Abstract
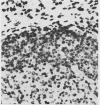
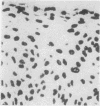
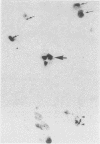
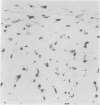
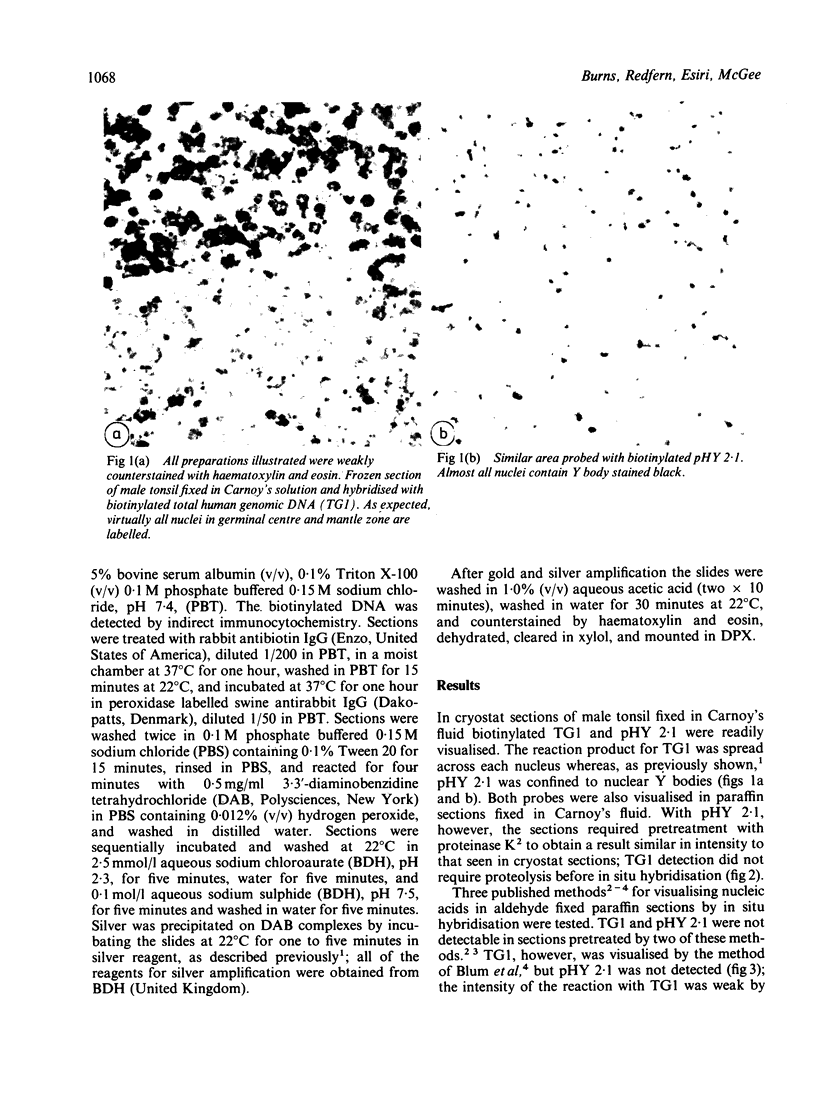
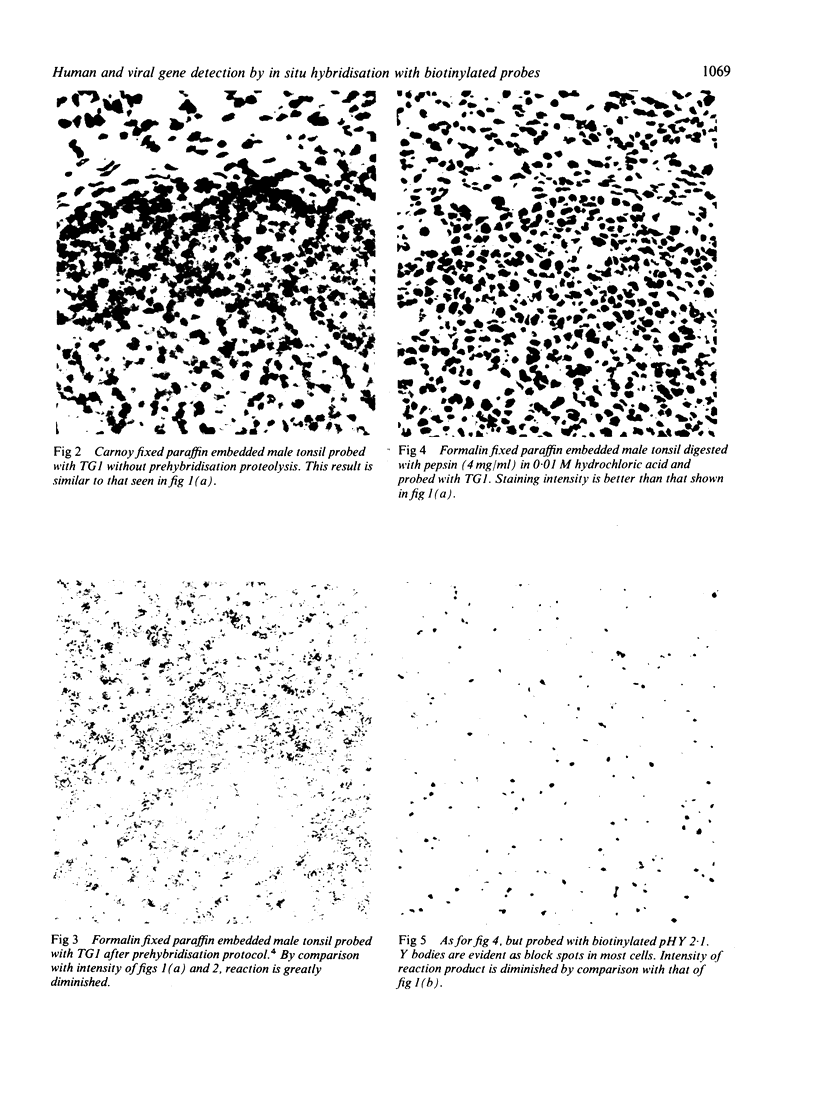
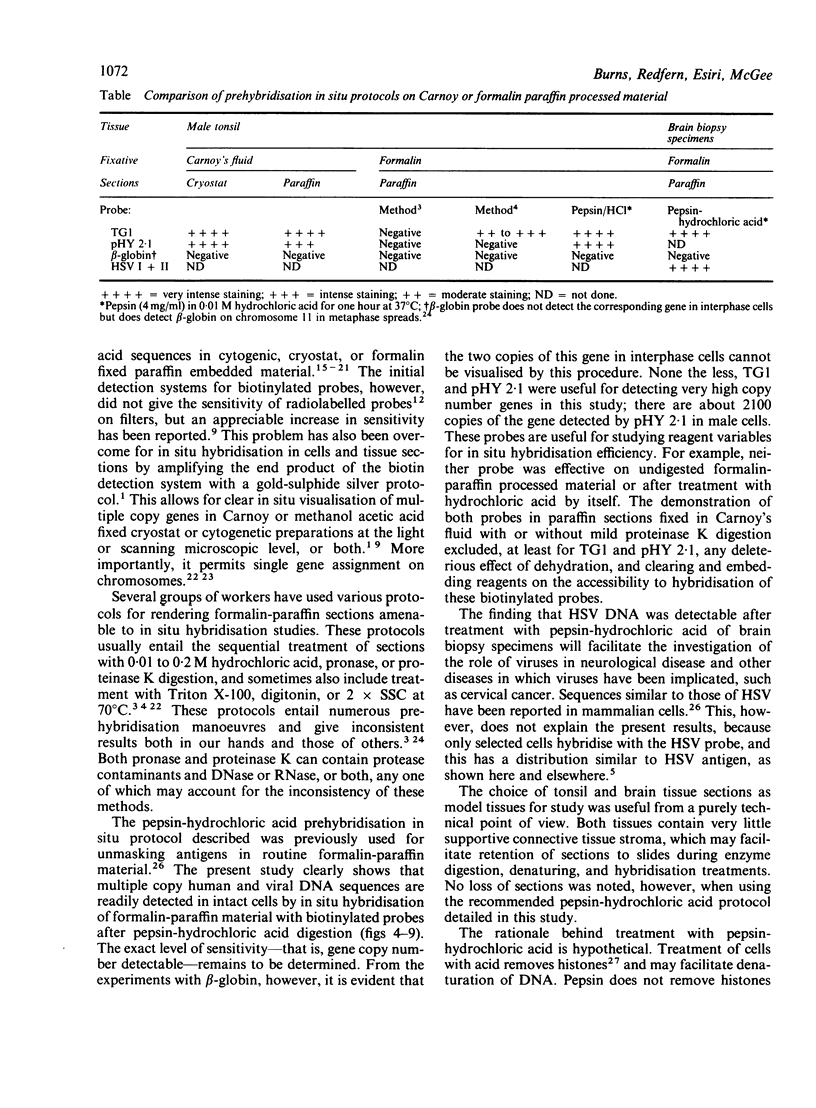
A simple reproducible protocol for detecting multiple copy human genes and viral DNA in routine formalin fixed paraffin embedded tonsil and brain, by in situ hybridisation with biotinylated probes, is described. The protocol consists of digestion of formalin fixed paraffin sections, with 0.4% pepsin in 0.01 M hydrochloric acid for one hour at 37 degrees C, followed by hybridisation with biotinylated probes. The biotinylated probes used for establishing the conditions for in situ localisation of DNA were total placental DNA (TG1), pHY 2.1 (a Y chromosome probe), and herpes simplex virus I and II. In human male tonsil TG1 labelled all nuclei and pHY 2.1 reacted only with nuclear Y bodies. In herpes encephalitis the virus was detected in some glial cells and neurones.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertson D. G. Mapping muscle protein genes by in situ hybridization using biotin-labeled probes. EMBO J. 1985 Oct;4(10):2493–2498. doi: 10.1002/j.1460-2075.1985.tb03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann A. M., Myerson D., Daling J. R., Kiviat N. B., Fenoglio C. M., McDougall J. K. Detection and localization of human papillomavirus DNA in human genital condylomas by in situ hybridization with biotinylated probes. J Med Virol. 1985 Jul;16(3):265–273. doi: 10.1002/jmv.1890160307. [DOI] [PubMed] [Google Scholar]
- Blum H. E., Haase A. T., Vyas G. N. Molecular pathogenesis of hepatitis B virus infection: simultaneous detection of viral DNA and antigens in paraffin-embedded liver sections. Lancet. 1984 Oct 6;2(8406):771–775. doi: 10.1016/s0140-6736(84)90703-7. [DOI] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Burns J., Chan V. T., Jonasson J. A., Fleming K. A., Taylor S., McGee J. O. Sensitive system for visualising biotinylated DNA probes hybridised in situ: rapid sex determination of intact cells. J Clin Pathol. 1985 Oct;38(10):1085–1092. doi: 10.1136/jcp.38.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V. T., Fleming K. A., McGee J. O. Detection of sub-picogram quantities of specific DNA sequences on blot hybridization with biotinylated probes. Nucleic Acids Res. 1985 Nov 25;13(22):8083–8091. doi: 10.1093/nar/13.22.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan J. P., Aldred P., Haralambidis J., Niall H. D., Penschow J. D., Tregear G. W. Hybridization histochemistry. Anal Biochem. 1985 Aug 15;149(1):1–28. doi: 10.1016/0003-2697(85)90472-5. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Schmidtke J., Gosden J. R. Characterisation of a human Y chromosome repeated sequence and related sequences in higher primates. Chromosoma. 1982;87(5):491–502. doi: 10.1007/BF00333470. [DOI] [PubMed] [Google Scholar]
- Esiri M. M. Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J Neurol Sci. 1982 May;54(2):209–226. doi: 10.1016/0022-510x(82)90183-6. [DOI] [PubMed] [Google Scholar]
- Forghani B., Dupuis K. W., Schmidt N. J. Rapid detection of herpes simplex virus DNA in human brain tissue by in situ hybridization. J Clin Microbiol. 1985 Oct;22(4):656–658. doi: 10.1128/jcm.22.4.656-658.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G., Pardue M. L. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci U S A. 1969 Jun;63(2):378–383. doi: 10.1073/pnas.63.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Parks C. L., Spector D. J., Hyman R. W. Hybridization of herpes simplex virus DNA and human ribosomal DNA and RNA. Virology. 1985 Jul 30;144(2):384–397. doi: 10.1016/0042-6822(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Kaufman R. E., Kretschmer P. J., Adams J. W., Coon H. C., Anderson W. F., Nienhuis A. W. Cloning and characterization of DNA sequences surrounding the human gamma-, delta-, and beta-globin genes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4229–4233. doi: 10.1073/pnas.77.7.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegent J. E., Jansen in de Wal N., van Ommen G. J., Baas F., de Vijlder J. J., van Duijn P., Van der Ploeg M. Chromosomal localization of a unique gene by non-autoradiographic in situ hybridization. Nature. 1985 Sep 12;317(6033):175–177. doi: 10.1038/317175a0. [DOI] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. F. Detection of Y-specific repeat sequences in normal and variant human chromosomes using in situ hybridization with biotinylated probes. Cytogenet Cell Genet. 1985;39(3):184–187. doi: 10.1159/000132132. [DOI] [PubMed] [Google Scholar]
- Myerson D., Hackman R. C., Nelson J. A., Ward D. C., McDougall J. K. Widespread presence of histologically occult cytomegalovirus. Hum Pathol. 1984 May;15(5):430–439. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- Negro F., Berninger M., Chiaberge E., Gugliotta P., Bussolati G., Actis G. C., Rizzetto M., Bonino F. Detection of HBV-DNA by in situ hybridization using a biotin-labeled probe. J Med Virol. 1985 Apr;15(4):373–382. doi: 10.1002/jmv.1890150407. [DOI] [PubMed] [Google Scholar]
- Royston M. E., Augenlicht L. H. Biotinated probe containing a long-terminal repeat hybridized to a mouse colon tumor and normal tissue. Science. 1983 Dec 23;222(4630):1339–1341. doi: 10.1126/science.6689218. [DOI] [PubMed] [Google Scholar]
- Unger E. R., Budgeon L. R., Myerson D., Brigati D. J. Viral diagnosis by in situ hybridization. Description of a rapid simplified colorimetric method. Am J Surg Pathol. 1986 Jan;10(1):1–8. doi: 10.1097/00000478-198601000-00001. [DOI] [PubMed] [Google Scholar]
- van der Ploeg M., van Duijn P., Bauman J. G., Landegent J. E., Raap A. K. Hybridocytochemistry as a tool for the investigation of chromatin organization. Basic Appl Histochem. 1985;29(3):181–189. [PubMed] [Google Scholar]