as for diversification of the cellular effects of the circulating hormones.
Testosterone is converted to estradiol in several areas of the brain, including the hippocampus (Naftolin and others 1975; Tabatadze and others 2014; Yague and others 2010). It is also metabolized, in both neurons and glia, to the powerful androgen, dihydrotestosterone (DHT). In type 1 astrocytes, DHT is reversibly converted to 5α-androstane-3α,17β-diol (Melcangi and others 1993), which is a weak ligand for nuclear androgen (AR) and estrogen (ER) receptors, but a potent modulator of the effects of GABA on GABAA receptors (Reddy 2004). The 3β isomer of androstandiol, 5α-androstan-3β,17β-diol, is also synthesized in the brain: whereas this steroid is not an agonist at either the androgen or GABAA receptor, it is a ligand for ERβ (Handa and others 2011). Thus, in both sexes, exposure of the brain to testosterone potentially exposes the hippocampus to a range of biologically active metabolites, all of which may contribute to the observed responses (reviewed in MacLusky and others 2006b; Scharfman and MacLusky 2014b).
Morphological Effects of Androgen in the Hippocampus
Effects of androgen on the morphology of the hippocampus have been known for more than 30 years. Much of the initial work focused on the developmental effects of androgen in the context of sexual differentiation. In some strains of mice, males have more granule cell neurons in the dentate gyrus than females (Wimer and Wimer 1989). Male rats have a larger and more asymmetric dentate gyrus than females (Roof 1993; Roof and Havens 1992) whereas sex differences have also been demonstrated in the apical dendritic structure and dendritic branching patterns of CA3 pyramidal neurons, consistent with the observation that males exhibit a significantly higher density of mossy fiber synapses (Parducz and Garcia-Segura 1993).
Responses to androgen are not confined to early development. Male mice exhibit a pronounced increase in the density of dendritic spines on the apical dendrites of CA1 and CA3 neurons at approximately the time of puberty, a response that is abrogated by prepubertal orchidectomy, suggesting that the changes in dendritic spine density may be a response to the pubertal rise in circulating testosterone levels (Meyer and others 1978). Given the pronounced positive effects of estradiol on both CA1 spine (Gould and others 1990) and spine synapse (Leranth and others 2000) density, it seemed possible that, in males, testosterone might exert effects on hippocampal structure analogous to those of estradiol in females. Numerous studies have since provided data consistent with this hypothesis, in rodents as well as nonhuman primates (Kovacs and others 2003; Leranth and others 2003; Leranth and others 2004b; Li and others 2012; MacLusky and others 2004). Indeed since, as indicated above, testosterone is converted to estradiol in the brain, it seemed reasonable to suppose that the effects of testosterone might be at least partially mediated via local estradiol formation, within the hippocampus itself. Whereas this does indeed appear to be the case in females (Fester and others 2012; Leranth and others 2004a; Vierk and others 2014) in males other mechanisms are clearly involved. In ovariectomized adult females, CA1 pyramidal cell spine synapse density increases after treatment with either estradiol or testosterone (Leranth and others 2004a), but in adult males estradiol has no significant effect (Leranth and others 2003). Treatment with the aromatase inhibitor letrozole, which blocks estradiol synthesis, decreases hippocampal synapse numbers in females but not in males (Fester and others 2012). Effects of orchidectomy on spine synapse density in area CA1 can be reversed by replacement with either testosterone or the non-aromatizable androgen, DHT (Leranth and others 2003), further supporting the view that synaptic responses to androgen in males do not require intermediate estrogen biosynthesis. Other experimental data also suggest that responses to androgen may differ fundamentally from those to estrogens. For example, in the original study of Meyer and others (1978) while there was a substantial increase in spine density in all segments of the dendritic tree in CA1 pyramidal cells over the period leading up to and including puberty (25–45 days of age), only 10 days later (at 55 days of age) the effect had almost disappeared. Circulating testosterone levels do not drop significantly in male rats between 45 and 55 days of age (Lee and others 1975), suggesting either that the effects of androgens are age-dependent or that normal adult male levels of testosterone do not elicit a sustained response in terms of dendritic spine density—even though there is a markedly higher density of spine synapses in the CA1 of intact males (Leranth and others 2003). Preliminary data from our own laboratories are consistent with the hypothesis that the effects of testosterone on hippocampal spine density in adult rats are in fact much more limited than those of estradiol, in females (Mendell and others, unpublished observations).
Androgen Effects on Hippocampal Neurogenesis
The effects of androgens on the hippocampus include changes in neurogenesis in the subgranular zone of the dentate gyrus. Like the effects of gonadal steroids in hippocampal spine synapse density, this response is sexually differentiated. Thus, estradiol modulates hippocampal neurogenesis and cell death in adult female rodents but not males (Galea 2008; Galea and others 2013). In contrast, testosterone enhances the survival of new dentate gyrus neurons in adult males, via an androgen receptor-dependent mechanism (Hamson and others 2013). Interestingly, the effects of androgens on neuronal survival may be mediated at a site other than in the dentate gyrus itself (Hamson and others 2013), which hints at the potential involvement of other regions of the brain in androgen responses (discussed further, below).
Glial Responses
The effects of androgens on hippocampal structure include changes in glia, in particular astrocytes. These effects are important not only because of the role of glia in neurosteroid biosynthesis (Melcangi and others 1993) but also because glia mediate some of the neuronal effects of steroid hormone action, responding to gonadal steroids by producing neurotrophic factors (Azcoitia and others 2010).
Several studies indicate that androgens have important effects on astroglia. Using immunocytochemistry for glial fibrillary acidic protein, astrocyte numbers were found to be significantly higher in androgen insensitive (Tfm) and hypogonadal mice when compared with normal males (McQueen and others 1992b). In wild-type mice, castration nearly doubled the expression of GFAP in CA1 and dentate gyrus, whereas with testosterone replacement, cell numbers returned to normal (McQueen and others 1992a). Similarly, Leranth and others (2008) found that administration of the environmental contaminant bisphenol-A, which has antiandrogenic properties, to male rats not only reduced spine synapse density but also increased the density of astroglial processes in the hippocampus and prefrontal cortex. Taken together, these observations suggest that whereas circulating androgens promote synapse formation in the hippocampus, they elicit a concomitant decrease in astroglial process density, which may be important in terms of the maintenance of long-term changes in synaptic plasticity (Bernardinelli and others 2014).
Mechanisms of Androgen Action
Local Mechanisms
Receptors for androgens and estrogens are present in hippocampal neurons. Androgen receptors as well as both the α and β subtypes of the ER are found in hippocampal neurons in both cell nuclei (Kerr and others 1995; Menard and Harlan 1993; Sar and others 1990; Shughrue and Merchenthaler 2000; Tabori and others 2005) and at extranuclear sites in the cell including the plasma membrane, mitochondria, and synaptic vesicles (Tabori and others 2005). Oligodendrocytes and astroglia (Finley and Kritzer 1999; Jung-Testas and Baulieu 1998) also appear to express androgen receptors and ER, indicating that they are direct targets for steroid action. The existence of these receptor systems is consistent with the hypothesis that the actions of androgens are, at least in part, mediated via actions within the hippocampus itself.
How these receptor systems may mediate effects of androgen on the hippocampus is not, however, well understood. There is substantial evidence that at least as far as androgen effects on hippocampal spine synapse density are concerned, the receptor systems involved may involve mechanisms different from those found in nonneural androgen target tissues. Treatment with comparable doses of DHEA, DHT, or testosterone induces almost identical CA1 spine synapse levels in adult males (Leranth and others 2003; MacLusky and others 2004), despite the fact that these steroids have very different androgenic potencies on androgen target tissues outside the central nervous system. Treatment with the androgen receptor antagonist, flutamide, which completely blocks the effects of androgens on the male reproductive tract, does not block the effects of DHT in male CA1 spine synapse density. In fact, flutamide has stimulatory effects of its own on hippocampal spine synapse density, which sum with those of co-administered androgens (MacLusky and others 2004). Consistent with these observations, flutamide and the structurally unrelated steroidal androgen receptor antagonist, cyproterone acetate, both also exert neuroprotective effects like those of DHT in cultured hippocampal neurons (Nguyen and others 2007). In adult male Tfm rats, which express a defective androgen receptor and therefore have greatly reduced sensitivity to testosterone in peripheral tissues, the effects of DHT and hydroxyflutamide on CA1 dendritic spine synapse density are indistinguishable from those observed in wild-type normal males (MacLusky and others 2006a). That the mutated androgen receptor found in Tfm rats remains capable of supporting some central androgen responses has also been demonstrated with respect to reinforcement behavior induced by anabolic-androgens (Sato and others 2010).
These findings suggest that the mechanisms mediating the effects of androgens on hippocampal structure and function may be different than those found in “classical” nonneural androgen target tissues. Although the precise mechanisms involved have yet to be established, one hypothesis is that the effects may be mediated via receptors acting outside the cell nucleus rather than via the transcriptional mechanisms that mediate many of the effects of gonadal steroids elsewhere in the body. Immunoreactivity for androgen receptors is found in spines on pyramidal and granule cell dendrites, associated with the synaptic vesicles of preterminal axons and axon terminals, particularly in the stratum lucidum of the CA3 (Tabori and others 2005). Activation of membrane-associated androgen receptors initiates kinase cascades that are known to be involved in CA1 pyramidal cell spine formation (Zadran and others 2009). Kinase-mediated effects of androgen also have been shown to be resistant to blockade with conventional antiandrogens. Thus, for example, in both breast (Zhu and others 1999) and prostate (Lee and others 2002) cancer cell lines, androgen activation of mitogen-activated protein kinase appears to be mimicked, rather than blocked, by flutamide. If similar response mechanisms operate in the brain, this could at least in part explain why flutamide exerts additive effects with those of DHT on CA1 pyramidal cell spine synapse formation.
Effects Mediated via Afferent Hippocampal Input
Whereas much of the work on androgen effects so far has focused on mechanisms intrinsic to the hippocampus, responses in other regions of the brain probably also play a role. In females, fimbria-fornix (FF) transection (Leranth and others 2000) or selective lesions of the basal forebrain cholinergic neurons via local injection of 192 IgG-saporin (Lam and Leranth 2003) essentially abolish the effects of estradiol on CA1 spine synapse density, ipsilateral to the site of the lesion (Fig. 1), suggesting that the effects of estradiol on spine synapse density are dependent on sub-cortical cholinergic input. Noncholinergic mechanisms could also be involved. GABAergic afferents originating from the septum innervate inhibitory interneurons in the hippocampus (Freund and Antal 1988), suggesting that hormonal activation of subcortical afferent inhibitory pathways could lead to rapid disinhibition of pyramidal neurons (Rudick and others 2001). Since, as mentioned above, androgens DHT and other natural androgens are converted in the brain to metabolites that potentiate GABAA mediated responses (Melcangi and others 1993; Reddy 2004), it seems possible that the effects of the androgens could be mediated in a similar fashion, via enhancement of GABA action on GABAergic interneurons—leading to disinhibition of the pyramidal cells. Such a mechanism could also contribute to the effects of high-dose flutamide, as flutamide has weak benzodiazepine-like activity (Ahmadiani and others 2003).
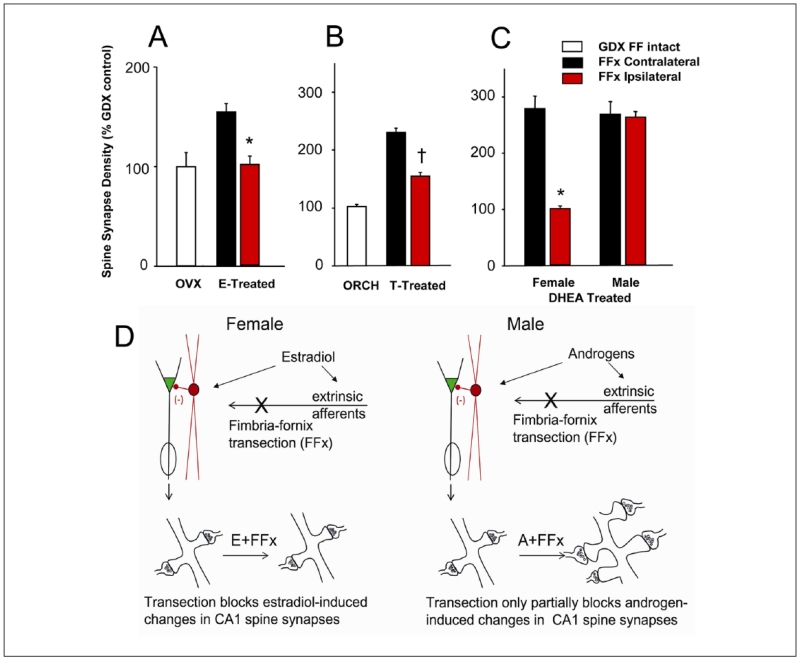
Figure 1.
Effects on synapse formation in response to gonadal steroid administration of unilateral transection of the subcortical afferents to the hippocampus, by cutting the right fimbria/fornix (FF), are dependent on sex, as well as the nature of the gonadal steroid treatment. (A) Morphometric estimation of the density of spine synapses in the ipsilateral (red bars) and contralateral (black bars) CA1 stratum radiatum of unilaterally fimbria/fornix transected female rats. Rats were either ovariectomized (OVX) or OVX and treated with estradiol benzoate (2 × 10 μg, 24 hours apart, s.c.). FF transection had no effect on CA1 synapse density in OVX rats, but completely abolished the increase in synapse density induced by estradiol ipsilateral to the transection. *Significantly different from results on the contralateral side of the brain. (B) Density of pyramidal cell spine synapses in the CA1 stratum radiatum of intact male, orchidectomized (ORCH) and ORCH testosterone (T)-treated rats. Approximately equal spine synapse densities are present in the hippocampi of control males, ORCH T replaced males, and FF-transected T-replaced males contralateral to the FF transection. The spine synapse response to T is partially inhibited ipsilateral to FF transection. *Significantly different from control, intact males; †Significantly different from both intact and ORCH males. (C) Spine synapses in the CA1 stratum radiatum of gonadectomized female and male rats after unilateral and dehydroepiandrosterone (DHEA) treatment. FF transection abolished the synaptic response to DHEA treatment ipsilateral to the transection in female, but not male, rats. In contrast to the results for T replacement, DHEA replacement in males was able to completely restore spine synapse density ipsilateral to FF transection. *Indicates a significant difference between the synapse densities in the ipsilateral and contralateral CA1. (D) Schematic illustration of the possible underlying mechanisms. In females, FF transection completely abolishes the ability of estradiol or the aromatizable androgen DHEA to increase CA1 spine synapse density, whereas in males the effects of androgen are only partially impaired by the elimination of subcortical afferents (adapted from data in Kovacs and others 2003; Leranth and others 2000; Mendell and others 2013).
The available evidence, however, suggests that CA1 structural plasticity in response to androgen administration in males may be less dependent on afferent subcortical input than is the case for estradiol in females. FF transection reduces the spine synapse density response to testosterone administration by only 50%, compared to control unoperated rats (Kovacs and others 2003). The magnitude of the effect also appears to be dependent on the androgen replacement used. Thus, if DHEA is administered instead of testosterone, FF transection in males has no significant effect on the resultant spine synapse density, ipsilateral to the surgery (Mendell and others 2013). This contrasts with the situation in ovariectomized females, in which FF transection completely abolishes the increase in spine synapse density observed after DHEA administration (Fig. 1). A reasonable hypothesis to explain these observations is that, unlike females in which subcortical estrogen-sensitive afferents play a critical role, in males there may be a greater contribution from intrahippocampal androgen-dependent mechanisms to the observed neurotrophic responses.
BDNF as a Mediator of Androgen Responses
Over the past few years, an increasing body of evidence has accumulated to suggest that BDNF may contribute to the effects of circulating gonadal steroids on the brain. In both central and peripheral androgen target tissues, numerous studies have demonstrated effects of androgen on BDNF expression, as well as actions of BDNF that appear to play a role in mediating the effects of changing androgen levels (reviewed in Scharfman and MacLusky 2014a). Given that hormone-sensitive pathways in the hippocampus, as well as afferent projections to the hippocampus from such regions of the brain as the entorhinal cortex may both contribute to intrahippocampal BDNF expression (Falkenberg and others 1993; Scharfman and Chao 2013), the control of BDNF synthesis and release clearly represent possible targets for the effects of circulating testosterone on hippocampal function.
A potential model for the effects of androgens on the hippocampus comes from studies of testosterone action in the spinal nucleus of the bulbocavernosus (SNB). SNB motorneurons in the lumbar spinal cord innervate muscles required for male copulatory behavior. BDNF has been implicated in the supportive role of androgen on SNB motorneurons, testosterone maintaining BDNF levels in the motorneuron dendrites (Ottem and others 2007). As up-regulation of BDNF has been shown to enhance hippocampal dendritic spine formation (Bennett and Lagopoulos 2014), one hypothesis to explain the trophic effects of androgen on hippocampal dendritic spines might be that androgen also induces BDNF in the hippocampus. Studies using hippocampal slices prepared from adult male mice have, indeed, provided data consistent with this hypothetical mechanism, with orchidectomy resulting in a significant decrease in the levels of BDNF protein in CA1 (Li and others 2012).
In rats, however, the regulation of hippocampal BDNF appears to be very different from that in either mice, or SNB motorneurons. Although local intrahippocampal levels of estrogens and androgens are high in intact males (Ooishi and others 2012), immunohistochemistry revealed very low intrahippocampal BDNF expression in male rats (Scharfman and others 2003). Orchidectomy did not decrease, but instead increased BDNF immunoreactivity in the mossy fiber pathway, examined 2 weeks or 2 months later (Fig. 2). As increases in BDNF have previously been shown to increase mossy fiber transmission, in both males and females (Scharfman and Maclusky 2005; Scharfman and others 1999; Scharfman and others 2003) a logical prediction from these observations was that orchidectomy might increase mossy fiber synaptic transmission. Indeed, mossy fiber transmission was enhanced in GDX rats compared to sham-operated controls (Skucas and others 2013). In addition, short-and long-term plasticity were also affected, paired pulse facilitation and mossy fiber LTP and post-tetanic potentiation (PTP) both increasing in orchidectomized animals (Fig. 3). The role of BDNF in these responses was supported by pharmacological blockade of the TrkB receptors mediating BDNF action, which reversed the orchidectomy-induced increase in mossy fiber transmission and LTP. That the effect of gonadectomy was indeed because of the removal of androgen was demonstrated in hippocampal slices from orchidectomized males, because addition of DHT normalizing mossy fiber transmission (Skucas and others 2013). Significantly, however, in view of the evidence cited above for actions of androgens mediated via conversion to neuroactive metabolites, mossy fiber transmission was not affected by addition of the GABA-active metabolite of DHT, 5α-androstane-3α, 7β-diol, suggesting that the effect was not mediated via enhancement of GABAA mediated responses (Skucas and others 2013).
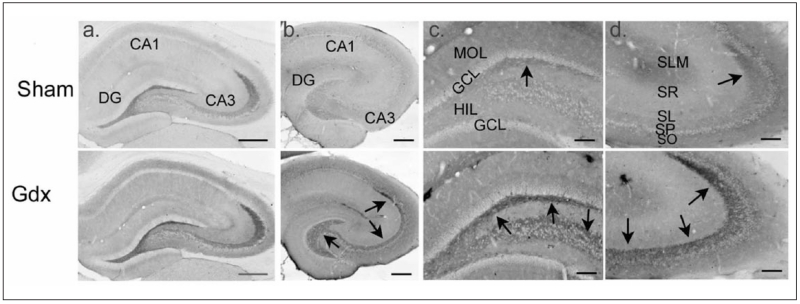
Figure 2.
Gonadectomized male rats exhibit increased immunoreactivity for BDNF protein in mossy fibers. Comparison of sham and gonadectomized male rats that were perfused and processed for immunocytochemistry using either a rabbit polyclonal BDNF antibody (a; antibody from Amgen Regeneron Partners), or a mouse monoclonal antibody to BDNF (b-d; antibody from Sigma). Arrows point to mossy fiber staining. DG = dentate gyrus; MOL = molecular layer; GCL = granule cell layer; HIL = hilus; SLM = stratum lacunosum moleculare; SR = stratum radiatum; SL = stratum lucidum; SP = stratum pyramidale; SO = stratum oriens. Calibration bars: a, b: 250 μm; c, d: 100 μm. Data from Skucas and others (2013).
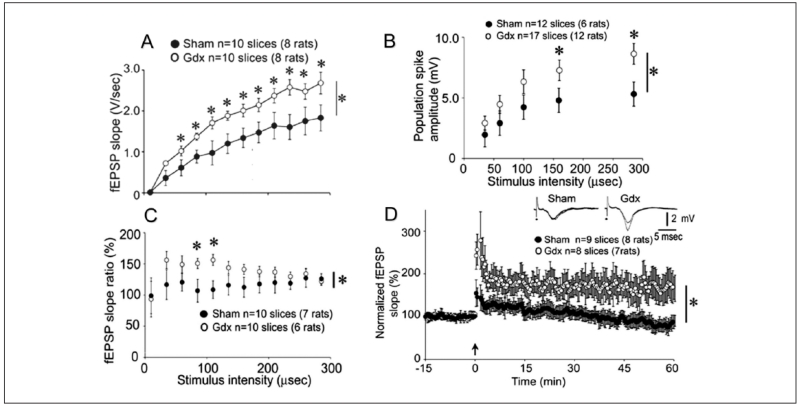
Figure 3.
Male gonadectomized rats exhibit increased mossy fiber transmission, paired pulse facilitation, and LTP. (A) Increasing stimulus strength to the mossy fibers produced field EPSPs (fEPSPs) with increasing slope and amplitude, recorded in stratum lucidum. Responses to stimuli are plotted for all sham (black circles) and gonadectomized (white) rats. Repeated-measures ANOVA showed that there was a significant effect of gonadectomy, and post hoc tests showed that most individual comparisons were significantly different (asterisks above the symbols). (B) Responses are shown for sham and gonadectomized rats with the recording electrode positioned in the pyramidal cell layer. Population spike amplitude was significantly larger in gonadectomized rats. (C) Recording electrodes were placed in stratum lucidum to examine paired pulse facilitation. Analysis of paired pulse facilitation with a range of interstimulus intervals from 20 to 200 ms showed that there was a significant effect of gonadectomy at intermediate intervals. (D) Comparison of LTP in gonadectomized (white circles) and sham rats (black circles) illustrates greater LTP in gonadectomized rats. Insets: representative examples of fEPSPs before and after LTP induction, with LTP evident only in the slice from the gonadectomized rat. Data from Skucas and others (2013).
The increased neuroplasticity induced by orchidectomy was not limited to increases in the magnitude of the field potentials recorded in CA3: it also included effects on the specificity of mossy fiber innervation of the CA3 pyramidal neurons. The first indication of this was seen in the electrophysiological data. In intact males, there is usually one specific, easily identifiable site in stratum lucidum where the largest field EPSP is observed after activation of mossy fiber axons. In orchidectomized males, however, mossy fiber–evoked fEPSPs could be recorded at multiple locations in stratum lucidum, stratum pyramidale, and stratum oriens (Skucas and others 2013). Given that BDNF has been reported to induce sprouting of the mossy fibers (Lowenstein and Arsenault 1996), we considered the possibility that this apparent spreading of the signal recorded in CA3 might be associated with mossy fiber outgrowth following removal of the testes. Immunocytochemistry for dynorphin (which specifically labels the mossy fibers) established that this was indeed the case. Sprouting increased in GDX rats compared to sham controls, particularly in stratum oriens (Fig. 4), suggesting that after gonadectomy, the increase in mossy fiber BDNF expression may lead to diffusion of the normal afferent specificity of the mossy fiber pathway (Scharfman and MacLusky 2014a; Skucas and others 2013).
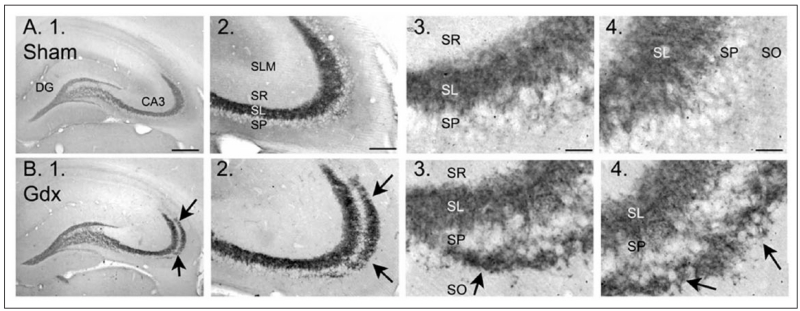
Figure 4.
Gonadectomized male rats exhibit mossy fiber sprouting. Examples of dynorphin immunocytochemistry in sham operated (A) and gonadectomized (B) male rats illustrate a novel band of immunoreactivity in stratum oriens (arrows) in gonadectomized rats. For abbreviations, see legend to Figure 2. Calibration bars: 500 μm (1); 100 μm (2), and 25 μm (3, 4). From Skucas and others (2013).
Physiological and Pathophysiological Implications
Age-related androgen deficiency is increasingly recognized as a clinically significant problem. Free circulating androgen levels decline substantially with age, in both men (Feldman and others 2002) and women (Davison and others 2005; Labrie and others 1997), which has led to an increase in the use of androgens in treatment of the effects of aging (Basaria 2013; Pluchino and others 2013). Whereas there are undeniable beneficial effects of such treatment, the long-term effects on the brain remain poorly understood. The data summarized above raise the possibility that, at least as far as the brain is concerned, androgen supplementation in the aging population may represent a mixed blessing. Whereas raising testosterone levels may enhance androgen-mediated changes in synaptic plasticity in the hippocampus, the downside could be a simultaneous reduction in BDNF-mediated neuroplasticity in the mossy fiber pathway.
Mood disturbances and aggressive behavior are an area of potential concern. Such problems are commonly reported in males abusing anabolic androgenic steroids (Oberlander and Henderson 2012). This may be related to the suppressive actions of androgens on BDNF expression in the mossy fibers (Skucas and others 2013), because of the known role of intrahippocampal BDNF in aggression and the maintenance of normal mood state. Conditional BDNF knockout mice, in which BDNF levels are selectively depleted in the dentate gyrus and CA3, exhibit enhanced aggressive behavior (Ito and others 2011) and reduced levels of hippocampal BDNF have been linked to depression (Duman and Li 2012). Thus, supraphysiological levels of androgens in men that abuse anabolic androgenic steroids may suppress the expression of BDNF in the mossy fiber plexus, leading to an adverse effect on mood and aggression. These effects could be exacerbated by other factors, such as physical inactivity (Cotman and Berchtold 2002) or stress (Smith and others 1995), both of which negatively affect hippocampal BDNF expression. Conversely, age-related increases in the incidence of epilepsy, in men, may in some cases be reversed by raising circulating androgen levels, because of the fact that increased BDNF expression causes hyperexcitability in the mossy fiber system, an effect that is opposed by testosterone (Skucas and others 2013).
Age-related androgen deficiency is not, however, a simple phenomenon. Over time, other endocrine changes as well as compensatory events within the brain itself may modulate the pattern of responses to androgen deprivation. As discussed previously, the experimental evidence does not provide a clear-cut indication that blocking the effects of androgen on the brain leads to significant deficits in cognitive function (Alibhai and others 2010; Joly and others 2006; Matousek and Sherwin 2010; Nelson and others 2008). It seems possible that potentially deleterious effects of declining androgen levels during normal ageing may at least in part be ameliorated by compensatory changes in BDNF expression, declining free testosterone reducing the dampening effect of androgens on mossy fiber transmission. However, the endocrine changes associated with ageing are complex and it may be naïve to assume that there is a simple relationship between BDNF and any individual circulating hormone in ageing subjects. Ageing in men is associated not only with a decline in free testosterone but also declining adrenal DHEA–cortisol ratio (Guazzo and others 1996; Labrie and others 1997). The overall effects on hippocampal function of age-related changes in free testosterone may thus reflect the outcome of a number of different mechanisms: loss of direct testosterone and DHEA-mediated effects on hippocampal neurons, positive effects on BDNF arising from reduced testosterone-mediated inhibition, as well as increased glucocorticoid-mediated inhibition of BDNF synthesis (Suri and Vaidya 2012). Other factors, besides circulating hormone levels, could also contribute to the central regulation of androgen-mediated hippocampal responses. Androgens are synthesized in the brain (Mukai and others 2006) where they have been implicated in the maintenance of normal hippocampal spine synapse density (Ooishi and others 2012) as well as normal behavioral responses to changing circulating hormone levels (Remage-Healey 2014; Soma and others 2014). Although there is currently very little information about how central nervous system androgen biosynthesis may change with age, there are hints that steroidogenesis in the brain may be age-dependent. StarD6, a member of the steroidogenic acute regulatory protein family that is expressed in the brain and appears to enhance de novo steroidogenesis, was recently reported to decrease in the cerebrum, but slightly increase in the hippocampus, of aged male rats, raising the possibility of age-related regional changes in neurosteroidogenesis (Chang and others 2014).
Future Directions
A central question that remains to be resolved is how androgens can apparently exert such a diversity of effects on different parameters of hippocampal neuroplasticity. Normal male circulating levels of testosterone promote spine (Li and others 2012; Meyer and others 1978) and spine synapse (Kovacs and others 2003; Leranth and others 2003) formation on the dendrites of pyramidal neurons in CA1 and CA3, as well as in other regions of the brain (Danzer and others 2001; Hajszan and others 2007; McGinnis and others 2007). At the same time, at least in rats, orchidectomy enhances BDNF expression in the mossy fiber system of CA3, leading to increased excitability and LTP (Skucas and others 2013). This contrasts with other regions of the brain and spinal cord, in which testosterone appears to induce BDNF. How can these apparently discordant results be reconciled? Is the regulation of BDNF in the brain region-specific? Are there species and/or age-dependent differences in the androgen regulation of hippocampal BDNF expression? Most important, to what extent can the observed changes in BDNF explain the morphological and functional consequences of androgen exposure in the hippocampus, as opposed to other potential mechanisms of androgen action?
At least in rats, the available evidence suggests that BDNF may indeed be regulated differently in the hippocampus than in other areas of the brain. Hill and colleagues (2012) studied serum androgen levels, BDNF mRNA (by Western blot), and BDNF protein (by ELISA) in the hippocampi of developing rats and found that while androgen levels increased with age, there was no change in BDNF mRNA or protein. In contrast, both BDNF mRNA and protein increased in striatum and frontal cortex. The results are consistent with the idea that effects of androgens on BDNF expression may vary regionally within the brain: in extrahippocampal sites, serum androgen levels correlate with BDNF expression, which is not the case in the hippocampus (Hill and others 2012).
Effects on tissue BDNF levels must, however, be interpreted carefully because, like the actions of testosterone itself, the consequences of changes in BDNF expression may be extensively modulated by local regulatory mechanisms. BDNF is synthesized as a precursor (pre-pro BDNF), which is processed initially to pro-BDNF and then to BDNF itself in the mossy fibers (Dieni and others 2012). Pro-BDNF has very different biological effects than BDNF, negatively regulating neuronal remodeling, synaptic transmission, and synaptic plasticity (Yang and others 2014). Cleavage of pro-BDNF in the brain involves tissue plasminogen activator (Pang and others 2004; Rodier and others 2014), an enzyme that is induced in endothelial cells by the action of testosterone (Jin and others 2007). Thus, changes in circulating testosterone levels may not only affect the rate of BDNF synthesis, they may also affect the ratio of pro-BDNF to BDNF, which could dramatically alter the nature and direction of the response. The effects of BDNF may be subject to local regulation at the receptor level and may include both positive and negative effects, depending on factors such as the relative expression of full-length and truncated trk-B receptors, as well as levels of other neuropeptides and neurotransmitters (reviewed in Scharfman and MacLusky 2014a). BDNF powerfully regulates the expression of NMDA receptor subunits in the hippocampus, potentially affecting both glutamate sensitivity (Kim and others 2012; Martin and Finsterwald 2011) and spine density (Murphy and others 2014; Woolley and McEwen 1994).
Within the context of the hippocampal circuitry, the overall effects of BDNF on the CA3 pyramidal neurons and mossy fiber mediated neurotransmission may be highly dependent on where, and how, BDNF is released. A large proportion of mossy fiber boutons terminate on GABAergic interneurons, where BDNF potentiates pre-synaptic GABA synthesis (Ohba and others 2005) rather than directly onto pyramidal cells (Acsady and others 1998). At low mossy fiber firing rates, increased BDNF expression in the mossy fiber system would be predicted to preferentially activate GABAergic interneurons, resulting in an overall negative effect on the pyramidal cell dendrites. Under circumstances of dramatically increased neural activity (e.g., during tetanic stimulation), however, local GABA-mediated inhibitory effects may be overwhelmed by the release of dense core vesicles (preferentially released in response to high frequencies of afferent input), frequency-facilitation of glutamate release from large mossy fiber boutons (Salin and others 1996), and up-regulation of BDNF in the giant boutons of the mossy fibers (Danzer and McNamara 2004). These giant boutons preferentially innervate pyramidal cells, not GABAergic neurons (Fig. 5).
Figure 5.
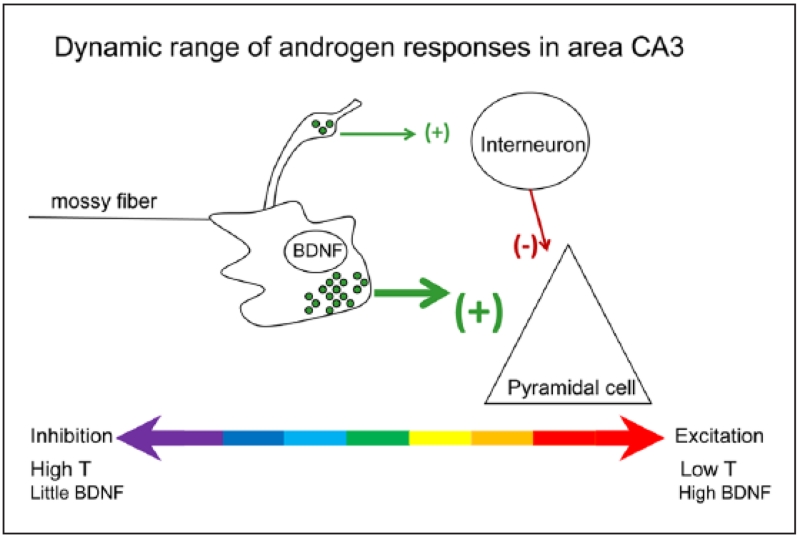
A schematic of the mossy fiber synapse shows a large bouton innervating a thorny excrescence of a CA3 pyramidal cell proximal apical dendrite. The large boutons have dense core vesicles that contain BDNF. A filamentous extension from the large bouton makes a smaller synaptic contact on a GABAergic neuron. Under normal conditions in the adult male rat, the mossy fiber system exerts a strong inhibitory tone because of the relatively low concentrations of BDNF (which normally facilitates glutamate release), the abundance of connections to the GABAergic interneurons, and high levels of the neurosteroid metabolite of testosterone, 5α-androstane-3α, 17β-diol, which facilitates GABA action at GABAA receptors. After gonadectomy, the mossy fiber pathway becomes more excitable, for two main reasons. First, there is a reduction in the effects of 5α-androstane-3α, 17β-diol at the GABAA receptor, because serum levels of its precursor (testosterone) fall. In addition, BDNF synthesis is increased while axonal sprouting occurs, so that more mossy fibers innervate area CA3 pyramidal cells (Skucas and others 2013).
Such a mechanism could potentially explain the apparent contradiction, noted above, of testosterone inducing hippocampal spine and spine synapse formation, while decreasing hippocampal BDNF expression and mossy fiber transmission. Increased BDNF expression following orchidectomy may activate GABAergic interneurons in Ammon’s horn, thereby decreasing activity-dependent spine and spine synapse formation on the pyramidal neurons (Murphy and others 1998; Skucas and others 2013). Conversely, the suppression of mossy fiber BDNF expression observed in response to testosterone could contribute to the increased spine density induced by this steroid, via a reduction in GABA interneuron-mediated inhibition, combined with increased postsynaptic NMDA receptor levels (Romeo and others 2005). However, plasticity in the mossy fiber pathway in response to an electrophysiological challenge (such as LTP) is simultaneously reduced, because of the testosterone-mediated reduction in BDNF synthesis (Skucas and others 2013). If this hypothesis is correct, physiological levels of testosterone may provide an overall stabilizing influence on the dentate gyrus-CA3 pathway, increasing the basal tone in the system, while reducing the likelihood of aberrant mossy fiber activation. Following orchidectomy, the dynamic range of hippocampal responses is increased, from tonic inhibition when activity in the mossy fibers is low, to powerful activation under conditions of increasing afferent stimulation, but at the cost of increased BDNF-mediated hippocampal instability (Scharfman and MacLusky 2014a) (Fig. 5).
A great deal more experimental work will need to be done before these proposed mechanisms can be accepted. Much of the above discussion remains speculative, with insufficient experimental evidence to determine whether key elements are anything more than hypothetical. We do not yet know how testosterone suppresses BDNF expression in the mossy fiber system or, indeed, whether this effect is in fact associated with increased activation of GABAergic interneurons in CA3. It remains to be demonstrated experimentally that what we predict in terms of an increased range of mossy fiber responses in orchidectomized, as compared to intact males, actually takes place. Nevertheless, the data so far provide tantalizing hints of possibilities yet to come, in terms of understanding how androgens may affect the brain, under both physiological and pathophysiological states. Androgens have been implicated in an extraordinary range of neurological disorders, ranging from developmental problems, such as autism (Baron-Cohen and others 2011), to epilepsy and depression (Herzog 1991; Seidman and Weiser 2013), and neurodegenerative disorders (Alsemari 2013; Barron and Pike 2012; Drummond and others 2009; Fuller and others 2007; Johnson and others 2010). Some of these androgen-sensitive conditions have been hypothesized to involve decreases in hippocampal neuroplasticity—for example, depression and schizophrenia, which have both been linked to abnormalities in tPA activity (Hoirisch-Clapauch and Nardi 2013; Hou and others 2009), raising the possibility that testosterone regulation of the relative amounts of pro-BDNF and mature BDNF in the brain (Jin and others 2007; Pang and others 2004; Rodier and others 2014) may contribute to sex differences in the incidence of these disorders. Unraveling the complex interplay between circulating and locally synthesized androgens, BDNF and neural activity in the hippocampus will be critical for development of a full understanding of how these steroids may affect hippocampal function, as well as the mechanisms underlying sex differences in response to changes in circulating androgen levels.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by NIH NS-37562 and the New York State Office of Mental Health (HS), and an NSERC Discovery Grant (NJM).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadiani A, Mandgary A, Sayyah M. Anticonvulsant effect of flutamide on seizures induced by pentylenetetrazole: involvement of benzodiazepine receptors. Epilepsia. 2003;44:629–35. doi: 10.1046/j.1528-1157.2003.36402.x. [DOI] [PubMed] [Google Scholar]
- Alibhai SM, Breunis H, Timilshina N, Marzouk S, Stewart D, Tannock I. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5030–7. doi: 10.1200/JCO.2010.30.8742. others. [DOI] [PubMed] [Google Scholar]
- Alsemari A. Hypogonadism and neurological diseases. Neurol Sci. 2013;34:629–38. doi: 10.1007/s10072-012-1278-4. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008;54:244–52. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur J Neurosci. 2010;32:1995–2002. doi: 10.1111/j.1460-9568.2010.07516.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011;9:e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:976–97. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S. Testosterone therapy in older men with late-onset hypogonadism: a counter-rationale. Endocr Pract. 2013;19:853–63. doi: 10.4158/EP13318.RA. [DOI] [PubMed] [Google Scholar]
- Beer TM, Bland LB, Bussiere JR, Neiss MB, Wersinger EM, Garzotto M. Testosterone loss and estradiol administration modify memory in men. J Urol. 2006;175:130–5. doi: 10.1016/S0022-5347(05)00049-2. others. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y, Muller D, Nikonenko I. Astrocyte-synapse structural plasticity. Neural Plast. 2014;2014:232105. doi: 10.1155/2014/232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchart J, Birch B, Bassily R, Wolfe L, Holmes C. Male sex hormones and systemic inflammation in Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27:153–6. doi: 10.1097/WAD.0b013e318258cd63. [DOI] [PubMed] [Google Scholar]
- Chang IY, Ohn T, Jeon YJ, Lee KH, Kim JW, Kim IY. A comparison of the steroidogenic acute regulatory protein-related lipid transfer (START) domain-containing 6 on the brain and testes between young and aged rats. Acta Histochem. 2014;116:551–8. doi: 10.1016/j.acthis.2013.11.008. others. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–8. doi: 10.1212/wnl.57.1.80. others. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Danzer SC, McNamara JO. Localization of brain-derived neurotrophic factor to distinct terminals of mossy fiber axons implies regulation of both excitation and feed-forward inhibition of CA3 pyramidal cells. J Neurosci. 2004;24:11346–55. doi: 10.1523/JNEUROSCI.3846-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, McMullen NT, Rance NE. Testosterone modulates the dendritic architecture of arcuate neuroendocrine neurons in adult male rats. Brain Res. 2001;890:78–85. doi: 10.1016/s0006-8993(00)03083-3. [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–53. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775–88. doi: 10.1083/jcb.201201038. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Resnick SM. Testosterone and cognition in normal aging and Alzheimer’s disease: an update. Curr Alzheimer Res. 2007;4:33–45. doi: 10.2174/156720507779939878. [DOI] [PubMed] [Google Scholar]
- Drummond ES, Harvey AR, Martins RN. Androgens and Alzheimer’s disease. Curr Opin Endocrinol Diabetes Obes. 2009;16:254–9. doi: 10.1097/MED.0b013e32832b101f. [DOI] [PubMed] [Google Scholar]
- Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci. 2012;367:2475–84. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg T, Metsis M, Timmusk T, Lindefors N. Entorhinal cortex regulation of multiple brain-derived neurotrophic factor promoters in the rat hippocampus. Neuroscience. 1993;57:891–6. doi: 10.1016/0306-4522(93)90034-d. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. others. [DOI] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol. 2012;131:24–9. doi: 10.1016/j.jsbmb.2011.11.010. others. [DOI] [PubMed] [Google Scholar]
- Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J Neurobiol. 1999;40:446–57. [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–3. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Fuller SJ, Tan RS, Martins RN. Androgens in the etiology of Alzheimer’s disease in aging men and possible therapeutic interventions. J Alzheimers Dis. 2007;12:129–42. doi: 10.3233/jad-2007-12202. [DOI] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–41. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25:1039–61. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Goudsmit E, Van de Poll NE, Swaab DF. Testosterone fails to reverse spatial memory decline in aged rats and impairs retention in young and middle-aged animals. Behav Neural Biol. 1990;53:6–20. doi: 10.1016/0163-1047(90)90729-p. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–91. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. J Clin Endocrinol Metab. 1996;81:3951–60. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology. 2007;148:1963–7. doi: 10.1210/en.2006-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–26. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LA. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology. 2013;154:3294–304. doi: 10.1210/en.2013-1129. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Sharma D, Uht R. A role for the androgen metabolite, 5alpha androstane 3beta, 17beta diol (3beta-diol) in the regulation of the hypothalamo-pituitary-adrenal axis. Front Endocrinol (Lausanne) 2011;2:65. doi: 10.3389/fendo.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden C, MacLusky NJ. Aromatase inhibition, testosterone, and seizures. Epilepsy Behav. 2004;5:260–3. doi: 10.1016/j.yebeh.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–7. doi: 10.1002/1098-1063(2000)10:6<693::AID-HIPO1007>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove L, MacLusky NJ, Scharfman HE. BDNF-estrogen interactions in hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63:559–65. doi: 10.1016/j.yhbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Reproductive endocrine considerations and hormonal therapy for men with epilepsy. Epilepsia. 1991;32(Suppl 6):S34–7. doi: 10.1111/j.1528-1157.1991.tb05890.x. [DOI] [PubMed] [Google Scholar]
- Hill RA, Wu YW, Kwek P, van den Buuse M. Modulatory effects of sex steroid hormones on brain-derived neurotrophic factor-tyrosine kinase B expression during adolescent development in C57Bl/6 mice. J Neuroendocrinol. 2012;24:774–88. doi: 10.1111/j.1365-2826.2012.02277.x. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Moffat SD. Increasing testosterone levels and effects on cognitive functions in elderly men and women: a review. Curr Drug Targets CNS Neurol Disord. 2005;4:531–40. doi: 10.2174/156800705774322049. [DOI] [PubMed] [Google Scholar]
- Hoirisch-Clapauch S, Nardi AE. Multiple roles of tissue plasminogen activator in schizophrenia pathophysiology. Semin Thromb Hemost. 2013;39:950–4. doi: 10.1055/s-0033-1357505. [DOI] [PubMed] [Google Scholar]
- Hou SJ, Yen FC, Tsai SJ. Is dysfunction of the tissue plasminogen activator (tPA)-plasmin pathway a link between major depression and cardiovascular disease? Med Hypotheses. 2009;72:166–8. doi: 10.1016/j.mehy.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Ito W, Chehab M, Thakur S, Li J, Morozov A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011;10:365–74. doi: 10.1111/j.1601-183X.2010.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–20. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 2005;96:48–53. doi: 10.1111/j.1464-410X.2005.05565.x. [DOI] [PubMed] [Google Scholar]
- Jin H, Lin J, Fu L, Mei YF, Peng G, Tan X. Physiological testosterone stimulates tissue plasminogen activator and tissue factor pathway inhibitor and inhibits plasminogen activator inhibitor type 1 release in endothelial cells. Biochem Cell Biol. 2007;85:246–51. doi: 10.1139/O07-011. others. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Day AE, Ho CC, Walker QD, Francis R, Kuhn CM. Androgen decreases dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22:238–47. doi: 10.1111/j.1365-2826.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly F, Alibhai SM, Galica J, Park A, Yi QL, Wagner L. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176:2443–7. doi: 10.1016/j.juro.2006.07.151. others. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Baulieu EE. Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1998;65:243–51. doi: 10.1016/s0960-0760(97)00191-x. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–21. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Khalil R, King MA, Soliman MR. Testosterone reverses ethanol-induced deficit in spatial reference memory in castrated rats. Pharmacology. 2005;75:87–92. doi: 10.1159/000087188. [DOI] [PubMed] [Google Scholar]
- Kim JH, Roberts DS, Hu Y, Lau GC, Brooks-Kayal AR, Farb DH. Brain-derived neurotrophic factor uses CREB and Egr3 to regulate NMDA receptor levels in cortical neurons. J Neurochem. 2012;120:210–9. doi: 10.1111/j.1471-4159.2011.07555.x. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EG, MacLusky NJ, Leranth C. Effects of testosterone on hippocampal CA1 spine synaptic density in the male rat are inhibited by fimbria/fornix transection. Neuroscience. 2003;122:807–10. doi: 10.1016/j.neuroscience.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Kurth F, Luders E, Sicotte NL, Gaser C, Giesser BS, Swerdloff RS. Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage Clin. 2014;4:454–60. doi: 10.1016/j.nicl.2014.03.001. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur J Neurosci. 2003;17:1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Lee VW, de Kretser DM, Hudson B, Wang C. Variations in serum FSH, LH and testosterone levels in male rats from birth to sexual maturity. J Reprod Fertil. 1975;42:121–6. doi: 10.1530/jrf.0.0420121. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004a;24:495–9. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Prange-Kiel J, Frick KM, Horvath TL. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb Cortex. 2004b;14:503–10. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–92. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–56. doi: 10.1016/s0306-4522(00)00369-9. [DOI] [PubMed] [Google Scholar]
- Leranth C, Szigeti-Buck K, MacLusky NJ, Hajszan T. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology. 2008;149:988–94. doi: 10.1210/en.2007-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M. Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res. 2012;1484:76–84. doi: 10.1016/j.brainres.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Arsenault L. Dentate granule cell layer collagen explant cultures: spontaneous axonal growth and induction by brain-derived neurotrophic factor or basic fibroblast growth factor. Neuroscience. 1996;74:1197–208. doi: 10.1016/0306-4522(96)00226-6. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006a;147:2392–8. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145:4154–61. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006b;138:957–65. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Martin JL, Finsterwald C. Cooperation between BDNF and glutamate in the regulation of synaptic transmission and neuronal development. Commun Integr Biol. 2011;4:14–6. doi: 10.4161/cib.4.1.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek RH, Sherwin BB. A randomized controlled trial of add-back estrogen or placebo on cognition in men with prostate cancer receiving an antiandrogen and a gonadotropin-releasing hormone analog. Psychoneuroendocrinology. 2010;35:215–25. doi: 10.1016/j.psyneuen.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav. 2007;92:1010–8. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen JK, Dow RC, Fink G. Gonadal steroids regulate number of astrocytes immunostained for glial fibrillary acidic protein in mouse hippocampus. Mol Cell Neurosci. 1992a;3:482–6. doi: 10.1016/1044-7431(92)90060-f. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wright AK, Arbuthnott GW, Fink G. Astrocytes immunoreactive for glial fibrillary acidic protein (GFAP) are increased in the mediobasal hypothalamus in hypogonadal (hpg) mice. Mol Cell Neurosci. 1992b;3:473–81. doi: 10.1016/1044-7431(92)90059-b. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Celotti F, Castano P, Martini L. Differential localization of the 5 alpha-reductase and the 3 alpha-hydroxysteroid dehydrogenase in neuronal and glial cultures. Endocrinology. 1993;132:1252–9. doi: 10.1210/endo.132.3.8440186. [DOI] [PubMed] [Google Scholar]
- Menard CS, Harlan RE. Up-regulation of androgen receptor immunoreactivity in the rat brain by androgenic-anabolic steroids. Brain Res. 1993;622:226–36. doi: 10.1016/0006-8993(93)90823-6. [DOI] [PubMed] [Google Scholar]
- Mendell AL, MacLusky NJ, Leranth C. Unilateral fimbria/fornix transection prevents the synaptoplastic effect of dehydroepiandrosterone in the hippocampus of female, but not male, rats. Neurosci Med. 2013;4:134. [Google Scholar]
- Meyer G, Ferres-Torres R, Mas M. The effects of puberty and castration on hippocampal dendritic spines of mice. A Golgi study. Brain Res. 1978;155:108–12. doi: 10.1016/0006-8993(78)90309-8. [DOI] [PubMed] [Google Scholar]
- Milner JS. Castration and amphetamine effects on acquisition of a Sidman avoidance task in rats. Physiol Behav. 1976;17:545–8. doi: 10.1016/0031-9384(76)90120-7. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Resnick SM. Long-term measures of free testosterone predict regional cerebral blood flow patterns in elderly men. Neurobiol Aging. 2007;28:914–20. doi: 10.1016/j.neurobiolaging.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser F, Raum WJ, Perry HM, 3rd, Flood JF, Jensen J. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A. 1997;94:7537–42. doi: 10.1073/pnas.94.14.7537. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Takata N, Ishii HT, Tanabe N, Hojo Y, Furukawa A. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience. 2006;138:757–64. doi: 10.1016/j.neuroscience.2005.09.010. others. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–9X. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JA, Stein IS, Lau CG, Peixoto RT, Aman TK, Kaneko N. Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J Neurosci. 2014;34:869–79. doi: 10.1523/JNEUROSCI.4538-13.2014. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z. The formation of estrogens by central neuroendocrine tissue. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. others. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 2001;897:44–51. doi: 10.1016/s0006-8993(00)03261-3. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer. 2008;113:1097–106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148:2936–43. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Henderson LP. The Sturm und Drang of anabolic steroid use: angst, anxiety, and aggression. Trends Neurosci. 2012;35:382–92. doi: 10.1016/j.tins.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba S, Ikeda T, Ikegaya Y, Nishiyama N, Matsuki N, Yamada MK. BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb Cortex. 2005;15:291–8. doi: 10.1093/cercor/bhh130. [DOI] [PubMed] [Google Scholar]
- Okun MS, Fernandez HH, Rodriguez RL, Romrell J, Suelter M, Munson S. Testosterone therapy in men with Parkinson disease: results of the TEST-PD Study. Arch Neurol. 2006;63:729–35. doi: 10.1001/archneur.63.5.729. others. [DOI] [PubMed] [Google Scholar]
- Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol. 2012;131:37–51. doi: 10.1016/j.jsbmb.2011.10.004. others. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148:3655–65. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–91. doi: 10.1126/science.1100135. others. [DOI] [PubMed] [Google Scholar]
- Parducz A, Garcia-Segura LM. Sexual differences in the synaptic connectivity in the rat dentate gyrus. Neurosci Lett. 1993;161:53–6. doi: 10.1016/0304-3940(93)90138-b. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Carmignani A, Cubeddu A, Santoro A, Cela V, Errasti T. Androgen therapy in women: for whom and when. Arch Gynecol Obstet. 2013;288:731–7. doi: 10.1007/s00404-013-2969-7. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha-androstanediol and 17beta-estradiol. Neuroscience. 2004;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L. Frank Beach Award Winner: Steroids as neuromodulators of brain circuits and behavior. Horm Behav. 2014;66:552–60. doi: 10.1016/j.yhbeh.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier M, Prigent-Tessier A, Bejot Y, Jacquin A, Mossiat C, Marie C. Exogenous t-PA administration increases hippocampal mature BDNF levels. plasmin-or NMDA-dependent mechanism? PLoS One. 2014;9:e92416. doi: 10.1371/journal.pone.0092416. others. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Romeo RD, Staub D, Jasnow AM, Karatsoreos IN, Thornton JE, McEwen BS. Dihydrotestosterone increases hippocampal N-methyl-D-aspartate binding but does not affect choline acetyltransferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology. 2005;146:2091–7. doi: 10.1210/en.2004-0886. [DOI] [PubMed] [Google Scholar]
- Roof RL. The dentate gyrus is sexually dimorphic in pre-pubescent rats: testosterone plays a significant role. Brain Res. 1993;610:148–51. doi: 10.1016/0006-8993(93)91228-k. [DOI] [PubMed] [Google Scholar]
- Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572:310–3. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–43. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–9. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 1990;127:3180–6. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Sato SM, Johansen JA, Jordan CL, Wood RI. Membrane androgen receptors may mediate androgen reinforcement. Psychoneuroendocrinology. 2010;35:1063–73. doi: 10.1016/j.psyneuen.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Chao MV. The entorhinal cortex and neurotrophin signaling in Alzheimer’s disease and other disorders. Cogn Neurosci. 2013;4:123–35. doi: 10.1080/17588928.2013.826184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Actions of brain-derived neurotrophic factor in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus. J Neurosci. 1999;19:5619–31. doi: 10.1523/JNEUROSCI.19-13-05619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology. 2014a;76(Pt C):696–708. doi: 10.1016/j.neuropharm.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis. 2014b doi: 10.1016/j.nbd.2014.07.004. Epub Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–52. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl JN, Massman PJ. Relationships between testosterone levels and cognition in patients with Alzheimer disease and nondemented elderly men. J Geriatr Psychiatry Neurol. 2014 doi: 10.1177/0891988714541872. Epub Jul 9. [DOI] [PubMed] [Google Scholar]
- Seidman SN, Weiser M. Testosterone and mood in aging men. Psychiatr Clin North Am. 2013;36:177–82. doi: 10.1016/j.psc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Steroid hormones and cognitive functioning in aging men: a mini-review. J Mol Neurosci. 2003;20:385–93. doi: 10.1385/JMN:20:3:385. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–12. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Skucas VA, Duffy AM, Harte-Hargrove LC, Magagna-Poveda A, Radman T, Chakraborty G. Testosterone depletion in adult male rats increases mossy fiber transmission, LTP, and sprouting in area CA3 of hippocampus. J Neurosci. 2013;33:2338–55. doi: 10.1523/JNEUROSCI.3857-12.2013. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Jones LS, Wilson MA. Sex differences in hippocampal slice excitability: role of testosterone. Neuroscience. 2002;109:517–30. doi: 10.1016/s0306-4522(01)00490-0. [DOI] [PubMed] [Google Scholar]
- Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE. DHEA effects on brain and behavior: Insights from comparative studies of aggression. J Steroid Biochem Mol Biol. 2014 doi: 10.1016/j.jsbmb.2014.05.011. E-pub Jun 11. [DOI] [PubMed] [Google Scholar]
- Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2012;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Sato SM, Woolley CS. Quantitative analysis of long-form aromatase mRNA in the male and female rat brain. PLoS One. 2014;9:e100628. doi: 10.1371/journal.pone.0100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–63. doi: 10.1016/j.neuroscience.2004.08.048. others. [DOI] [PubMed] [Google Scholar]
- Vierk R, Brandt N, Rune GM. Hippocampal estradiol synthesis and its significance for hippocampal synaptic stability in male and female animals. Neuroscience. 2014;274C:24–32. doi: 10.1016/j.neuroscience.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Wimer CC, Wimer RE. On the sources of strain and sex differences in granule cell number in the dentate area of house mice. Brain Res Dev Brain Res. 1989;48:167–76. doi: 10.1016/0165-3806(89)90073-4. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. doi: 10.1016/j.brainres.2009.09.111. [DOI] [PubMed] [Google Scholar]
- Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014;7:796–806. doi: 10.1016/j.celrep.2014.03.040. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, Baudry M. 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci U S A. 2009;106:21936–21941. doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Li H, Liu JP, Funder JW. Androgen stimulates mitogen-activated protein kinase in human breast cancer cells. Mol Cell Endocrinol. 1999;152:199–206. doi: 10.1016/s0303-7207(99)00031-3. [DOI] [PubMed] [Google Scholar]