Abstract
Background
Pressure drift (PD), resulting from differences between room and body temperature, reduces the accuracy of pressure measurements with the Manoscan high resolution manometry (HRM) system. Our aims were to assess PD during anorectal HRM.
Methods
Defined as the residual pressure measured immediately after the catheter was removed, PD was calculated for each sensor and averaged across all 12 sensors in 454 anorectal consecutive studies recorded with 3 HRM catheters. The relationship between PD and study duration, number of prior uses of a catheter, and peak and average pressure exposure during a study were evaluated. The correction of PD with a software algorithm (thermal compensation) was evaluated in 76 studies where the most distal sensor was outside the body.
Key Results
The PD varied among sensors and across catheters. The average PD (7.3±0.2 mm Hg) was significantly greater for newer catheters, during longer studies, or when sensors were exposed to higher pressures. Together, these factors explained 81% of the variance in overall PD. After thermal compensation, the uncorrected median PD for the most distal sensor was 2.5–5 mm Hg over the study duration. Correcting this changed the interpretation (e.g., as abnormal instead of normal) of at least 1 anorectal parameter in 8 of 76 studies.
Conclusions & Inferences
During anorectal HRM, PD declines with catheter use and is greater for newer catheters, when sensors are exposed to higher pressures, and for studies of longer duration. While PD is partially corrected with thermal compensation algorithms, the impact on interpretation is modest.
Keywords: anorectal manometry, constipation, fecal incontinence
Anorectal manometry (ARM) is used to diagnose defecatory disorders and identify anal weakness in fecal incontinence (FI) (1–3). While high resolution manometry (HRM) catheters provide better spatial resolution, the pressures recorded with ManoscanTM HRM catheters vary over time (4, 5). Although pressure drift (PD) has been attributed to the difference between the temperature at which the catheter is calibrated (i.e., room temperature) and the body temperature, in vitro experiments in a water bath at constant temperature and an in vivo study with esophageal HRM observed that the drift increased linearly over time (4, 5). Moreover, PD is not remedied by the software algorithm (‘thermal compensation’) designed to correct this when the catheter is removed from the body at the end of the study. Uncorrected PD reduces the accuracy of pressure measurements. The aims of this study were to evaluate PD during anorectal HRM, factors contributing to PD, and the thermal compensation algorithm.
MATERIALS AND METHODS
Experimental Design
This Institutional Review Board at Mayo Clinic approved this study. All consecutive 482 clinical anorectal HRM studies were conducted with 3 randomly selected Manoscan 360 (Medtronics Inc, Shorewood, MN) catheters by a single, trained, experienced, registered nurse between August 2013 and August 2014 without the disposable sanitary sheath. All catheters had been appropriately maintained and were within the 2- year warranty period with less than 200 uses. Pressures were evaluated at rest, during three squeeze maneuvers, and during simulated evacuation maneuvers before and during rectal distention (6–8).
Twenty two studies in which patients had not provided research authorization and 6 studies in which the recording did not include the time when the catheter was removed from the anorectum were excluded, leaving 454 for analysis. As detailed previously (5), the PD was defined as the residual pressure measurement immediately after the catheter was removed; theoretically this should be atmospheric pressure (Figure 1). At this moment, thermal compensation was applied. This compensation subtracted the measured instantaneous pressure of each sensor uniformly from the entire in vivo recording of that sensor (Figure 1). These residual pressures and the entire pressure dataset for each study were saved as American Standard Code for Information Interchange (ASCII) files and exported to Microsoft Excel (Microsoft Inc, Redmond, WA).
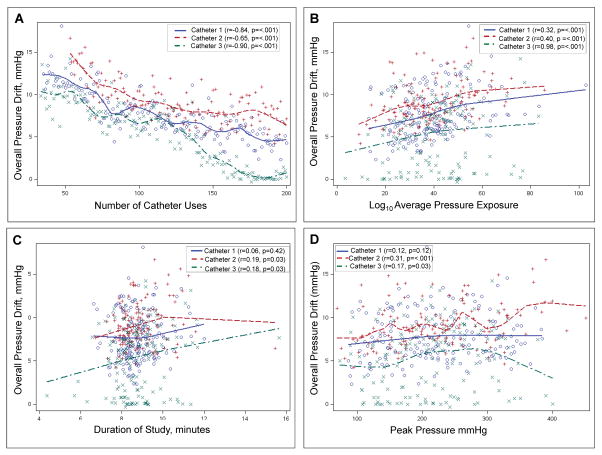
Figure 1. Factors influencing overall pressure drift.
Average pressure drift across sensors in a study is inversely correlated with (A) Number of prior catheter uses, and directly correlated with (B) Average pressure exposure across sensors, (C) Length of study, and (D) Peak pressure exposure across sensors.
In 76 studies, the most distal sensor was outside the anus throughout the study. After thermal compensation, this sensor should record a pressure of 0 mm Hg. Values deviating from 0 mm Hg for this sensor reflect partially uncorrected or residual PD. This residual PD was applied to all sensors, and the impact of this adjustment on the interpretation of the following anorectal parameters (i.e., anal pressure at rest and during squeeze) and the following measures during evacuation (i.e., anal pressure, anal relaxation, rectal pressure increase during evacuation, and rectoanal gradient) as normal or abnormal was assessed.
Statistical Analysis
The relationship between PD and the number of catheter uses, the average and peak pressures recorded with each sensor, and the duration of each study was evaluated univariately with Spearman correlation coefficients. Significant predictors were included in a multiple variable autoregressive error model. Data are summarized as Mean±SEM unless stated otherwise.
RESULTS
The manometric studies lasted 8.8±0.1 minutes. The overall PD was positive in 445 (98%) of studies and greater than 5 mm Hg, which exceeds the reported accuracy of 1–2 mm Hg, in 80% of studies. The overall PD, averaged across all sensors, was 7.3±0.2 mm Hg. However, for individual sensors, the PD ranged from −16 to +26 mm Hg. For the sensor with the greatest drift in each study, the PD in all studies was 12.3±0.2 mm Hg.
The overall PD was inversely correlated with the number of uses and directly correlated with the average and peak pressures to which sensors were exposed and the duration of the study (Figure 1). Except for study duration, all other variables remained significant and explained 81% of the variance in the overall PD in the multiple variable model (Table 1). This model suggests that the PD declined by an average of 0.05 mm Hg after every study. This translates to an average reduction of 5 mm Hg after 100 uses, which is 50% of the guaranteed 200 uses for each catheter. The PD was generally the highest in sensors 5 and 6 (data not shown).
Table 1.
Univariate and Multiple Variable Analyses of Factors Associated With Pressure Drift
| Variable | Association with overall pressure drift a | Multiple variable model b | ||
|---|---|---|---|---|
| Catheter 1 | Catheter 2 | Catheter 3 | ||
| Use no., per use | −0.84** | −0.65** | −0.90 | −0.05±0.002 ** |
| Catheter 2 c | 1.9±0.3 ** | |||
| Catheter 3 c | −2.3±0.3 ** | |||
| Duration, per min. | 0.06 | 0.19* | 0.18* | 0.07±0.07 |
| Log10 Average Pressure | 0.32** | 0.40** | 0.19* | 4.3±0.5 ** |
| Peak Pressure, per mmHg | 0.12** | 0.31** | 0.17* | 0.002±0.001 |
| Total variance explained (%) | 81 | |||
Spearman correlation coefficient
Dependent (outcome) variable was overall pressure drift; All values are regression coefficients
Compared to catheter 1
P<.05,
P<.001
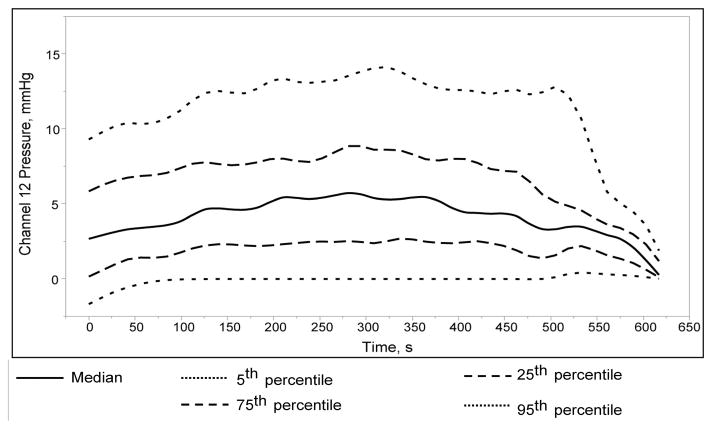
Among 76 studies in which the most distal sensor was outside the anus throughout the study, the median distribution of uncorrected pressure drift (i.e., even after thermal compensation) recorded by this sensor was between 2.5 and 5 mm Hg (Figure 2). Correcting for this residual PD changed the interpretation in 8 of 76 patients – anal pressure at rest was normal instead of high in 3, anal pressure during squeeze was low instead of normal in 4, and anal pressure during evacuation was normal instead of high in 1 patient.
Figure 2. Distribution of pressure recorded by the last sensor in studies where it was confirmed to be outside the body for the entire duration of the study (N=76).
Even after thermal compensation, the pressure recorded was >5 mm Hg in >25% of studies.
DISCUSSION
There are 4 main observations. First, anorectal pressures recorded with a Manoscan catheter drift over time. While the overall PD across all sensors was relatively small, for individual sensors, the PD ranged from −16 to +26 mm Hg. Second, the magnitude of PD varied among sensors in a catheter and also for the same sensor across catheters. Nearly 80% of the measured catheter drift can be explained, in order of magnitude, by the following factors: prior number of uses of the catheter, the average pressure to which the catheter was exposed during the study, and the duration of the study. Of note, PD decreased substantially with increasing use of a given catheters, consistent with the ‘break in’ effect suggested by Babaei et al (5). Third, the software-based thermal compensation algorithm only partly compensated the PD. Fourth, the incomplete correction of PD by this algorithm infrequently altered the diagnostic interpretation.
Save for minor differences in the predictor variables (factors) contributing to PD, these findings are very similar to PD during esophageal HRM (5). Indeed, the contribution of these predictor variables to PD was comparable in the esophagus and anorectum. Sensor location (i.e., upper or lower esophageal sphincter, esophagus, or stomach) is less relevant in the anorectum. In HRM catheters, pressure affects the distance between 2 electrical capacitors which comprise the sensor element (personal communication, Medtronics Inc.). When the catheter is inserted into the body, the air between these 2 capacitors expands because of the temperature difference between room and body temperature, which affects the pressure recorded by the catheter. Two components contribute to PD, i.e., an immediate component, which is likely attributable to the temperature difference between room and body temperature, that occurs instantaneously when the catheter is inserted into the anorectum; and a second component, i.e., increasing PD over time during the study.
HRM catheters are calibrated every week in a water bath at 37° C. This process identifies major differences in sensor performance over time. Before each study, the catheters are calibrated in a pressurized chamber. Lastly, thermal compensation is applied when the catheter is withdrawn from the body at the end of the study. This feature instructs the software to set that point to zero pressure. The offset or difference between the pressure recorded by the catheter and 0 mm Hg (atmospheric pressure) after the algorithm is applied is deducted from each sensor throughout the study. This assumes that the thermally driven offset occurs within a short timeframe after the catheter is introduced into the body and explains why the algorithm is biased toward adequately correcting the PD towards the end of the study at the cost of over correcting for PD at the beginning of the study. The thermally compensated pressure recorded by this most distal sensor was outside the reported performance boundaries (±2 mm Hg for less than 50% of the duration of the study in 76% of studies. This sensor may not have been exposed to room temperature because the adjacent air could have absorbed heat radiating from the buttocks. Because the other sensors were not exposed to atmospheric pressure during the study, the accuracy of the thermal compensation algorithm for these sensors could not be evaluated. However, assuming that this residual PD obtained from the most distal sensor could also be applied to the other sensors, the clinical interpretation would change in 8 of 76 studies.
These findings provide some insights into the possible mechanisms of PD. Because the PD declined with increasing catheter use, it seems unlikely that wear and tear contributes to PD. The PD was greater for catheters that were exposed to higher pressures in the body. However, because the peak pressure to which catheters were exposed and study duration had a small effect, maneuvers which expose catheters to high pressures (e.g., during placement or removal, or manual pressure) are unlikely to markedly impact catheter drift. PD was not uniform across sensors, suggesting additional effects beyond temperature and usage.
Key Messages.
Similar to esophageal high resolution manometry (HRM), there is pressure drift (PD), resulting in part from differences between the temperature at which the catheter is calibrated (room temperature) and body temperature, during anorectal HRM.
The overall PD, i.e., averaged across all sensors, was 7.3±0.2 mm Hg. The number of uses, average and peak pressures to which sensors were exposed, and the duration of the study explained 81% of the variance in overall PD.
Even after thermal compensation, the median distribution of pressure recorded by the sensors in atmosphere ranged from 2.5 to 5 mm Hg over the duration of the study; ideally, this should be 0 mm Hg. In 8 of 76 studies, the partial correction of PD could potentially have altered the clinical impression of rectoanal pressures at rest, during squeeze and evacuation.
Acknowledgments
Funding: This study was supported by USPHS NIH Grant R01 DK078924.
Abbreviations
- ARM
anorectal manometry
- HRM
high resolution manometry
- PD
pressure drift
Footnotes
Author Contributions: AEB, GP, and JM designed the research study.
KF, GP, and JM analyzed the data.
ARZ conducted the statistical analysis.
AEB, JM, and GP wrote the paper.
All authors approved the final version of this manuscript.
Disclosure: No competing interests declared.
References
- 1.Bharucha AE, Rao SS. An update on anorectal disorders for gastroenterologists. Gastroenterology. 2014;146:37–45. e32. doi: 10.1053/j.gastro.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141–1157. doi: 10.1038/ajg.2014.190. (Quiz) 1058. [DOI] [PubMed] [Google Scholar]
- 3.Lee TH, Bharucha AE. How to Perform and Interpret a High-resolution Anorectal Manometry Test. J Neurogastroenterol Motil. 2016;22:46–59. doi: 10.5056/jnm15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson EV, Lee YY, Derakhshan MH, et al. High-resolution esophageal manometry: addressing thermal drift of the manoscan system. Neurogastroenterol Motil. 2012;24:61–64. e11. doi: 10.1111/j.1365-2982.2011.01817.x. [DOI] [PubMed] [Google Scholar]
- 5.Babaei A, Lin EC, Szabo A, Massey BT. Determinants of pressure drift in Manoscan(TM) esophageal high-resolution manometry system. Neurogastroenterol Motil. 2015;27:277–284. doi: 10.1111/nmo.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noelting J, Ratuapli SK, Bharucha AE, Harvey DM, Ravi K, Zinsmeister AR. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012;107:1530–1536. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratuapli SK, Bharucha AE, Noelting J, Harvey DM, Zinsmeister AR. Phenotypic identification and classification of functional defecatory disorders using high-resolution anorectal manometry. Gastroenterology. 2013;144:314–322. e312. doi: 10.1053/j.gastro.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharucha AE, Stroetz R, Feuerhak K, Szarka LA, Zinsmeister AR. A novel technique for bedside anorectal manometry in humans. Neurogastroenterol Motil. 2015;27:1504–1508. doi: 10.1111/nmo.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]