Abstract
Objectives
The aim of this study was to establish the predictive role of obstetric variables for obstetric outcomes and birth related levator ani muscle trauma.
Methods
In this prospective study, women underwent 3D pelvic floor ultrasound at their first appointment at 36 weeks and also 3 months postpartum. The measurements included Minimal Levator Hiatus Circumference (MLHC), and the ratio of fetal head circumference (FHC) to MLHC = head induced stretch ratio (HISR) as an indicator of the discrepancy between passage and passing canal. To derive the true impact of baby’s mass on the levator ani musculature, we devised the levator ani stretch ratio (LASR), which was calculated by multiplying the HISR and the baby’s weight.
Results
Dataset of 173 women were available for analysis. Mean HISR and LASR values were statistically different across all binary outcome categories, with one exception for HISR and levator ani injury. The odds ratios for LASR indicated positive and statistically significant associations with all obstetric outcomes examined. The probability of the LASR correctly classifying those with the adverse obstetric outcome, as estimated by the area under the curve (AUC), ranged from 0.64 to 0.80 with the strongest discriminatory ability observed for severe levator ani muscle trauma.
Conclusions
Fetal head circumference/ mother minimal levator hiatus circumference ratio (HISR) is associated with longer length of second stage of labor, assisted delivery and increased severity of perineal trauma. Similar associations were observed for LASR, but in addition, LASR had good discriminatory ability to identify severe levator ani muscle trauma.
Keywords: pelvic floor trauma, Endovaginal ultrasound, vaginal birth
Introduction
The anatomical and functional integrity of levator ani muscle (LAM) plays a fundamental role in pelvic organ support. Throughout the whole female life span, the LAM closes the pelvic floor. It is only during vaginal delivery that it undergoes an enormous stretching, to allow the passage of the newborn (1). There have been attempts at defining the distension required for vaginal childbirth with help of biomechanical engineering and Finite Element modeling. These models suggest that some muscle damage during the second stage of labor may come from overstretching because those parts of the muscle that are stretched the most are those parts that are seen to be injured (2). Researchers have calculated maximum stretch ratios of 2.28– 3.26 at different muscle regions, which considerably exceeds the maximum stretch ratio of 1.5 tolerated by striated muscle in non-pregnant animal preparations (3). On the other hand, all women sustain stretching of their pelvic floor during birth, but only some experience injury. Prospective studies have shown that LAM injuries occur in 13–36% of women who deliver vaginally (4–6). Antepartum prediction of levator muscle trauma has been difficult or even impossible (7) but there are reports of obstetric risk factors associated with LAM trauma including operative vaginal delivery (8–10), episiotomy (10), prolonged second stage of labor (4, 9, 10), increased fetal head circumference (4) and increased maternal age (10).
The aim of the current study was to establish the predictive role of variables for levator ani muscle injury due to vaginal birth. We used the ratio of fetal head circumference (HC) to mother’s minimal levator hiatus circumference (MLHC) as an indicator of LAM distension ratio and assessed the association of HC/MLHC with levator ani trauma and known obstetric risk factors for LAM trauma including prolonged second stage of labor, operative delivery, episiotomy and perineal laceration.
Methods
This study was a secondary analysis of data collected from a previously published prospective observational study on “ levator ani muscle avulsion during childbirth: a risk prediction model” (8). Institutional Review Board approval was obtained from National Research Ethics Service South West London committee and the University of Oklahoma Health Sciences Center. Nulliparous women from the antenatal clinic and parent craft classes at Croydon University Hospital, London, UK were invited to participate in the parent study between January 2011 and May 2012. At the initial contact, patients were informed about the project, an information leaflet was given and contact details were collected. At 34 weeks of gestation the researcher telephoned eligible patients to inquire if they were interested in participating in the study. The inclusion criteria were a singleton pregnancy, maternal age >18 years, no previous history of pregnancy of more than 20 weeks of gestation, and being able to read and understand English. All nulliparous women were approached to participate to create a sample representative of the general population. The recruitment process has previously been described in detail (11). All women gave written informed consent during enrollment at 36 weeks of gestation. Subsequently, participants were invited by telephone, postal mail and electronic mail to book a follow-up appointment three months following childbirth. Demographic and obstetric details were collected from the hospital confidential notes. The current study was limited to participants of the parent study who delivered vaginally.
Ultrasound protocol
Levator ani muscle avulsion
Imaging was obtained using the BK Medical Ultrafocus (Peabody, MA, USA) and a 2052 12 MHz transducer. All postnatal ultrasound exams were performed in the office setting, with the patient in dorsal lithotomy position, with hips flexed and abducted. No preparation was required and the patient was recommended to have a comfortable volume of urine in the bladder. No rectal or vaginal contrast was used. To avoid excessive pressure on surrounding structures that might distort the anatomy, the probe was inserted into the vagina in a neutral position with no pressure on vaginal walls. US volumes were digitally stored for further analysis.
US volumes were evaluated blinded to patient symptoms, and muscles were scored according to levator ani deficiency (LAD) scoring system, which has been previously validated (12, 13). The levator muscle was divided into two subgroups; the puborectalis [PR], and iliococcygeus/pubococcygeus [PV] (14). Subgroups were evaluated and scored (1=no defect, 2=minimal defect with < 50% muscle loss, 3=major defect with >50% muscle loss, 4=total absence of the muscle) on each side based on thickness and detachment from the pubic bone. Partial and complete muscle loss was diagnosed if each muscle subgroup score was 3 or 4, respectively. Scores for the two muscle subgroups on both sides were summed and categorized as mild to moderate (scores 0–13) or extreme (scores 14–16) LAD.
Minimal levator hiatus area and circumference
Minimal levator hiatus area was measured in US volumes at 36 weeks of gestation. To obtain the measurement, US volumes were rotated to position them in an anatomically correct orientation as if the patient were lying down with the mid-sagittal view facing the reader. In order to find the minimal levator hiatus, we located the shortest line between the pubic symphysis and the levator plate prominence in the sagittal view. The anterior posterior (AP) line of the minimal levator hiatus was drawn. The axial plane was tilted posteriorly and was advanced cephalad parallel to the AP line. The mid-sagittal plane was expanded to make the whole volume visible. The minimal levator measurements (height A, width B, and area) were obtained in this plane, the “minimal levator hiatus plane” (Figure 1). Circumference was calculated using the following formula:
Circumference ≈
(pi/2)(A+B)[1 + H2/4 + H4/64 + H6/256 + 25H8/16384]
Where H = |A−B|/(A+B)
We used an online tool to convert area to circumference.
Figure 1.
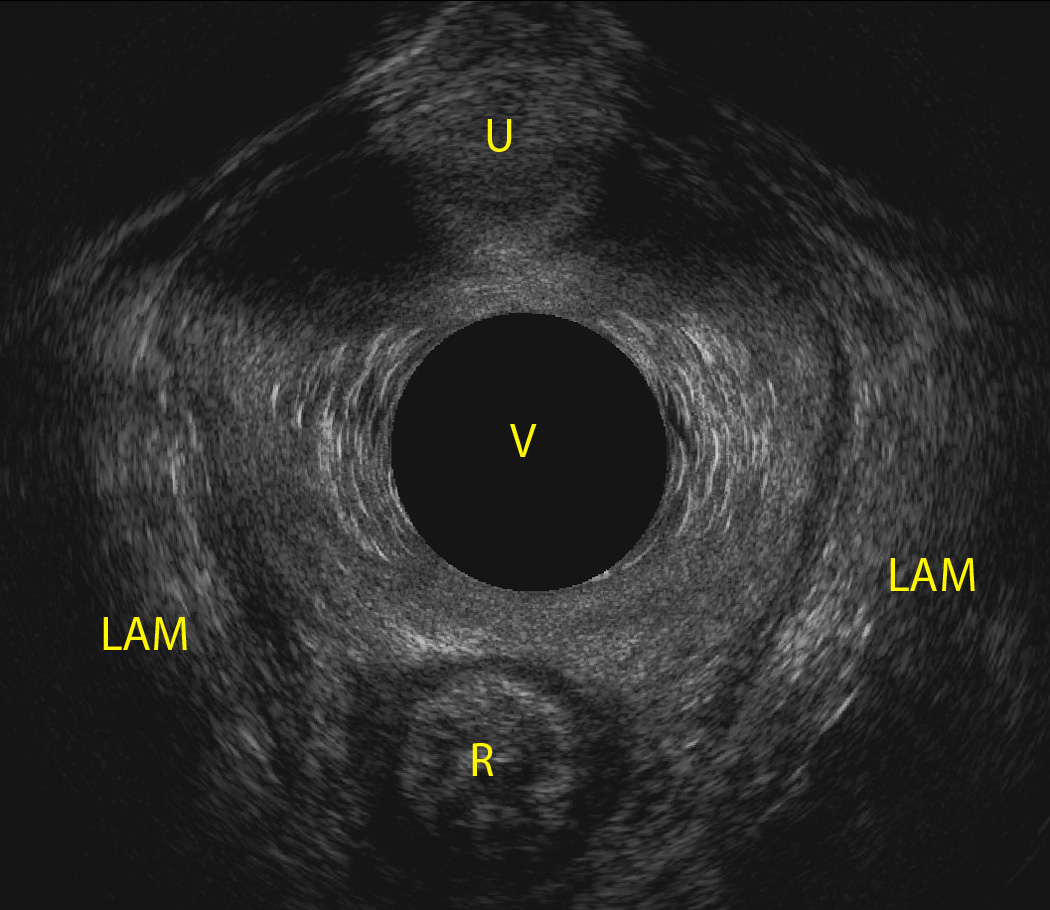
Minimal Levator hiatus surrounded by levator ani muscles and pubic arc, AM: levator ani muscle, R: Rectum, U: urethra, V: vagina
New levator ani muscle distention ratio indicator
Minimal levator hiatus circumference (MLHC) is the smallest muscular pelvic floor dimension during fetal passage. The ratio of fetal head circumference (FHC) to MLHC reflects the discrepancy between fetal head size and the passage canal and can be an indicator for pelvic floor stretch ratio associated with the initial passage of the head. The head induced stretch ratio (HISR) therefore is calculated by FHC/MLHC. Since we wanted to take into account not only the baby’s head, but also the baby’s size as another variable that stretched minimal levator hiatus beyond its stretch threshold, we multiplied FHC/MLHC and baby’s weight in kg (BW). Therefore the resultant levator ani stretch ratio (LASR) could be calculated by multiplying FHC/MLHC × BW in kg.
Obstetric outcomes
Obstetric outcomes were collected from the medical record. Length of second stage of labor was defined as ≥ 90 minutes and < 90 minutes between cervical dilation to 10 cm and delivery. Method of vaginal delivery was defined as unassisted or assisted (i.e., forceps and/or vacuum). Presence or absence of episiotomy was recorded as a binary indicator. A dichotomous measure of third degree laceration was defined as Subclass 3a, 3b or 3c compared to absent, first-degree or second-degree lacerations. No participants in this study experienced fourth-degree lacerations.
Statistical methods
Analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC). Mean HISR and LASR measures were compared across obstetric outcome categories (length of second stage of labor, method of delivery, episiotomy, third-degree laceration) and severe pelvic trauma using Student’s t-tests. ROC curves analyses were conducted to estimate the discriminatory ability of the HISR and LASR measures, as well as the individual components of these measures (baby weight, fetal head circumference and the minimal levator hiatus circumference), for predicting obstetric outcomes and extreme levator ani deficiency. The area under the curve (AUC) and 95% confidence intervals are reported for each measure. Differences in the areas under the ROC curves were compared using the non-parametric approach of DeLong, DeLong, and Clarke-Pearson (15). For each outcome, the optimal thresholds for the HISR and LASR measures were defined by the value maximizing sensitivity – (1-specificity). After considering the models for reporting ORs or RRs, it became clear that it would not be useful to estimate an association with the continuous value of the ratio measure since an increased risk associated with a one unit change in the ratio measure would not be very meaningful to the reader.
We concluded that it would be more useful to take the optimal threshold for the ratio measure and create an indicator variable. Then we used this as the independent variable in the model. Since we had a cohort study, we used the modified Poisson model to directly calculate a risk ratio (rather than a logistic regression which provides an odds ratio that only approximates the RR). Since each ratio measure had a different optimal cut point for each outcome (which becomes very complicated to describe and to report), we streamlined this by only reporting the RR result for the most promising association (between LASR and extreme LAD). Using the optimal threshold to create a binary indicator of the LASR, a modified Poisson regression model was used to report crude and adjusted risk ratios and 95% confidence interval for the association between LASR and severe pelvic floor trauma. Adjusted risk ratios were controlled for maternal age at enrollment, race/ethnicity and body mass index.
Results
Of the 269 participants in the parent study, 187 delivered vaginally and were eligible for the current study. Of these, 173 women had complete data on head circumference and MLHC measures and were included in this analysis. Study participants had a mean age of 29.6 (SD 5.7) and median BMI of 24 (range 16–46). Mean HISR and LASR were 2.42 (SD 0.29, range 1.72– 3.56) and 8.08 (SD 1.58, range 3.65– 14.05), respectively.
In all instances, mean values for HISR and LASR were greater among those with adverse outcomes (Table 1). Mean differences were statistically significant for all comparisons, with the exception of mean HISR by severe levator ani muscle trauma.
Table 1.
Comparison of mean measures of the head induced stretch ratio and levator ani stretch ratio by delivery outcomes and severity of pelvic floor trauma
| T-tests for Comparisons of Mean HISR and Mean LASR by Outcomes | |||||
|---|---|---|---|---|---|
| n | HISR Mean (std) |
p | LASR Mean (std) |
p | |
| Length of Second Stage | |||||
| ≤ 90 | 137 | 2.39 (0.28) | 7.88 (1.56) | ||
| >90 | 36 | 2.58 (0.27) | 0.0003 | 8.85 (1.42) | 0.0009 |
| Mode of Delivery | |||||
| Unassisted Vaginal | 105 | 2.35 (0.25) | 7.60 (1.37) | ||
| Assisted Vaginal | 68 | 2.54 (0.30) | <0.0001 | 8.67 (1.71) | 0.0001 |
| Laceration | |||||
| No Tear | 13 | 2.26 (0.26) | 6.90 (1.63) | ||
| Tear | 160 | 2.44 (0.28) | 0.03 | 8.17 (1.55) | 0.007 |
| Episiotomy | |||||
| No | 87 | 2.33 (0.24) | 7.61 (1.36) | ||
| Yes | 86 | 2.52 (0.30) | <0.0001 | 8.56 (1.65) | <0.0001 |
| LAD Score | |||||
| < 7 | 108 | 2.30 (.028) | 7.98 (1.45) | ||
| ≥ 7 | 26 | 2.40 (0.33) | 0.11 | 8.75 (1.70) | 0.03 |
| LAD Score | |||||
| < 14 | 128 | 2.40 (0.29) | 8.05 (1.54) | ||
| ≥ 14 | 6 | 2.62 (0.38) | 0.08 | 9.89 (2.00) | 0.005 |
The probability of the LASR correctly classifying those with the adverse outcomes, as estimated by the area under the curve (AUC), ranged from 0.66 to 0.80 with the strongest discriminatory ability observed for severe levator ani muscle trauma (Table 2 and Figure 2). For length of second stage of labor, assisted delivery, third and fourth degree lacerations and episiotomy, the HISR and LASR performed similarly for correctly classifying adverse outcomes. Based on the magnitude of the AUCs, overall the ratio measures had improved discriminatory ability over the individual measures of head circumference, birth weight and MLHC. However, the HISR did not outperform birth weight as an indicator of pelvic trauma. Statistical comparisons of the areas under the ROC curves indicated that LASR differed significantly from head circumference as an indicator of severe levator ani muscle trauma (p=0.04) but did not differ significantly from the other measures. Similarly, as a predictor of all other outcomes, LASR had significantly better discriminatory ability than birth weight (p<0.05). As a predictor of third/fourth degree lacerations, LASR also performed better than head circumference alone (p=0.04).
Table 2.
Area under the curve (AUC) and 95% confidence intervals for measures assessed as predictors of delivery outcomes and severe pelvic floor trauma
| Delivery Outcome | Head Induced Stretch Ratio AUC (95% CI) |
Levator Ani Stretch Ratio AUC (95% CI) |
Head Circumference AUC (95% CI_ |
MLHC AUC (95% CI)_ |
Birth Weight AUC (95% CI) |
|---|---|---|---|---|---|
| Second Stage of Labor >90 min | 0.70 (0.61–0.79) | 0.68 (0.60–0.77) | 0.60 (0.50–0.71) | 0.65 (0.56–0.75) | 0.57 (0.48–0.67) |
| Assisted Delivery | 0.68 (0.60–0.76) | 0.66 (0.57–0.74) | 0.62 (0.53–0.70) | 0.63 (0.55–0.72) | 0.57 (0.49–0.66) |
| Third/Fourth Degree Laceration | 0.75 (0.64–0.86) | 0.75 (0.64–0.86) | 0.61 (0.48–0.75) | 0.70 (0.58–0.82) | 0.65 (0.51–0.78) |
| Episiotomy | 0.69 (0.61–0.77) | 0.67 (0.59–0.75) | 0.61 (0.53–0.70) | 0.63 (0.55–0.72) | 0.58 (0.49–0.66) |
| Extreme LAD | 0.67 (0.48–0.87) | 0.80 (0.66–0.94) | 0.62 (0.34–0.90) | 0.66 (0.40–0.92) | 0.74 (0.51–0.99) |
Figure 2.
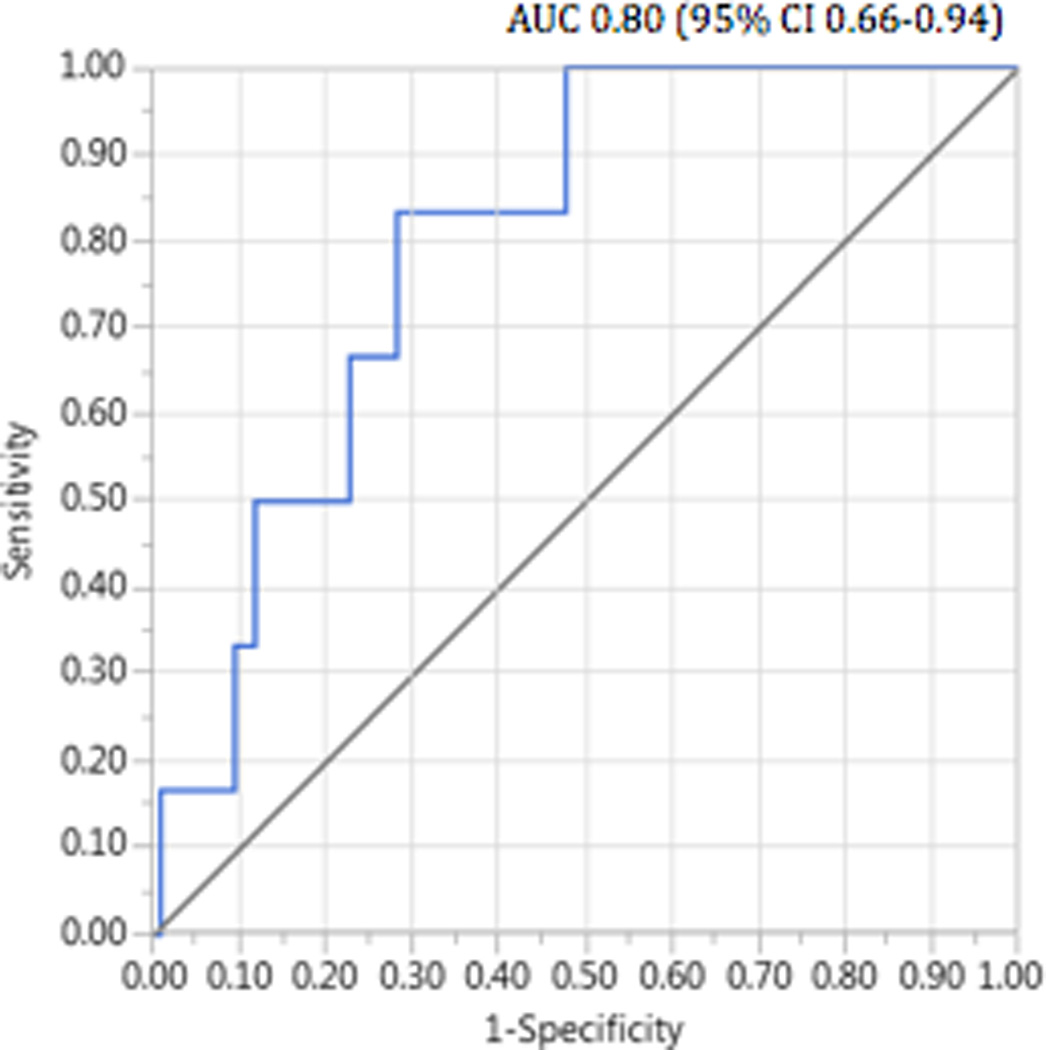
Receiver operating characteristic (ROC) curve for levator ani stretch ratio as a predictor of severe pelvic floor traum
When creating a binary indicator of the LASR score using the optimal threshold of the LASR (Table 3), the risk of severe levator ani muscle trauma was 6.6 times greater (RR 6.6, 95% CI 1.4–31.5) among those with a “positive” LASR (>8.86) compared to those with a “negative” LASR score, controlling for age. When controlling for age and body mass index, the risk ratio decreased to 5.4 (95% CI 0.96–30.35), but with only six events of severe levator ani muscle trauma the stability of the model may be comprised by exceeding the general rule of ten events per covariate.
Table 3.
Optimal thresholds and related sensitivity and specificity for the head induced stretch ratio and the levator ani stretch ratio as predictors of delivery outcomes and severe pelvic floor trauma
|
Delivery Outcome |
Head Induced Stretch Ratio Optimal Threshold (Se, Sp) |
Levator Ani Stretch Ratio Optimal Threshold (Se, Sp) |
|---|---|---|
| Second Stage of Labor >90 min | 2.36 (0.86, 0.50) |
7.68 (0.89, 0.46) |
| Assisted Delivery | 2.38 (0.75, 0.58) |
7.75 (0.74, 0.51) |
| 3rd /4thDegree Laceration | 2.50 (0.74, 0.69) |
8.05 (0.84, 0.56) |
| Episiotomy | 2.43 (0.63, 0.74) |
7.72 (0.76, 0.55) |
| Extreme LAD | 2.33 (1.00, 0.39) |
8.86 (0.83, 0.72) |
Conclusions
Fetal head circumference/mother’s minimal levator hiatus circumference ratio (HISR) is associated with longer length of second stage of labor, assisted delivery and increased severity of perineal trauma. When taking baby’s weight into account, similar associations were observed, but in addition, LASR demonstrated good discriminatory ability to predict severe levator ani muscle trauma.
Every experienced obstetrician can admit that exact prediction of labor process antenatally is impossible and that is due to multiple factors that play a role in that process. In an attempt to identify risk factors for complicated vaginal delivery, prior studies have shown that levator ani trauma at the time of first delivery is associated with forceps delivery and a longer second stage. Epidural pain relief may exert a protective effect (16). It is likely that birth weight, length of second stage, size of fetal head and forceps delivery increase the probability of muscle injury (17–20). Valsky showed an OR of 2.27 for muscle injury detected by ultrasound when the second stage was more than 110 minutes and OR of 3.34 for muscle injury when fetal head circumference was more than 35.5 cm (19). Falkert et al. also found a positive correlation with weight and head circumference of baby and area of levator hiatus (21). There are controversies regarding maternal age at first delivery. In some studies increased maternal age contributes to muscle injury (22), but not in some other studies (7, 19). Shek et al. found that women with lower BMI were at higher risk of sustaining muscle injury, but its clinical significance is questionable as BMI was set at 27.85 vs 30.01 kg/m2 (23). Additionally, this association has not been confirmed in another study (21). Ultrasound enables us to identify and evaluate the interpubic gap and the infrapubic arc with a high interrater agreement. It has been shown that infrapubic angle is not associated with length of second stage of labor and the occurrence of levator ani defects (24).
Our study demonstrated that discrepancy between baby’s head circumference and baby’s weight with mother’s pelvic floor biometry measured by pelvic floor ultrasound can predict the need for lengthened second stage of labor, assisted vaginal delivery, and also extent of pelvic floor and perineal trauma.
Our study was limited by a relatively small sample size. Additionally, we used the baby’s measurements immediately following birth, which is reasonably precise and readily available for research purposes; however, clinical application of these ratio measures would require an antenatal prediction model that uses fetal ultrasound measurements. Because fetal ultrasound measurement may be prone to some degree of measurement error, it is necessary to replicate this study using antenatal components. We are in the process of a prospective study to validate our model using antenatal measures and we will report the results in the near future.
Ability to predict the labor outcome has been the aim of many studies in the history of obstetrics. Recently with the advent of modern imaging techniques, valuable information regarding the birth canal biometry paved the way for more comprehensive understanding of the labor mechanism. The current study demonstrates how the assessment of the postnatal measures may inform the development of prenatal indicators of risk, which may ultimately guide efforts to prevent pelvic floor trauma during the vaginal delivery.
Acknowledgments
Financial Disclaimers/Conflict of interest: Dr. Peck was funded by NIH grant and Kim van Delft was funded by the Mayday Childbirth Charity Fund.
Abbreviation
- EVUS
endovagial ultrasound
- HC
head circumference
- MLHC
minimal levator hiatus circumference
- HISR
head induced stretch ratio
- LAM
levator ani muscle
- LASR
levator ani stretch ratio
References
- 1.Albrich SB, Laterza RM, Skala C, et al. Impact of mode of delivery on levator morphology: a prospective observational study with three-dimensional ultrasound early in the postpartum period. BJOG. 2012 Jan 2012;119(1):51–60. doi: 10.1111/j.1471-0528.2011.03152.x. PubMed PMID: 21985531. eng. [DOI] [PubMed] [Google Scholar]
- 2.Lien KC, Mooney B, DeLancey JO, et al. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004 Jan 2004;103(1):31–40. doi: 10.1097/01.AOG.0000109207.22354.65. PubMed PMID: 14704241. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashton-Miller JA, Delancey JO. On the biomechanics of vaginal birth and common sequelae. Annu Rev Biomed Eng. 2009;11:163–176. doi: 10.1146/annurev-bioeng-061008-124823. PubMed PMID: 19591614. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valsky DV, Lipschuetz M, Bord A, et al. Fetal head circumference and length of second stage of labor are risk factors for levator ani muscle injury, diagnosed by 3-dimensional transperineal ultrasound in primiparous women. Am J Obstet Gynecol. 2009 Jul 2009;201(1):91. doi: 10.1016/j.ajog.2009.03.028. PubMed PMID: 19481726. eng. [DOI] [PubMed] [Google Scholar]
- 5.Dietz HP, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol. 2005 Oct 2005;106(4):707–712. doi: 10.1097/01.AOG.0000178779.62181.01. PubMed PMID: 16199625. eng. [DOI] [PubMed] [Google Scholar]
- 6.Blasi I, Fuchs I, D'Amico R, et al. Intrapartum translabial three-dimensional ultrasound visualization of levator trauma. Ultrasound Obstet Gynecol. 2011 Jan 2011;37(1):88–92. doi: 10.1002/uog.8818. PubMed PMID: 20814872. eng. [DOI] [PubMed] [Google Scholar]
- 7.Shek KL, Dietz HP. Can levator avulsion be predicted antenatally? American Journal of Obstetrics & Gynecology. 2010 Jun;202(6):586.e1–586.e6. doi: 10.1016/j.ajog.2009.11.038. PubMed PMID: 20079479. English. [DOI] [PubMed] [Google Scholar]
- 8.van Delft K, Thakar R, Sultan A, et al. Levator ani muscle avulsion during childbirth: a risk prediction model. BJOG. 2014 Aug 2014;121(9):1155–1163. doi: 10.1111/1471-0528.12676. PubMed PMID: 24593314. eng. [DOI] [PubMed] [Google Scholar]
- 9.Shek KL, Dietz HP. Can levator avulsion be predicted antenatally? Am J Obstet Gynecol. 2010 Jun 2010;202(6):586. doi: 10.1016/j.ajog.2009.11.038. PubMed PMID: 20079479. eng. [DOI] [PubMed] [Google Scholar]
- 10.Kearney R, Miller JM, Ashton-Miller JA, DeLancey JO. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006 Jan 2006;107(1):144–149. doi: 10.1097/01.AOG.0000194063.63206.1c. PubMed PMID: 16394052. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Delft K, Schwertner-Tiepelmann N, Thakar R, et al. Recruitment of pregnant women in research. J Obstet Gynaecol. 2013 Jul 2013;33(5):442–446. doi: 10.3109/01443615.2013.767787. PubMed PMID: 23815192. eng. [DOI] [PubMed] [Google Scholar]
- 12.Rostaminia G, Manonai J, Leclaire E, Omoumi F, et al. Interrater reliability of assessing levator ani deficiency with 360 degrees 3D endovaginal ultrasound. Int Urogynecol J. 2014 Jun 2014;25(6):761–766. doi: 10.1007/s00192-013-2286-5. PubMed PMID: 24337615. eng. [DOI] [PubMed] [Google Scholar]
- 13.Rostaminia G, White D, Hegde A, et al. Levator ani deficiency and pelvic organ prolapse severity. Obstet Gynecol. 2013 May 2013;121(5):1017–1024. doi: 10.1097/AOG.0b013e31828ce97d. PubMed PMID: 23635738. eng. [DOI] [PubMed] [Google Scholar]
- 14.Shobeiri SA, Leclaire E, Nihira MA, et al. Appearance of the levator ani muscle subdivisions in endovaginal three-dimensional ultrasonography. Obstetrics & Gynecology. 2009;114:66–72. doi: 10.1097/AOG.0b013e3181aa2c89. PubMed PMID: 19546760. [DOI] [PubMed] [Google Scholar]
- 15.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep 1988;44(3):837–845. PubMed PMID: 3203132. eng. [PubMed] [Google Scholar]
- 16.Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. BJOG. 2010 Nov;117(12):1485–1492. doi: 10.1111/j.1471-0528.2010.02704.x. Epub 2010/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 17.Krofta L, Otcenasek M, Kasikova E, et al. Pubococcygeus-puborectalis trauma after forceps delivery: evaluation of the levator ani muscle with 3D/4D ultrasound. International urogynecology journal and pelvic floor dysfunction. 2009 Oct;20(10):1175–1181. doi: 10.1007/s00192-009-0837-6. Epub 2009/07/30. eng. [DOI] [PubMed] [Google Scholar]
- 18.Albrich SB, Laterza RM, Skala C, et al. Impact of mode of delivery on levator morphology: a prospective observational study with three-dimensional ultrasound early in the postpartum period. BJOG. 2012 Jan;119(1):51–60. doi: 10.1111/j.1471-0528.2011.03152.x. Epub 2011/10/12. eng. [DOI] [PubMed] [Google Scholar]
- 19.Valsky DV, Lipschuetz M, Bord A, Messing B, Hochner-Celnikier D, et al. Fetal head circumference and length of second stage of labor are risk factors for levator ani muscle injury, diagnosed by 3-dimensional transperineal ultrasound in primiparous women. American journal of obstetrics and gynecology. 2009 Jul;201(1):91, e1–e7. doi: 10.1016/j.ajog.2009.03.028. Epub 2009/06/02. eng. [DOI] [PubMed] [Google Scholar]
- 20.Cassado Garriga J, Pessarrodona Isern A, Espuna Pons M, et al. Tridimensional sonographic anatomical changes on pelvic floor muscle according to the type of delivery. International urogynecology journal. 2011 Aug;22(8):1011–1018. doi: 10.1007/s00192-011-1413-4. Epub 2011/04/07. eng. [DOI] [PubMed] [Google Scholar]
- 21.Falkert A, Endress E, Weigl M, et al. Three-dimensional ultrasound of the pelvic floor 2 days after first delivery: influence of constitutional and obstetric factors. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010 May;35(5):583–588. doi: 10.1002/uog.7563. Epub 2010/01/20. eng. [DOI] [PubMed] [Google Scholar]
- 22.Dietz HP, Simpson JM. Does delayed child-bearing increase the risk of levator injury in labour? Aust N Z J Obstet Gynaecol. 2007 Dec;47(6):491–495. doi: 10.1111/j.1479-828X.2007.00785.x. Epub 2007/11/10. eng. [DOI] [PubMed] [Google Scholar]
- 23.Shek KL, Dietz HP. Can levator avulsion be predicted antenatally? American journal of obstetrics and gynecology. 2010 Jun;202(6):586 e1–586 e6. doi: 10.1016/j.ajog.2009.11.038. Epub 2010/01/19. eng. [DOI] [PubMed] [Google Scholar]
- 24.Albrich S, Laterza RM, Merinsky A, et al. Measurement of the infrapubic angle using 3D perineal ultrasound and its relationship to obstetrical parameters. Ultraschall in der Medizin (Stuttgart, Germany : 1980) 2012 Dec;33(7):E95–E100. doi: 10.1055/s-0031-1299053. Epub 2012/06/23. Die Messung des infrapubischen Winkels in der 3-D-Perineal-Sonografie und seine Beziehung zu geburtshilflichen Parametern. ger. [DOI] [PubMed] [Google Scholar]


