Abstract
Background
Early life adversity (ELA) is a risk factor for the later-life onset of gastrointestinal (GI) diseases such as irritable bowel syndrome (IBS); however, the mechanisms are poorly understood. Here, we utilized a porcine model of ELA, early weaning stress (EWS), to investigate the influence of ELA on the development and function of the enteric nervous system (ENS).
Methods
Female and castrated male (Male-C) piglets were weaned from their sow either at 15 d of age (EWS) or 28 d of age (late weaning control; LWC). At 60 d and 170 d of age, ileal mucosa-submucosa preparations were mounted in Ussing chambers and veratridine- and corticotropin releasing factor (CRF)-evoked short circuit current (Isc) responses were recorded as indices of secretomotor neuron function. Enteric neuron numbers and the expression of select neurotransmitters and their receptors were also measured.
Key Results
Compared with LWC pigs, female, but not Male-C EWS pigs exhibited heightened veratridine-induced Isc responses at 60 d and 170 d of age, that were blocked with tetrodotoxin (TTX) and atropine. Ileum from EWS pigs had higher numbers of enteric neurons that were choline acetyltransferase-positive (ChAT+). Markers of increased cholinergic signaling (increased acetylcholinesterase (AchE) and down-regulated mucosal muscarinic receptor 3 gene expression were also observed in EWS pigs.
Conclusions & Inferences
This study demonstrated that EWS in pigs induces lasting and sex-specific hypersensitivity of secretomotor neuron function and upregulation of the cholinergic ENS. These findings may represent a mechanistic link between ELA and lifelong susceptibility to GI diseases such as IBS.
Keywords: Cholinergic neurons, early life adversity, enteric nervous system development, irritable bowel syndrome, porcine model
Introduction
The adult onset and severity of gastrointestinal (GI) diseases, including Irritable Bowel Syndrome (IBS) and the inflammatory bowel diseases (IBD), are associated with early life adversity (ELA), including loss of caregiver, and emotional and physical abuse (1–6). Although the link between ELA and GI disease has been established in humans and in animal models of early life stress (7–10), the mechanistic link between ELA and long-term GI disease susceptibility remains poorly understood.
In early postnatal life, the GI system continues to undergo major developmental and adaptive changes (11). In particular, the enteric nervous system (ENS) undergoes a number of major developmental changes in postnatal life including ENS neuronal sprouting, synthesis of neurochemicals, synapse formation, and neuronal-pruning, which together shape the adult ENS phenotype (12–15). The early postnatal period is also a period of tremendous ENS plasticity and therefore injurious events that occur in early life can have long-lasting functional outcomes such as visceral hypersensitivity (a surrogate for abdominal pain), as demonstrated by animal models of neonatal colonic irritation (16–18). Likewise, rodent models of ELA, such as neonatal maternal separation stress (NMS) and limited nesting stress, were shown to induce long-term increases in visceral hypersensitivity, a surrogate function marker of abdominal pain that exists into adulthood (7, 19). In another study, NMS in rats increased mucosal nerve fiber density (20). Together, studies in rodent models of early life stress and chemical injury demonstrate the profound influence of early life events on long-term ENS function. This paradigm correlates to functional bowel disorders in humans, such as IBS, in which ELA is a significant risk factor as described above. How early life stress influence ENS develop and function has not been extensively explored.
Although a majority of stress research has been conducted with rodent models, pigs represent a model that is uniquely poised to model the human GI response to early life stress. It is well known that pigs possess a more highly developed brain and CNS and a more similar neuroanatomy and cognition to humans than rodents (21, 22). Furthermore, pigs possess a comparable GI system to human, in terms of size, anatomy, development, and diet preference (omnivores) compared vs. rodents (23–26). Compared with rodents, pigs possess a more similar ENS phenotype and structure compared with humans as the human and porcine gut have more complex inter-neuronal connections and plexi that are not observed in rodents (27), and exhibit a co-localization of specific neurotransmitters that is more similar between pigs and humans as compared with rodents (23). We have developed and characterized an ELA model in pigs, termed EWS. In this model, piglets are early weaned from their sow and littermates (15–19 d weaning age; EWS pigs) or allowed to remain with the sow for an additional period and weaned at a later age (23–28 d weaning age; LWC pigs). After weaning both EWS and LWC are grown up in controlled nursery environments until they reach adulthood. The stress associated with weaning in pigs is multifactorial (including removal from the mother and most littermates, introduction to a new environment, changes in diet source and feed intake etc.) and thus is considered more similar to the nature the ELA or early weaning experienced by humans. Previous investigations with the EWS model have demonstrated that EWS pigs exhibit clinical and pathophysiological hallmarks of stress-related that are comparable to human GI disease including persistent intestinal permeability, elevated baseline intestinal electrogenic transport activity, chronic, relapsing diarrhea, heightened intestinal mast cell activity (10, 28, 29), and increased severity of clinical and intestinal injury responses to a later life infectious enteric E. coli challenge (30). Furthermore, we demonstrated that many of the intestinal changes observed in EWS are mediated by corticotropin releasing factor (CRF) signaling pathways thus confirming that stress signaling is play a central role in GI disturbances in this model. Utilizing a porcine EWS model of ELA, the objective of the present study was to determine how EWS alters the development and long-term function of the submucosal and mucosal ENS.
Materials and Methods
Animals and experimental design
Experiments were conducted under approval of the Institutional Animal Care and Use Committee (IACUC). Yorkshire-duroc cross, female and castrated male piglets (Male-C, castrated at 9 d of age) were weaned from their sow at 15 d of age (EWS) or 28 d of age (LWC). Weaned pigs were housed in pens with tenderfoot flooring (12 ft × 6 ft pens, n=6 pigs per pen.) in the same environmentally controlled room. All pigs were offered ad libitum access to the same diet and water. The diets were formulated to meet or exceed the nutrient requirements of pigs (31). At 60 d and 170 d of age, representing juvenile and adulthood periods, respectively, n=12 pigs/weaning age group (6 female, 6 Male-C) were euthanized via captive bolt penetration and intestinal tissues were immediately harvested for experiments.
Ussing chamber experiments
Ileum was harvested from each animal immediately following euthanasia and opened lengthwise along the anti-mesenteric border. Samples were taken approximately 6 cm from the termination of the ileocecal ligament (identified by following the ileocecal ligament to the ileocecal junction). In oxygenated (95% O2, 5% CO2) ringer solution (154 Na+ mM, 6.3 K+ mM, 137 Cl− mM, 0.3 H2PO3 mM, 1.2 Ca2+ mM, 0.7 Mg2+ mM, 24 HCO3− at pH 7.4) at 37°C, the seromuscular layer was removed from the tissue by blunt dissection. Tissue free of Peyer’s patches was then mounted in a 0.3cm2 –aperture on Ussing chambers (Physiologic Instruments, Inc., Sand Diego, CA) as described in previous studies (10). The tissue was bathed on both the mucosal and serosal sides in Ringer’s solution containing 10 mM glucose (serosal side) that was balanced with 10 mM mannitol on the mucosal side. Bathing solutions were oxygenated (95% O2, 5% CO2) and maintained at 37°C. The viability of intestinal tissue mounted on Ussing chambers was confirmed during and at the end of the experiment by monitoring transepithelial electrical resistance (TER) and voltage continuously. At the end of the experiment, a subset of tissue were formalin-fixed, stained with H&E, and visualized on a light microscope to confirm that the intestinal epithelium was intact and that no major histopathological defects were evident.
Veratridine- and CRF- evoked short circuit current (Isc) experiments
After a 30-minute equilibration period, veratridine (Abcam, Cambridge, MA) (5 µM, 30 µM or 100 µM) in DMSO (1% of total chamber volume, previously demonstrated to have no measureable effect on Isc) was added to the serosal side of ileal mucosa mounted on Ussing chamber and ISC was recorded at 5-second intervals. Tissue was stimulated and responses were recorded for the proceeding 60 minutes. In select experiments, tissues were pre-treated with atropine (Med-Pharmex, Pomona, CA) (1 µM, serosal side of ileal mucosa), or TTX (Abcam, Cambridge, MA) (0.5 µM, Added to the serosal chamber) and allowed to equilibrate for 30 minutes, prior to veratridine stimulation. CRF (a generous gift from J.E.F. Rivier, Sentia Medical Sciences) was added to the serosal chamber and Isc was recorded as described for veratridine experiments.
Immunofluorescence
Ileum of full thickness (collected using the same anatomical landmarks as for the Ussing chamber experiments) was removed from the animals immediately after euthanasia. Five transverse samples, several centimeters apart, and representing the length of the ileum were washed with phosphate-buffered saline (PBS), pinned flat and fixed in 4% Paraformaldehyde solution (Thermo Fischer Scientific, Waltham, MA) for 3 hours at room temperature. The tissue was then washed with PBS and stored in a solution of 10% sucrose and 0.5% sodium azide in PBS at 4°C for 24 hours. Tissue was then embedded in Tissue-TEK OCT (Sakura, Torrance, CA), frozen (stored at −80°C) and sectioned transversely at 5 µm thickness on a cryostat on to Superfrost+ slides (Thermo Fischer Scientific, Waltham, MA) and stored at −80°C.
Slides were washed in PBS (2× 5 minutes), and then treated with 0.1% triton-X (Sigma Aldrich, St Louis, MO) in PBS for 10 minutes at room temperature. After washing again with PBS (2× 5 minutes), slides were treated with 20% normal donkey serum (Sigma Aldrich, St Louis, MO) and 0.5% bovine serum albumin (Sigma Aldrich, St Louis, MO) in PBS for 45 minutes at room temperature. Slides were then washed with 0.5% bovine serum albumin in PBS (5× 5 minutes) before being incubated for 24 hours at 4°C with primary antibodies (Supplemental Table 1) in PBS with 0.5% bovine serum albumin. Slides were washed with 0.5% bovine serum albumin in PBS (5× 5 minutes) and then incubated with the secondary antibodies (Supplemental Table 2) and a nuclear stain, ToPro (Invitrogen, Carlsbad, CA), at 1:1000 for 60 minutes at room temperature with 0.5% bovine serum albumin in PBS. The slides were then washed with PBS (5× 5 minutes) and cover-slipped with Faramount mounting media (Dako, Carpinteria, CA). Confocal images of stained sections were captured using a Nikon Eclipse C1 confocal microscope.
Image analysis
Images were captured in tissue areas free from Peyer’s patches. Neuron cell bodies were counted if a ToPro-stained nucleus was visible within a protein gene product 9.5 (PGP9.5) positive cell body. Tissue area was measured using ImageJ (NIH) and measurements were expressed as neurotransmitter positive neurons as a percentage of total counted neurons, or neurons/mm2. Mucosal and submucosal neurons were defined as PGP-positive cells between the muscularis mucosa and epithelium (mucosal) and between the circular muscle and muscularis mucosa (submucosal), respectively.
Acetylcholinesterase Assay
Ileal mucosal protein (taken from ileum identified in the same manner as in the Ussing chamber experiments) was extracted in mammalian protein extraction reagent (MPER) buffer (Thermo Fischer Scientific, Waltham, MA) and homogenized. Protein concentration was determined using Pierce bicinchoninic acid assay (BCA) protein assay kit (Thermo Fischer Scientific, Waltham, MA). Acetylcholinesterase (AchE) activity of tissue was determined in two technical replicates using Amplex® Red Acetylcholine/Acetylcholinesterase Assay Kit (Invitrogen, Carlsbad, CA) and expressed on a per mg protein basis.
RNA extraction and RT-qPCR analysis
Ileum was identified via the same protocol as for the Ussing chamber experiments, and total RNA was isolated from dissected mucosal and submucosal layers of the tissue using the RNeasy® Mini Kit (Qiagen, Valencia, CA). cDNA was derived from 3.5 ug total RNA using the Maxima First Strand cDNA synthesis kit for RT-qPCR with dsDNAse (Thermo Fischer Scientific, Waltham, MA). Quantitative real-time PCR, using LightCycler® 480SYBR Green I Master Mix (Roche, Nutley, NJ) and 30ng of cDNA, was performed in two technical replicates using a 20 uL reaction volume with a LightCycler® 480 Instrument according to Roche protocols. All target genes were normalized to the RPL4 gene. All primer sequences are listed below in Table 3.
Statistical Analysis
Data are reported as mean ± standard error (SE) based on experimental number (n). Data were analyzed using a Student’s T-Test or a standard 2-way ANOVA using GraphPad Prism (GraphPad Software, San Diego CA) with weaning age, treatment, and sex as the main effects. A post-hoc Tukey's test was used to determine differences between treatment groups following ANOVA. p<0.05 was considered significant and p values ≥ 0.05 and ≤0.1 were reported as trends.
Results
Overall health of animals
Upon physical examinations performed weekly by a university veterinarian, all animals remained clinically healthy throughout the study. There were no significant differences between LWC and EWS pigs with regards to final body weight (BW) (108.9 ± 4 kg vs. 103.7 ± 8 kg in EWS and LWC pigs, respectively). Despite similar final BW and general health appearance, we did find that EWS pigs, particularly females exhibited looser stools (as indicated by increased fecal scores) compared with LWC pigs (data to be reported in another manuscript).
EWS induces long-term, heightened neural-evoked Isc responses
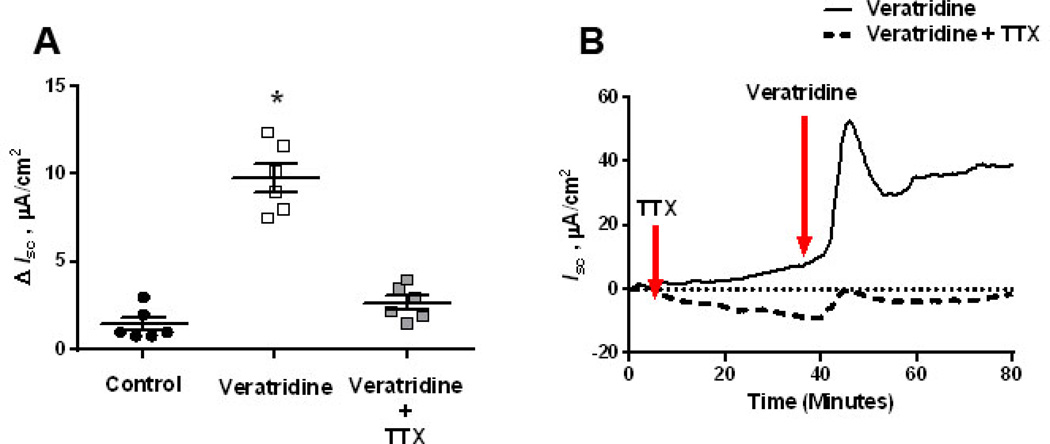
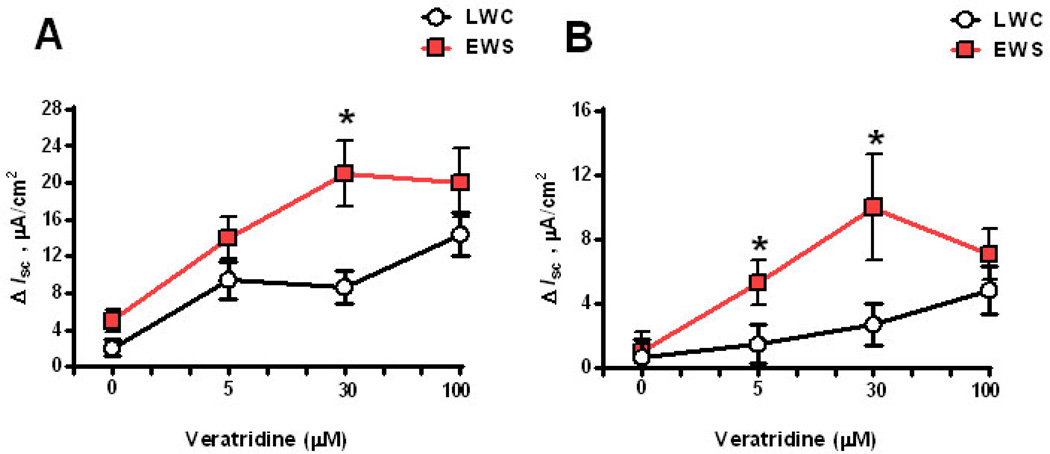
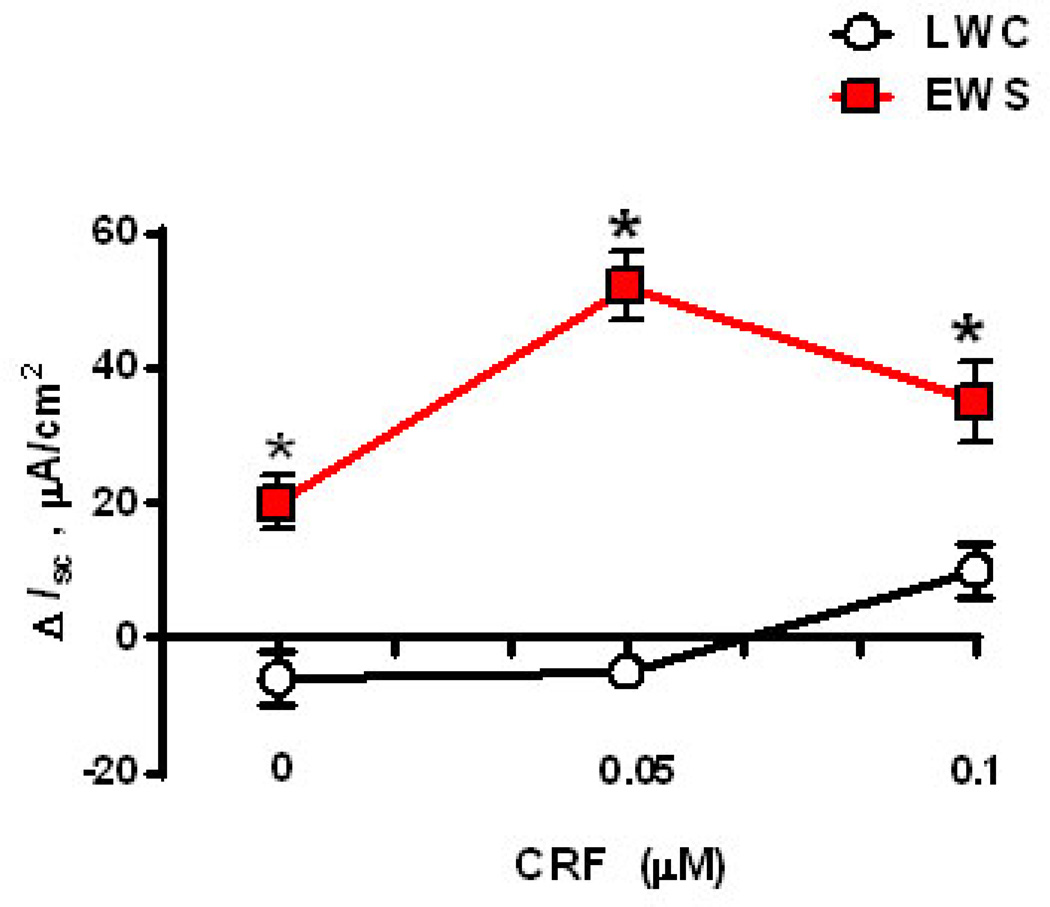
To determine whether EWS influenced submucosal/mucosal ENS function, we measured Isc responses to veratridine in EWS and LWC porcine ileum at 60 d and 170 d age. Veratridine activates voltage-gated sodium channels leading to neuron depolarization (32) and therefore, veratridine-evoked Isc responses are an index of electrogenic ion transport induced by secretomotor neurons. Exposure of ileal mucosa to veratridine evoked an increase in Isc (Figure 1A) that was ablated with the neuronal blocker tetrodotoxin, thus confirming the neuronal specificity of the veratridine response in the porcine ileum (Figure 1B). We next compared veratridine-evoked Isc responses between LWC and EWS ileum at 60 d and 170 d of age. Overall, we observed that the magnitude of the veratridine-induced ileal Isc responses decreased between 60 and 170 d of age in both LWC and EWS pigs (Figures 2A&B) which is in agreement with previous studies demonstrating a decline in intestinal secretory capacity with age (33). More importantly, we observed that ileum from EWS pigs exhibited greater (by ~2.5-fold) veratridine-evoked Isc responses (30 µM veratridine dose) compared with LWC ileum at both 60 d of age (Figure 2A) and 170 d of age (Figure 2B). To confirm that heightened Isc responses were observed in response to other physiological agonists, we compared ileal Isc responses in LWC and EWS pigs to CRF. We have previously shown that CRF induces Isc responses and increased permeability in the porcine intestine in a TTX-sensitive manner (34). Concentrations of 0.05 and 0.1 µM CRF induced elevations in Isc in EWS ileum, but not LWC ileum (Figure 3). Overall, these data demonstrate that EWS in pigs induces long-term hypersensitivity of ileal secretomotor neuron responses.
Figure 1. Veratridine-evoked Isc in the porcine ileum.
Porcine ileum (mucosa and submucosa) was mounted on Ussing chambers. After a 30 minute equilibration period, the serosal side of the tissue was exposed to veratridine (30 µM), in the presence or absence of a pre-treatment with tetrodotoxin (TTX), on the serosal side of tissues. Isc was recorded at 1 minute intervals over a 50-minute period. Data is presented as the absolute peak change in Isc in response to veratridine (A) or as a representative line tracing of treatment responses (B). Data were analyzed using a 1-Way ANOVA. *P<0.05 compared with other treatments.
Figure 2. Influence of early weaning stress (EWS) on veratridine-evoked Isc responses in juvenile and adult pigs.
Porcine ileum was obtained from late weaned control (LWC) pigs and EWS pigs at 60 d (A) and 170 d (B) of age and exposed to dose concentrations of veratridine. Isc responses were recorded and data were presented as the absolute peak change in Isc (ΔIsc) in response to veratridine. Values represent means (±SE) for n=12 pigs/treatment. *P<0.05 LWC vs. EWS within each veratridine dose; Data were analyzed with a Student’s T-test.
Figure 3. Influence of early weaning stress (EWS) on CRF-evoked Isc responses in juvenile pigs.
Porcine ileum was obtained from late weaned control (LWC) pigs and EWS pigs at 60 d of age and exposed to increasing doses of CRF (0 µM, 0.05 µM, and 0.1 µM. Isc responses were recorded and data were presented as the absolute peak change in Isc (ΔIsc) in response to CRF. Values represent means (±SE) for n=6 pigs/treatment. #P<0.05 vs. 0 µM CRF dose within weaning age group (LWC and EWS). Values represent means (±SE) for n=6 pigs/treatment. *P<0.05 LWC vs. EWS within each CRF concentration; Data were analyzed with a Student’s T-test.
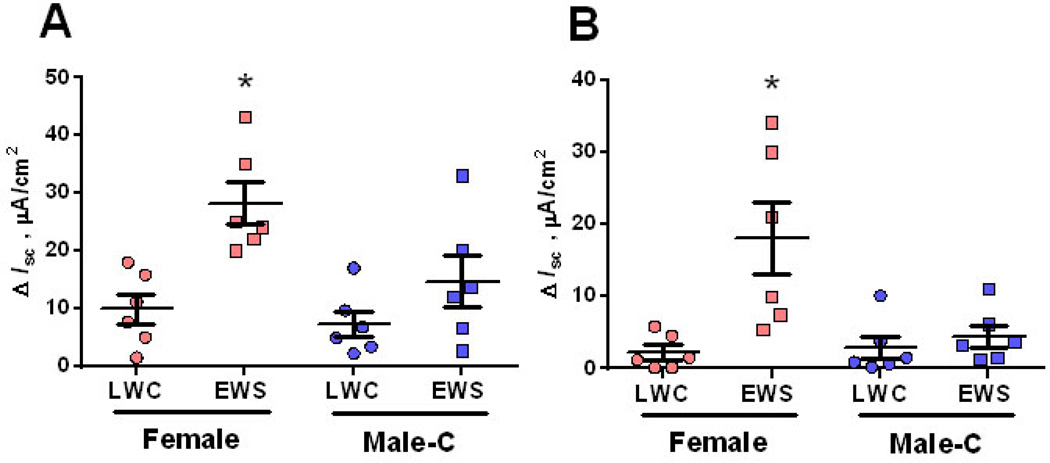
Ileum from EWS females exhibit greater neural-evoked Isc responses
Women are up to four times more likely to develop IBS than males (35–37). Therefore, we next determined whether female pigs were more susceptible to EWS-induced ENS sensitivity compared with Male-C pigs. In LWC pigs, veratridine-evoked Isc responses were similar between Male-C and females at 60 d or 170 d of age, suggesting no inherent sex-specific differences in veratridine-induced Isc responses (Figure 4A&B). In contrast, in EWS pigs, ileum from female pigs exhibited greater veratridine-evoked Isc responses at both 60 d (Figure 4A) and 170 d (Figure 4B) of age, compared with Male-C. Collectively, these data indicate that EWS female pigs exhibited heightened, neural-evoked mucosal secretory responses compared with castrated Male-C pigs.
Figure 4. Influence of sex and early weaning stress (EWS) on veratridine-evoked Isc responses in juvenile and adult pigs.
Porcine ileum was obtained from late weaned control (LWC) pigs (n=6 female; n=6 Male-C) and EWS pigs (n=6 Female; n=6 Male-C) at 60 d (A) and 170 d (B) of age and exposed to veratridine (30 µM). Isc responses were recorded and data were presented as the absolute peak change in Isc (ΔIsc) in response to veratridine. Data were analyzed using a 2-way ANOVA (Factors: Sex and weaning age group). *P<0.05 compared with other treatments.
Heightened neural-evoked Isc responses in EWS ileum is mediated via muscarinic cholinergic receptors
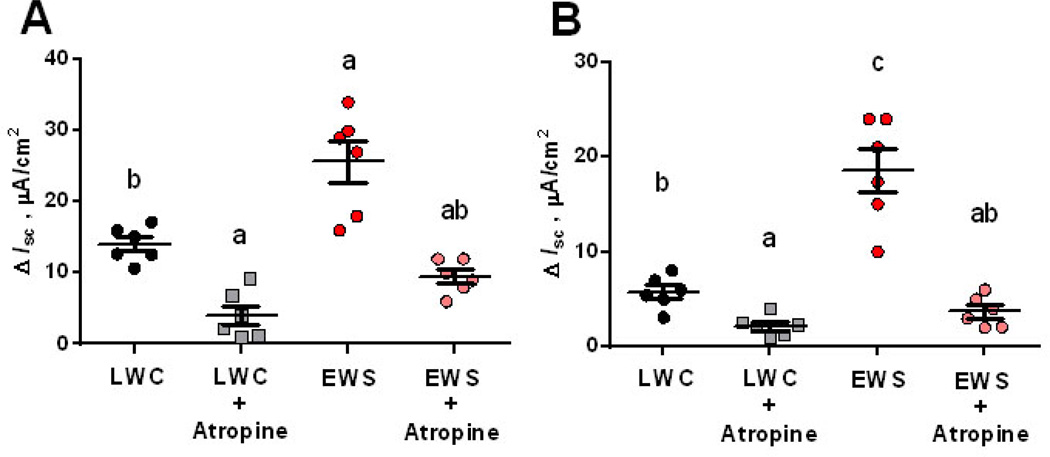
Acetylcholine (Ach) is the major excitatory neurotransmitter in the GI tract that induces electrogenic ion and fluid secretion via activation of muscarinic cholinergic receptors expressed on intestinal epithelial cells (38). We investigated whether heightened neural-evoked Isc responses in EWS pigs were mediated via acetylcholine/muscarinic cholinergic receptor pathways. To do this, we evaluated veratridine-evoked Isc responses in the absence or presence of the muscarinic cholinergic receptor antagonist atropine in LWC and EWS ileum. At 60 d of age, atropine inhibited veratridine-evoked Isc responses in both LWC and EWS (Fig 5A), indicating that a significant portion of the neural-evoked Isc responses in both LWC and EWS were via activation of muscarinic cholinergic receptors. Similarly, at 170 d of age, atropine blocked neural-evoked Isc responses in both LWC and EWS porcine ileum (Fig 5B). Together, these data indicate that the heightened neural-evoked secretomotor responses observed in EWS ileum were in large part mediated via muscarinic cholinergic receptor pathways.
Figure 5. Effects of muscarinic receptor blockade on veratridine-evoked Isc responses in late weaned control (LWC) and early weaning stress (EWS) pig ileum.
Porcine ileum was obtained from female LWC pigs and EWS pigs at 60 d (A) and 170 d (B) of age and exposed to veratridine (100 µM), in the presence or absence of a pre-treatment with the muscarinic cholinergic receptor antagonist atropine (1 µM). Isc responses were recorded and data were presented as the absolute peak change in Isc (ΔIsc) in response to veratridine. Data were analyzed using a 2-way ANOVA (Factors: Sex and weaning age group). Different letters (a,b,c) differ by P<0.05.
EWS influences enteric neuron number and neurotransmitter expression
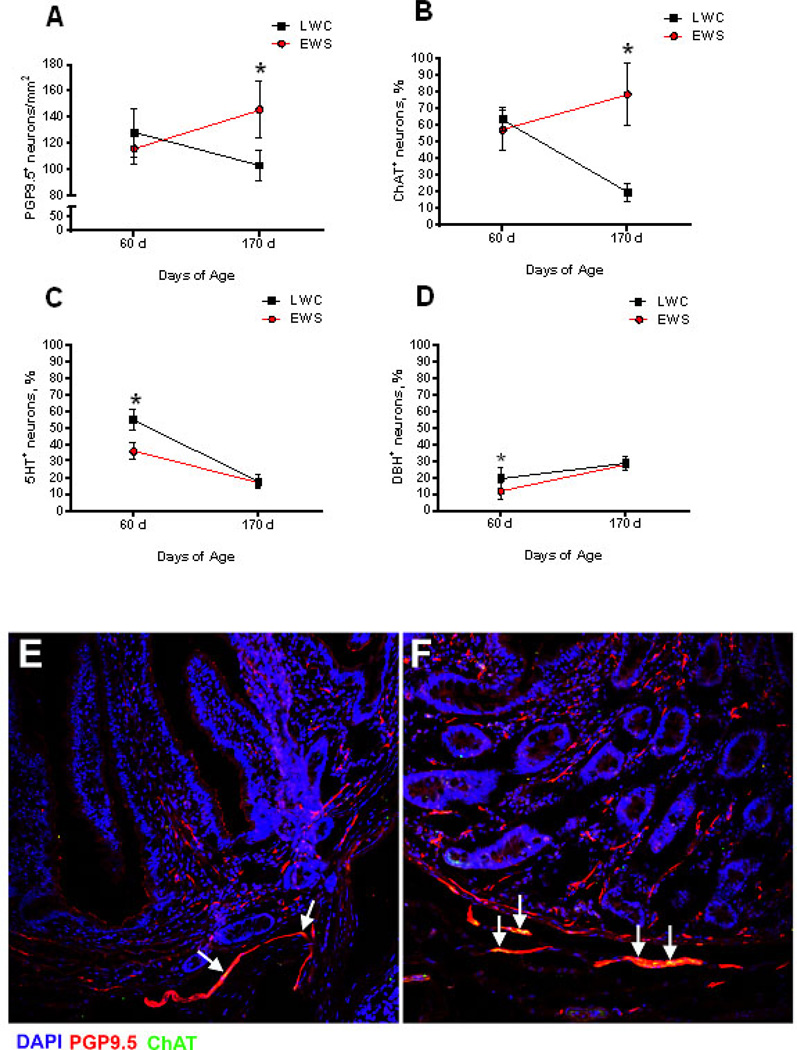
We next sought to determine whether heightened neural-evoked Isc responses were accompanied by changes in the phenotype of the ENS. First, we investigated whether EWS influenced the number of enteric neurons in the ileum. Using immunofluorescence, we quantified the number of PGP9.5 positive (PGP9.5+) neurons (PGP9.5+ cells) in the ileal submucosal plexus. At 60 d of age, the number of PGP9.5+ ileal submucosal neurons were comparable between EWS and LWC pigs (Figure 6A). Between 60–170 d of age, populations of submucosal neurons declined in the LWC ileum. In contrast, in EWS ileum, the numbers of PGP9.5+ submucosal neurons persisted between 60–170 d of age, resulting in a higher (p<0.05) number of submucosal neurons, compared with LWC, at 170 d of age.
Figure 6. Influence of early weaning stress (EWS) on enteric neuron numbers and phenotype.
The numbers of enteric neurons were quantified counting the number of PGP9.5-positive cells and expressing them as neurons/mm2. The proportion of ChAT-, 5HT- and DBH-positive neurons were measured by immunohistochemistry in the submucosal plexus of ileum of LWC and EWS pigs at 60 d and 170 d of age and were expressed as a percentage relative to PGP9.5-positive neurons. (A) Number of PGP9.5-positive enteric neurons. (B) percentage of ChAT-positive/PGP-positive neurons. (C) percentage of 5HT-positive/PGP-positive neurons. (D) percentage of DβH -positive/PGP-positive neurons. (E and F), representative example of PGP9.5-ChAT double positive in LWC (E) and EWS (F) porcine ileum at 170 d of age. White arrows indicate double-positive cells. Values represent means (±SE) for n=12pigs/treatment. *P<0.05 LWC vs. EWS within each age time point; Data were analyzed with a Student’s T-test.
We next investigated whether there were differences in the neurotransmitter expression of enteric submucosal neurons between LWC and EWS pigs by quantifying the percentage of PGP9.5+ neurons expressing choline acetyltransferase (ChAT) (a marker of cholinergic neurons), 5-hydroxy tryptophan (5-HT) (a marker of serotonergic neurons), and dopamine beta hydroxylase (DβH) (a marker of noradrenergic neurons). At 60 d of age, the percentage of ChAT+ neurons was similar between the mucosa and submucosa of LWC and EWS pigs (Figure 6B). However, differences between LWC and EWS pigs were observed in the percentage of ChAT+ neurons over time between 60 d and 170 d of age. In LWC pigs, the percentage ChAT+ neurons declined between 60–170 d of age (Fig 6B). In contrast, a decline in the percentage of ChAT+ neurons was not observed in EWS ileum resulting in a higher percentage of ChAT+ in EWS ileum (50% versus 22% in EWS and LWC ileum, p<0.05) at 170 d of age. Together, these findings suggested a persistence of ChAT+ submucosal neurons in the EWS pig ileum. At 60 d of age, ileum from EWS pigs had a lower percentage of 5HT+ submucosal neurons (58% versus 34% in LWC and EWS ileum, respectively, p<0.05; Figure 6C); however, unlike the percentage of ChAT+ neurons, the % of 5HT+ enteric neurons declined between 60–170 d of age in both EWS and LWC pigs and there were no differences in the numbers of 5HT+ enteric neurons at 170 d of age. Submucosal noradrenergic neurons, defined as the percentage of DβH+ neurons, were higher in EWS compared with LWC (20% versus 11% in LWC and EWS ileum, respectively, p<0.05; Figure 6D) at 60 d of age. The percentage of DβH+ enteric neurons increased between 60–170 d of age in both EWS and LWC ileum, and no differences in DβH+ enteric neurons were observed between LWC and EWS at 170 d of age.
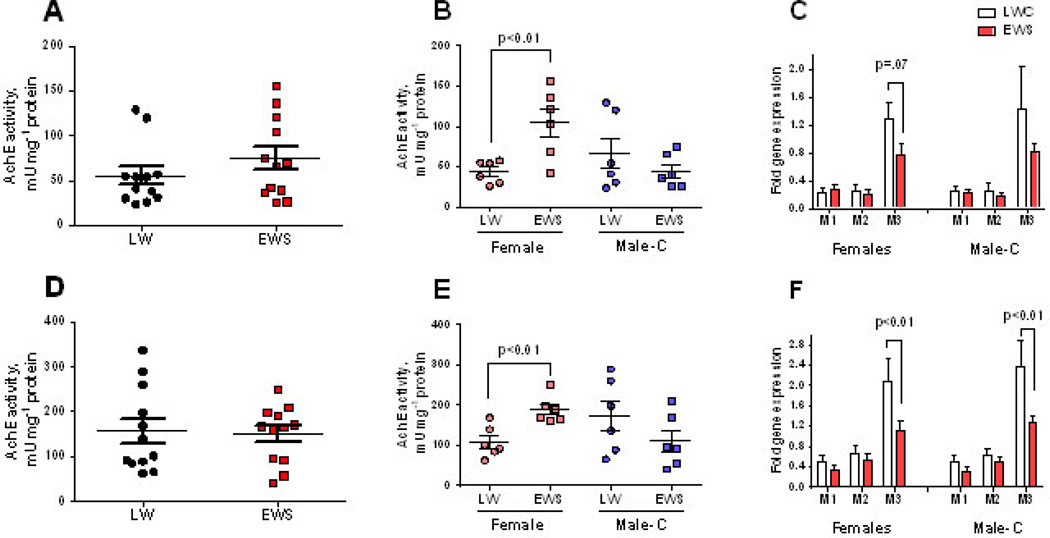
Cholinergic activity is upregulated in EWS pig ileum
Given the heightened cholinergic-mediated Isc responses and the persistence of ChAT+ neurons in the EWS ileum, we further characterized the changes in intestinal cholinergic activity in the EWS intestine by measuring mucosal AchE activity (an indirect measure of acetylcholine levels) and muscarinic receptor subtype gene expression. Using a 2-Way ANOVA, there were no significant differences in mucosal AchE activity with regards to weaning age or sex either at 60d or 170 d of age (Figure 7). However, a student’s T-test revealed differences in how Male-C and female pigs responded to EWS. Compared with Male-C, LWC pigs, EWS did not significantly influence ileal mucosal AchE activity measured at 60 d (Figure 7B) and 170 d (Figure 7E) of age. However, in EWS females, higher ileal mucosal AchE activity at 60 d and 170 d of age, compared with LWC female pigs.
Figure 7. Impact of Early weaning stress (EWS) on enteric cholinergic markers in juvenile and adult pigs.
Mucosal acetycholinesterase (AcHE) activity in LWC and EWS pigs presented as combined Male-C + female data at 60 d (A) and 170 d (D) of age or as individual Male-C and female data at 60 d (B) and 170 d (E) of age. Quantitative PCR was performed to measure the gene expression for muscarinic cholinergic receptor subtypes 1(CHRM1), 2 (CHRM2) and 3 (CHRM3) in the mucosa from 60 d (C) and 170 d (F) old pigs. Student’s T-tests were performed for each variable to detect differences between LWC and EWS pig groups. *P<0.05.
We next evaluated the gene expression of mucosal muscarinic acetylcholine receptor subtypes (CHRM1–CHRM3), which are responsible for mediating physiologic effects on target cells (39–41). While there were no significant changes in the gene expression of ileal mucosal CHRM1 or CHRM2 subtypes between LWC and EWS pigs, there was a trend (p=0.07) for down-regulation of mucosal CHRM3 muscarinic receptor gene (by 1.6 Fold) at 60 d of age in both LWC and EWS pigs (Figure 7C). The down-regulation in CHRM3 gene expression in EWS castrated male and female pigs was significant (p<0.01) at 170 d of age (Figure 7F). In contrast to mucosa, there were no differences in gene expression of CHRM1–3 receptor in the ileal submucosa (Supplemental Figure 1). In addition to cholinergic muscarinic receptor gene expression, we measured the mucosal and submucosal gene expression of TPH1 (encodes for tryptophan hydroxylase 1), which is the rate limiting enzyme for 5HT synthesis, and the α-adrenergic receptor subtypes 1 (ADR1A) and 2 (ADR2A). There were no significant differences in mucosal TPH1 or ADRA1A gene expression between LWC and EWS pigs (Supplemental Figure 2A–C). However, mucosal ADR2A gene expression was down-regulated in EWS pigs at 60 d and 170 d of age (Supplemental figure 2A&B). Furthermore, EWS-induced down-regulation of ADRA2 gene expression was only observed in Male-C pigs and not female pigs, indicating a sex-specific effect of EWS on ileal ADRA2 gene expression. No differences in gene expression of TPH, ADRA1, or ADR2A were observed in the submucosal tissues (Supplemental Figure 2C).
Discussion
Early life adversity is a major risk factor for later life susceptibility to major GI disorders including IBS and IBD, however the underlying mechanisms remain poorly understood. In the present study, EWS in pigs was used as a model for investigating the influence of ELA on the development and function of the ENS. Here we demonstrate that pigs subjected to EWS at 15 d of age exhibit lasting and sex-specific alterations in the development and function of the ENS. Specifically, EWS induced alterations in ENS function were highlighted by a persistence of enteric cholinergic neuron populations, upregulation of markers of cholinergic activity, and heightened sensitivity and magnitude of cholinergic-mediated secretomotor neuron responses. Together, these finding could have major implications to the pathogenesis of human and animal GI diseases such as IBS and IBD.
In the present study, piglets subjected to EWS exhibited long-lasting changes in ENS function as demonstrated by increased sensitivity and heightened veratridine- and CRF-evoked ileal Isc responses, indicative of a heightened secretomotor neuron response in the EWS ileum. The precise mechanism for heightened neural-evoked secretomotor responses in EWS pigs is presently unclear. The secretomotor neuron response involves activation of submucosal plexus neurons and the subsequent release of acetylcholine and vasoactive intestinal peptide (VIP) at the neuro-epithelial junction, triggering electrogenic Cl− secretion, reflected by increased Isc (42, 43). The heightened Isc responses in EWS pigs in the present study were inhibited in the presence of the muscarinic cholinergic receptor antagonist atropine, demonstrating that that acetylcholine was a major mediator in the hyperactive secretomotor responses in EWS pigs. Quantification of ileal enteric neurons revealed a persistence of enteric neuron numbers, specifically ChAT+ neurons in EWS pigs. However, increased ChAT+ neurons did not completely explain the increased sensitivity to neural-evoked Isc in the present study as heightened secretomotor neuron responses were observed at 60 d of age when enteric neuron numbers were similar between LWC and EWS pigs. While the number of enteric neurons may not have correlated with increased secretomotor activity in the EWS ileum in the present study, it is possible that increased neuronal sensitivity (as opposed to neuron number) could have contributed to increased heightened responses in EWS ileum in the present study. Another potential explanation for heightened neural-evoked Isc responses could be related to possible changes in voltage-gated sodium channels in the EWS porcine intestine. There is evidence implicating voltage-gated sodium channel dysfunction (both in expression and function) in both IBS in humans (44, 45) and animal models such as NMS and neonatal colonic inflammation in rats (46–48). The role of voltage-gated sodium channels in the porcine EWS model neural-evoked Isc remains to be elucidated.
A major factor that was associated with hypersensitive neural-evoked Isc responses in EWS pigs was the sex of the pigs. Female EWS pigs exhibited increased sensitivity to neural-evoked stimuli compared with Male-C pigs at 60 d and 170 d of age. While the sex-specific sensitivity of the ENS secretomotor neurons has not been specifically addressed in humans, it is well established that females experience IBS at a ratio of 2:1 to 5:1 compared with males (36, 37) and thus the present findings may represent a potential mechanism for the sexual dimorphism observed in human stress-related GI diseases such as IBS. It has also been hypothesized that ovarian hormones are a potential modulator of sex-specific disease response. In the GI tract, ovarian hormones are known to affect intestinal functional and GI transit time, with cyclic IBS flares in female IBS patients influenced by the menstrual cycle (36). Furthermore, differences between incidences of IBS in males and females have been associated with puberty and increase into early adulthood (37). Rodent models demonstrated that visceral hypersensitivity in females was prevented by ovariectomy (49, 50). However, in the present study, heightened veratridine-induced Isc in female pigs was observed early at 60 d of age which is well before the onset of sexual maturity in pigs which typically occurs between 155–170 d of age in most commercial lines of pigs. While female sex hormones are thought to play a role in disease initiation in females, there also exists evidence to suggest that male sex hormones may be protective against IBS (51). In the present study, male pigs were castrated at a young age, making the potential protective influence of male sex hormones on EWS-associated GI dysfunction difficult to discern. It is possible that the sex-specific differences between EWS female and non-castrated males could have been even more substantial than Male-C pigs. Regardless, given that sex-specific differences in the present study were observed relatively early and prior to the onset of sexual maturity, these data suggests that sex-specific difference in response to EWS may not be solely dependent on sex hormones.
In the present study, sex-specific differences in GI responses to EWS were not associated with enteric neuron numbers, percentage of ChAT+ neurons, or down-regulation of mucosal CHRM3 receptor gene expression. However, sex-specific changes in select enteric neural signaling responses were observed. First, females, but not Male-C pigs, exhibited the highest AchE activity, suggestive of higher acetylcholine levels which could have contributed to heightened neural-evoked secretory responses. Second, while muscarinic cholinergic receptor gene expression was not different between Male-C and female pigs in the present study, Male-C pigs responded to EWS with a more pronounced down-regulation of ileal mucosal α-adrenergic receptor 2A gene, ADRA2. Similar to muscarinic cholinergic receptors, adrenergic receptors are G-protein coupled receptors that regulate their activity and sensitivity when exposed to high ligand concentrations by receptor internalization and decreased gene expression (52). Therefore, the downregulation of ADRA2 may reflect increased α-adrenergic receptor signaling which has been shown to be inhibitory to intestinal mucosal electrogenic ion transport (53), and thus may contribute to reduced secretomotor Isc responses in castrated EWS males, compared with females.
In LWC pigs, an age-dependent decline in the number of enteric submucosal neurons was observed between 60 and 170 d of age. The observed neuronal senescence with age in the present study is in agreement with existing data in other species, including humans (54–56), and is associated with a process of “pruning” neurons generated in excess (55, 57). In contrast, enteric neuron numbers in the EWS pig ileum did not decline with age. The persistence of enteric submucosal neuron numbers in EWS resulted in an increased number of neurons at 170 d of age, compared with LWC pigs. A further characterization of the enteric neurons revealed a persistent population of ChAT+ neurons into adulthood in the EWS ileum. Gareau et al (2007) demonstrated increased cholinergic neurons in the colon of weanling rats subjected to NMS (58); however, the long-term cholinergic changes into adulthood were not investigated in that study. In another study, NMS in rats was shown to increase mucosal nerve fiber density (20). In line with animal model data, mucosal biopsies from IBS patients exhibited neuronal outgrowth (59). Overall, results from the present study demonstrate for the first time that ELA in a porcine model of EWS, induces a persistence of enteric cholinergic neurons that lasts into adulthood. The mechanisms for cholinergic neuron persistence remains to be fully elucidated. It is known that neuron survival and neurite outgrowth in the postnatal ENS are controlled in part by a number of secreted survival factors and loss of such factors will result in the “pruning” and elimination of neurons (57). Some examples of specific neuron growth factors include glial cell line derived neurotrophic factor (GDNF) (60), and pro-inflammatory cytokines such as IL-1β and TNFα (61). GDNF secretion is also increased in response to inflammation (62) and has also been shown to increase choline uptake (an initial step involved in the conversion to acetylcholine), and increase acetylcholine release from neurons (63). Whether these inflammatory and glial-derived factors are influencing ENS cholinergic development in EWS pigs remains to be investigated.
In the present study, we focused on the effects of EWS in the porcine ileum, but did examine changes in the colon. However, we have demonstrated in previous studies that intestinal permeability and heightened baseline Isc levels are observed in both the small intestine and colon of EWS pigs (64, 65). While many human IBS investigations have focused on the colon, there are several lines of evidence of small intestinal involvement with IBS. For example, Di NArdo et al (2014) demonstrated that the number of mast cells in close proximity to nerves in both ileal and colonic mucosa was increased in IBS children with IBS and were related to the intensity and frequency of abdominal pain (66). In support of this, there are other studies demonstrating small intestinal (jejunum and ileum) involvement in IBS (67–69). Therefore, findings presented here in the EWS ileum have relevance to human stress-related GI diseases such as IBS.
Findings from the present study demonstrated that ELA induced by EWS in pigs, resulted in developmental changes in the ENS highlighted by long-lasting, heightened cholinergic secretomotor tone. It is well-known that the cholinergic nervous system is a major regulator of intestinal mucosal secretory mechanisms and barrier function and has received considerable attention as a critical modulator of intestinal inflammation (70–72). Therefore, heightened cholinergic tone observed in EWS pigs and further investigation into the associated mechanisms could have significant implications for a number of important GI diseases, especially GI diseases associated with ELA. For example, several studies have shown that cholinergic input plays a stimulatory role in infectious, secretory diarrhea as muscarinic cholinergic receptor blockade inhibited intestinal secretion induced by bacterial enterotoxins (73, 74). Cholinergic input was shown to synergize with cholera toxin to augment intestinal secretory responses in human intestinal cells (75). Additionally, Krane et al (1990) demonstrated that carbachol, an acetylcholine analogue, enhanced E. coli heat-stable enterotoxin (STa)-induced cGMP production by up to 100% (76). In line with the pro-secretory influences of cholinergic input and the heightened cholinergic, neural-evoked secretory responses observed in EWS pigs, we have previously demonstrated that EWS pigs exhibit heightened secretory diarrheal disease in response to a subsequent challenge with enterotoxigenic E. coli (30). Taken together, the heightened cholinergic tone induced by EWS could be an important influence in modulating life-long enteric secretory diarrheal disease severity. In addition to regulation of intestinal ion transport, it has also become evident that the cholinergic nervous system plays a major role in the regulation of GI inflammation (71, 72, 77). For example, several investigations demonstrated that vagotomized animals exhibit exaggerated dextran sulphate sodium (DSS)-induced colitis and inflammatory cytokine responses, including IL-1β, IL-6, and TNF, compared with sham controls (78, 79). Furthermore, it was shown that α7-nicotinic receptor (α7nAchR) knock-out mice exhibit more severe DSS-induced colitis, while choline chloride, a α7nAchR agonist, ameliorated colitis (79), overall indicating that the α7nAchR was a major receptor involved in the cholinergic anti-inflammatory mechanism. The impact of heightened cholinergic tone observed in the EWS intestine on intestinal inflammatory responses has not been investigated and thus remains to be elucidated.
In conclusion, data from this study demonstrates that ELA in a porcine model of EWS induces long-term, heightened ileal secretomotor responses in sex-specific manner. Furthermore, EWS induced a marked upregulation of the cholinergic ENS characterized by persistence and elevated ChAT+ neurons and mucosal AchE activity. These findings may represent an important mechanistic link between ELA and life-long GI disease susceptibility.
Supplementary Material
Key Messages.
The onset and severity of gastrointestinal (GI) diseases such as IBS and inflammatory bowel disease (IBD) are associated with early life adversity (ELA). The underlying mechanisms linking ELA and GI disease remain poorly understood.
Utilizing a porcine model of ELA, early weaning stress (EWS), this study aimed to investigate whether EWS influenced the long-term development and function of the enteric nervous system (ENS), as a potential underlying mechanism for ELA-associated GI disease.
Ileal mucosa-submucosa preparations from EWS and late weaned control (LWC) pigs at 60 and 170 d of age, were mounted in Ussing chambers and veratridine- and CRF-evoked short circuit current (Isc) responses were recorded as indices of secretomotor neuron function. Immunofluorescence microscopy, ELISA, and qPCR were used to characterize the phenotype of the ENS.
Results from this study showed that EWS induced developmental changes in the ENS characterized by heightened, sex-specific secretomotor responses, persistence of ChAT+ enteric neurons, and upregulated enteric cholinergic activity. Furthermore, female EWS pigs exhibited heightened secretomotor responses compared with male pigs
These findings provide potentially new mechanistic insights into how ELA influences lifelong susceptibility to GI disorders, such as IBS.
Acknowledgments
The authors thank Dr. J.E.F Rivier for generously providing the CRF compound. J. E. F. Rivier is Founder of Sentia Medical Sciences. Dr. Adam J. Moeser is the Matilda R. Wilson Endowed Chair at the College of Veterinary Medicine, Michigan State University.
Funding
This work was supported by grants from the National Institutes of Health, USA (DK084313 to AJM), (DK097462 to AJM), and (HD072968 to AJM)
Footnotes
Disclosure
The authors declare no conflicts of interest.
Author contribution
AJM designed the research study; JEM, CSP, LLE, KB, YL, SFA, AJM performed the experiments; JEM, AJM analyzed the data; and JEM, AJM wrote the paper.
References
- 1.Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390. e381–e383. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107:1040–1049. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–833. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 4.Ercolani MFM, Agostini A, Baldoni F, Baracchini F, Ravegnani G, Bortolotti M. Gastroesophageal reflux diases (GERD) and inflammatory bowel disease (IBD): attachment styles and parental bonding. Perceptual and Motor Skills. 2010;111:625–630. doi: 10.2466/02.09.15.21.PMS.111.5.625-630. [DOI] [PubMed] [Google Scholar]
- 5.Agostini A, Rizzello F, Ravegnani G, et al. Adult attachment and early parental experiences in patients with Crohn's disease. Psychosomatics. 2010;51:208–215. doi: 10.1176/appi.psy.51.3.208. [DOI] [PubMed] [Google Scholar]
- 6.Agostini A, Rizzello F, Ravegnani G, et al. Parental bonding and inflammatory bowel disease. Psychosomatics. 2010;51:14–21. doi: 10.1176/appi.psy.51.1.14. [DOI] [PubMed] [Google Scholar]
- 7.Chung EK, Zhang X, Li Z, Zhang H, Xu H, Bian Z. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain Res. 2007;1153:68–77. doi: 10.1016/j.brainres.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 8.Lennon EM, Maharshak N, Elloumi H, Borst L, Plevy SE, Moeser AJ. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflamm Bowel Dis. 2013;19:712–719. doi: 10.1097/MIB.0b013e3182802a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. American journal of physiology Gastrointestinal and liver physiology. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- 10.Smith F, Clark JE, Overman BL, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. American journal of physiology Gastrointestinal and liver physiology. 2010;298:G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohl CS, Medland JE, Moeser AJ. Early-life stress origins of gastrointestinal disease: animal models, intestinal pathophysiology, and translational implications. Am J Physiol Gastrointest Liver Physiol. 2015;309:G927–G941. doi: 10.1152/ajpgi.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns AJ, Roberts RR, Bornstein JC, Young HM. Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin Pediatr Surg. 2009;18:196–205. doi: 10.1053/j.sempedsurg.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Chalazonitis A, Gershon MD, Greene LA. Cell death and the developing enteric nervous system. Neurochem Int. 2012;61:839–847. doi: 10.1016/j.neuint.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foong JP, Nguyen TV, Furness JB, Bornstein JC, Young HM. Myenteric neurons of the mouse small intestine undergo significant electrophysiological and morphological changes during postnatal development. J Physiol. 2012;590:2375–2390. doi: 10.1113/jphysiol.2011.225938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao MM, Bornstein JC, Young HM. Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP mice. J Comp Neurol. 2013;521:3358–3370. doi: 10.1002/cne.23354. [DOI] [PubMed] [Google Scholar]
- 16.Al-Chaer EdKMPPJ. A new model of chronic visceral hypersensitivty in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 17.Chaloner ARA, Al-Chaer ED, Greenwood-Van Meerveld B. Importance of neural mechanisms in colonic mucosal and muscular dysfunction in adult rats following neonatal colonic irritation. Internation Journal of Developmental Neuroscience. 2010;28:99–103. doi: 10.1016/j.ijdevneu.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CA-CE. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain research. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Wang Z, Mayer EA, Holschneider DP. Neonatal stress from limited bedding elicits visceral hyperalgesia in adult rats. Neuroreport. 2015;26:13–16. doi: 10.1097/WNR.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582–590. doi: 10.1136/gut.2007.126680. [DOI] [PubMed] [Google Scholar]
- 21.Gieling ET, Nordquist RE, van der Staay FJ. Assessing learning and memory in pigs. Animal Cognition. 2011;14:151–173. doi: 10.1007/s10071-010-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gieling ET, Schuurman T, Nordquist RE, van der Staay FJ. The Pig as a Model Animal for Studying Cognition and Neurobehavioral Disorders. Curr Top Behav Neuro. 2011;7:359–383. doi: 10.1007/7854_2010_112. [DOI] [PubMed] [Google Scholar]
- 23.Brown DR, Timmermans JP. Lessons from the porcine enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 1):50–54. doi: 10.1111/j.1743-3150.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 24.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 25.Guilloteau P, Zabielski R, Hammon HM, Metges CC. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr Res Rev. 2010;23:4–22. doi: 10.1017/S0954422410000077. [DOI] [PubMed] [Google Scholar]
- 26.Wernersson R, Schierup MH, Jorgensen FG, et al. Pigs in sequence space: a 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genomics. 2005;6:70. doi: 10.1186/1471-2164-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmermans JP, Hens J, Adriaensen D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat Rec. 2001;262:71–78. doi: 10.1002/1097-0185(20010101)262:1<71::AID-AR1012>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Moeser AJ, Klok CV, Ryan KA, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. American journal of physiology Gastrointestinal and liver physiology. 2007;292:G173–G181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- 29.Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. American journal of physiology Gastrointestinal and liver physiology. 2007;293:G413–G421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- 30.McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS One. 2013;8:e59838. doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.8. NRC. Nutrient requirements of swine. 11th. Washington, DC: National Academy Press; 2012. [Google Scholar]
- 32.Ulbrich W. Effects of veratridine on sodium currents and fluxes. Reviews of Physiology, Biochemistry and Pharmacology. 1998;133:1–54. doi: 10.1007/BFb0000612. [DOI] [PubMed] [Google Scholar]
- 33.McEwan GT, Schousboe B, Nielsen CG, Skadhauge E. Effect of age on the secretory capacity of pig small intestine in vivo and in vitro. Am J Physiol. 1990;259:G474–G480. doi: 10.1152/ajpgi.1990.259.3.G474. [DOI] [PubMed] [Google Scholar]
- 34.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovell RMFA. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. American Journal of Gastroenterology. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 36.Meleine MMJ. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World Journal of Gastroenterology. 2014;20:6725–6743. doi: 10.3748/wjg.v20.i22.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulak ATY, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World Journal of Gastroenterology. 2014;20:2433–2448. doi: 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol. 2006;149:463–479. doi: 10.1038/sj.bjp.0706889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandan RHK, Seybold VS, Soldani G, Brown DR. Cholinergic neurons and muscarinic receptors regulate anion secretion in pig distal jejunum. European Journal of Pharmacology. 1991;193:265–273. doi: 10.1016/0014-2999(91)90139-h. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman TWBH. Muscarinic receptors on rat isolated colonic epithelial cells. A correlation between inhibition of [3H]quinuclidinyl benzilate binding and alteration in ion transport. Gastroenterology. 1982;83:1244–1251. [PubMed] [Google Scholar]
- 41.Khan MRAA, Sembra S, Ma Y, Uwada J, Hayashi H, Suzuki Y, Takano T, Ikeuchi H, Uchino M, Maemoto A, Ushikubi F, Muramatsu I, Taniguchi T. M1 is a major subtype of musacrinic acetylcholine receptors on mouse colonic epithelial cells. Journal of Gastroenterology. 2013;48:885–896. doi: 10.1007/s00535-012-0718-5. [DOI] [PubMed] [Google Scholar]
- 42.Baldassano S, Liu SM, Qu MH, Mule F, Wood JD. Glucagon-like peptide-2 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;297:G800–G805. doi: 10.1152/ajpgi.00170.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldassano S, Wang GD, Mule F, Wood JD. Glucagon-like peptide-1 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;302:G352–G358. doi: 10.1152/ajpgi.00333.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito YA, Strege PR, Tester DJ, et al. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G211–G218. doi: 10.1152/ajpgi.90571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beyder A, Mazzone A, Strege PR, et al. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology. 2014;146:1659–1668. doi: 10.1053/j.gastro.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu S, Xiao Y, Zhu L, et al. Neonatal maternal deprivation sensitizes voltage-gated sodium channel currents in colon-specific dorsal root ganglion neurons in rats. American journal of physiology Gastrointestinal and liver physiology. 2013;304:G311–G321. doi: 10.1152/ajpgi.00338.2012. [DOI] [PubMed] [Google Scholar]
- 47.Hu S, Xu W, Miao X, et al. Sensitization of sodium channels by cystathionine beta-synthetase activation in colon sensory neurons in adult rats with neonatal maternal deprivation. Exp Neurol. 2013;248:275–285. doi: 10.1016/j.expneurol.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 48.Qu R, Tao J, Wang Y, et al. Neonatal colonic inflammation sensitizes voltage-gated Na(+) channels via upregulation of cystathionine beta-synthetase expression in rat primary sensory neurons. American journal of physiology Gastrointestinal and liver physiology. 2013;304:G763–G772. doi: 10.1152/ajpgi.00466.2012. [DOI] [PubMed] [Google Scholar]
- 49.Chaloner A, Greenwood-Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J Pain. 2013;14:270–280. doi: 10.1016/j.jpain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Rosztoczy A, Fioramonti J, Jarmay K, Barreau F, Wittmann T, Bueno L. Influence of sex and experimental protocol on the effect of maternal deprivation on rectal sensitivity to distension in the adult rat. Neurogastroenterol Motil. 2003;15:679–686. doi: 10.1046/j.1350-1925.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 51.Houghton LA, Jackson NA, Whorwell PJ, Morris J. Do male sex hormones protect from irritable bowel syndrome? Am J Gastroenterol. 2000;95:2296–2300. doi: 10.1111/j.1572-0241.2000.02314.x. [DOI] [PubMed] [Google Scholar]
- 52.Haga T. Molecular properties of muscarinic acetylcholine receptors. Proceedings of the Japan academy, Series B Physical and Biological Sciences. 2013;89:226–256. doi: 10.2183/pjab.89.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam RS, App EM, Nahirney D, et al. Regulation of Cl− secretion by alpha2-adrenergic receptors in mouse colonic epithelium. J Physiol. 2003;548:475–484. doi: 10.1113/jphysiol.2002.036806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gabella G. Neuron size and number in the myenteric plexus of the newborn and adult rat. Journal of Anatomy. 1971;109:81–95. [PMC free article] [PubMed] [Google Scholar]
- 55.Schaefer KHHA, Mestres P. Morphological changes of the myenteric plexus during early postnatal development of the rat. The anatomical record. 1999;256:20–28. doi: 10.1002/(SICI)1097-0185(19990901)256:1<20::AID-AR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 56.Aoki TJA, Litsuka Y, Isono K, Tokuhisa T, Hatano M. Ncx (Enx, Hox11L1) is rewuired for neuronal cell death in enteric ganglia of mice. Journal of pediatric surgery. 2007;42:1081–1088. doi: 10.1016/j.jpedsurg.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 57.Chalazonitis AGM, Greene LA. Cell death and the developing enteric nervous system. Neurochemistry International. 2012;61:839–847. doi: 10.1016/j.neuint.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gareau MGJJ, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2007;293:G198–G203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- 59.Dothel GBM, Boudin H, Vasina V, Cremon C, Gargano L, Bellacosa L, De Giorgio R, Le Berre-Scoul C, Aubert P, Neunlist M, De Ponti F, Stanghellini V, Barbara G. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 60.Huang ERL. Neurotrophins: roles in neuronal development and function. Annual Reviews in Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gougeon PYLS, Han TY, Nair DG, Ropeleski MJ, Blennerhassett MG. The pro-inflammatory cytokines IL-1b and TNFa are neurotrophic for enteric neurons. The Journal of Neuroscience. 2013;33:3339–3351. doi: 10.1523/JNEUROSCI.3564-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han TYLS, Miller KG, Blennerhassett MG. Intestinal smooth muscle phenotype determines enteric neuronal survival via GDNF expression. Neuroscience. 2015;290:357–368. doi: 10.1016/j.neuroscience.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 63.Rodrigues DMLA, Nair DG, Blennerhassett MG. Glial cell line-derived neurotrophic factor is a key neurotrophin in the postnatal enteric nervous system. Neurogastroenterology and Motility. 2011;23:e44–e56. doi: 10.1111/j.1365-2982.2010.01626.x. [DOI] [PubMed] [Google Scholar]
- 64.Moeser AJKC, Ryan KA, Wooten JG, Little D, Cook VL, Blikslager AT. Stress signaling pathways activated by weaning mediated intestinal dysfunction in the pig. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2007;292:G173–G181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- 65.Moeser AjRKANPKBAT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2007;293:G413–G421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- 66.Di Nardo G, Barbara G, Cucchiara S, et al. Neuroimmune interactions at different intestinal sites are related to abdominal pain symptoms in children with IBS. Neurogastroenterol Motil. 2014;26:196–204. doi: 10.1111/nmo.12250. [DOI] [PubMed] [Google Scholar]
- 67.Guilarte M, Santos J, de Torres I, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez C, Vicario M, Ramos L, et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736–746. doi: 10.1038/ajg.2011.472. [DOI] [PubMed] [Google Scholar]
- 69.Wang SH, Dong L, Luo JY, et al. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. doi: 10.3748/wjg.v13.45.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2014;7:335–347. doi: 10.1038/mi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ju Z, Chavan SS, Antoine DJ, et al. Sequestering HMGB1 via DNA-conjugated beads ameliorates murine colitis. PLoS One. 2014;9:e103992. doi: 10.1371/journal.pone.0103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munyaka P, Rabbi MF, Pavlov VA, Tracey KJ, Khafipour E, Ghia JE. Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+CD25− T cells in experimental colitis. PLoS One. 2014;9:e109272. doi: 10.1371/journal.pone.0109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaur T, Singh S, Gorowara S, Ganguly NK. Role of enteric nervous system in Shigella dysenteriae type 1 toxin-induced fluid secretion in rabbit ileum. J Diarrhoeal Dis Res. 1995;13:159–165. [PubMed] [Google Scholar]
- 74.Young A, Levin RJ. Segmental heterogeneity of rat colonic electrogenic secretion in response to the bacterial enterotoxin Escherichia coli STa in vitro. Exp Physiol. 1991;76:979–982. doi: 10.1113/expphysiol.1991.sp003561. [DOI] [PubMed] [Google Scholar]
- 75.Banks MR, Golder M, Farthing MJ, Burleigh DE. Intracellular potentiation between two second messenger systems may contribute to cholera toxin induced intestinal secretion in humans. Gut. 2004;53:50–57. doi: 10.1136/gut.53.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crane JK, Burrell LL, Weikel CS, Guerrant RL. Carbachol mimics phorbol esters in its ability to enhance cyclic GMP production by STa, the heat-stable toxin of Escherichia coli. FEBS Lett. 1990;274:199–202. doi: 10.1016/0014-5793(90)81363-s. [DOI] [PubMed] [Google Scholar]
- 77.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–1222. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–2218. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghia JE, Blennerhassett P, Deng Y, Verdu EF, Khan WI, Collins SM. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136:2280–2288. e2281–e2284. doi: 10.1053/j.gastro.2009.02.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.