Abstract
Background
As the effect of biomedical prevention interventions on the natural history of HIV-1 infection in participants who seroconvert is unknown, the Microbicide Trials Network (MTN) established a longitudinal study (MTN-015) to monitor virologic, immunological, and clinical outcomes, as well as behavioral changes among women who become HIV-infected during MTN trials. We describe the rationale, study design, implementation and enrollment of the initial group of participants in the MTN seroconverter cohort.
Methods
Initiated in 2008, MTN-015 is an ongoing observational cohort study enrolling participants who acquire HIV-1 infection during effectiveness studies of candidate microbicides. Eligible participants from recently completed and ongoing MTN trials are enrolled after seroconversion and return for regular follow-up visits with clinical and behavioral data collection. Biologic samples including blood and genital fluids are stored for future testing.
Results
MTN-015 was implemented initially at 6 African sites and enrolled 100/139 (72%) of eligible women who seroconverted in HIV Prevention Trials Network protocol 035 (HPTN 035, conducted by the MTN). The median time from seroconversion in HPTN 035 to enrollment in MTN-015 was 18 months. Retention was good with >70% of visits completed. Implementation challenges included regulatory reviews, translation and testing of questionnaires, and site readiness.
Conclusions
Enrollment of HIV-seroconverters into a longitudinal observational follow up study is feasible and acceptable to participants. Data and samples collected in this protocol will be used to assess safety of investigational HIV microbicides and answer other important public health questions for HIV infected women.
Keywords: HIV, Clinical Trial Design, Cohort Study, Prevention, Microbicides, Pre-exposure Prophylaxis, Seroconversion, Drug resistance
Introduction
Randomized clinical trials of biomedical HIV prevention interventions, including topical microbicides and oral pre-exposure prophylaxis (PrEP) with antiretrovirals (ARVs), are necessary to identify effective products. In the course of such trials, HIV infections may be observed among study participants in the intervention arm due to lack of efficacy or poor adherence to potentially efficacious study products. The development of studies for the longitudinal follow up of the HIV seroconverters from effectiveness trials of biomedical interventions for HIV prevention has been recommended in order to fully understand and describe the impact of novel prevention modalities on subsequent HIV disease.1 Carefully collected longitudinal data on seroconverters may identify differences in the clinical course of HIV infection among individuals exposed to different biomedical prevention modalities and inform guidance for policy makers and prescribers. Additionally, prospective assessment of study participants with recent HIV infection and known dates of seroconversion from clinical trials provides a valuable resource to evaluate important biologic and behavioral public health research questions.2
Although not observed with products evaluated to date, it is possible that use of microbicides or oral PrEP at the time of HIV infection may result in a beneficial effect, such as slower disease progression.2,3 An ongoing concern with ARV-based prevention is the possibility that topical or oral ARVs will select for drug resistant HIV in those who become infected or are unknowingly already infected while using the prevention agent, and that this in turn may result in poorer outcomes of subsequent antiretroviral therapy (ART). Clinical trials of oral PrEP have reported a low incidence of acquired resistance to tenofovir and emtricitabine with the greatest risk among individuals who are unknowingly infected at the time of study enrollment.4-9 The risk of resistance with topically applied products may be lower due to high tissue concentrations; however, data are limited.9-11
In order to assess the impact of investigational ARV-based products for HIV prevention on the natural history of HIV infection in women who become infected or are unknowingly already infected while receiving an investigational product, the Microbicide Trials Network (MTN) developed and implemented a longitudinal cohort study (MTN-015) that enrolls participants who acquire HIV while participating in MTN trials. In this paper we describe the design and implementation of this HIV seroconverter protocol at sites in Sub-Saharan Africa.
Methods
Study Design
MTN-015 is an ongoing, multi-site, prospective observational cohort study enrolling participants who acquire HIV-1 infection during effectiveness studies of candidate microbicides conducted by the MTN. Potential participants are offered enrollment in MTN-015 following identification of HIV seroconversion in the parent trial (the prevention trial during which they were identified as HIV-infected). The diagnosis and confirmation of HIV seroconversion is performed in the parent trial. Participants are offered the opportunity to be enrolled concurrently in the MTN parent trial and MTN-015 for the duration of the parent trial and subsequently continue in MTN-015 for longer term follow up. The minimum planned duration of follow-up is 12 months from seroconversion; additional follow up is dependent on the results of the prevention trial and the availability of funding. The eligible parent studies to date include HIV Prevention Trials Network protocol 035 (HPTN 035), MTN-003 (VOICE) and MTN-020 (ASPIRE); findings from only the first protocol HPTN 035 will be reported on here. HPTN 035 was a randomized, Phase II/IIb, safety and effectiveness trial of BufferGel and 0.5% PRO2000 microbicide gels for HIV prevention. HPTN 035 was conducted from February, 2005 to January, 2009, and was initiated by the HPTN and completed by the MTN.12 HPTN 035 study sites conducting MTN-015 were located in Malawi (Lilongwe and Blantyre), South Africa (Durban), Zambia (Lusaka), and Zimbabwe (Harare and Chitungwiza).
Potential participants are recruited for MTN-015 as soon as possible after identification of HIV seroconversion. In order to provide adequate comparison groups for specific analyses of interest, all participants with seroconversion during microbicide study participation are eligible for enrollment into MTN-015 regardless of the assigned study arm (intervention or placebo). All participants provide written informed consent prior to participation. All consent forms, participant education materials, and questionnaires are translated into local languages. The protocol was reviewed and approved by each site’s Institutional Review Board (IRB) or Ethics Committee (EC) prior to study implementation. The MTN-015 study is registered at www.clinicaltrials.gov (NCT00514098) and the protocol can be accessed at http://www.mtnstopshiv.org.
Study Procedures
Follow up visits are scheduled based on the seroconversion date and ART initiation date, if applicable. The enrollment visit is completed as soon as possible following identification of HIV infection. Follow-up visits occur at 1, 3, and 6 months after seroconversion, and every 6 months thereafter. When participants initiate ART, the ART start date is used as the basis for scheduling visits. Figure 1 provides a schematic representation of three examples of visit schedules. The purpose of the ART visit schedule is to capture early time points for virologic outcomes after ART initiation. Past or current use of any ARV (excluding the use of single-dose nevirapine for prevention of mother to child transmission (PMTCT)) is considered to be initiation of ART. Participants who initiate other single or dual ARV for PMTCT are followed on the ART schedule but are considered separately for analysis of outcomes.
Figure 1.
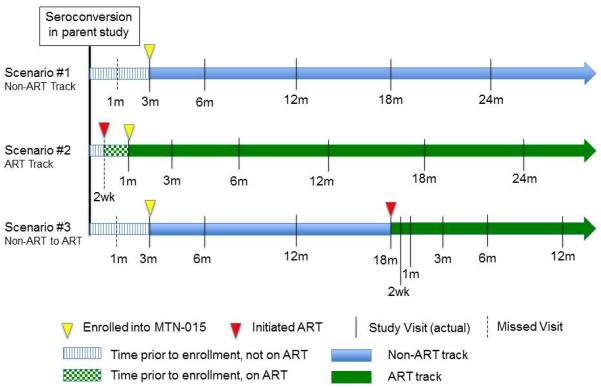
Examples of MTN-015 visit schedules. In Scenario 1, a participant is enrolled 3 months after seroconversion; the month 1 visit is missed. In Scenario 2, a participant begins ART prior to enrollment into MTN-015. The target dates for the visit schedule are based on the ART initiation date. In Scenario 3, a participant is enrolled 3 months after seroconversion and begins ART 18 months after seroconversion. The target dates for subsequent study visits are reset to be based on the ART initiation date.
Study visit procedures are summarized in Table 1. Demographics, locator information, ART status, sexual behaviors, contraceptive use, and seroconversion symptoms are collected at the enrollment visit via case-report forms. Medical history including all medications and HIV-related and AIDS-defining conditions (obtained by participant self-report and confirmed when possible by medical records) is collected at every visit.
Table 1.
Summary of study procedures
| Enrollment | M1 Post- Seroconversion |
M3 Post - Seroconversion |
M 6/every 6 M Post- Seroconversion |
W2, M 1, M 3 Post-ART Initiation |
M6 and every 6 M Post-ART Initiation |
|
|---|---|---|---|---|---|---|
| Clinical | ||||||
| Acute Seroconversion Assessment | X | |||||
| Concomitant Medications Assessment | X | X | X | X | X | X |
| Antiretroviral Treatment Record | ▲ | X | X | |||
| Physical Exam | X | X | X | X | X | X |
| Behavioral | ||||||
| Baseline Behavioral Questionnaire | X | |||||
| Follow-Up Behavioral Questionnaire | X | X (M12 and M 24) |
X (M 3 Post-ART Initiation) |
X (M12 and M 24 Post-ART Initiation) |
||
| ART Adherence Questionnaire | ▲ | X (M 3) | X | |||
| Laboratory Procedures | ||||||
| Total Blood Volume Collected (mL) | 83 | 72 | 77 | 77 | 72 | 77 |
| Urine Pregnancy Test | X | ▲ | ▲ | ▲ | ▲ | ▲ |
| Sexually Transmitted Infection testing1 | X | ▲ | ▲ | X (every 12 M) |
▲ | X (every 12 M) |
| CBC, Liver and Renal Function | X | X | X | ▲ | X | |
| CD4 T-Cell Count | X | X | X | X | X | X |
| Plasma HIV-1 RNA | X | X | X | X | X | X |
| HIV-1 Genotypic Resistance Test | X | ▲ | ▲ | |||
| HIV Serology | ▲ | |||||
| PAP Smear2 | X | ▲ | ▲ | X (every 12 M) |
▲ | X (every 12 M) |
| Specimens for Storage | ||||||
| Vaginal Swabs | X | X | X | X | X | X |
| Cervicovaginal Lavage | X | X | X | X | X (M 3) | X |
| Plasma | X | X | X | X | X | X |
| Serum | X | |||||
| PBMC 2 | X | X | X | X | X (W 2 and M 3) |
X |
▲: performed if clinically indicated
M: Month; W: Week; ART: Antiretroviral therapy; CBC: Complete Blood Count;
Urine SDA for Chlamydia, GC, Syphilis Serology, Wet Mount for BV, Candida, Trichomonas
Performed at sites with capacity
Laboratory procedures
Laboratory procedures include CD4 T-cell count and plasma HIV-1 RNA (at every visit); and complete blood count, creatinine, liver enzymes, and urine pregnancy testing (Table 1). Testing for sexually transmitted infections (STI) is performed at enrollment, annually, and when clinically indicated based on symptoms at or between scheduled visits (syphilis serology, urine nucleic acid amplification testing for Chlamydia trachomatis and Neisseria gonorrhea, and saline microscopy or rapid antigen test for Trichomonas vaginalis). Plasma HIV genotypic resistance testing is performed by the MTN Network Laboratory on samples with HIV RNA ≥ 200 copies/ml using ViroSeq, Version 2.0 (Celera Diagnostics, Alameda, CA) or in-house modifications as described previously.20 Samples are tested at enrollment if results are not available from the parent study, and if clinically indicated per the site investigator during participant follow-up (for example, poor response to ART). Follow-up resistance testing is also done on plasma samples from participants who had resistance in the parent trial to learn about the persistence of mutations once active product is stopped and the potential impact of resistance selected from microbicide/PrEP use on future ART. Additionally, repository samples including serum (at enrollment only), plasma, peripheral blood mononuclear cells, vaginal swabs and cervicovaginal lavage are collected at most study visits (Table 1).
Behavioral assessments
Participants complete behavioral questionnaires at enrollment and at months 3, 12, and 24 following seroconversion. The questionnaires include assessments of sexual practices, partnership status, disclosure of HIV status, availability and use of health care and social services, and depression (using the 8 item subset of the Hopkins Symptom Checklist (HSCL-25).13,14 Additionally a social harms assessment is completed at every visit. The behavioral assessments were conducted by face-to-face interview initially but were changed to audio-computer-assisted self-interviews (ACASI) with an abbreviated face-to-face interview in Version 2 of the protocol (implemented in May 2013) and assessments for substance use were added. Behavioral questionnaires were pre-tested at a number of sites in multiple languages through a formal process including staff and proxy participant cognitive interviewing. For participants who initiate ART, behavioral questionnaires are done at months 3, 12, and 24 following ART initiation, and a brief ART adherence assessment is completed at each visit.
Clinical care
Physical examinations including pelvic exams, and counseling for contraceptive options, STI risk reduction and HIV-1 secondary prevention are provided at enrollment and each 6 month interval. Family planning services and STI treatment are provided on-site. Each site has established referral mechanisms for HIV related care, and active referral including scheduling appointments and accompanying participants, as needed, is used to promote uptake of care, especially ART for participants who meet local guidelines for ART initiation. Pregnant women are counseled extensively and referred for antenatal care, including PMTCT. Study laboratory results are provided to the participants and other care providers (with permission of the participant).
Data Management and Analytic Objectives
All data are recorded on case report forms and transmitted to the MTN Statistical and Data Management Center (SDMC) using the DataFax data management system (Clinical DataFax Systems Inc., Ontario, Canada). The SDMC reviews data for accuracy and sends quality control queries to the study sites on a routine basis. The data from the seroconverters are reviewed by the parent study Data Safety and Monitoring Committee during the conduct of effectiveness studies. After completion of each parent study, the MTN-015 study data are reviewed approximately annually by a study monitoring committee convened by the MTN. The primary analytic objectives of MTN-015 are to compare HIV disease progression post-seroconversion among participants assigned to an active agent compared to placebo/control participants 12 months following seroconversion (minimum planned duration of follow-up for each participant) and over the total duration of study follow-up. Secondary objectives include comparisons of virologic and immunologic responses following initiation of ART by study arm, HIV-1 drug resistance profiles among ART recipients at the time of virologic failure by study arm, and assessments of post-seroconversion changes in sexual behaviors and partnership status. In this report, descriptive statistics were used to summarize participant characteristics using SAS (SAS, Inc; Cary, North Carolina, USA).
Results
Study Implementation
MTN-015 protocol development was initiated after the formation of the MTN in 2006, and the protocol was finalized in June, 2007 with the first site activation in August, 2008. The time from finalization of the protocol to site activation for this initial group of six sites that participated in HPTN 035 ranged from 14 to 21 months. The causes of delay in site activation were several and included regulatory reviews by IRBs and ECs, including the requirement for review by a United States based university IRB in addition to the local site EC for most sites. The total time required for regulatory approvals ranged from approximately 4 to 12 months. Additionally, time was required for the translation, back-translation, and review of the informed consent and behavioral questionnaires from English into 5 languages at the initial trial sites (currently forms are available in 9 languages). For most sites, MTN-015 was the first study for which HIV-infected participants were enrolled, thus development of site-specific plans for review and management of laboratory results and referral mechanisms for HIV care in the community was required. Centralized staff training was conducted including clinical training in HIV staging and laboratory assays, opportunistic infections, ART and drug resistance, and prevention of maternal to child transmission (PMTCT).
Characteristics and Follow Up of MTN-015 Participants Enrolled from HPTN 035
Among the 194 women with HIV seroconversion in HPTN 035, 139 were at sites conducting subsequent MTN trials and 100/139 (72%) of these eligible women were enrolled into MTN-015. The median time from the first positive rapid test for HIV-1 in HPTN 035 to enrollment was 18.2 months (range 5.0 to 45.7). As a result of the enrollment time frame, the analysis of HIV disease progression 12 months after seroconversion could not be performed for the HPTN 035 participants. The median follow up time was 48 months from enrollment (66 months from first positive rapid HIV test in HPTN 035). The median age at enrollment was 27 years (range 20 to 45). The majority of participants had at least some secondary education (59%) and 56% earned an income on their own; 48% were married, 38% were unmarried but with a male sexual partner, and 14% were unmarried and without a partner. More participants from South Africa were unmarried (94%) than those from the other 3 countries (28-33%).
The retention rate for the non-ART track was >70% at each visit through month 48 after seroconversion. For the ART track, adherence to the visit schedule was 98%, 98%, and 91% of participants retained at 3, 6 and 12 months after initiation of ART, respectively.
Select Behavioral Outcomes
Baseline behavioral questionnaires were completed by all 100 participants from HPTN 035. Most of the women reported having disclosed their HIV status to someone (86%) and mostly to a husband (51%) or regular partner/boyfriend (33%). Reactions from their husbands/regular partners included positive responses such as suggestions to seek care from a doctor (36% of the husbands and 36% of the regular partners) and to use condoms regularly (63% and 75%, respectively); however, 9% reported husbands made the participant leave their house, 5% of husbands and 3% of partners refused to continue sex with the participant, and 14% of husbands took another wife/partner. When asked about the consequence of disclosing their HIV status many women reported a response of sadness from their male partners (36% of those reporting a husband and 46% of those reporting regular partners) and 16% of women with husbands and 11% of those with regular partners reported the men responded with anger. Sixty percent of the participants were depressed (using a cut-off score of 1.06 or greater from the Johns Hopkins Index).
Antiretroviral Therapy
The majority of participants from HPTN 035 were enrolled into MTN-015 prior to receipt of ARV, however 18 women were ARV-experienced: 14 were on combination ART and 4 received ARV for PMTCT. For the participants who were ARV-naïve, the median CD4 T-cell count was 405 cells/mm3 (interquartile range [IQR] 273, 657), and median plasma HIV-1 RNA was 3.9 log10 copies/ml (IQR 3.3, 4.6) (N=81). Additionally, although the median time from diagnosis to MTN-015 enrollment was approximately 18 months, at the enrollment visit only 50 (50%) reported that they had ever seen a health care provider for HIV care or treatment.
A total of 68/100 (68%) women initiated any ARV prior to or during participation in MTN-015; 54 initiated combination ART (3 or more medications) and 14 received a nucleoside reverse transcriptase inhibitor (NRTI) as PMTCT. Country-level and/or World Health Organization (WHO) guidelines were closely adhered to for the choices of combination ART. The combination ART regimens were most commonly non-nucleoside reverse transcriptase (NNRTI) based (nevirapine with 2 NRTIs in 36 participants, efavirenz with 2 NRTI in 15 participants); only 3 women initiated therapy with a protease inhibitor (lopinavir/ritonavir in all cases). Regimens containing nevirapine and stavudine were frequently used in the 2007-2010 period with a shift to efavirenz and tenofovir (with lamivudine or emtricitabine) in the subsequent years reflecting changes in WHO guidance and ARV availability.15
Pregnancies and Pregnancy outcomes
Thirty-four pregnancies were reported among 28 participants; 6 participants were pregnant at enrollment into MTN-015, 21 participants became pregnant during follow-up and 1 participant was pregnant at enrollment and had 1 additional pregnancy detected during follow-up. Outcomes were reported for 32 of 34 pregnancies. For the 32 pregnancy outcomes assessed, 30 were singleton births and 2 were twins. Of the 32 pregnancy outcomes assessed, 29 (85%) were full term births, 2 (6%) were premature births, and 3 (9%) were spontaneous abortions.
ARV use was assessed for all 28 participants (34 pregnancies). Four women (4 pregnancies) reported no ARV for PMTCT, 12 women (13 pregnancies) received single NRTI ARV for PMTCT with zidovudine (12 pregnancies) or stavudine (1 pregnancy) as per the standard guidelines at the time of the pregnancies, and 12 women were on combination ART during pregnancy (total of 17 pregnancies).
HIV Subtypes and Genotypic Drug Resistance
HIV genotypic resistance test results were available from 85/100 participants from the enrollment visit. Results were not available for 15 participants due to insufficient HIV-1 RNA (<200 copies/mL) (14 samples) and sequencing failure (1 sample). Of the 85 participants with HIV-1 sequence results, 84 were infected with Subtype C HIV-1 and 1 was infected with an unusual subtype (B/K). Several resistance-associated polymorphisms were identified (F77F/L, V90I/V, E138E/A, and V179A/D/T in reverse transcriptase; M46I/L in protease) however none were associated with reductions in susceptibility to ARV medications. Only one participant had HIV-1 with a major NNRTI mutation, K103K/N – this participant had initiated ART with a nevirapine-based regimen 9 days prior to collection of this sample. A follow up plasma sample tested for resistance after approximately 19 months of ART (nevirapine, tenofovir and emtricitabine) revealed that the early-ART K103K/N was no longer detectable and was replaced by A62V/K65R/M184V with V108I and G190A.
Follow up plasma specimens for resistance testing were available for 3 additional participants who had a pre-ART baseline sample and subsequently failed ART with a viral load of ≥200 copies/mL. All three initiated ART with stavudine, lamivudine and an NNRTI (nevirapine or efavirenz). One participant had no resistance mutations pre-ART, but had M184M/I post-ART. One participant had E138A both pre- and post-ART, and the third participant had E138A pre-ART which remained post-ART in addition to K101K/E, K103K/N, and V106V/M.
AIDS-Defining Conditions
WHO-defined advanced HIV infection conditions (Clinical Stage 3 or 4)16 were observed during study follow up in 16 participants (24 events total). The most common events were severe bacterial infection (7 events), severe unexplained weight loss of greater than 10% of body weight (5 events), and tuberculosis [5; extrapulmonary (2) and pulmonary (3)].
Additional Microbicide Parent Studies
For each additional effectiveness trial conducted by the MTN, implementation of MTN-015 was initiated in parallel with the parent study. In addition to HPTN 035, participants with seroconversion have been enrolled from _ENREF_9MTN-003 (VOICE, conducted September 2009 to August 2012)9, and MTN-020 (ASPIRE, conducted August 2012 to June 2015).17 The median time from the first positive HIV-1 test for MTN-003 participants to enrollment into MTN-015 was 2.1 months (Table 2). Follow up of seroconverters from HPTN 035 and MTN-003 was discontinued in May, 2013 and June, 2014, respectively; enrollment and follow up of participants from MTN-020 is ongoing.
Table 2.
Parent microbicide studies enrolling HIV-1 seroconverters to MTN-015
| Parent study | Study short title | Parent study arms | Proportion eligible participants enrolled |
Median time from HIV seroconversion to MTN-015 enrollment |
Parent study reference |
|---|---|---|---|---|---|
| HPTN 035 | Safety and effectiveness of BufferGel and PRO2000 |
|
72% | 18 months | Abdool Karim, et al., 2011 |
| MTN-003 | Tenofovir-based HIV Preexposure Prophylaxis (VOICE) |
|
72% | 2 months | Marrazzo, et al., 2015 |
| MTN-020 | Safety and effectiveness of a dapivirine vaginal ring (ASPIRE) |
|
71%* | NA* | Palanee-Phillips, et al., 2015 |
Accrual ongoing; NA not available
Discussion
We describe the implementation of MTN-015, an observational cohort study enrolling participants who acquire HIV-1 infection during effectiveness studies of investigational products conducted by the Microbicide Trials Network. Our experience with the HPTN 035 participants illustrate some of the challenges that may be encountered in the implementation of seroconverter protocols as well as the potential value of the data collected and capacity to have excellent retention of a cohort facing many personal, physical, and social concerns. Despite the observational nature of the study, significant delays in implementation resulted from prolonged regulatory reviews, translation and testing of questionnaires, and site readiness issues. We recommend early planning for similar studies to include pre-trial discussions with local regulatory committees, establishment of mechanisms for site testing of behavioral questionnaires, and dedicated site staffing for the seroconverter trial.
The enrollment of this initial cohort of participants from HPTN 035 provided the MTN and the trial sites with valuable insights. For example, despite participation in HPTN 035 and direct post-study referral to community clinics, approximately one-half of the women were not in care for HIV infection at the time of MTN-015 enrollment. The reasons for the low uptake of referral to the HIV clinics are not clear. At the time of the completion of HPTN 035, ART availability was limited to individuals with CD4 T-cells ≤200 cells/mm3 (per WHO guidelines) thus women may not have perceived any benefit of going to the clinic. Another possible reason was depression which has been shown to be associated with linkage to and retention in care in some, but not all, studies.18-20 The reported prevalence of depression in HIV-infected African women varies with the instrument and cutoff used. We observed 60% of women to be depressed at enrollment, similar to that reported by Antelman et al. for Tanzanian women using the same 8-item subscale and cutoff.21 During MTN-015 follow up, site staff reported continued resistance of some participants for attending the ART clinics but were able to encourage and support uptake of care over time. Additionally, when WHO guidelines were modified in 2010 to recommend ART for CD4 T-cells ≤350 cells/mm3, this information was incorporated into counseling messages for participants.
The difficulties in the initial implementation have been largely overcome. For the MTN-003 (VOICE) study, the protocol was implemented all 15 sites and most study sites were able to begin enrollment concurrently or shortly after initiation of the parent study. The median time from the first positive rapid HIV-1 test to MTN-015 enrollment for VOICE was 2.1 months compared to 18 months for HPTN 035, thus the data from the VOICE participants is more comprehensive and complete. Improved integration of the VOICE and MTN-015 protocols have allowed for linkage of data from pre- to early- to post-seroconversion. More recently, the study initiated audio computer-assisted self-interview for the behavioral questionnaire, a methodology that will improve disclosure of sensitive information such as reports of sexual activity that may be stigmatizing particularly if the interviewer is a male (i.e. having multiple partners and transactional partners) as demonstrated by an ancillary study conducted in HPTN 035.22 This study will provide valuable information for how some African women’s patterns of disclosure, experience of depression, and relationships with their partners will change in the period following seroconversion and how this may change over time as availability of ART expands in Africa.
In summary, we have demonstrated that structured, comprehensive, longitudinal follow up of HIV seroconverters is feasible, acceptable, and beneficial to participants as evidenced by successful implementation of the protocol, enrollment of >70% of eligible participants and excellent retention. The participants benefit by remaining connected to a familiar study environment with supportive staff and close clinical observation including risk-reduction counseling, STI testing, and monitoring of HIV RNA and CD4 cell counts. MTN-015 provides key data for researchers and policy makers for understanding the implications of HIV transmission in the setting of a prevention trial.
Acknowledgements
The MTN-015 Team is grateful for the dedication of the participants in this study, and for the work of the staff from all of the MTN sites. The authors would like to acknowledge Karen Patterson for data management, and Krista Eskay, Russell Hardesty and Amy Opest for resistance testing and data analysis. The Microbicide Trials Network is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Etter P, Landovitz R, Sibeko S, et al. Recommendations for the follow-up of study participants with breakthrough HIV infections during HIV/AIDS biomedical prevention studies. Aids. 2013 Apr 24;27(7):1119–1128. doi: 10.1097/QAD.0b013e32835dc08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrett NJ, Werner L, Naicker N, et al. HIV disease progression in seroconvertors from the CAPRISA 004 tenofovir gel pre-exposure prophylaxis trial. J Acquir Immune Defic Syndr. 2015 Jan 1;68(1):55–61. doi: 10.1097/QAI.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kersh EN, Luo W, Zheng Q, et al. Reduced inflammation and CD4 loss in acute SHIV infection during oral pre-exposure prophylaxis. The Journal of infectious diseases. 2012 Sep 1;206(5):770–779. doi: 10.1093/infdis/jis422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New England Journal of Medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Liegler T, Defechereux P, et al. Drug resistance and plasma viral RNA level after ineffective use of oral pre-exposure prophylaxis in women. Aids. 2015 Jan 28;29(3):331–337. doi: 10.1097/QAD.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 7.Liegler T, Abdel-Mohsen M, Bentley LG, et al. HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. The Journal of infectious diseases. 2014 Oct 15;210(8):1217–1227. doi: 10.1093/infdis/jiu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012 Aug 2;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 9.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. New England Journal of Medicine. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X, Hunt G, Abdool Karim SS, et al. Sensitive tenofovir resistance screening of HIV-1 from the genital and blood compartments of women with breakthrough infections in the CAPRISA 004 tenofovir gel trial. The Journal of infectious diseases. 2014 Jun 15;209(12):1916–1920. doi: 10.1093/infdis/jiu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdool Karim SS, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. Aids. 2011 Apr 24;25(7):957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesbacher PT, Rickels K, Morris RJ, Newman H, Rosenfeld H. Psychiatric illness in family practice. The Journal of clinical psychiatry. 1980 Jan;41(1):6–10. [PubMed] [Google Scholar]
- 14.Kaaya SF, Fawzi MC, Mbwambo JK, Lee B, Msamanga GI, Fawzi W. Validity of the Hopkins Symptom Checklist-25 amongst HIV-positive pregnant women in Tanzania. Acta psychiatrica Scandinavica. 2002 Jul;106(1):9–19. doi: 10.1034/j.1600-0447.2002.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. http://www.who.int/hiv/pub/guidelines/en/. Accessed 12/4/2015.
- 16.Organization WH WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-related Disease in Adults and Children. 2007 [Google Scholar]
- 17.Palanee-Phillips T, Schwartz K, Brown ER, et al. Characteristics of Women Enrolled into a Randomized Clinical Trial of Dapivirine Vaginal Ring for HIV-1 Prevention. PloS one. 2015;10(6):e0128857. doi: 10.1371/journal.pone.0128857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naik R, Doherty T, Jackson D, et al. Linkage to care following a home-based HIV counselling and testing intervention in rural South Africa. Journal of the International AIDS Society. 2015;18:19843. doi: 10.7448/IAS.18.1.19843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turan B, Stringer KL, Onono M, et al. Linkage to HIV care, postpartum depression, and HIV-related stigma in newly diagnosed pregnant women living with HIV in Kenya: a longitudinal observational study. BMC pregnancy and childbirth. 2014;14:400. doi: 10.1186/s12884-014-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenney JL. Behavior change and other factors related to HIV transmission among female sero-converters in Microbicide Trials. Retrieved from: http://escholarship.org/uc/item/7kj8z5wc.
- 21.Antelman G, Kaaya S, Wei R, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007 Apr 1;44(4):470–477. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorbach PM, Mensch BS, Husnik M, et al. Effect of computer-assisted interviewing on self-reported sexual behavior data in a microbicide clinical trial. AIDS and behavior. 2013 Feb;17(2):790–800. doi: 10.1007/s10461-012-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


