Abstract
Background
Surgeon and hospital volume are both known to affect outcomes for patients undergoing pancreatic resection. The objective was to evaluate the relative effects of surgeon and hospital volume on 30-day mortality and 30-day complications after pancreatic resection among older patients.
Materials and Methods
The study used Texas Medicare data (2000–2012), identifying high-volume surgeons as those performing ≥4 pancreatic resections/year, and high-volume hospitals as those performing ≥11 pancreatic resections/year, on Medicare patients. Three-level hierarchical logistic regression models were used to evaluate the relative effects of surgeon and hospital volumes on mortality and complications, after adjusting for case mix differences.
Results
There were 2,453 pancreatic resections performed by 490 surgeons operating in 138 hospitals. 4.5% of surgeons and 6.5% of hospitals were high-volume. The overall 30-day mortality was 9.0%, and the 30-day complication rate was 40.6%. Overall, 8.9% of the variance in 30-day mortality was attributed to surgeon factors and 9.8% to hospital factors. For 30-day complications, 4.7% of the variance was attributed to surgeon factors and 1.2% to hospital factors. After adjusting for patient, surgeon and hospital characteristics, high surgeon volume (OR 0.54, 95% CI 0.33–0.87) and high hospital volume (OR, 0.52; 95% CI, 0.30–0.92) were associated with lower risk of mortality; high surgeon volume (OR 0.71, 95% CI 0.55–0.93) was also associated lower risk of 30-day complications.
Conclusions
Both hospital and surgeon factors contributed significantly to the observed variance in mortality, but only surgeon factors impacted complications.
Keywords: pancreatic resection, multilevel models, mortality, complications, surgeon volume, hospital volume
INTRODUCTION
For complex surgical procedures, the influence of high surgeon and hospital volume on improved perioperative and postoperative outcomes is well established. Since the 1970s, a growing body of evidence has demonstrated an absolute mortality benefit when complex surgical procedures are performed at high-volume centers or by high-volume providers.1–4 These benefits have been identified in patients undergoing total hip replacement5, ovarian cancer resection6, and complex oncologic resections7, 8 including pancreatic resection.9, 10
While studies have attempted to understand the relative contribution of surgeon and hospital volume on perioperative outcomes in patients undergoing pancreatic resection, the results have been discordant.9–13 Most studies that evaluate surgeon and hospital volume have focused exclusively on in-hospital or 30-day mortality and concluded that both hospital and surgeon volume affect mortality independently, however, some studies have suggested little difference between the impacts of hospital or surgeon volume, or that surgeon volume may be more influential.3, 9, 10 In addition, isolated studies have even demonstrated excellent outcomes at individual low-volume centers, or with low-volume surgeons.13 Finally, even among high-volume centers, significant variability in outcomes exist, suggesting that other factors are at play.14, 15 Therefore, it remains unclear how much of the observed variation in mortality and complications is explained by hospital and surgeon volume, separately or in concert. Current recommendations from the Leapfrog group16 emphasize increased hospital volume (≥ 11 pancreatic resection per year), but not surgeon volume, as a necessary component to improve operative outcomes for all complex surgical patients. In addition, no previous studies have addressed the relative effect of hospital and surgeon volume on complications after pancreatectomy.
We used Texas Medicare claims data (2000–2012) to determine the relative effects of surgeon and hospital volume factors on 30-day mortality and 30-day complications among patients aged 66 and older undergoing pancreatic resection. We further partitioned the variance to understand how much of the variation in outcomes between surgeons and hospitals was explained by surgeon and hospital volume.
METHODS
This study involved analysis of secondary data and was not considered human subjects research. It was thus exempt from review by the Institutional Review Board at the University of Texas Medical Branch.
Data Source and Study Cohort
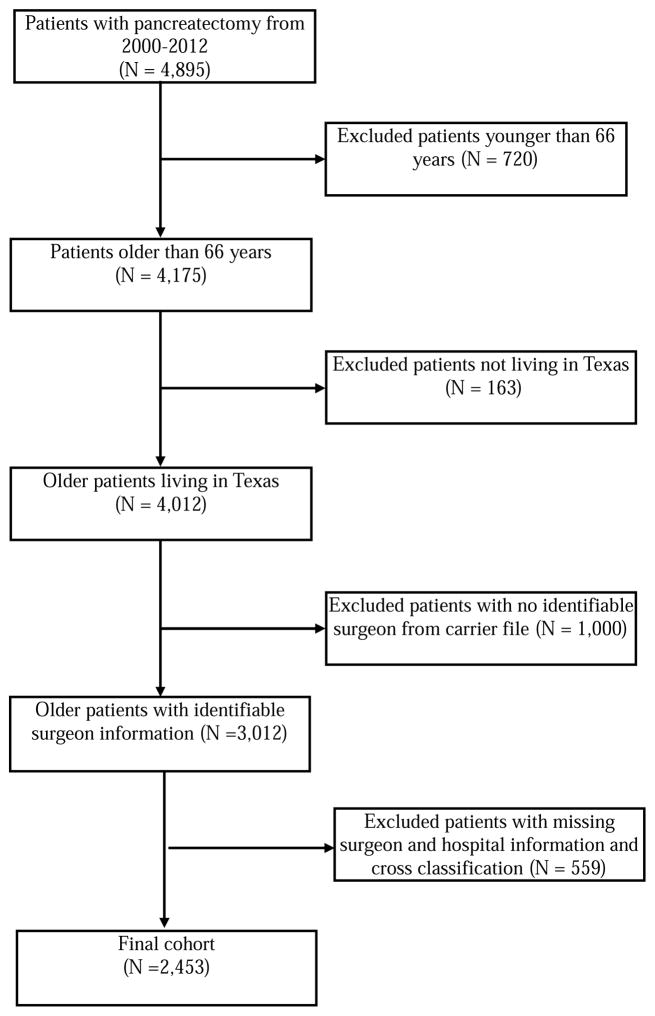
We performed a retrospective cohort study of all patients aged 66 years and older who underwent pancreatic resection including pancreaticoduodenectomy, distal pancreatectomy, total pancreatectomy, and other pancreatectomies (International Classification of Diseases, Ninth Edition codes: 52.6, 52.7, 52.51–52.53, 52.59) in Texas between 2000 and 2012. Data were obtained from the Texas Medicare claims data. Medicare data does not include older adults who underwent pancreatic resections at Veterans Affairs hospitals and therefore these patients were not included in the cohort. Medicare files used for this study included the Denominator file, the Medicare Provider Analysis and Review file (MedPAR) for inpatient claims, the Carrier claims file, and the Outpatient Standard Analytical File. We excluded the following from the study cohort: 1) patients younger than 66 years at the date of surgery; 2) patients not living in Texas; 3) patients with no identifiable surgeon from Carrier file; and 4) patients with missing surgeon and hospital information.
Outcome Measure
The study outcomes were 30-day mortality and 30-day complications. Both outcomes were defined within 30 days from the date of surgery. We also considered 90-day mortality outcome for sensitivity analysis. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes used to identify complications are presented in Appendix 1.
Provider Volume
Identifying the Operating Surgeon
MedPAR inpatient stay records do not include physician Unique Provider Identification Numbers (UPIN) or National provider Identifiers (NPI). Therefore, the operating surgeon was identified using the UPIN or NPI and specialty code from Carrier files. We linked the inpatient pancreatic resection record from MedPAR to Carrier claims by date of surgery and procedure codes. All carrier claims filed by surgeons including general surgeons, surgical oncologists and other surgical specialty were retained. If multiple surgeons had claims in the carrier file, the surgeon who billed the highest amount was designated as the performing surgeon. To handle new or retired surgeons, we identified the first and last claims for each surgeon by scanning all the claims associated with the surgeon in the Carrier file for each year. If we did not find any claims from a surgeon in an entire calendar year, we assumed surgeon stopped practicing or performing pancreatectomies. We only considered the active time period for the surgeon to define surgeon volume. American Medical Association (AMA) Masterfile was used to find surgeon characteristics through the crosswalk with UPIN.
Surgeon Volume
Surgeons with an average Medicare volume of ≥ 4 pancreatic resections per year over the study period were considered high-volume surgeons. Previous studies have classified surgeons as high-volume if they performed ≥ 5 pancreatic resections in a given year.10 However, only 15 surgeons in Texas performed ≥ 5 pancreatic resections on Medicare patients in any given year. The Leapfrog Group evidence-based surgeon high-volume safety standard criterion for pancreatic resection is 2 per year.16 Since our volume estimates were based on Medicare patients, and are therefore slightly lower than they would be if patients outside Medicare were included, we chose ≥ 4 as the definition of high-volume. We also performed sensitivity analysis by considering 5 as a cutoff value for high-volume surgeons.
Hospital Volume
Hospitals with an average Medicare volume of ≥ 11 pancreatic resections per year over the study period were considered high-volume hospitals. The Leapfrog Group evidence-based hospital referral Safety Standard criterion for pancreatic resection volume is ≥ 11 per year.16 Medicare volume and total volume for 14 procedures including pancreatic resection have been shown to be highly correlated at the hospital level (overall correlation coefficient = 0.97).2 Provider of Service (POS) file from CMS was used to find hospital charcacteristics, including hospital type (profit, non-profit, government), number of beds, and teaching status.
Covariates
Covariates included patient age, sex, and race/ethnicity (white, black, Hispanic/Other). Clinical characteristics included Elixhauser comorbidity index, indication for surgery (periampullary cancer vs. other) and procedure type (pancreatic head resection, distal pancreatectomy, or pancreatectomy not otherwise specified). We used Walraven weights to derive a summary Elixhauser comorbidity score.17, 18 Surgeon characteristics included surgeon’s age, gender, specialty and years of practice. Hospital characteristics included hospital type (profit, non-profit, government), number of beds, and teaching status.
Statistical Analysis
Patient characteristics were compared across surgeon and hospital volume (high vs. low) using the chi-square test for categorical variables and the t-test for continuous variables. 30-day mortality and 30-day complications were also compared across surgeon and hospital volume using the chi-square test and the Cochrane-Armitage trend test.
Hierarchical logistic regression models were used to account for the multilevel structure of the data and to adjust for clustering of patients clustered within surgeons, clustered within hospitals. Hierarchical modeling allows for the estimation and partitioning of variance in 30-day mortality and 30-day complication between the patient, surgeon, and hospital levels. To address issues with cross-classification in the 3-level model, surgeons who operated in more than one hospital were assigned to the hospital where they did the plurality of their cases. Cases performed by surgeon outside the assigned hospital were excluded. First, we constructed two separate null (not controlling for any covariates) 2-level hierarchical models (model 1, patients clustered within surgeons; model 2, patients clustered within hospitals) to determine variance attributable to the surgeon and hospital level while ignoring the other level. The intraclass correlation coefficient (ICC) was calculated using the threshold model assumption for binary outcomes for the null model.19 The ICCs represent the percentage of the total variance in the outcome attributable to each level of the model; in this case patients, surgeons and hospitals. We next constructed a 3-level hierarchical model with patients clustered within surgeons, and surgeons clustered within hospitals, to partition variance at both the surgeon and hospital level. In contrast to 2-level models, the 3-level hierarchical model will truly partition variance at surgeon and hospital level as it correctly accounts for clustering. To the 3-level null hierarchical model, we first added patient characteristics, surgeon and hospital volumes; in the next model, we additionally added surgeon and hospital characteristics. Residual ICC was reported at surgeon and hospital level for each model to determine how much variance is attributed to the surgeon and hospital level after controlling for these other characteristics. The association of surgeon volume and hospital volume with outcome was reported using odds ratio after controlling for patient, surgeon and hospital characteristics. In the full model, a cross-level interaction between surgeon and hospital volume was tested to determine if the effect of surgeon volume varied by hospital volume, but the interaction term was not statistically significant. We repeated these analysis separately for 30-day mortality and 30-day complications. We compared characteristics of the final cohort with excluded patients to address concerns of selection bias. We performed three sensitivity analyses (i) by modeling surgeon and hospital volume as continuous variables, (ii) using 5 as a cutoff value to define surgeon volume, and (iii) for 90-day mortality.
All p-values were from two-sided tests. All statistical analyses were performed with SAS version 9.4 (SAS Inc., Cary, NC) and STATA 13, (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
The study cohort included 2,453 patients who underwent pancreatic resection in Texas from 2000 to 2012 (Figure 1). Overall the mean age of the cohort was 74.5±5.6 years, 50.4% were males, and 84.8% were non-Hispanic Whites. 87.2% of patients had at least one Elixahuser comorbidity. Table 1 demonstrates patient characteristics by surgeon and hospital volume. Patients operated on by high-volume surgeons were more likely to be white, more likely to have periampullary cancer and have pancreatic head resection than low-volume surgeons. Similar patterns of difference were observed between high- and low-volume hospitals.
Figure 1.
Cohort Selection
Table 1.
Patient Characteristics across Surgeon and Hospital Volume
| Characteristics (%) | Surgeon Volume | P value | Hospital Volume | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Low- volume (< 4) | High- volume (≥ 4) | Low- volume (< 11) | High- volume (≥ 11) | |||
| Sample size, N | 1,309 | 1,144 | 1,023 | 1,430 | ||
| Age group | ||||||
| < 70 | 22.1 | 21.2 | 0.763 | 20.7 | 22.5 | 0.376 |
| 70–74 | 30.5 | 32.1 | 31.3 | 31.2 | ||
| 75–79 | 27.7 | 28.2 | 27.4 | 28.3 | ||
| > 80 | 19.6 | 18.5 | 20.6 | 18.0 | ||
| Male | 51.3 | 49.5 | 0.378 | 51.2 | 49.9 | 0.506 |
| Race/ethnicity | ||||||
| Whites | 82.2 | 87.8 | 0.0005 | 81.2 | 87.3 | 0.0002 |
| Black | 9.4 | 6.9 | 10.2 | 6.9 | ||
| Hispanic/others | 8.4 | 5.3 | 8.6 | 5.8 | ||
| Periampullary cancer | 68.2 | 71.1 | 0.127 | 68.1 | 70.6 | 0.198 |
| Procedure | ||||||
| Pancreatic head resection | 60.0 | 67.7 | <0.0001 | 58.6 | 67.1 | <0.0001 |
| Distal Pancreatic resection | 29.4 | 20.5 | 31.2 | 21.0 | ||
| Pancreatectomy not specified | 10.6 | 11.8 | 10.2 | 11.9 | ||
| Summary Elixhauser comorbidity score, mean ± sd | 4.5 ± 6.2 | 4.4 ± 5.7 | 0.812 | 4.2 ± 5.9 | 4.6 ± 6.0 | 0.068 |
| Individual Elixhauser comorbidities* | ||||||
| Congestive heart failure | 7.9 | 5.0 | 0.004 | 7.9 | 5.5 | 0.018 |
| Hypertension | 64.3 | 70.4 | 0.002 | 62.5 | 70.5 | <0.0001 |
| Hypothyroidism | 14.1 | 18.4 | 0.004 | 13.1 | 18.2 | 0.0007 |
| Solid tumor without metastasis | 28.7 | 35.0 | 0.0008 | 26.5 | 35.3 | <0.0001 |
| Coagulopathy | 2.7 | 4.4 | 0.022 | 2.6 | 4.1 | 0.059 |
Only significant comorbidities are presented in this table. Please refer Appendix 2 for the full list of comorbidities.
Hospital and Surgeon Volume
Within the 138 hospitals, 490 surgeons performed pancreatectomies, with a median surgeon volume of 3 cases (interquartile range (IQR), 1–11). The median hospital volume for pancreactomy was 18 cases (IQR, 5–27). While 4.5% of the surgeons were classified as high-volume (≥4 cases per year), they performed 46.6% of all pancreatectomies. Similarly, 6.5% of the hospitals were classified as high-volume hospitals; however, they accounted for 58.3% of all pancreatic resections performed. Of the overall cohort, 39.1% of patients were operated on by low-volume surgeons in low-volume hospitals, 2.6% by high-volume surgeons in low-volume hospitals, 14.2% by low-volume surgeons in high-volume hospitals, and 44.1% by high-volume surgeons in high-volume hospitals. The proportion of pancreatic resections performed by high-volume surgeons at high-volume hospital increased from 23.2% in 2000 to 54.1% in 2012 (Appendix 3).
30-Day Mortality and Complications
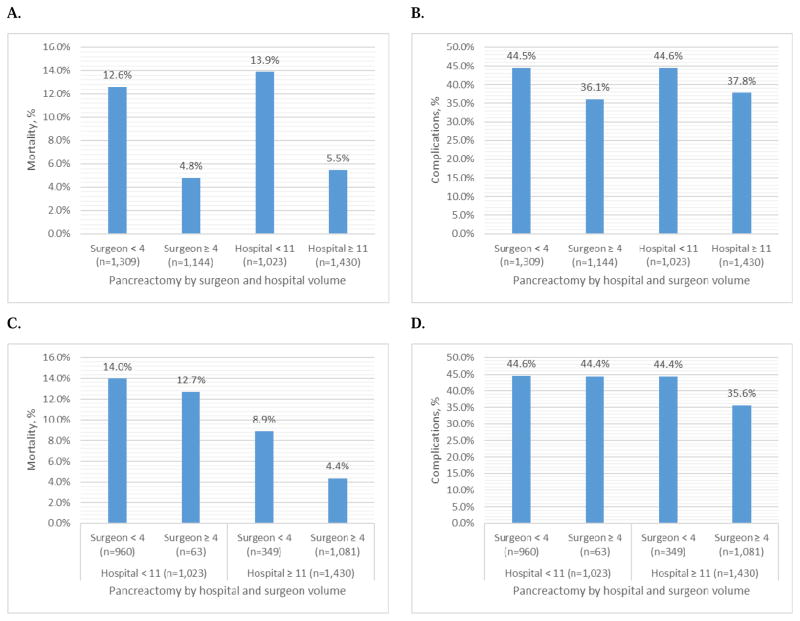
The overall 30-day mortality was 9.0%, and the 30-day complication rate was 40.6%. Figure 2 A–D report the 30-day mortality and 30-day complication rate by surgeon and hospital volume. Unadjusted 30-day mortality was higher in low-volume surgeons compared to high-volume surgeons (12.6% vs. 4.8%, p<0.0001), and low-volume hospitals compared to high-volume hospitals (13.9% vs. 5.5%, p<0.0001). (Figure 2A) Similarly, the unadjusted rate of 30-day complications was higher in low-volume surgeons (44.5% vs. 36.1%, p<0.0001) and low-volume hospitals (44.6% vs. 37.8%, p=0.0007) compared to their high-volume counterparts. (Figure 2B) Figure 2C–D show the 30-day mortality and complication rates stratified by hospital volume and surgeon volume. The 30-day mortality was 14.0% for low-volume surgeons in low-volume hospitals; this decreased to 12.7% for high-volume surgeons in low-volume hospitals, 8.9% for low-volume surgeons in high-volume hospitals, and 4.4% for high-volume surgeons in high-volume hospitals (p<0.0001). (Figure 2C) The 30-day complications were lowest for high- volume surgeons in high-volume hospitals (p<0.0001). (Figure 2D)
Figure 2.
30-day Mortality and Complications by Hospital and Surgeon Volume
Hierarchical Logistic Regression Models
30-Day Mortality
Table 2 reports the proportion of the variance in hospital mortality that is attributable to the surgeon and hospital level (all measureable and unmeasurable surgeon and hospital characteristics), and how much is explained by hospital and surgeon volume. In the null 2-level hierarchical models, 21.8% of the variance in mortality was attributed to the surgeon factors (patients clustered within surgeons) and 13.7% of the variance was attributed to the hospital factors (patients clustered within hospitals). The 3-level null model accurately accounted for clustering of patients, surgeons and hospitals, and found that 8.9% and 9.8% of the variance in mortality was attributed to surgeon and hospital factors, respectively. This indicates that variation in mortality was attributed to both surgeon and hospital level factors equally. The remaining 81.3% variance was attributed at patient level. In the 3-level hierarchical model, controlling for measurable patient characteristics had little impact on residual ICC at both the surgeon and hospital level compared to the null model. Controlling for surgeon and hospital volume explained most of the variation at surgeon level (residual ICC=1.1%) and some at hospital level (residual ICC=5.2%). This shows that 88.3% (9.4–1.1/9.4) and 42.9% (9.1–5.2/9.1) of variance in mortality was explained at surgeon and hospital level, respectively, when both surgeon and hospital volume were added to the model. Controlling for additional surgeon and hospital characteristics further reduced ICC at surgeon and hospital level. (Table 2)
Table 2.
Hierarchical Multilevel Models to Partition Percentage of Variance in 30-day Mortality at Surgeon and Hospital Level
| Null model | Controlling for patient characteristics | Controlling for patient characteristics, surgeon volume, hospital volume | Controlling for patient characteristics, surgeon volume, hospital volume, surgeon and hospital characteristics | |
|---|---|---|---|---|
| 2-level model (patient, surgeon) | ||||
| Residual ICC (% variance) – surgeon | 21.8% | - | - | - |
|
| ||||
| 2-level model (patient, hospital) | ||||
| Residual ICC (% variance) – hospital | 13.7% | - | - | - |
|
| ||||
| 3-level model (patient, surgeon, hospital) | ||||
| Residual ICC (% variance) – surgeon | 8.9% | 9.4% | 1.1% | 0.4% |
| Residual ICC (% variance) – hospital | 9.8% | 9.1% | 5.2% | 3.9% |
When all patient, surgeon and hospital characteristics were included in the model, high surgeon volume was associated with 46% reduction in odds of mortality (OR 0.54, 95% CI 0.33–0.87) compared to low-volume surgeons, and high hospital volume was associated with 48% reduction in the odds of 30-day mortality (OR, 0.52; 95% CI, 0.30–0.92) compared to low-volume hospitals. (Table 3)
Table 3.
Three-level Hierarchical Logistic Regression Models to Determine the Association of Surgeon and Hospital Volume with of 30-day Mortality and 30-day Complications
| 30-day mortality | 30-day complications | |||||||
|---|---|---|---|---|---|---|---|---|
| Model controlled for patient characteristics | Model controlled for patient, surgeon and hospital characteristics | Model controlled for patient characteristics | Model controlled for patient, surgeon and hospital characteristics | |||||
|
| ||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Surgeon volume | ||||||||
| Low (< 4) | Ref | Ref | Ref | Ref | ||||
| High (≥ 4) | 0.54 | 0.33–0.86 | 0.54 | 0.33–0.87 | 0.70 | 0.53–0.94 | 0.71 | 0.55–0.93 |
| Hospital volume | ||||||||
| Low (≤ 10) | Ref | Ref | Ref | Ref | ||||
| High (> 10) | 0.50 | 0.30–0.84 | 0.52 | 0.30–0.92 | 0.92 | 0.68–1.23 | 0.95 | 0.69–1.30 |
| Patient characteristics | ||||||||
| Age group | ||||||||
| < 70 | Ref | Ref | Ref | Ref | ||||
| 70–74 | 1.24 | 0.78–1.95 | 1.22 | 0.77–1.92 | 1.03 | 0.82–1.30 | 1.03 | 0.82–1.30 |
| 75–79 | 1.51 | 0.96–2.37 | 1.49 | 0.95–2.34 | 1.06 | 0.83–1.34 | 1.05 | 0.83–1.33 |
| > 80 | 2.60 | 1.64–4.10 | 2.56 | 1.62–4.05 | 1.40 | 1.08–1.82 | 1.36 | 1.05–1.77 |
| Patient gender | ||||||||
| Male | Ref | Ref | Ref | Ref | ||||
| Female | 0.80 | 0.60–1.07 | 0.79 | 0.59–1.06 | 0.88 | 0.74–1.04 | 0.88 | 0.74–1.04 |
| Race/ethnicity | ||||||||
| Whites | Ref | Ref | Ref | Ref | ||||
| Black | 1.08 | 0.65–1.80 | 1.08 | 0.65–1.80 | 1.17 | 0.86–1.59 | 1.13 | 0.84–1.54 |
| Hispanic/others | 1.01 | 0.59–1.74 | 1.04 | 0.60–1.81 | 0.88 | 0.63–1.22 | 0.88 | 0.63–1.23 |
| Periampullary cancer | 0.63 | 0.45–0.88 | 0.63 | 0.45–0.88 | 0.67 | 0.55–0.82 | 0.68 | 0.56–0.83 |
| Procedure | ||||||||
| Pancreatic head resection | Ref | Ref | Ref | Ref | ||||
| Distal Pancreatic resection | 0.76 | 0.52–1.11 | 0.76 | 0.52–1.11 | 0.68 | 0.55–0.85 | 0.71 | 0.57–0.88 |
| Pancreatectomy not specified | 1.22 | 0.77–1.94 | 1.23 | 0.78–1.96 | 0.87 | 0.66–1.14 | 0.90 | 0.68–1.11 |
| Summary Elixhauser comorbidity score | 1.01 | 0.98–1.03 | 1.01 | 0.99–1.03 | 1.02 | 1.01–1.03 | 1.02 | 1.01–1.04 |
| Surgeon characteristics | ||||||||
| US trained (ref: yes) | 0.88 | 0.54–1.46 | 0.71 | 0.52–0.97 | ||||
| Surgeon gender | ||||||||
| Male | Ref | Ref | ||||||
| Female | 0.84 | 0.36–1.98 | 1.52 | 0.94–2.46 | ||||
| Surgeon specialty | ||||||||
| General surgery | Ref | Ref | ||||||
| Other | 0.84 | 0.52–1.36 | 0.76 | 0.58–1.00 | ||||
| Surgeon’s age | 0.96 | 0.89–1.04 | 1.02 | 0.98–1.07 | ||||
| Surgeon’s year of practice | 1.04 | 0.97–1.11 | 0.99 | 0.95–1.03 | ||||
| Hospital Characteristics | ||||||||
| Hospital type | ||||||||
| Non-profit | Ref | Ref | ||||||
| Profit | 0.85 | 0.51–1.42 | 1.26 | 0.89–1.77 | ||||
| Government | 0.86 | 0.47–1.56 | 0.99 | 0.70–1.41 | ||||
| Hospital bed size | ||||||||
| <200 | Ref | Ref | ||||||
| 200–350 | 1.06 | 0.59–1.91 | 0.79 | 0.52–1.20 | ||||
| 351–500 | 0.75 | 0.40–1.40 | 0.76 | 0.51–1.14 | ||||
| >500 | 0.75 | 0.40–1.38 | 0.62 | 0.41–0.93 | ||||
| Hospital status | ||||||||
| Major | Ref | Ref | ||||||
| Minor | 0.67 | 0.38–1.17 | 0.62 | 0.44–0.88 | ||||
| No-teaching | 0.73 | 0.40–1.33 | 0.53 | 0.37–0.78 | ||||
30-Day Complications
The unadjusted 3-level hierarchical model showed that 4.2% of variation in 30-day complications was attributed to the surgeon level, and 1.7% of variation was attributed to the hospital level. Because very low variation was attributed to surgeon and hospital levels, we did not further partition the variance. In the model that controlled for all covariates, high surgeon volume was associated with lower odds of 30-day complications (OR 0.71, 95% CI 0.55–0.93), but hospital volume was not associated with 30-day complication (OR 0.95, 95% CI 0.69–1.30). (Table 3)
Sensitivity Analyses
We performed sensitivity analysis by considering surgeon and hospital volume as continuous variables. Results are similar to our main analysis: surgeon and hospital volume are associated with 30-day mortality and only surgeon volume is associated with complications (Appendix 4). Using 5 as a cutoff to define high-volume surgeon showed similar results compared to 4 cutoff. In the final model that controlled for patient, surgeon and hospital characteristics, high-volume surgeons (OR 0.47, 95% CI 0.28–0.77) and high-volume hospitals (OR 0.54, 95% CI 0.31–0.93) were associated with lower odds of 30-day mortality; high surgeon volume (OR 0.71, 95% CI 0.54–0.93) was associated with lower 30-day complications and high hospital volume (OR 0.93, 95% CI 0.68–1.27) had no effect on 30-day complications. For 90-day mortality, both surgeon (either 4 or 5 cutoff to define high surgeon volume) and hospital volume were associated with lower odds of mortality. Adjusted 30-day and 90-day mortality variation by hospitals is reported in Appendix 5.
DISCUSSION
Our study used multilevel modeling to evaluate the association of surgeon and hospital volume with 30-day mortality and complications following pancreatectomy. Furthermore, this is the first study that partitions the variance at both the surgeon and hospital levels to better understand the relative contribution of both levels in the observed variation in outcomes. In multilevel models, both hospital and surgeon volume contributed significantly to the observed variance in mortality and only surgeon volume contributed for complications. Our study demonstrates that, in Texas Medicare beneficiaries undergoing pancreatectomy, high hospital volume and high surgeon volume is associated with lower 30-day mortality and high surgeon volume is associated with lower 30-day complications.
Multiple prior studies have identified the volume-outcomes relationship in pancreatic surgery and results are contradictory. A systematic review and meta-analysis which evaluated the effect of surgeon and hospital volume on postoperative mortality reported that higher hospital volume was associated with lower postoperative mortality (OR, 0.32; 95% CI, 0.16–0.64) and surgeon volume was not significantly associated with postoperative mortality (OR, 0.46; 95% CI, 0.17–1.26).20 Nathan et al used multilevel modeling to assess the relative contributions of the two volume groups for complex hepatobiliary surgery, including pancreatectomy. Using the State Inpatient Databases across three states, the authors observed an effect of both surgeon (OR, 0.30; p<0.001) and hospital (OR, 0.32; p<0.001) volume on mortality for patients who underwent pancreatectomy. However, after adjusting for surgeon volume, the association between hospital volume and mortality was no longer observed, suggesting that surgeon volume played a greater role in determining mortality.9
Birkmeyer et al. used hierarchical regression modeling to identify the effect of surgeon volume on operative mortality for pancreatic resection using hospital volume as a fixed effect in a cohort of national Medicare patients and found that both surgeon (low-volume OR, 2.31; 95% CI, 1.43–3.72) and hospital (low-volume OR, 2.34; 95% CI, 1.38–3.99) volume were significant predictor of operative mortality.3 Eppsteiner et al. used national inpatient sample data and performed propensity score matching to reduce selection bias. The authors reported that high-volume surgeons (OR, 0.49; 95% CI, 0.28–0.83) and high-volume hospitals (OR, 0.55; 95% CI, 0.32–0.97) were associated with lower in-hospital mortality.10 Our study results were consistent with these two studies and showed that both surgeon and hospital volume were associated with lower risk of 30-day mortality.
Pancreatic surgery demands both technical competency as well as extensive, adjunctive perioperative care. Birkmeyer et al. proposed that in surgery there is a balance of the technical skill of the surgeon and the need for intensive perioperative care.3 For some procedures (carotid endarterectomy), technical skill outweighs the need for specific hospital-based resources, whereas in others (lung lobectomy), the likelihood for postoperative complications outweighs intraoperative technical demands. Pancreatic surgery lies in the middle of this spectrum, demanding both technical expertise and vigilant postoperative care.
Our findings regarding the effect of hospital and surgeon volume on complications is novel and adds additional insight. We found that surgeon volume played an important role in reducing complications whereas hospital volume was not significantly associated with lower rates of complications. This suggests that lower mortality observed at high-volume hospitals occurs for two reasons. First, high-volume hospitals have more high volume surgeons and, as a result, lower complication rates.
Second, hospital factors are likely play a critical role in “rescuing” patients, with higher failure to rescue rates at low-volume hospitals. Failure to rescue, defined as the proportion of patients who died from a complication in those who suffered a complication, has been identified as a viable measure of quality of care.21, 22 Previous studies illustrate that because of the high likelihood for postoperative complications, hospital resources and surgeon expertise in identifying and managing pancreatic surgery pancreatic surgery complications is essential in lowering failure to rescue rates.20, 22–24 The high-volume hospitals’ ability to better rescue patients is supported by our findings. Low-volume surgeons at high-volume hosptials had lower mortality rates (8.9%) than high-volume surgeons in low-volume hospitals (12.7%). This indicates that hospital resources are more important in rescuing patients regardless of surgeon expertise.
Our study has several limitations. Unlike previous studies, we used a cohort of Medicare beneficiaries within a single state and our findings may not be generalizable to patients undergoing pancreatic resection elsewhere across the country. In addition, while our study is limited to a discussion of hospital volume as an isolated quality metric, these findings should be taken in the context of likely improved access to resources amongst high-volume centers; volume may be a proxy for other factors impacting care. Medicare volume is a proxy of total hospital volume, and therefore, we may have misclassified the true volume of the hospitals. However, several prior studies have showed that Medicare volume reasonably reflect overall volume of the hospital.2, 25, 26 From the initial cohort, we excluded nearly half of the patients while applying different exclusion criteria. We excluded 38.8% of patients (1,559 of 4,012) because of lack of surgeon data due to no enrollment of beneficiary in part B (1,000), missing information on surgeons and removal of cross-classified surgeons (559). This may have led to selection bias. Therefore, we compared patient characteristics of the final cohort (n=2,453) with excluded patients (n=1,559) and both groups were comparable, thus alleviating some concerns of selection bias (Appendix 6). Multiple prior studies have illustrated that hospital volume can be associated with improved outcomes but only when it is related to increased staffing and hospital supportive measures.27–29 Pancreatectomy-specific complications such as pancreatic fistula and delayed gastric emptying were not analyzed as it is difficult to identify specific complications from claims data.30 Unlike previous studies, we could not classify surgeons and hospitals into low, medium and high-volume due to small sample size.3, 9
CONCLUSIONS
With increasing attention on outcomes-based performance coupled with the need to improve the delivery of quality care, it is imperative to identify the distinction between hospital and surgeon volume effects. Our study contributes to the existing literature on the complex interplay between the impacts of hospital volume, surgeon volume, and traditional outcomes for patients undergoing pancreatic resection. Both hospital and surgeon factors contributed significantly to the observed variance in mortality, but not complications. Surgeon volume and hospital volume were associated with lower mortality but only surgeon volume was associated with lower complication rates. The small proportion of the variance in complications due to hospital factors and the lack of association of hospital volume with complications suggests that patient- and disease-level factors, and surgeon volume primarily contribute to complications and that observed differences in mortality are due to hospital and surgeon ability to rescue a patient once complications occur.
Supplementary Material
Acknowledgments
Funding: Cancer Prevention Research Institute of Texas Grant # #RP101207-P03, UTMB Clinical and Translational Science Award #UL1TR000071, NIH T-32 Grant # T32DK007639, AHRQ Grant # 1R24HS022134.
Footnotes
Author Disclosures: None
Author contributions: HM, AP and TR are responsible for study concept and design. HM, DA and DJ are responsible for statistical analysis. AP, NT, FD and TR are responsible for providing clinical inputs. All authors are responsible for interpretation of results and critical inputs to improve the research. All authors contributed in drafting the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301(25):1364–9. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 4.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83-A(11):1622–9. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Schrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst. 2006;98(3):163–71. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]
- 7.Hannan EL, Radzyner M, Rubin D, et al. The influence of hospital and surgeon volume on inhospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002;131(1):6–15. doi: 10.1067/msy.2002.120238. [DOI] [PubMed] [Google Scholar]
- 8.Ho V, Heslin MJ, Yun H, et al. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol. 2006;13(6):851–8. doi: 10.1245/ASO.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Nathan H, Cameron JL, Choti MA, et al. The volume-outcomes effect in hepato-pancreato-biliary surgery: hospital versus surgeon contributions and specificity of the relationship. J Am Coll Surg. 2009;208(4):528–38. doi: 10.1016/j.jamcollsurg.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Eppsteiner RW, Csikesz NG, McPhee JT, et al. eppn. Ann Surg. 2009;249(4):635–40. doi: 10.1097/SLA.0b013e31819ed958. [DOI] [PubMed] [Google Scholar]
- 11.Learn PA, Bach PB. A decade of mortality reductions in major oncologic surgery: the impact of centralization and quality improvement. Med Care. 2010;48(12):1041–9. doi: 10.1097/MLR.0b013e3181f37d5f. [DOI] [PubMed] [Google Scholar]
- 12.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747–51. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham JD, O’Donnell N, Starker P. Surgical outcomes following pancreatic resection at a low-volume community hospital: do all patients need to be sent to a regional cancer center? Am J Surg. 2009;198(2):227–30. doi: 10.1016/j.amjsurg.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Riall TS, Nealon WH, Goodwin JS, et al. Outcomes following pancreatic resection: variability among high-volume providers. Surgery. 2008;144(2):133–40. doi: 10.1016/j.surg.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamirisa NP, Parmar AD, Vargas GM, et al. Relative Contributions of Complications and Failure to Rescue on Mortality in Older Patients Undergoing Pancreatectomy. Ann Surg. 2016;263(2):385–91. doi: 10.1097/SLA.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Leapfrog Group. [Accessed April 15, 2016];Evidence Based Hospital Referral. Available at: http://www.leapfroggroup.org/for_hospitals/leapfrog_safety_practices/evidence-based_hospital_referral.
- 17.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 18.Mehta HB, Dimou F, Adhikari D, et al. Comparison of Comorbidity Scores in Predicting Surgical Outcomes. Medical Care. 2016;54(2):180–7. doi: 10.1097/MLR.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snijders TAB, Bosker RJ. Multilevel Analysis : an introduction to basic and advanced multilevel modeling. 2. London: Sage Publishers; 2012. [Google Scholar]
- 20.Gooiker GA, van Gijn W, Wouters MW, et al. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98(4):485–94. doi: 10.1002/bjs.7413. [DOI] [PubMed] [Google Scholar]
- 21.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250(6):1029–34. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 22.Gordon TA, Bowman HM, Tielsch JM, et al. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228(1):71–8. doi: 10.1097/00000658-199807000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003;237(4):509–14. doi: 10.1097/01.SLA.0000059981.13160.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janes RH, Jr, Niederhuber JE, Chmiel JS, et al. National patterns of care for pancreatic cancer. Results of a survey by the Commission on Cancer. Ann Surg. 1996;223(3):261–72. doi: 10.1097/00000658-199603000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 26.Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245(5):777–83. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135(6):569–75. doi: 10.1016/j.surg.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Joseph B, Morton JM, Hernandez-Boussard T, et al. Relationship between hospital volume, system clinical resources, and mortality in pancreatic resection. J Am Coll Surg. 2009;208(4):520–7. doi: 10.1016/j.jamcollsurg.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Glance LG. [Accessed April 15, 2016];Regionalizing Health Care: Volume Standards vs. Risk-Adjusted Mortality Rate. 2008 doi: 10.1093/intqhc/mzm020. Available at: http://archive.ahrq.gov/news/events/conference/2008/Glance.html. [DOI] [PubMed]
- 30.Reddy DM, Townsend CM, Jr, Kuo YF, et al. Readmission after pancreatectomy for pancreatic cancer in Medicare patients. J Gastrointest Surg. 2009;13(11):1963–74. 1974–5. doi: 10.1007/s11605-009-1006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.