Abstract
Purpose
There are limited data regarding clinical and treatment factors associated with radiation pneumonitis (RP) in patients receiving Taxane-based tri-modality therapy for esophageal cancer. The purpose of this study was to identify predictors of RP in patients undergoing tri-modality therapy.
Materials and Methods
We retrospectively reviewed patients undergoing chemoradiation followed by esophagectomy between 2006-2011. The association between clinical and dosimetric factors with RP was assessed using Chi-square test and Mann-Whitney U test. Multivariable (MVA) regression was used to assess the relationship between grade 2+ RP and clinical/dosimetric factors. Receiver operator curves were generated to identify threshold doses for RP.
Results
A total of 139 patients were included; 19 (13.7%) patients experienced grade 2+ RP. Patients with upper/middle thoracic tumors (p=0.038) and receiving higher radiation doses (p=0.038) were more likely to develop grade 2+ RP. There was no association between taxane-based therapy and grade 2+ RP (p=0.728). The total lung V5 (p<0.001), V10 (p<0.001), V20 (p<0.001), and V30 (p<0.001) were associated with an increased risk of grade 2+ RP. On MVA, the lung V5 (OR 1.101 95% CI 1.1014-1.195) and V20 (OR 1.149 95% CI 1.1015-1.301) remained associated with grade 2+ RP. A V5≤65% and V20≤ 25% were identified as optimal thresholds for increased grade 2+ RP.
Conclusions
Dosimetric parameters are strong predictors of symptomatic RP in patients undergoing tri-modality therapy for esophageal cancer. Mitigating the risk of RP in these patients should be an important consideration during treatment planning.
Keywords: Radiation pneumonitis, esophageal, tri-modality, dosimetric, taxane
Introduction
Multimodality therapy involving neoadjuvant chemoradiation followed by esophagectomy is the standard of care in the management of locally advanced esophageal cancer. Due to the aggressive approach, patients are at risk for surgical complications and radiation related toxicity. With improving survival in these patients, minimizing potential late toxicity is becoming increasingly important.
Radiation pneumonitis (RP) is one of the dose limiting factors in the delivery of radiation for thoracic malignancies. In addition to clinical factors such as pre-existing pulmonary comorbidities and smoking history, radiation dosimetric factors are a known predictor of RP 1, 2. Furthermore, prolonged lung ventilation during surgical procedures can cause lung injury due to multiple mechanisms 3. Limiting radiation dose below certain thresholds has been advocated to minimize the potential risk for RP. The Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) study recommended a lung V20 ≤ 30-35% and MLD ≤ 20-23 Gray (Gy) to limit the risk of RP to ≤ 20% in definitive radiation for non-small cell lung cancer with conventional fractionation 2.
Literature regarding the impact of radiation dose on pulmonary toxicity in patients undergoing multimodality therapy for esophageal radiation is limited. Furthermore, despite the increasing adoption of taxane-based chemoradiation regimens, data regarding its impact on radiation-related toxicity is limited. The purpose of this study was to identify predictors of RP in a cohort of patients undergoing multimodality therapy for esophageal cancer.
Materials and Methods
Patient Selection
Following IRB approval, we identified all patients undergoing neoadjuvant chemoradiation followed by esophagectomy for esophageal cancer at an NCI-designated cancer center between 2006 and 2011. Exclusion criteria were surgery or radiation at an outside facility or patients who did not complete tri-modality therapy. Patients with incomplete treatment information were not included in the analysis.
All patients underwent staging pre- and postoperatively according to the tumor-node-metastasis classification of the American Joint Committee for Cancer Staging Seventh edition 4. Pretreatment clinical staging routinely included positron emission tomography (PET)/CT scan, esophagogastroduodenoscopy and biopsy, bronchoscopy, and endoscopic ultrasound (EUS).. Patient (age, gender, diagnosis date, pathology) and treatment characteristics (type of chemotherapy, radiation dose, type of surgery) were extracted using patient medical records. Extracted data included age, gender, tumor staging, and location of tumor.
Treatment
The radiation treatment technique was at the discretion of the treating radiation oncologist. During radiation planning, patients typically underwent CT simulation with IV and oral contrast, supine in an immobilization cast. Most patients underwent four-dimension computed tomography (CT) to account for respiratory motion. Radiation treatment was delivered via 3D-conformal radiotherapy (3D-CRT) or intensity modulated radiotherapy (IMRT). 3D-CRT was typically delivered via a three to four beam arrangement at the discretion of the treating radiation oncologist. IMRT was typically delivered using seven to nine step and shoot fields.
The gross tumor volume (GTV) consisted of the gross tumor and involved lymph nodes as identified on imaging studies and delineated per the endoscopy report. In general, for patients with gastroesophageal (GE) junction tumors, the celiac axis was electively treated. For proximal esophageal tumors, the supraclavicular lymph nodes were treated. The clinical target volume (CTV) generally consisted of the GTV plus 4-5 cm longitudinal expansion and 1-2 cm radial expansion. An additional 0.5-1 cm expansion was used for the planning target volume (PTV). Median radiation dose was 50.4 Gy in 1.8 Gy fractions (range 45-60 Gy).
The chemotherapy regimen used was at the discretion of the treating medical oncologist. Chemotherapy was delivered concurrently and most commonly consisted of either carboplatin/paclitaxel or cisplatin/5-fluorouracil (5-FU). All patients were scheduled to undergo esophagectomy approximately 4-8 weeks after completion of neoadjuvant therapy. The surgery technique was at the discretion of the operating physician and typically employed a trans-hiatal or trans-thoracic approach. Prior to undergoing surgical resection, patients underwent re-staging studies.
Statistical Analysis
RP was evaluated retrospectively using patients' medical records and imaging studies. RP grading was defined per Common Terminology Criteria for Adverse Events (CTCAE) version 4.0: grade 2+ RP (symptomatic) was considered clinically significant (Table 1) 5. The time to RP was identified according to the date of radiation completion. Dosimetric data was extracted from cumulative dose-volume histogram. The Vn was defined as the percent volume of lung receiving at least n Gy of radiation. Extracted data included the V5, V10, V20, V30, V40, V50 and the MLD. In addition to dosimetric parameters, clinical factors (age, gender, tumor location, stage, treatment technique, histology, chemotherapy regimen, pulmonary function test, performance status) were examined for their potential relationship with RP. Following completion of treatment, patients were followed up every 3-6 months for the first two years, after which patients were followed up every year.
Table 1. Common Terminology Criteria for Adverse Events Version 4.0 Pneumonitis Grading.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; medical intervention indicated; limiting instrumental ADL | Severe symptoms; limiting self-care ADL; oxygen indicated | Life-threatening respiratory compromise; urgent intervention indicated (eg, tracheostomy or intubation) | Death |
The association between clinical and dosimetric factors with RP was assessed using the Chi-square test for categorical variables and Student's t-test and Mann-Whitney U test for continuous variables. The probability of developing grade 2+ RP was assessed using the Kaplan-Meier method with patients lost to follow-up or passing away being censored. Multivariable logistic regression was used to assess the relationship between grade 2+ RP and clinical/dosimetric factors significant on univariable analysis. Threshold doses for grade 2+ RP and doses corresponding to the optimal point of the receiver operator curve (ROC) were determined. For each model, the likelihood chi-squared values were obtained and tested for significance. All statistical tests were two-sided, p-values ≤0.05 were considered significant.
Results
Patient Characteristics
A total of 139 patients met the inclusion criteria. The median age was 62 (range 36-80). The majority of patients were male (83.5%), had lower/GE junction tumors (91.4%), and had adenocarcinomas (84.9%). In terms of treatment characteristics, the median radiation dose was 50.4 Gy (range 45-60) and patients were most frequently treated using 3D-CRT (56.1%). Nearly half of all patients were treated with concurrent taxane-based therapy (48.9%). Further patient and treatment characteristics are demonstrated in Table 1.
Clinical and Dosimetric Predictors (Univariable)
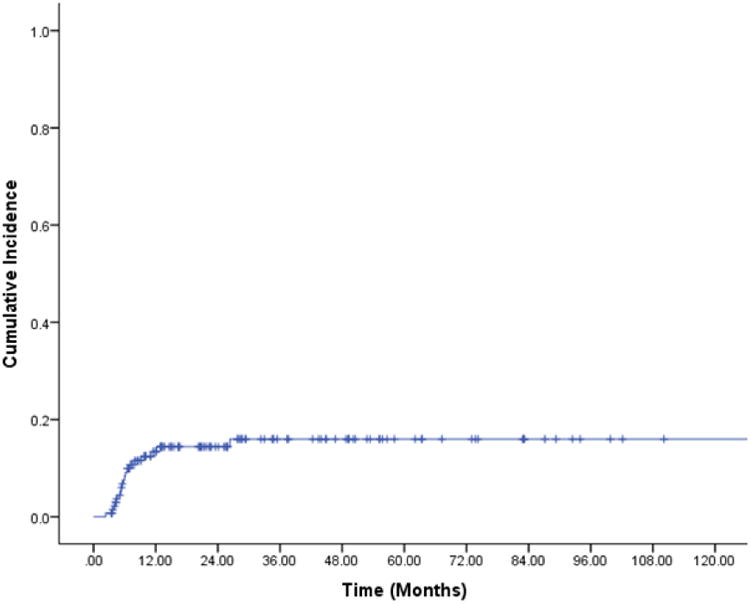
A total of 20 patients (23%) experienced grade 2+ RP; 19 (13.7%) experienced grade 2 RP, and one (0.7%) experienced grade 3 RP. The 9- and 12-month actuarial rates of grade 2+ RP were 11.6% and 13.4%, respectively (Figure 1). No patients experienced grade 4 or 5 RP. The median time to RP was 5.3 months from end of radiotherapy (range 3-23.8 months). Patients with upper/middle thoracic tumors (p=0.038) and receiving a higher radiation dose (p=0.038) were more likely to develop grade 2+ RP. There were no other patient or non-dosimetric treatment factors associated with grade 2+ RP (Table 1).
Figure 1. Cumulative incidence of grade 2+ radiation pneumonitis.
Dose-volume metrics are listed in Table 2. For patients developing symptomatic (grade 2+) RP, the MLD was 14.27% +/- 5.49% versus 9.38% +/- 3.89% for patients without grade 2+ RP. The mean V5 was 66.47% +/- 17.63% versus 47.08% +/- 13.54% for patients without grade 2+ RP. The mean V10 was 47.53% +/- 19.51% for patients with grade 2+ RP versus 31.52% +/- 12.07% for patients without grade 2+ RP. The mean V20 was 28.05% +/- 14.14% for patients with grade 2+ RP versus 17.13% +/- 9.51 for patients without grade 2+ RP. All p-values for these comparisons were <0.001. The mean V30 was also associated with grade 2+ RP (p=0.016).
Table 2. Patient and treatment characteristics.
| All | < Grade 2 RP | ≥ Grade 2 RP | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | p-value | ||
| Number of Patients | 139 | 120 | 19 | |||||
| Age (Years) | ||||||||
| Median (Range) | 62 (36-80) | 63 (37-80) | 57 (36-75) | 0.133 | ||||
| ≥65 | 83 | 59.7% | 70 | 58.3% | 13 | 68.4% | 0.405 | |
| <65 | 56 | 40.3% | 50 | 41.7% | 6 | 31.6% | ||
| Sex | ||||||||
| Male | 116 | 83.5% | 99 | 82.5% | 17 | 89.5% | 0.724 | |
| Female | 22 | 15.8% | 21 | 17.5% | 2 | 10.5% | ||
| Tumor Location | ||||||||
| Upper/Middle Thoracic | 12 | 8.6% | 8 | 6.7% | 4 | 21.1% | 0.038 | |
| Lower/GE Junction | 127 | 91.4% | 112 | 93.3% | 15 | 78.9% | ||
| Clinical T Stage | ||||||||
| 1 | 2 | 1.4% | 2 | 1.7% | 0 | 0.0% | 0.178 | |
| 2 | 12 | 8.6% | 8 | 6.7% | 4 | 21.1% | ||
| 3 | 122 | 87.8% | 107 | 89.2% | 15 | 78.9% | ||
| 4 | 3 | 2.2% | 3 | 2.5% | 0 | 0.0% | ||
| Nodal Positivity | ||||||||
| Yes | 96 | 69.1% | 81 | 67.5% | 15 | 78.9% | 0.316 | |
| No | 43 | 30.9% | 39 | 32.5% | 4 | 21.1% | ||
| Radiation Technique | ||||||||
| 3D-CRT | 78 | 56.1% | 67 | 55.8% | 11 | 57.9% | 0.916 | |
| IMRT | 60 | 43.2% | 52 | 43.3% | 8 | 42.1% | ||
| Histology | ||||||||
| SCC | 21 | 15.1% | 17 | 14.2% | 4 | 21.1% | ||
| Adenocarcinoma | 118 | 84.9% | 103 | 85.8% | 15 | 78.9% | 0.436 | |
| Chemotherapy | ||||||||
| Taxane Based | 68 | 48.9% | 58 | 48.3% | 10 | 52.6% | 0.728 | |
| Non-Taxane Based | 71 | 51.1% | 62 | 51.7% | 9 | 47.4% | ||
| Radiation Dose (Gy) | ||||||||
| ≤ 50.4 | 127 | 91.4% | 112 | 93.3% | 15 | 78.9% | 0.038 | |
| > 50.4 | 12 | 8.6% | 8 | 6.7% | 4 | 21.1% | ||
| ECOG PS | 0.179 | |||||||
| 0-1 | 88 | 63.3% | 74 | 61.7% | 14 | 73.7% | ||
| 2+ | 7 | 3.6% | 5 | 4.2% | 7 | 36.8% | ||
| Not Available | 44 | 31.7% | 41 | 34.2% | 3 | 15.8% | ||
Dose-Volume Thresholds
ROC curves were generated to identify potential threshold values significantly associated with symptomatic RP. Predictors of grade 2+ RP are listed in Table 3. The MLD, V5, V10, V20, and V30 were associated with an increased risk of symptomatic RP as continuous variables. For MLD, a value ≥13 Gy (OR 17.83, 95% CI 5.79-54.97) was the most significant predictor. For V5, a value ≥65% (OR 19.25 95% CI 6.04-61.36) was the most significant predictor. For V10, a value ≥40% was the most significant predictor (OR 9.65 95% CI 3.30-28.20). For V20, the most significant predictor was ≥25% (OR 8.92 95% CI 2.99-26.61). For V30, the most significant predictor was ≥20% (OR 4.41 95% CI 1.29-15.02). For each dose level associated with a statistically significant risk of symptomatic RP (V5-V30), cut-points were used to determine the volume threshold for developing a 15% and 20% risk of developing symptomatic RP (Figure 2).
Table 3. Dosimetric values correlated with symptomatic radiation pneumonitis.
| Variable | < Grade 2 RP (Mean +/- SD) |
> Grade 2 RP (Mean +/- SD) |
|
|---|---|---|---|
| Mean Lung Dose (Gy) | 9.38 +/- 3.89 | 14.27 +/- 5.49 | <0.001 |
| V5 (%) | 47.08 +/- 13.54 | 66.47 +/- 17.63 | <0.001 |
| V10 (%) | 31.52 +/- 12.07 | 47.53 +/- 19.51 | <0.001 |
| V20 (%) | 17.13 +/- 9.52 | 28.05 +/- 14.14 | <0.001 |
| V30 (%) | 10.59 +/- 9.09 | 16.47 +/- 13.61 | 0.016 |
| V40 (%) | 6.02 +/- 7.41 | 8.84 +/- 10.19 | 0.146 |
| V50 (%) | 1.61 +/- 5.72 | 0.79 +/- 1.32 | 0.536 |
Figure 2. Threshold dose curve estimating the risk of symptomatic radiation pneumonitis (grade 2+).

Multivariable Analysis
Table 4 demonstrates the multivariable analysis for clinical and dosimetric factors. On multivariable analysis, after controlling for dosimetric variables, tumor location, and radiation dose, the lung V5 (OR 1.101 95% CI 1.1014-1.195) and V20 (OR 1.149 95% CI 1.1015-1.301) remained significantly associated with symptomatic RP.
Table 4. Univariable analysis for development of grade 2+ radiation pneumonitis.
| Factor | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Mean Lung Dose | |||
| Continuous | 1.00 | 1.00-1.00 | 0.001 |
| ≥10 Gy | 15.22 | 3.36-69.03 | <0.001 |
| ≥11 Gy | 15.31 | 4.18-56.13 | <0.001 |
| ≥12 Gy | 10.83 | 3.68-31.90 | <0.001 |
| ≥13 Gy | 17.83 | 5.79-54.97 | <0.001 |
| ≥14 Gy | 16.96 | 5.45-52.82 | <0.001 |
| ≥15 Gy | 13.82 | 4.06-47.09 | <0.001 |
| V5 | |||
| Continuous | 1.09 | 1.05-1.14 | <0.001 |
| ≥55% | 6.72 | 2.10-21.51 | 0.001 |
| ≥60% | 18.20 | 5.77-57.41 | <0.001 |
| ≥65% | 19.25 | 6.04-61.4 | <0.001 |
| ≥70% | 14.53 | 4.46-47.31 | <0.001 |
| V10 | |||
| Continuous | 1.07 | 1.04-1.11 | <0.001 |
| ≥35% | 7.23 | 2.25-23.18 | 0.001 |
| ≥40% | 9.65 | 3.30-28.20 | <0.001 |
| ≥45% | 8.92 | 2.99-26.61 | <0.001 |
| ≥50% | 8.00 | 2.62-24.45 | <0.001 |
| V20 | |||
| Continuous | 1.07 | 1.03-1.12 | 0.001 |
| ≥20% | 6.80 | 2.37-19.50 | <0.001 |
| ≥25% | 8.92 | 2.99-26.61 | <0.001 |
| ≥30% | 8.77 | 2.47-31.19 | 0.001 |
| ≥35% | 5.44 | 1.11-26.55 | 0.036 |
| V30 | |||
| Continuous | 1.04 | 1.00-1.09 | 0.034 |
| ≥10% | 2.14 | 0.80-5.70 | 0.130 |
| ≥15% | 2.28 | 0.84-6.22 | 0.107 |
| ≥20% | 4.41 | 1.29-15.02 | 0.018 |
| ≥25% | 3.41 | 0.58-20.07 | 0.175 |
| V40 | |||
| Continuous | 1.04 | 0.99-1.09 | 0.171 |
| V50 | |||
| Continuous | 0.92 | 0.71-1.20 | 0.540 |
Discussion
Tri-modality therapy consisting of neoadjuvant chemoradiation followed by esophagectomy is associated with a risk of acute and chronic treatment related toxicity 6-8. The development of RP after irradiation can cause significant morbidity and mortality and is a dose limiting factor in the management of thoracic malignancies. Despite multiple series investigating clinical factors associated with RP in patients receiving lung irradiation, there are limited data regarding potential predictors of RP in patients receiving tri-modality therapy for esophageal cancer. In our analysis, dosimetric factors (MLD, V5, V10, V20, and V30) and clinical factors (upper/middle location, radiation dose) were associated with an increased risk for symptomatic RP in patients completing tri-modality therapy. Minimizing the amount of lung irradiated should be an important consideration during treatment planning.
In the presented dataset, a total of 20 patients experienced symptomatic RP with only one person developing grade 3 RP. Our results are consistent with other series in the literature 9, 10. Dosimetric values were a strong predictor of the development of symptomatic RP. In particular, MLD, V5, V10, V20, and V30 were associated with an increased risk of symptomatic RP. Clinical factors associated with RP included tumor location and radiation dose. On multivariable analysis, V5 and V20 remained associated with an increased risk for RP. Our results are consistent with multiple reports in the literature demonstrating an association between low dose radiation and RP 1, 11. In particular, V20 has been a strong predictor of RP in multiple series 1, 11, 12. In order to identify clinically meaningful guidelines, we performed a robust analysis to identify threshold values of predictors for increased risk of symptomatic RP. Based on our analysis, a V5 ≤ 65% and V20 ≤ 25% can be used as threshold values in the clinical setting. Minimizing low dose radiation appears to decrease the risk of RP in patients with localized esophageal cancer undergoing concurrent chemoradiation. The above dosimetric thresholds may be meaningful quality metrics in these patients.
To our knowledge, the presented series is the only analysis examining RP in a modern cohort of patients receiving taxane-based chemoradiation. Multiple series have examined potential predictors of RP in patients receiving definitive chemoradiation for esophageal cancer. Asakura et al performed a retrospective review of 37 patients who received definitive chemoradiation with 5-FU and cisplatin 13. In their analysis, dosimetric parameters were strongly associated with an increased risk of symptomatic RP. Other series have demonstrated similar relationships 14. There are limited data investigating predictors of RP in patients receiving tri-modality therapy for esophageal cancer. Wang et al examined potential predictors of surgical complications in patients receiving tri-modality therapy for esophageal cancer 15. In their analysis, MLD was strongly associated with increased pulmonary complications. Our series confirms the relationship between dosimetric parameters and RP.
Since the publication of the CROSS trial, taxane-based chemotherapy consisting of carboplatin/paclitaxel has become increasingly adopted as a standard chemotherapy option in patients receiving concurrent chemoradiation for esophageal cancer 16. Carboplatin/paclitaxel is often advocated due to the improved tolerability versus other chemotherapy regimens such as cisplatin/5-FU. A possible disadvantageous effect of taxane-based concurrent chemoradiation is the radiosensitization of normal tissue and potential increased risk for toxicity. Palma et al performed a large multi-national meta-analysis to assess potential predictors of RP in patients receiving definitive lung 12. In their analysis, increasing age, carboplatin/paclitaxel chemotherapy, and V20 were the most significant predictors of RP. In the presented series, V20 remained associated with an increased risk of RP after controlling for other clinical and treatment factors. There was no association between chemotherapy regimen or age with risk of developing RP. Other series have also demonstrated an association between RP risk and taxane-based therapy 17, 18. Despite the lack of significance in our analysis, minimizing lung dose may be particularly important in patients receiving taxane-based chemoradiation for esophageal cancer. Further analysis is warranted in this subset of patients to better identify the impact of chemotherapy regimens on radiation related toxicity.
Our analysis has both strengths and limitations. Strengths of our dataset include a contemporary cohort of patients treated using modern radiation techniques and chemotherapy regimens. As a result, our results are applicable to patients treated at many institutions in the modern era. Our analysis is limited due its retrospective nature and inability to control for all potential confounders. Furthermore, there was limited data on smoking history, comorbidities, or chemotherapy dosing which may have impacted the risk of RP in this cohort of patients. Despite the limitations, our analysis presents potential factors which may be associated with an increased risk for RP. Limiting radiation dose to the lung should continue to be an important consideration during treatment planning.
RP is a potentially lethal toxicity associated with radiotherapy for thoracic malignancies. The presented analysis is one of the first studies looking at a contemporary cohort of patients undergoing tri-modality therapy consisting of concurrent taxane-based chemoradiation followed by esophagectomy using modern radiation techniques. In the presented analysis, taxane-based therapy was not associated with an increased risk of RP. Minimizing low dose spread to the lung can decrease the potential risk for RP in patients undergoing tri-modality therapy for locally advanced esophageal cancer and should be considered as a quality metric in patients receiving tri-modality therapy. Further research is warranted to mitigate the risk of RP in patients receiving tri-modality therapy for locally advanced esophageal cancer.
Table 5. Multivariable analysis of predictors of grade 2+ radiation pneumonitis.
| Variable | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Radiation Dose (≤5040 vs. >5040) | 1.14 | 0.20-6.51 | 0.886 |
| Lung V5 | 1.10 | 1.01-1.20 | 0.022 |
| Lung V10 | 0.93 | 0.08-1.03 | 0.169 |
| Lung V20 | 1.15 | 1.02-1.30 | 0.028 |
| Lung V30 | 0.88 | 0.77-1.01 | 0.061 |
| Mean Lung Dose | 1.00 | 1.00-1.01 | 0.515 |
| Tumor Location (Upper/Middle vs. Lower) | 3.37 | 0.68-16.66 | 0.136 |
Acknowledgments
This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflicts of Interest Statement: None to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) International journal of radiation oncology, biology, physics. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 2.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. International journal of radiation oncology, biology, physics. 2010;76:S70–76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn PF. Physiology of the lateral decubitus position and one-lung ventilation. Int Anesthesiol Clin. 2000;38:25–53. doi: 10.1097/00004311-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB. AJCC cancer staging handbook : from the AJCC cancer staging manual. 7th. New York: Springer; 2010. American Joint Committee on Cancer., American Cancer Society. [Google Scholar]
- 5.National Cancer Institute (U.S.) Common terminology criteria for adverse events (CTCAE) Bethesda, Md: U.S. Dept of Health and Human Services, National Institutes of Health, National Cancer Institute; 2015. Rev. ed. [Google Scholar]
- 6.Reynolds JV, Ravi N, Hollywood D, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. The Journal of thoracic and cardiovascular surgery. 2006;132:549–555. doi: 10.1016/j.jtcvs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Kumagai K, Rouvelas I, Tsai JA, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. The British journal of surgery. 2014;101:321–338. doi: 10.1002/bjs.9418. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh T, Thomay A, Ruth K, Cohen SJ, Meyer JE. Association of treatment factors with surgical outcomes in tri-modality therapy for esophageal cancer. Journal of surgical oncology. 2015 doi: 10.1002/jso.24060. [DOI] [PubMed] [Google Scholar]
- 9.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. The lancet oncology. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 10.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. The New England journal of medicine. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 11.Tsujino K, Hirota S, Endo M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. International journal of radiation oncology, biology, physics. 2003;55:110–115. doi: 10.1016/s0360-3016(02)03807-5. [DOI] [PubMed] [Google Scholar]
- 12.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asakura H, Hashimoto T, Zenda S, et al. Analysis of dose-volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2010;95:240–244. doi: 10.1016/j.radonc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Nomura M, Kodaira T, Furutani K, Tachibana H, Tomita N, Goto Y. Predictive factors for radiation pneumonitis in oesophageal cancer patients treated with chemoradiotherapy without prophylactic nodal irradiation. Br J Radiol. 2012;85:813–818. doi: 10.1259/bjr/13604628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi K, Fujiwara Y, Nomura M, et al. Predictive factors for pericardial effusion identified by heart dose-volume histogram analysis in oesophageal cancer patients treated with chemoradiotherapy. Br J Radiol. 2015;88:20140168. doi: 10.1259/bjr.20140168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England journal of medicine. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 17.Parashar B, Edwards A, Mehta R, et al. Chemotherapy significantly increases the risk of radiation pneumonitis in radiation therapy of advanced lung cancer. American journal of clinical oncology. 2011;34:160–164. doi: 10.1097/COC.0b013e3181d6b40f. [DOI] [PubMed] [Google Scholar]
- 18.Onishi H, Kuriyama K, Yamaguchi M, et al. Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: a good local response but no good survival due to radiation pneumonitis. Lung Cancer. 2003;40:79–84. doi: 10.1016/s0169-5002(02)00532-9. [DOI] [PubMed] [Google Scholar]


