Abstract
γδ T cells function at the interface between innate and adaptive immunity and have well-demonstrated roles in response to infection, autoimmunity and tumors. A common characteristic of these seemingly disparate conditions may be cellular stress or death. However, the conditions under which ligands for γδ T cells are induced or exposed remain largely undefined. We observed that induction of necroptosis of murine or human dendritic cells (DC) by inhibition of caspase activity paradoxically augments their ability to activate γδ T cells. Furthermore, upregulation of the stabilizer of caspase-8 activity, c-FLIP, by IL-4, not only greatly reduced the susceptibility of DC to necroptosis, but also considerably decreased their ability to activate γδ T cells. Collectively, these findings suggest that the induction of necroptosis in DC upregulates or exposes the expression of γδ T cell ligands, and they support the view that γδ T cells function in the immune surveillance of cell stress.
Key Words: γδ T cells, Dendritic cells, Necroptosis, Caspases, c-FLIP
Introduction
γδ T cells represent a unique lymphoid lineage that functions at the interface between the innate and adaptive immune responses [1, 2]. Clues to their specificity and function derive, in part, from the observation that they are frequently localized to selected anatomical areas, including epithelial barriers and sites of inflammation, such as the synovium in Lyme and rheumatoid arthritis [3, 4]. This is particularly striking in mice where a monoclonal γδ T cell subset expressing an invariant and canonical Vγ5/Vδ1 populates exclusively the epidermis whereas another subset of Vγ6/Vδ1 T cells colonizes specifically the female reproductive tract, the tongue and the lung [2]. The limited diversity of expressed TCR-γδ, despite the potential for gene rearrangement of a large diversity of receptors, suggests a limited repertoire of TCR-γδ ligands. In striking contrast to αβ T cells or B cells, which eliminate self-reactive cells during development, γδ T cells appear to be biased toward the recognition of self-antigens [1]. Furthermore, growing evidence suggests that many of these self-ligands may be induced under conditions of stress in a variety of cell types [1]. As such, γδ T cells may have evolved to respond to certain host-derived molecules that are exposed and/or upregulated during the early phases of infection, sterile inflammation, tumor development or even cell death [5].
It has become clear that many danger-associated molecular patterns (DAMPs) are exposed during cell stress or death, and they can activate innate immune receptors. Caspase-8 activity is critical for the survival and proliferation of T lymphocytes as well as a variety of other cell types [6, 7]. Caspase-8 is known to promote cell survival through the cleavage of RIPK1 (receptor interacting protein kinase 1) [8, 9]. Cleaved RIPK1 acts as an inhibitor of full-length RIPK1 in the formation of the Ripoptosome, containing FADD, caspase-8 and RIPK1, which induces cell death, called necroptosis, via RIPK3 and MLKL (mixed lineage kinase domain-like) [10]. Caspase-8 activity in proliferating cells is maintained by the caspase-8 paralog, c-FLIP, which lacks caspase enzymatic activity but instead contains a C-terminal loop that activates caspase-8 when these molecules heterodimerize [11]. Thus, the interactions of caspase-8, c-FLIP and RIPK1 can profoundly influence cell fate.
Given the known expression of c-FLIP in certain myeloid dendritic cells (DC), particularly in the presence of IL-4 [12, 13], and the potential role of necroptosis in general cell stress, we examined the susceptibility of murine and human DC to necroptosis and how this might influence their ability to activate γδ T cells. We observed that DC cultivated in the absence of IL-4 were highly susceptible to necroptosis induced by the pan-caspase blocker zVAD, but paradoxically augmented the activation of both murine and human γδ T cells. IL-4 induced the expression of c-FLIP in DC that led to resistance to necroptosis and a greatly reduced ability to activate γδ T cells. These findings are consistent with the role of γδ T cells in innate immune surveillance of cellular stress.
Materials and Methods
Mice
C57BL/6J wild-type (WT) and TNF-α−/− mice were housed and bred in the animal facility at the University of Vermont approved by the Association for Assessment and Accreditation of Laboratory Animal Care and according to protocols approved by the University Institutional Animal Care and Use Committee. Mice were used at 8-12 weeks of age for the harvesting of T cells from their lymph nodes and spleens as well as the harvesting of bone marrow cells. Original breeders were obtained from Jackson Laboratory (Bar Harbor, Maine, USA).
Murine γδ T Cell Purification and Culture
Spleens were isolated and disrupted through nylon mesh in RPMI 1640 with 25 mm HEPES (MediaTech, Herndon, Va., USA) containing 5% (v/v) bovine calf serum (HyClone, Logan, Utah, USA). Erythrocyte lysis of splenocytes was performed using Geys solution. γδ T lymphocytes were enriched by negative/positive selection using a magnetic bead system (Miltenyi Biotec, Auburn, Calif., USA) Briefly, non-γδ T cells were labeled with CD45R (B220) and CD11b magnetic microbeads, and γδ T cells were labeled with anti-TCR-γδ-biotin. Magnetic separation was then performed to initially remove the non-γδ T cells. The TCR-γδ-positive T cells were then further bound with antibiotin magnetic microbeads. Separation was done using a magnetic separation column, and the γδ T cells were collected. The purity of γδ T cells was on average 84%. Finally, enriched γδ T cells were resuspended in complete medium (RPMI 1640, Mediatech), 2.5 mg/ml glucose (Sigma, St. Louis, Mo., USA), 10 mg/ml folate (Invitrogen, Carlsbad, Calif., USA), 110 μg/ml pyruvate (Invitrogen), 5 × 10-5M 2-mercaptoethanol (Sigma), 292.3 μg/ml glutamine (Invitrogen), 100 units/ml penicillin-streptomycin (Invitrogen) and 5% bovine calf serum. Purified γδ T cells were initially activated at a density of 1 × 106 cells/ml by plate-bound anti-TCR-γδ (5 μg/ml, clone GL-3) and recombinant human IL-2 (50 units/ml, Cetus). After 2 days, the cells were removed from anti-TCR-γδ stimulation, supplied with fresh medium plus IL-2, and returned to culture at a density of 0.3 × 106 cells/ml. Cells were counted daily and supplied with fresh media containing 50 units/ml IL-2. At the time of the experiments, typically day 7, cultures were routinely >95% γδ T cells by pan-anti-γδ antibody staining, and approximately 45% Vγ1-positive and negative for Vγ2, Vδ4 and Vδ6 by flow cytometry (not shown). In addition, in some experiments, freshly isolated splenic γδ T cells were prepared using anti-γδ-coated magnetic beads (Miltenyi Biotec) and magnet purification.
Human γδ T Cell Clones
γδ T cell clones were derived from synovial fluid T cells stimulated with Borrelia burgdorferi sonicate (10 μg/ml) and then cloned at limiting dilution as previously described [14]. All clones cultured with B. burgdorferi were of the Vδ1 subset according to antibody staining and DNA sequencing [14]. The HD.108 clone expresses the Vγ2Vδ2 TCR and was derived from a normal adult human donor by stimulation of peripheral blood mononuclear cells with PPD for a short period followed by purification of γδ T cells by magnetic beads and cloning at limiting dilution with PHA-P mitogen stimulation. Clones were restimulated every 10-14 days in the presence of irradiated peripheral blood lymphocytes (3 × 105/well), human recombinant IL-2 (100 U/ml) and either 10 μg/ml of B. burgdorferi for Vδ1 clones or PHA-P (1:1,000) for HD.108.
Fresh Human γδ T Cell Purification
γδ T lymphocytes were enriched by negative selection using a magnetic bead system (Miltenyi Biotec). Non-γδ T cells (αβ T cells, NK cells, B cells, DC, granulocytes, monocytes, stem cells and erythroid cells) were labeled using a cocktail of biotin-conjugated antibodies and anti-biotin magnetic microbeads. The non-γδ T cells were retained in the column in a magnetic field, while the unlabeled γδ T cells flowed through and were collected. This selection method yielded 82% purity.
Bone Marrow DC
The preparation of bone marrow-derived DC (BMDC) was done according to the method of Lutz et al. [15] using GM-CSF (10 ng/ml PeproTech, Rocky Hill, N.J., USA) or GM-CSF plus IL-4 (10 ng/ml, PeproTech). Cells were used on day 7.
Human DC
Human monocytes were obtained as CD14-positive cells by magnetic bead purification (Miltenyi Biotech) from peripheral blood of healthy volunteers. Myeloid DC were prepared by culture of monocytes in AIM V media plus 10% fetal calf serum (HyClone) with 800 U/ml of GM-CSF (BioLegend, San Diego, Calif., USA) with or without 500 U/ml IL-4 (BioLegend). Cells were used on day 7.
Mixed Cultures
Day 7 γδ T cells and DC were cultured either individually or together at a 1:1 ratio (106 cells/ml each). To some cultures, the following reagents were added: a sonicate of B. burgdorferi (10 μg/ml), zVAD-fmk (MP Biomedical, Santa Ana, Calif., USA) at the doses indicated, necrostatin (50 μM, R&D Systems, Minneapolis, Minn., USA), anti-TNF-α (10 μg/ml, Calbiochem, Darmstadt, Germany), the anti-IL-1 receptor antagonist, Anakinra (200 ng/ml, Amgen, Thousand Oaks, Calif., USA), anti-IL-12 (10 μg/ml, BioLegend), anti-IL-18 (10 μg/ml, MBL, Woburn, Mass., USA) or rat IgG (10 μg/ml Jackson Immunoresearch, West Grove, Pa., USA). Transwell assays were performed using transparent collagen-treated microporous membranes (Corning Cat. No. 3495, Corning, N.Y., USA). γδ T cells (1 × 106) in 1 ml of complete medium + IL-2 were placed in the lower chamber and 5 × 105 DC in 100 μl were placed in the upper chamber. Supernatants were collected after 20 h for cytokine analysis, and the surface expression of CD25 by γδ T cells was determined by flow cytometry.
Cytokine/Chemokine Detection by the Multi-Plex Assay
Cytokine levels of IFN-γ, IL-1β, IL-12p40, IL-12p70, IL-17 and TNF-α were detected using the Bio-Plex, MilliPlex or Luminex immunoassay (Bio-Rad; Millipore-EMD and R&D Systems) according to the manufacturer's protocol. Briefly, samples were run undiluted or diluted 1:10 in RPMI complete media; 50 μl of the magnetic-bead working solution was added to each well, then 50 μl of appropriate samples or standards were then added to wells and incubated at room temperature for 30-120 min at 800 r.p.m. on an IKA MS 3 digital shaker. After 3 washes with 100 μl Bio-Plex wash buffer, incubation with 25 μl of detection antibody solution was done at room temperature for 30-60 min on the shaker. Following another set of 3 washes, 50 μl of streptavidin-phycoerythrin in assay buffer was added to each well and incubated as described for the previous step. After an additional 3 washes, 125 μl of assay buffer was added. Sample data were analyzed with Bio-Plex Manager software.
Flow Cytometry
The following monoclonal antibodies to murine cell surface proteins were purchased from BioLegend: phycoerythrin-conjugated anti-CD25, APC-conjugated anti-TCR-γδ and TNF-α neutralizing antibody. Rat IgG was purchased from Jackson Immunoresearch. For direct staining, single-cell suspensions (1 × 106 total cells per staining condition) were washed with cold (4°C) PBS containing 1% (w/v) BSA fraction V (Sigma; PBS/1% BSA), incubated with unconjugated rat IgG (50 μg/ml) for 15 min at 4°C, washed and then incubated with the appropriate antibodies in PBS/1% BSA. After washing, the cells were fixed with freshly made 1% (v/v) methanol-free formaldehyde (Ted Pella Inc., Redding, Calif., USA) in PBS/1% BSA.
Cell death was examined by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). Briefly, cells were stained for the expression of cell surface molecules, and then fixed with 1% methanol-free formaldehyde followed by 70% ethanol. After washing, the TUNEL reaction was performed by incubating cells in 50 µl of reaction mix containing 10 units of terminal deoxyribosyl transferase (TdT), 2.5 mM cobalt chloride in 1× TdT buffer and 0.2 pmol/µl of FITC-dUTP (Roche, Indianapolis, Ind., USA) at 37°C for 1 h. The cells were washed twice and fixed in 1% methanol-free formaldehyde. Murine thymocytes were included as a positive control for apoptotic cells. Flow cytometry was performed on an LSRII (BD Biosciences, San José, Calif., USA) calibrated with compensation beads (BD Biosciences). Analysis was performed using FlowJo software (Tree Star, Inc., Ashland, Oreg., USA).
Detection of Active Caspases by Immunoblot
On day 7, DC were lysed in Tris-buffer containing Complete Protease Inhibitor (Roche), 0.2% NP-40 (Roche) and biotin-VAD-fmk (10 μM, MP Biomedicals). Insoluble cell fragments were removed by centrifugation, and the protein concentration of the resulting whole-cell lysates was determined by Bradford assay (BioRad, Hercules, Calif., USA). Whole-cell lysates (30 μg/lane) were separated by SDS-PAGE. Precipitation of active caspases was performed by rocking 600 μg of whole-cell lysates initially with sepharose 4B beads (Sigma) at 4°C for 2 h to remove nonspecific binding of proteins to the beads, followed by overnight rocking incubation with streptavidin-conjugated sepharose beads (Invitrogen) at 4°C. Release of biotin-VAD-fmk-labeled caspases from the streptavidin beads was achieved by heating to 95°C for 10 min in Laemmli buffer. After gel electrophoresis, proteins were transferred to PVDF membranes (0.2-μm pore size, Bio-Rad), and immunoblots were performed using antibodies (1 μg/ml) against caspase-3, caspase-8 (Cell Signaling, Danvers, Mass., USA), caspase-9 (Stressgen, Farmingdale, N.Y., USA) and RIPK1 (BD Biosciences). Densitometry was assessed by ImageStudio software (LI-COR Biotechnology, Lincoln, Nebr., USA). Intensity differences between groups were tested using the Mann-Whitney U test.
Statistical Analyses
Normal distribution of data was first determined using the Shapiro-Wilk test, followed by one-way ANOVA and Tukey's post hoc test to assess the differences among the various conditions in the production of the indicated cytokines. p < 0.05 was considered to be significant.
Results
Inhibition of Caspase Activity in Murine DC Results in Cell Death but an Enhanced Ability to Activate γδ T Cells
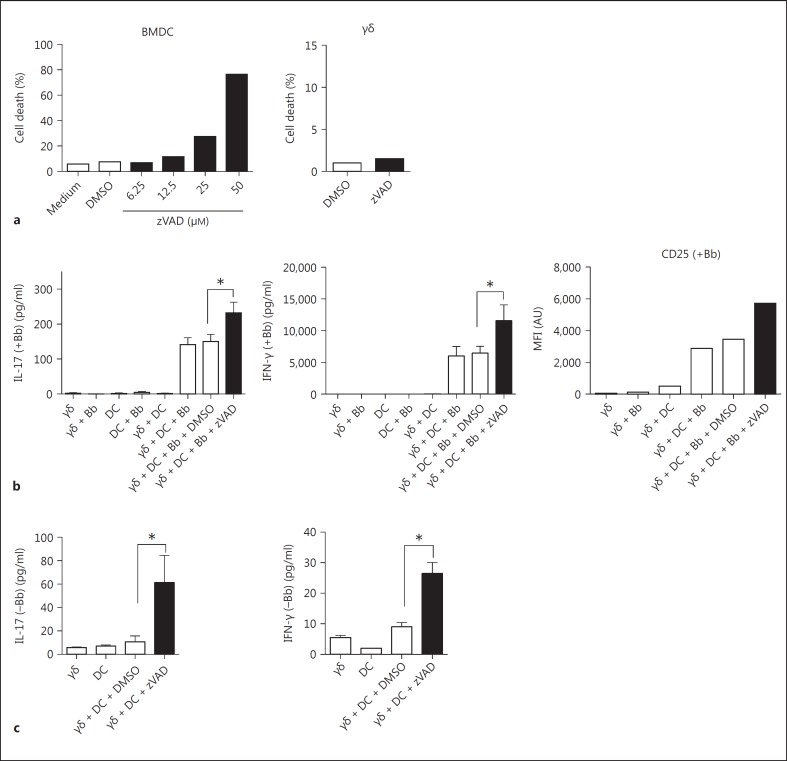
Both murine and certain human γδ T cells are activated by B. burgdorferi in the presence of DC [4, 16]. Given the growing number of cell types in which caspase activity is critical for survival [7], we examined the effect of caspase inhibition on the survival of DC. As shown in figure 1a, murine BMDC grown in GM-CSF were exquisitely sensitive to the pan-caspase blocker zVAD, which was dose-dependent, yielding nearly 80% dead DC at 50 μM zVAD. By contrast, cultured murine splenic γδ T cells in the same 50 μM dose of zVAD manifested no increase in cell death (fig. 1a). Despite the death of DC with zVAD, their ability to activate B. burgdorferi-stimulated γδ T cells was paradoxically enhanced, as indicated by the augmented production of IL-17 and IFN-γ by the γδ T cells, and their increased expression of surface CD25 (fig. 1b). In fact, even in the absence of B. burgdorferi, zVAD treatment of DC was able to promote production of IL-17 and IFN-γ by γδ T cells, albeit at less absolute levels than in the presence of B. burgdorferi (fig. 1c). This suggested that inhibition of caspase activity and induction of cell death in DC might induce the exposure of a self-antigen(s) that could activate the γδ T cells. Of interest was that induction of DC death using staurosporine did not augment the activation of γδ T cells (data not shown).
Fig. 1.
Caspase inhibition induces cell death of murine DC but enhances their ability to activate cultured γδ T cells. a BMDC were cultured using GM-CSF and were incubated on day 6 with the indicated concentrations of the pan-caspase inhibitor, zVAD or vehicle control DMSO. Cultured γδ T cells were also treated with zVAD at 50 μM. After 20 h, cells were analyzed for cell death by TUNEL assay. b γδ T cells and DC were cultured separately or together at a 1:1 ratio in the absence or presence of B. burgdorferi sonicate (Bb; 10 μg/ml). Either zVAD (50 μM) or control DMSO was added to cultures as indicated. After 20 h, supernatants were assessed for production of the γδ T cell cytokines IL-17 and IFN-γ by Bio-Plex, and the γδ T cells were assessed for CD25 expression using flow cytometry. c Cell cultures were as described in b except B. burgdorferi was deleted, in order to reveal the ability of zVAD-treated DC alone to partially activate γδ T cells (* p < 0.05 by one-way ANOVA followed by Tukey's post hoc test). Data are representative of 3-5 experiments, using pooled cells from 2-3 mice in each experiment. Cytokine results represent the mean ± SD of triplicate wells from 1 experiment. Flow cytometry results for TUNEL and CD25 represent single cultures of 1/3 experiments conducted.
Freshly isolated splenic γδ T cells behaved similarly to cultured γδ T cells. In the presence of zVAD-treated DC and B. burgdorferi, fresh γδ T cells also manifested a greatly augmented production IL-17 and IFN-γ (fig. 2a), and this also occurred even in the absence of B. burgdorferi (fig. 2b).
Fig. 2.
Caspase inhibition of DC also activates freshly isolated murine γδ T cells. Freshly isolated splenic γδ T cells (84% purity) and DC were cultured separately or together at a 1:1 ratio in the presence (a) or absence (b) of B. burgdorferi sonicate (Bb; 10 μg/ml). Either zVAD (50 μM) or control DMSO was added to cultures as indicated. After 20 h, supernatants were assessed for production of the γδ T cell cytokines IL-17 and IFN-γ by Bio-Plex assay (* p < 0.05 by one-way ANOVA followed by Tukey's post hoc test). Data represent triplicate wells from 1/2 experiments using 3-4 mice in each experiment.
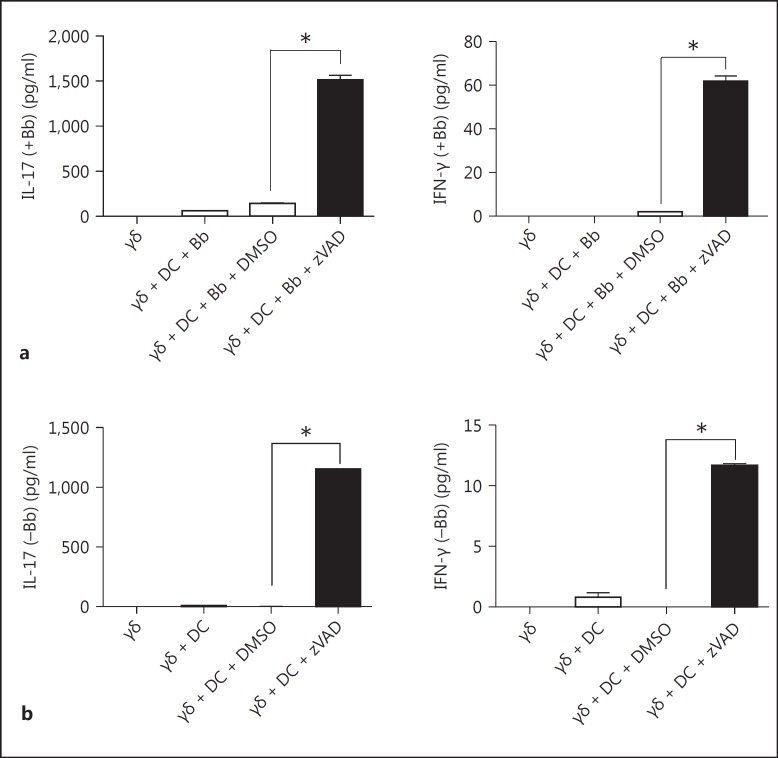
To further investigate how zVAD-treated DC might activate γδ T cells, we examined whether this involved a soluble factor or direct cell-to-cell contact. Initial examination of cytokine production by the DC revealed that zVAD did not augment, but actually partially inhibited, the production of IL-12 p40 or p70 by B. burgdorferi-stimulated DC (fig. 3a). Not surprisingly, DC production of IL-1β, which requires caspase-1 to cleave it into an active form, was completely inhibited by zVAD. In addition, blocking of IL-1β, IL-12 and IL-18, individually or together, did not affect the activation of γδ T cells, nor did the addition of these cytokines, individually or in combination (data not shown).
Fig. 3.
Augmented activation of γδ T cells by caspase-inhibited DC is mediated only partly by TNF-α. a BMDC were cultured without or with B. burgdorferi in the absence or presence of zVAD. After 20 h, supernatants were assayed by Bio-Plex for the DC cytokines IL-12 p40, IL-12 p70, IL-1β and TNF-α. b DC from WT or TNFα−/− mice were cultivated in GM-CSF. On day 6, DC were incubated with 50 μM zVAD or vehicle control DMSO. After 20 h, cells were analyzed for cell death. c WT γδ T cells were cultured with DC from WT or TNFα−/− mice, plus B. burgdorferi sonicate (Bb) either in the presence of DMSO or zVAD. After 20 h, supernatants were collected and assayed for TNF-α, IL-17 and IFN-γ. d γδ T cells cultured with WT DC and B. burgdorferi were incubated with control IgG or anti-TNF-α (both 10 μg/ml) or without B. burgdorferi but with exogenous TNF-α (2.5 μg/ml). Supernatants were harvested after 20 h and assayed for IL-17 and IFN-γ (* p < 0.05 by one-way ANOVA followed by Tukey's post hoc test). Data were consistent in 3 (a-c) and 2 (d) experiments; 3-4 mice per experiment were used.
By contrast, murine DC production of TNF-α was considerably augmented in the presence of zVAD (fig. 3a). We thus further investigated the possible contribution of TNF-α to activation of the γδ T cells by first examining the ability of TNF-α-deficient (TNF-α−/−) DC to activate γδ T cells. TNF-α−/− DC were also susceptible to zVAD-induced cell death, but were not as sensitive as WT DC (fig. 3b). In parallel, TNF-α−/− DC treated with zVAD also augmented the stimulation of γδ T cells, though not quite as strongly as WT DC (fig. 3c). These findings suggested that the reduced ability of TNF-α−/− DC to activate γδ T cells with zVAD might not be due to the lack of TNF-α per se, but rather their reduced death with zVAD. To further explore this possibility, we either blocked TNF-α or added it to cultures of γδ T cells and DC, with or without B. burgdorferi. As shown in figure 3d, anti-TNF-α only partly inhibited IFN-γ production, and there was no effect on IL-17 secretion. Furthermore, the addition of TNF-α to cultures did not augment production of either IFN-γ or IL-17. These findings suggested that a factor(s) in addition to or instead of TNF-α, was produced by DC + zVAD, that could activate γδ T cells.
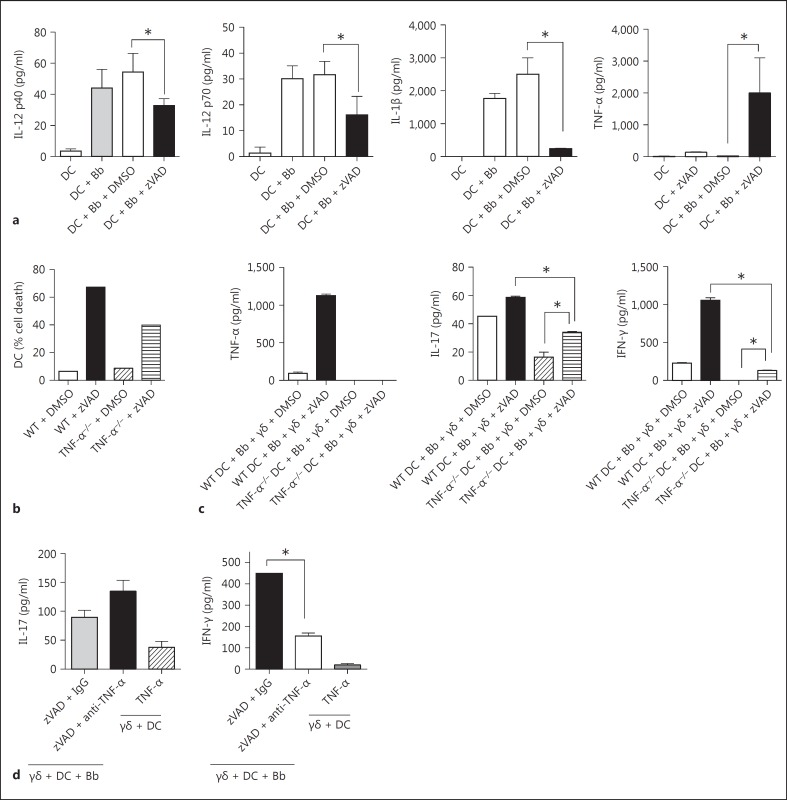
Further analyses suggested that the factor(s) from necroptotic DC that activated the γδ T cells was soluble. First, supernatants alone from cultures containing DC + B. burgdorferi + zVAD could partially augment activation of the γδ T cells in the absence of added DC or B. burgdorferi (fig. 4a). Second, transwell assays revealed that, in the absence of cell contact between the DC and γδ T cells, the γδ response to B. burgdorferi was lost when using intact DC but was still present when using zVAD-treated DC (fig. 4b). In addition to soluble cytokines secreted by DC, it is also possible that a ligand(s) for the γδ T cells was induced or exposed by the DC via caspase inhibition-induced cell stress that could pass through the rather large restriction size (0.4 μm) of the transwell system.
Fig. 4.
Augmented activation of γδ T cells by caspase-inhibited DC involves a soluble factor. a γδ T cells were cultured with supernatants (SN) previously obtained from 20-hour cultures of DC plus B. burgdorferi (Bb) with either DMSO or zVAD (50 μM). After an additional 20 h, the γδ T cell supernatants were assayed for IL-17 and IFN-γ. b Transwell cultures were established in which γδ T cells were cultured in the lower chamber and DC cultured with B. burgdorferi in the upper chamber in the absence or presence of zVAD. After 20 h, supernatants from triplicate cultures (mean ± SD) were evaluated for IL-17 and IFN-γ (* p < 0.05 by one-way ANOVA followed by Tukey's post hoc test). Data were consistent in 3 experiments; 2 mice were used in each experiment.
Caspase-8 Activity Is Detectable in DC and Is Increased by the IL-4 Induction of c-FLIP
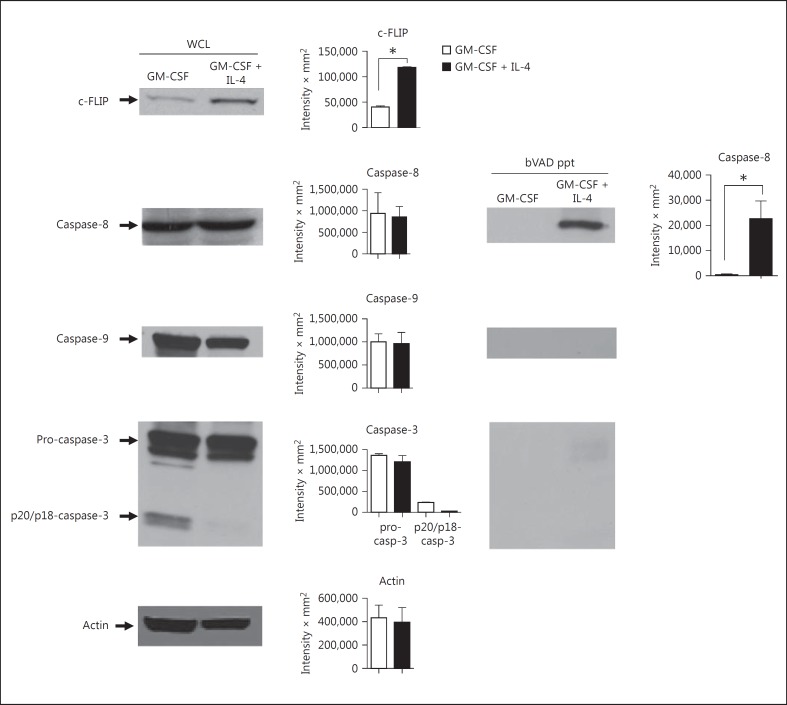
Given the extreme sensitivity of GM-CSF-derived DC to zVAD-mediated cell death, and the known requirement for caspase-8 in the survival of many cell types, we examined the levels of specific caspases and their activity using biotin-VAD (bVAD) to selectively bind only the active caspase fraction, which was then precipitated with avidin-sepharose. In addition, we monitored the levels of the caspase-8 paralog, c-FLIP, which regulates caspase-8 activity. Although c-FLIP was originally described as a competitive inhibitor of caspase-8 following Fas-induced cell death [17], subsequent studies revealed that it is also an activator of moderate caspase-8 activity, which is essential for cell survival [11, 18]. In addition, we and others have observed that c-FLIP levels increase during the maturation of DC in the presence of IL-4 [12, 13]. As shown in figure 5, there was no difference in the levels of total cellular caspase-8, caspase-9 or pro-caspase-3 in whole-cell lysates from DC grown with GM-CSF vs GM-CSF + IL-4. However, as observed previously [12, 13], IL-4 considerably increased the level of c-FLIP (fig. 5). This paralleled an increase in the level of active caspase-8 in DC cultured in GM-CSF + IL-4 (fig. 5). However, active caspase-9 and caspase-3 were undetectable in bVAD precipitates, even at long exposures. Thus, the active caspase-8 signal did not propagate to the downstream caspases.
Fig. 5.
IL-4 induces c-FLIP and caspase-8 activity in DC. DC were cultured in GM-CSF in the absence or presence of IL-4. After 7 days, lysates were made containing biotin-VAD (bVAD) to bind active caspases. Active caspases were then selectively precipitated using avidin-sepharose (bVAD ppt) and compared to whole-cell lysates (WCL) by Western blot for c-FLIP, caspase-8, caspase-9 and caspase-3. The results were consistent in 3 experiments; 3 mice were used in each experiment. Densitometry represents a compilation of the 3 experiments.
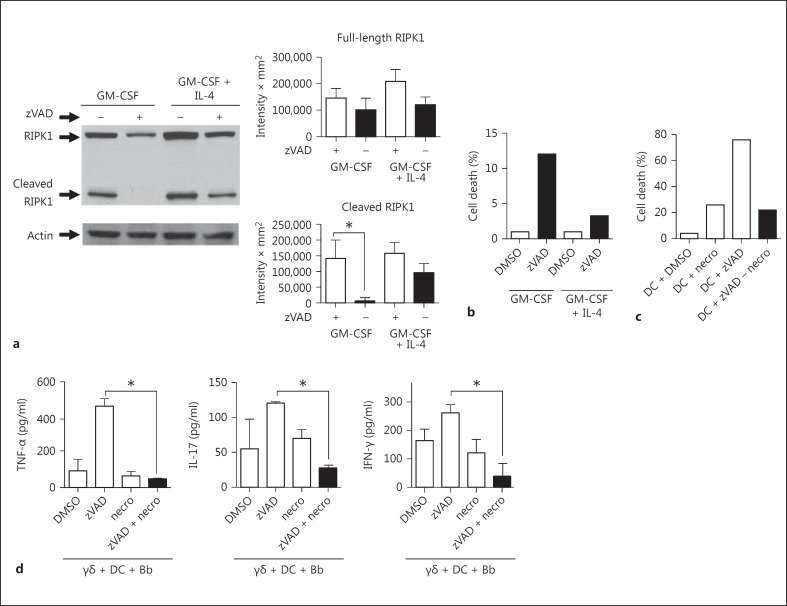
Cell death resulting from the absence of caspase-8 or FADD can be rescued by the loss of RIPK3 or RIPK1 [8, 9]. Caspase-8 activity is required in the proliferating cells to cleave RIPK1, which prevents the formation of the Ripoptosome and subsequent cell death by necroptosis [19]. Consistent with this model, DC exhibited cleaved RIPK1 in both growth conditions (fig. 6a). The addition of zVAD completely inhibited cleavage of RIPK1 in DC cultured with GM-CSF only, but in the presence of GM-CSF + IL-4, it did not effectively inhibit RIPK1 cleavage. This is consistent with both the increased caspase-8 activity in DC with IL-4 as well as the known ability of c-FLIP to reduce the accessibility of zVAD to the enzymatic pocket of caspase-8 [20]. The persistence of cleaved RIPK1 despite zVAD in GM-CSF + IL-4-cultured DC was paralleled by greatly reduced cell death in the presence of zVAD compared to DC cultured with only GM-CSF (fig. 6b).
Fig. 6.
zVAD inhibits caspase-mediated RIPK1 cleavage and induces DC cell death with GM-CSF but not with IL-4. DC cultured for 7 days in GM-CSF or GM-CSF + IL-4 were analyzed in the absence (DMSO) or presence of zVAD for cleavage of RIPK1 by Western blot of whole-cell lysates (a) or cell death (b). c Cell death of GM-CSF-cultivated DC was assessed following culture for 20 h in the absence (DMSO) or presence of the RIPK1-inhibitor, necrostatin (necro; 50 μM), in the absence or presence of zVAD (50 μM). d Cultures containing γδ T cells, DC and B. burgdorferi (Bb) were treated with either control DMSO, zVAD alone or zVAD + necrostatin. Supernatants were assayed after 20 h for the indicated cytokines (* p < 0.05 by one-way ANOVA followed by Tukey's post hoc test). Data were similar in 3 experiments; 4 mice were used in each experiment. Densitometry represents a compilation of the 3 experiments.
Given these findings, we used the RIPK1 inhibitor, necrostatin [21], to examine whether RIPK1 activity influenced either cell death mediated by zVAD or the ability of zVAD-treated DC to augment γδ T cell stimulation. The addition of necrostatin to GM-CSF-cultured DC treated with zVAD rescued them from cell death (fig. 6c). Furthermore, Necrostatin also reversed the ability of zVAD-treated DC to produce TNF-α or to augment the activation of γδ T cells (fig. 6d). Necrostatin was not simply toxic to the cells, as it did not block production of these cytokines by DC exposed to B. burgdorferi in the absence of zVAD (fig. 6d). Thus, RIPK1-mediated necroptosis of DC enhanced γδ T cell activation.
IL-4 Reduces the Ability of zVAD-Treated DC to Activate γδ T Cells
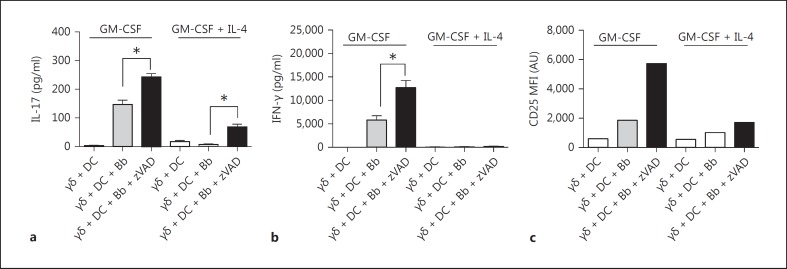
Although zVAD treatment of GM-CSF-cultured DC augmented their ability to activate γδ T cells, it was not certain that this was due to cell death. DC cultured with GM-CSF + IL-4 afforded an opportunity to test this, given their greater caspase-8 activity and resistance to zVAD-induced cell death. In fact, DC grown with GM-CSF + IL-4 displayed greatly reduced ability to activate B. burgdorferi-stimulated γδ T cells in the presence of zVAD (fig. 7). This further supports the view that the actual cell stress or death caused by the caspase inhibition of DC was responsible for the induction of a factor(s) that could promote the activation of γδ T cells.
Fig. 7.
IL-4 inhibits the ability of DC to activate γδ T cells. DC grown with either GM-CSF or GM-CSF + IL-4 were mixed at a 1:1 ratio with γδ T cells in the absence or presence of B. burgdorferi (Bb) and zVAD. After 20 h, supernatants from triplicate cultures (mean ± SD) were analyzed by Bio-Plex for IL-17 (a), IFN-γ (b) and surface CD25 (c) on the γδ T cells measured by flow cytometry (* p < 0.05 by one-way ANOVA followed by Tukey's post hoc test). Findings were consistent in 3 experiments; 3-4 mice were used in each experiment. AU = Arbitrary units; MFI = mean fluorescence intesity.
Necroptosis of DC Also Enhances the Activation of Human Vδ1 γδ T Cells
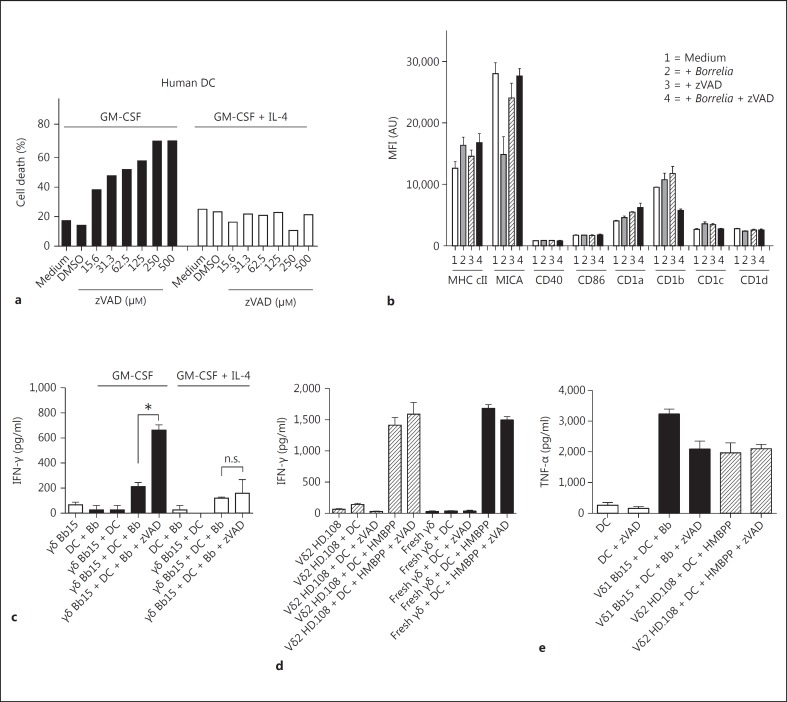
The ability of necroptotic DC to augment γδ T cell activation also extended to human γδ T cells. Human DC from peripheral blood monocytes cultured with GM-CSF were highly susceptible to death with zVAD at doses very similar to murine DC, and this was also blocked by the addition of IL-4 (fig. 8a). We have previously reported that IL-4 upregulates c-FLIP expression in human DC as it does in murine DC [12]. In addition, neither B. burgdorferi, zVAD, or both, altered the surface phenotype of GM-CSF-cultivated DC (fig. 8b). We further examined the ability of zVAD-treated human DC to promote the activation of human B. burgdorferi-reactive synovial Vδ1 γδ T cell clones from Lyme arthritis. Figure 8c shows an example of 1 of 3 synovial Vδ1 clones examined, Bb15, revealing increased IFN-γ production (the synovial γδ T cell clones do not produce IL-17) when cultured with B. burgdorferi, plus zVAD-treated DC cultured with GM-CSF alone but not when cultured with IL-4. In contrast, the response of Vδ2 γδ T cells, either as synovial-fluid T cell clones or freshly isolated from peripheral blood to the prenyl phosphate (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), was not augmented by zVAD (fig. 8d). Unlike murine DC, zVAD did not augment the production of TNF-α from human GM-CSF-cultivated DC, and so was not the source of augmented activation of Vδ1 T cells (fig. 8e). These findings establish the ability of necroptotic DC to augment subssets of human γδ T cell immune responses.
Fig. 8.
Caspase inhibition of DC also promotes activation of human Vδ1 but not Vδ2 T cells. a Human DC, cultured from peripheral blood monocytes with GM-CSF in the presence or absence of IL-4, were exposed to the indicated concentrations of zVAD for 20 h and then assessed for the percentage of dead cells. b Surface phenotype of human DC cultured with GM-CSF following stimulation for 20 h with Borrelia, zVAD or Borrelia + zVAD. The mean fluorescence intensities (MFI) of the indicated surface marker are shown. AU = Arbitrary units; cII = class II. c Human synovial Vδ1 clone Bb15 was cultured with human DC cultured with GM-CSF alone or GM-CSF + IL-4 in a 1:1 ratio with B. burgdorferi (Bb; 10 μg/ml) in the absence or presence of zVAD (50 μM). After 20 h, supernatants were assessed for IFN-γ production (human synovial γδ T cells do not produce IL-17). n.s. = Not significant. d IFN-γ production by the human Vδ2 clone HD.108 (hatched bars) or human fresh peripheral blood γδ T cells (black bars) in the presence of human GM-CSF-cultivated DC without or with zVAD, HMBPP or HMBPP + zVAD. Supernatants from triplicate cultures (mean ± SD) were harvested after 20 h. e TNF-α production by GM-CSF-cultivated DC in the absence or presence of zVAD (white bars), Vδ1 clone Bb15 + Borrelia (black bars) or Vδ2 clone HD.108 + HMBPP (hatched bars) (* p < 0.05 by one-way ANOVA followed by Tukey's post hoc test). Data are representative of 2 experiments.
Discussion
Our findings support a model in which murine and human γδ T cells respond to DC dying by necroptosis, consistent with the notion that γδ T cells function in a stress-surveillance immune response. Of further interest is that since the ability of necroptotic DC to activate γδ T cells was not entirely mediated by TNF-α or other known DC-derived cytokines, it raises the possibility that ≥1 ligands for γδ T cell receptors are produced or exposed during the necroptosis of DC. This study further extends the growing list of cells that have been shown to require caspase-8 activity for survival. Additionally, our findings demonstrate that IL-4-induced upregulation of the caspase-8 paralog, c-FLIP, parallels an increase in caspase-8 activity in DC. This is consistent with the ability of c-FLIP to activate caspase-8 in its full-length form [11]. Interestingly, IL-4-induced c-FLIP also reduces both the sensitivity of DC to zVAD-mediated cell death as well as the ability of DC to activate γδ T cells, even in the presence of zVAD. As such, c-FLIP expression may be central to the regulation of inflammatory versus tolerogenic DC function. This is consistent with findings in mice in which c-FLIP was deleted in the DC, resulting in reduced numbers of DC, consistent with the requirement of c-FLIP for their survival [22]. Of particular interest was that these mice spontaneously developed autoreactive T cells and inflammatory arthritis.
To date, only a limited number of ligands has been identified for murine or human γδ T cells, and they lack a common structure or any evidence of classical MHC restriction. The MHC class I-like molecule T22 is recognized by the murine γδ T cell clone G8, almost exclusively through the δ-chain [23]. By contrast, endothelial protein C receptor was recently shown to be a ligand for a human γδ T cell clone from a CMV-infected individual, largely through the γ-chain [24]. The stress-inducible MHC class I-like molecules, MICA in humans [25] and Rae-1 in the mouse [26], engage the activating receptor NKG2D, found on most γδ T cells, as well as engaging some TCR-γδ [27]. CD1d complexed with sulfatide was also recently reported to be a ligand for some human γδ T cells [28]. In addition, phosphoantigens from infectious agents and alkylamines can activate certain human Vδ1 T cells but not murine γδ T cells [29]. Many of these potential ligands are induced during infections or various types of cell stress. This has proffered the notion that γδ T cells are inherently biased toward the recognition of autologous molecules exposed or upregulated during cell stress or death [1]. Our previous findings of the γδ T cell response to B. burgdorferi are consistent with this view. We found that both murine and human γδ T cells respond to B. burgdorferi indirectly in a TLR2-dependent manner [30]. It is of interest to note that B. burgdorferi has been reported to induce cell death of monocytes [31]. We have also observed that a soluble human TCR-γδ tetramer derived from a Lyme arthritis synovial γδ T cell clone reacts with the intracellular components of B. burgdorferi-stimulated monocytes (C.C., unpubl. observation). Collectively, these findings support a model in which γδ T cells respond to infection- or stress-induced cellular self-components. The findings are also consistent with our observations that reduced DC death with zVAD by 2 different means, either a lack of TNF-α or the upregulation of c-FLIP; both resulted in a reduced ability to activate γδ T cells. The fact that necroptotic DC could activate γδ T cells, even in the absence of B. burgdorferi, albeit perhaps to a lesser extent, implies that such activation of γδ T cells could occur with necroptosis during sterile inflammation, as occurs during wound repair, inflammatory synovitis or tumor immunology, all of which provoke γδ T cell responses.
Since the early descriptions of caspase-8 activity being required for T cell proliferation [6], a growing list of cell types has been identified that require caspase-8 activity for cell survival and growth. This includes endothelial cells, bone marrow cells and hepatocytes, among others [7]. It has thus become of considerable interest how caspase-8 activity is regulated between the modest levels in membrane lipid rafts that are required for cell growth versus the large cytoplasmic levels of active caspase-8 initiated following the engagement of cell death receptors such as Fas (CD95) [32]. The caspase-8 paralog, c-FLIP, is a critical regulator of caspase-8 activity [11]. c-FLIP can inhibit caspase-8 activation via Fas by competing with caspase-8 for recruitment to FADD in the death-inducing signal complex [17]. Paradoxically, c-FLIP can also heterodimerize with caspase-8 independently of death receptor engagement, in which case c-FLIP provides the activation of full-length caspase-8 during cell growth [11]. The lower level of caspase-8 activity that we observed in DC grown with only GM-CSF likely reflected the reduced levels of c-FLIP, as we have observed that deletion of c-FLIP in T cells or mouse embryonic fibroblasts results in a loss of active caspase-8 [33] whereas increased c-FLIP augments caspase-8 activity [34]. It has recently been appreciated that caspase-8 activity is required to maintain cleavage of RIPK1 [9]. In the absence of active caspase-8, full-length RIPK1 can form a complex with FADD, caspase-8 and c-FLIP, known as the Ripoptosome, which induces caspase-independent cell death or necroptosis via downstream RIPK3 and MLKL [10, 19]. Thus, c-FLIP emerges as a key regulator of the Ripoptosome. Hence, the production of the endogenous self-ligand(s) recognized by γδ T cells may occur through activation of the Ripoptosome. Future studies will determine to what extent c-FLIP and caspase-8 levels differ in various in vivo DC subsets, as well as their susceptibility to zVAD-mediated cell death.
The finding that IL-4 upregulates c-FLIP in DC adds a new dimension to the known ability of IL-4 to promote ‘alternate activation’ of M2 macrophages [35]. Classically activated M1 macrophages, following exposure to IFN-γ, acquire tumoricidal activity and can elicit tissue-destructive reactions. By contrast, IL-4 and IL-13 can induce the alternative activation of M2 macrophages oriented more toward tissue repair and remodeling, immunoregulation and tumor promotion [36]. This is accompanied by upregulation of the mannose receptor, certain chemokines such as CCL22 and intracellular enzymes such as arginase, which are implicated in cell recruitment and granuloma formation [36]. As previously observed in T cells from c-FLIP-transgenic mice [34], we found that the increased expression of c-FLIP in DC with IL-4 paralleled the increased caspase-8 activity and hence resistance to zVAD-induced cell death. c-FLIP can also inhibit Fas-mediated cell death and divert signals toward the NF-κB and ERK pathways, by virtue of its ability to associate with TRAF2 and Raf-1 [37, 38]. γδ T cells are known to express high levels of FasL [39], and hence the upregulation of c-FLIP in DC could promote cross-talk of γδ T cells and DC via FasL-Fas interactions. Future studies will explore the potential contribution of γδ T cells to the activation of inflammatory versus immunoregulatory DC via FasL. Our findings reveal the ability of necroptotic DC to augment the activation of a subset of γδ T cells and to potentially upregulate ligands for γδ T cells, underscoring their role in the immune surveillance of cell stress.
Disclosure Statement
C.T.M. is a coinventor of US Patent 8,012,466 on the development of live bacterial vaccines for activating γδ T cells. The other authors declare no financial or commercial conflict of interest.
Acknowledgements
This work was supported by grants AR43520, AI36333 and AI45666 from the National Institutes of Health to R.C.B. and from the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Grant 2I01 BX000972-05) and the National Cancer Institute, National Institutes of Health [Grant CA097274 (University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence) and P30CA086862 (Holden Comprehensive Cancer Center Core Support)] to C.T.M. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies.
References
- 1.Kalyan S, Kabelitz D. Defining the nature of human gamma delta T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol. 2013;10:21–29. doi: 10.1038/cmi.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan FM, Londei M, Jackson AM, Hercend T, Brenner MB, Maini RN, et al. T cells expressing gamma delta chain receptors in rheumatoid arthritis. J Autoimmun. 1988;1:319–326. doi: 10.1016/0896-8411(88)90002-9. [DOI] [PubMed] [Google Scholar]
- 4.Vincent M, Roessner K, Lynch D, Cooper SM, Sigal LH, Budd RC. Apoptosis of Fas high CD4+ Synovial T cells by Borrelia reactive Fas Ligand high gamma delta T cells in Lyme arthritis. J Exp Med. 1996;184:2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Acker HH, Anguille S, Van Tendeloo VF, Lion E. Empowering gamma delta T cells with antitumor immunity by dendritic cell-based immunotherapy. Oncoimmunology. 2015;4:e1021538. doi: 10.1080/2162402X.2015.1021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 12.Collins C, Wolfe J, Roessner K, Shi C, Sigal LH, Budd RC. Lyme arthritis synovial gamma delta T cells instruct dendritic cells via Fas ligand. J Immunol. 2005;175:5656–5665. doi: 10.4049/jimmunol.175.9.5656. [DOI] [PubMed] [Google Scholar]
- 13.Ashany D, Savir A, Bhardwaj N, Elkon KB. Dendritic cells are resistant to apoptosis through the Fas (CD95/APO-1) pathway. J Immunol. 1999;163:5303–5311. [PubMed] [Google Scholar]
- 14.Vincent MS, Roessner K, Sellati T, Huston CD, Sigal LH, Behar SM, et al. Lyme arthritis synovial gamma delta T cells respond to Borrelia burgdorferi lipoproteins and lipidated hexapeptides. J Immunol. 1998;161:5762–5771. [PubMed] [Google Scholar]
- 15.Lutz MB, Kukutsch N, Ogilvie ALJ, Robner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 16.Shi C, Sahay B, Russell JQ, Fortner KA, Hardin N, Sellati TJ, et al. Reduced immune response to Borrelia burgdorferi in the absence of γδ T cells. Infect Immun. 2011;79:3940–3946. doi: 10.1128/IAI.00148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 18.Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 19.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McStay GP, Salvesen GS, Green DR. Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Diff. 2008;15:322–331. doi: 10.1038/sj.cdd.4402260. [DOI] [PubMed] [Google Scholar]
- 21.Linkermann A, Brasen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, et al. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-alpha-induced shock. Mol Med. 2012;18:577–586. doi: 10.2119/molmed.2011.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang QQ, Perlman H, Birkett R, Doyle R, Fang D, Haines GK, et al. CD11c-mediated deletion of Flip promotes autoreactivity and inflammatory arthritis. Nat Commun. 2015;6:7086. doi: 10.1038/ncomms8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams EJ, Chien YH, Garcia KC. Structure of a gamma delta T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- 24.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, Moreau J-F, Hayday AC, Willcox BE, Dechanet-Merville J. Cytomegalovirus and tumor stress surveillance by binding of a human gamma delta T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13:872–880. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 25.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and γδ T cells by NKG2D, a receptor for stress- inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 26.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gamma delta T cells. Science. 2001;294:605–609. [PubMed] [Google Scholar]
- 27.Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, et al. Crystal structure of a gamma delta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci USA. 2011;108:2414–2419. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, et al. Crystal structure of V1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human T cells. Immunity. 2013;39:1032–1042. doi: 10.1016/j.immuni.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 30.Collins C, Shi C, Russell JQ, Fortner KA, Budd RC. Activation of gamma delta T cells by Borrelia burgdorferi is indirect via a TLR- and caspase-dependent pathway. J Immunol. 2008;181:2392–2398. doi: 10.4049/jimmunol.181.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz AR, Moore MW, La Vake CJ, Eggers CH, Salazar JC, Radolf JD. Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect Immun. 2008;76:56–70. doi: 10.1128/IAI.01039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 33.Koenig A, Buskiewicz IA, Fortner KA, Russell JQ, Asaoka T, He YW, et al. The c-FLIPL cleavage product p43FLIP promotes activation of extracellular signal-regulated kinase (ERK), nuclear factor κB (NF-κB), and caspase-8 and T cell survival. J Biol Chem. 2014;289:1183–1191. doi: 10.1074/jbc.M113.506428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dohrman A, Russell JQ, Cuenin S, Fortner K, Tschopp J, Budd RC. Cellular FLIP long form augments caspase activity and death of T cells through heterodimerization with and activation of caspase-8. J Immunol. 2005;175:311–318. doi: 10.4049/jimmunol.175.1.311. [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, et al. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–2346. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-κB signaling pathway. Mol Cell Biol. 2004;24:2627–2636. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dohrman A, Kataoka T, Cuenin S, Russell JQ, Tschopp J, Budd RC. Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-kappa B activation. J Immunol. 2005;174:5270–5278. doi: 10.4049/jimmunol.174.9.5270. [DOI] [PubMed] [Google Scholar]
- 39.Roessner K, Wolfe J, Shi C, Sigal LH, Huber S, Budd RC. High expression of Fas ligand by synovial fluid-derived gamma delta T cells in Lyme arthritis. J Immunol. 2003;170:2702–2710. doi: 10.4049/jimmunol.170.5.2702. [DOI] [PubMed] [Google Scholar]