Abstract
Background
Extremity rhabdomyosarcomas do not always show satisfactory outcomes. We analyzed data from 643 patients treated in 14 studies conducted by European and North American groups between 1983 and 2004 to identify factors predictive of outcome.
Procedure
Clinical factors, including age; histology; site of primary (hand and foot vs. other); size; invasiveness (T stage); nodal involvement (N stage); and treatment factors, including post-surgical group; chemotherapy type and duration; radiotherapy; and treatment (before or after 1995); were evaluated for impact on overall survival (OS).
Results
5-year OS were 67% (se 1.8). Multivariate analysis showed that lower OS correlated with age >3 years, T2 and N1 stage, incomplete initial surgery, treatment before 1995, and European cooperative group treatment. Patients with gross residual disease after initial incomplete resection/biopsy had similar outcomes in both continental groups. The better global survival of patients treated in American studies was accounted for by differences in outcome in the subset of those with grossly resected tumors (OS 86% [se 3] for COG patients vs. 68% [se 4] for European patients (P = 0.004)). When excluding chemotherapy duration from the model, analysis in this subset of patients showed that cooperative group (P = 0.001), site (P = 0.001), and T stage (P = 0.05) were all significant. However, after adding duration of chemotherapy (≥ 27 weeks) to the model, only primary site remained significant (P = 0.006).
Conclusion
This meta-analysis confirms the role of many established prognostic factors but identifies for the first time that chemotherapy duration may have an impact on outcome in patients with grossly resected tumors.
Keywords: extremity, pediatric, prognosis, rhabdomyosarcoma
INTRODUCTION
Rhabdomyosarcoma (RMS) arises in a multiplicity of sites, and primary location in the extremities accounts for approximately 15% of localized RMS. Sequential clinical studies by international collaborative groups have failed to improve the outcome for children with extremity RMS, which remains suboptimal compared with that of children with RMS at more favorable sites.[1–5] Several adverse factors are frequently associated with extremity RMS, including older age, alveolar subtype (ARMS), and nodal involvement.[5–7]
We describe the results of collaboration between four cooperative study groups in Europe and North America to evaluate prognostic factors for extremity RMS and offer data on the largest number of prospectively treated extremity RMS patients reported to date.
MATERIALS AND METHODS
Data were collected from the files of the Intergroup Rhabdomyosarcoma Study (IRS) Group, now the Soft Tissue Sarcoma Committee of the Children's Oncology Group (COG), in North America; and the Cooperative Weichteilsarkom Studiengruppe (CWS), Italian Cooperative Group for Pediatric Soft Tissue Sarcomas (ICG) and the International Society of Paediatric Oncology Malignant Mesenchymal Tumour Committee (SIOP) in Europe.
Extremity site was defined as any part of the upper and lower extremity, buttock, and shoulder girdle. Data were collected from 643 patients, diagnosed between 1983 and 2004, treated within the following protocols: IRS/COG: IRS-III, IRS-IVP, IRS-IV, D9803 (n = 300 patients);[2,8–10] ICG RMS-79, −88, −96 (n = 44);[1] CWS-81, −86, −91, −96 (n = 139);[3,11,12] and SIOP MMT-84, −89, −95 (n = 160).[4,5,13]
All complied with the Helsinki Declaration regarding human experimentation. Informed consent was obtained in line with research ethics requirements at each participating institution. Completeness of initial surgical resection is reported using the IRS Clinical Grouping system (Supplemental Table I).
All of these protocols utilized similar chemotherapy regimens, with either VA (vincristine, actinomycin D), VAC (vincristine, actinomycin D, cyclophosphamide), IVA (ifosfamide + VA), VAIA (adriamycin + IVA), EVAIA (etoposide + VAiA), VACA (VAC + adriamycin), or CEVAIE (carboplatin, epirubicin + EVAIA) at approximately equivalent myelotoxic doses. Some patients on COG D9803 also received topotecan (Supplemental Table II).
Duration of the chemotherapy varied between groups: duration of European chemotherapy protocols was 24–27 weeks, compared with 46 weeks for the chemotherapy regimens used by the COG protocols (Supplemental Figure 1).
Radiotherapy (RT) requirements also varied between treatment groups. COG protocols recommended systematic local therapy including radiation therapy for all patients except those with primary completely resected embryonal RMS (ERMS). SIOP and ICG protocols limited the indication for systematic use of RT to patients who did not achieve complete remission with chemotherapy with/without secondary surgery. In the CWS studies, radiation dose was stratified depending on the results of second-look surgery. Only patients who did not undergo second-look surgery or who had a residue were irradiated (Supplementary Data 1).
Statistical Methods
Intergroup consensus determined the data items for analysis (Supplemental Table III). Data were pooled in a master database at Gustave Roussy where analyses were performed.
Progression free survival (PFS) was defined as the time from the start of treatment to disease progression/relapse or death from any cause. Overall survival (OS) was defined as the time from the start of treatment to death from any cause. The Kaplan–Meier method was used to estimate the PFS and OS distributions. Differences between survival curves were analyzed by the log-rank test. The distributions of categorical participant characteristics were compared between the groups using χ2 or Fisher's exact tests. Multivariate comparisons of time to event distributions were made using the Cox proportional hazards model.
RESULTS
The characteristics of patients and tumors are described in Table I.
TABLE I.
Characteristics of the 643 Patients and Overall Survival Rates by Prognostic Variables
| Variables | No | 5-year OS (%) | 10-year OS (%) | RR of death | Log-rank test (P) |
|---|---|---|---|---|---|
| Total | 643 | 67 | 62 | ||
| Sex | |||||
| Male | 327 | 66 | 62 | 1.1 | NS |
| Female | 316 | 69 | 62 | 1 | |
| Age | |||||
| <3 | 189 | 79 | 71 | 1 | |
| 3–9 | 232 | 65 | 61 | 1.6 | 0.03 |
| 10+ | 222 | 59 | 54 | 1.9 | |
| Age | |||||
| <3 | 189 | 79 | 71 | 1 | <0.001 |
| 3+ | 454 | 62 | 58 | 1.7 | |
| Site of primary | |||||
| Upper limb | 264 | 66 | 61 | ||
| Lower limb | 373 | 68 | 62 | NS | |
| Site of primary | |||||
| Hand and foot | 108 | 57 | 55 | 1.3 | |
| Other | 529 | 69 | 63 | 1 | 0.08 |
| T status | |||||
| T1 | 398 | 75 | 70 | 1 | |
| T2 | 222 | 54 | 49 | 2.1 | <0.001 |
| Unknown | 23 | ||||
| Tumor size | |||||
| <5 cm | 270 | 75 | 70 | 1 | |
| >5 cm | 364 | 62 | 56 | 1.7 | 0.002 |
| Unknown | 9 | ||||
| Clinical stage | |||||
| I | 346 | 78 | 71 | 1 | |
| II | 133 | 58 | 53 | 2.1 | <0.001 |
| III | 131 | 51 | 46 | 2.5 | |
| Unknown | 33 | ||||
| Regional node involvement | |||||
| No | 495 | 72 | 66 | 1 | <0.001 |
| Yes | 131 | 51 | 46 | 1.9 | |
| Unknown | 17 | ||||
| Pathology | |||||
| Non alveolar | 223 | 72 | 68 | 1 | 0.06 |
| Alveolar | 420 | 65 | 58 | 1.4 | |
| Initial surgery | |||||
| Complete | 148 | 82 | 77 | 1 | |
| Micro residue | 153 | 71 | 66 | 1.6 | <0.001 |
| Macro residue or biopsy | 101+239 | 59 | 52 | 2.8 | |
| Chemotherapy | |||||
| VA | 39 | 84 | 81 | 1 | |
| Alkyating agents (AA) | 307 | 69 | 64 | 2 | 0.04 |
| AA + any anthracyclines | 197 | 64 | 57 | 2.4 | |
| 6 drugs (CEVAIA) | 94 | 57 | 55 | 2.7 | |
| Unknown | 6 | ||||
| Initial radiation | |||||
| No | 231 | 68 | 64 | 1 | |
| Yes | 388 | 66 | 59 | 1.1 | NS |
| Unknown or amputation | 24 | ||||
| Cooperative groups | |||||
| COG | 300 | 72 | 68 | 1 | |
| CWS | 139 | 63 | 54 | 1.5 | 0.02 |
| ICG | 44 | 64 | 59 | 1.5 | |
| SIOP | 160 | 61 | 56 | 1.5 | |
| Cooperative groups | |||||
| COG | 300 | 72 | 68 | 1 | 0.002 |
| Europe | 343 | 62 | 56 | 1.5 | |
| Era of treatment | |||||
| 1984–1994 | 355 | 62 | 57 | 1.5 | 0.004 |
| 1995+ | 288 | 73 | 68 | 1 |
NS, not statistically significant.
Patient Demographics and Tumor Characteristics
Median age at diagnosis was 6.3 years (0–20.8). Nearly one-third (29%) of the patients were <3 years of age and one-third (35%) were >10 years. Based on morphology, 420 patients (65%) had alveolar RMS, with a similar proportion in each cooperative group. However, diagnostic definition of alveolar subtype ARMS has evolved with time and tumors diagnosed before 1995 were significantly less often identified as alveolar than tumors diagnosed later on (61% vs. 70%, P < 0.03).
Primary sites were located in lower limbs in 58% patients and in upper limbs in 41% (not specified in one); 49% were located proximally and 51% distally. Overall 17% patients had primaries in the hand (n = 55) or foot (n = 53). Tumor characteristics according to cooperative groups are described in Supplemental Table IV.
Clinical, radiological, or pathological evidence of proximal nodal involvement of proximal lymph nodes was detected recorded in 21% patients and was significantly more frequent in patients with ARMS (26.3% vs. 10.6% in ERMS, P < 0.02). Histologically proven involvement was also more frequent for ARMS than ERMS (40% vs. 16%, P < 0.001).
Lymph node sampling was recommended but not required in all studies and only required on COG D9803. Differences in recommended/required investigations may account for variations in rates of nodal involvement identified between groups, ranging from 24% (COG) to 14% (SIOP).
Initial Surgery
All patients had at least a biopsy and 58% underwent an initial attempt at surgical resection, although this varied between the groups from 63% (COG) to 43% (ICG) (P = 0.001). Initial complete excision was achieved in 23% (IRS Group I) whereas 24% had microscopic positive margins (Group II) and 53% had macroscopic residue after resection or were only biopsied (Group III). Completeness of surgery was more often achieved in COG patients (30%) compared to other groups (range: 13–23%) (P < 0.002). Amputation was performed as a primary procedure in 28 (9%) COG patients; this included 11 with digit/ray amputations in hands/feet (Supplemental Table V).
Chemotherapy
All patients received multiagent chemotherapy in various combinations according to groups and treatment eras (Supplemental Table II).
Radiation Therapy
Overall, 376 (59%) patients received RT as part of primary treatment. This varied significantly between groups: only 29% were irradiated in SIOP studies compared to 67% (IRS/COG), 73% (CWS), and 73% (ICG).
Patients who received RT were older and there was further variation in the delivery of RT to very young (<3years) patients (IRS/COG, 25%; CWS, 13%; ICG, 19%; SIOP, 13%) (Supplemental Table VI).
Radiation doses ranged from 16.2 to 66 Grays (median 45 Grays). Radiation was delivered only to the primary in 84% and to primary site with regional nodes in 16% (field data were available for 72% of irradiated patients).
Remission, Survival, and Relapse
Median follow-up of survivors was 8.2 years (1.9–22 years). Five- and ten-year OS rates were 67% (standard error (SE) 1.8) and 62% (SE 2), respectively.
Twenty-four (3.7%) patients progressed and died without achieving tumor control and 258 (40%) relapsed. Median time to relapse was 17 months, only 3% occurring ≥5 years from diagnosis. Five- and ten-year PFS rates were 54% (se 1.9) and 51% (se 2.0), respectively.
Loco-regional failure occurred in 163 patients (63% of all relapses), with simultaneous metastasis in 41. Site of failure (details specified in 118 patients) was primary site in 57%, regional lymph nodes in 33%, and both primary and nodal sites in 10%. Local failure occurred more often in patients who did not receive initial irradiation (31% vs. 22% in those receiving RT as part of primary treatment, P = 0.02). Radiation dose had no impact on the risk of locoregional relapse. Metastatic relapse was documented in 95 patients (37% of all relapses). Histological subtype was not associated with the pattern of relapse (Supplemental Table VII). Ten patients developed a second tumor as first event.
Overall, 91% (230/252) of all deaths were attributed to the primary disease; 10 died from treatment toxicity, six from second malignancy, and four from other, unrelated causes. The cause of death was unknown for two patients.
Survival after relapse was very poor: 5-year survival was only 32% after isolated local relapse, 12% after metastatic relapse, and 9% after combined relapse. Survival after relapse was significantly better for patients who did not receive RT as part of the initial treatment (33% vs. 16% for patients who had received RT, P < 0.001).
Details of treatment for relapse were not available to allow analysis of overall burden of therapy, but within the COG studies; 76% of the surviving patients had received radiation therapy or had undergone amputation during initial treatment of the primary disease. Among the European groups, the use of RT and/or amputation varied widely from 24% in surviving SIOP patients to 79% in CWS and 81% in ICG patients.
Prognostic Factors
Analysis on the whole population
Table I shows OS by prognostic variable. Survival differed by age: age 3 years or more being associated with a less favorable outcome (P < 0.001). Patients less than 1 year had the same survival as patients 1–3 years old. The risk for patients 3–9 years old appeared to be intermediate to that of patients between 0 and 3 years old and those who were ≥10 years or older (P = 0.02). (Supplemental Figures 2A and B).
Tumor invasiveness (T2 stage (P < 0.001), large tumor size (P = 0.002), lymph node involvement (P < 0.001), and incomplete initial surgery (P < 0.001)) were all strongly correlated with lower survival. Pathological subtype had only a borderline impact on OS (P = 0.06) and tumors of hand and foot (P = 0.08) tended to do worse than other sites. RT as part of the initial treatment did not correlate with OS. Period of treatment had an impact, with patients treated more recently doing better. Overall survival was identical in the European cooperative groups (62% at 5 years) but significantly lower than that seen in the IRS/COG studies (P = 0.002).
Multivariate analysis on 592 patients with complete data (Table II) demonstrated that age (P = 0.002), T status (P = 0.02), nodal involvement (P = 0.04), completeness of initial surgery (P = 0.001), and era of treatment (P = 0.001) were independently predictive of OS. Cooperative group maintained an independent prognostic impact (P = 0.001).
TABLE II.
Cox Model for Overall Survival (n = 592 Patients With Complete Data)
| Prognostic variables | Relative risk of death | P |
|---|---|---|
| Age (years) | ||
| <3 | 1 | |
| 3+ | 1.7 (1.2–2.4) | 0.002 |
| T status | ||
| T1 | 1 | |
| T2 | 1.4 (1.1–2) | 0.02 |
| Tumor size | ||
| <5cm | NS | |
| >5cm | ||
| Site of the Primary Tumour | ||
| Hand and foot | NS | |
| Other | ||
| Lymph Node involvement | 1 | |
| No | 1.4 (1–1.9) | 0.04 |
| Yes | ||
| Initial surgery | ||
| Complete | 1 | |
| Micro residue | 1.5 (0.9–2.3) | |
| Macro residue | 2.3 (1.5–3.6) | 0.001 |
| Initial radiation | ||
| No | ||
| Yes | NS | |
| Cooperative groups | ||
| COG | 1 | |
| Europe | 1.6 (1.2–2.2) | 0.001 |
| Era of treatment | ||
| 1984–1994 | 1.7 (1.3–2.3) | |
| 1995+ | 1 | 0.001 |
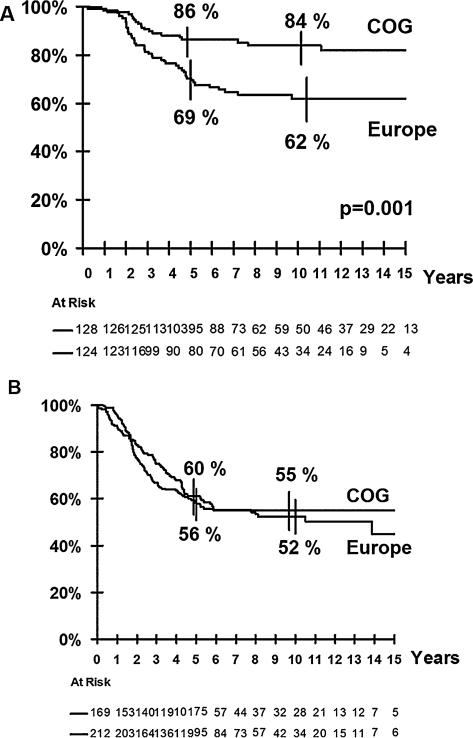
Further analysis of=this difference showed that the outcome was different only for patients with grossly resected tumors after initial surgery (IRS Groups I and II). The outcome of IRS Group III patients (macroscopic residual/biopsy alone) was identical for all groups (Fig. 1A and B).
Fig. 1.
Overall survival by continental groups. (A) Overall survival for grossly resected tumors: complete surgery (IRS group 1) or microscopic residue (IRS group 2). (B) Overall survivals for unresected tumors or resected tumor with macroscopic residue (IRS group 3).
Analysis for patients with grossly resected primaries (IRS Groups I and II) without nodal involvement
In this subset of 252 patients, univariate analysis (Table III) showed that, in addition to the influence of cooperative group (P = 0.0004), completeness of initial surgery (P = 0.05), T status (P = 0.02), and primary site (hand/foot vs. other extremity sites, P = 0.01) all correlated with OS. Furthermore, the duration of chemotherapy was strongly associated with OS (P = 0.002), with lower local and metastatic relapse rates in those treated 27 weeks or more. Histology, age at diagnosis, size of the tumor, radiation therapy as part of the initial treatment, and treatment period had no impact.
TABLE III.
Overall Survival Rates by Prognostic Variables for Grossly Resected Tumors Without Nodes
| Variables | No | 5 year OS (%) | RR of death | Log-rank test (P) |
|---|---|---|---|---|
| Total | 252 | 78 (73–83) | ||
| Age | ||||
| <3 | 72 | 86 (76–92) | 1 | NS |
| 3+ | 180 | 75 (69–81) | 1.51 | −0.17 |
| Site of the primary tumour | ||||
| Upper limb | 112 | 75 (66–82) | 1.34 | NS |
| Lower limb | 136 | 81 (74–87) | 1 | −0.25 |
| Site of the primary tumour | ||||
| Hand and foot | 43 | 64 (48–77) | 2.09 | 0.01 |
| Other | 205 | 82 (75–86) | 1 | |
| T status | ||||
| T1 | 203 | 82 (76–86) | 1 | 0.02 |
| T2 | 43 | 67 (52–79) | 1.92 | |
| Unknown | 6 | |||
| Tumor size | ||||
| <5 cm | 151 | 80 (72–85) | 1 | NS |
| >5 cm | 96 | 77 (67–84) | 1.29 | −0.32 |
| Unknown | 5 | |||
| Pathology | ||||
| Non alveolar | 90 | 83 (74–89) | 1 | NS |
| Alveolar | 162 | 76 (69–82) | 1.47 | −0.16 |
| Initial surgery | ||||
| Complete (IRS I) | 136 | 83 (76–88) | 1 | 0.05 |
| Micro residue (IRS II) | 116 | 73 (64–23) | 1.65 | |
| Chemotherapy | ||||
| VA | 36 | 86 (71–94) | 1 | NS |
| Alkyating agents (AA) | 103 | 85 (77–91) | 1.02 | −0.07 |
| AA + any anthracyclines | 94 | 70 (60–79) | 1.98 | |
| 6 drugs (CEVAIA) | 18 | 66 (43–84) | 2.04 | |
| Unknown | 1 | |||
| Chemotherapy duration | ||||
| < 23 weeks | 52 | 70 (56–81) | 2.39 | 0.004 |
| 24–26 weeks | 59 | 69 (56–79) | 2.39 | |
| >27 weeks | 141 | 85 (79–90) | 1 | |
| Initial radiation | ||||
| No | 104 | 79 (70–85) | 1 | NS |
| Yes | 129 | 77 (69–84) | 0.99 | |
| Unknown or amputation | 19 | |||
| Amputation | ||||
| No | 234 | 78 (72–83) | 0.86 | NS |
| Yes | 18 | 83 (59–94) | 1 | |
| Cooperative groups | ||||
| COG | 128 | 86 (79–90) | 1 | 0.004 |
| Europe | 124 | 70 (62–78) | 2.51 | |
| Era of treatment | NS | |||
| 1984–1994 | 171 | 76 (69–82) | 0.91 | −0.1 |
| 1995+ | 81 | 83 (74–90) | 1 |
In multivariate analysis only tumor site (P = 0.006) and duration of chemotherapy (P < 0.001) were independently predictive of OS (Table IV). The strong association between cooperative group and duration of chemotherapy (IRS/COG trials uniformly using longer duration of therapy) did not allow further analysis.
TABLE IV.
Cox Model for Overall Survival for Grossly Resected Tumors Without Nodes, IRS I and II (n = 252)
| Model without chemotherapy duration |
Model including chemotherapy duration |
|||
|---|---|---|---|---|
| Prognostic variables | Relative risk of death | P | Relative risk of death | P |
| Initial surgery | ||||
| Complete | ||||
| Micro residue | NS | |||
| Site of the PT | ||||
| Hand and foot | 2.2 | 0.01 | 2.2 | 0.006 |
| Others | 1 | 1 | ||
| T status | ||||
| T1 | 1 | |||
| T2 | 1.8 | 0.05 | NS | |
| Cooperative groups | ||||
| COG | 1 | |||
| Europe | 2.6 | 0.001 | NS | |
| Duration of chemotherapy | ||||
| <27 weeks | 2.6 | |||
| >27 weeks | 1 | <0.001 | ||
DISCUSSION
The results of this analysis of a large international cohort of patients show that extremity RMS in patients without distant metastatic disease occurs at a median age (6.3 years) similar to parameningeal or orbital tumors[14,15] but older than in other sites such as bladder/prostate (2.5 years).[16]
The 10-year OS rate of 62% confirms the relatively less favorable prognosis of extremity RMS as compared to other sites. [2,3,5,12] Age is a prognostic factor and patients ≥10 years old or more had a poorer survival than younger patients, consistent with data from other studies.[14,17–19] However, patients less than 3 years had a better survival than patients between 3- and 9-years-old despite the challenges of delivering local therapy to very young children. Other adverse prognostic factors measurable at diagnosis included, tumor invasiveness (T2 stage), tumor size (>5 cm), and locoregional nodal involvement, findings similar to those reported for RMS regardless of tumor site.[4,6,14,20]
Nodal involvement was common (21%) and more common for alveolar than for non-alveolar tumors. This is in line with data in a detailed study of patterns of nodal involvement in extremity RMS which also raised the issue of managing potential/confirmed involvement of in-transit nodes.[21] In that study, patients who underwent complete lymph node staging with appropriate radiotherapy to the in-transit nodal site were at slightly lower risk of in-transit failure. However, the delivery of optimal local therapy to limit failure in in-transit nodes in patients with extremity tumors remains a challenge.[22] Assessment using sentinel lymph node sampling and FDG-PET scanning may help refine the diagnosis of lymph node involvement at diagnosis but the place of such technologies is not yet fully established in the management of RMS.[23–25]
ARMS is frequent in extremity sites and was found in 65% of the tumors, a proportion similar across all the groups despite long-standing discussions about its definition.[26] Although alveolar subtype has been identified as a poor prognostic factor in many studies[2,4,6,9,15,16,27] it did not correlate with a worse outcome in this series, either in univariate or multivariate analysis, nor was it associated with a specific pattern of failure. This possibly reflects increased treatment intensity and routine use of radiation for ARMS.
However, similar findings emerged from an analysis of metastatic RMS[19] reflecting the fact that ARMS not only occurs more often in extremity sites but is also more often metastatic at diagnosis than ERMS, but is not per se an independent prognostic factor. The new definition based on molecular biology and currently used in RMS protocols might impact the prognostic value of RMS alveolar subtype in future studies.[28]
Overall, patients treated in North American studies fared better than patients treated in Europe, even after adjustment for other prognostic factors. This was accounted for by differences in outcome for grossly resected (IRS Group I–II) tumors without node involvement. In this subset of patients, multivariate analysis showed that tumor invasiveness and location in hands/feet were prognostically important in addition to cooperative group. There was no effect from the use of RT during initial treatment and although the difference seen in outcome might reflect differences in initial surgical approach, an unexpected finding was attributable to the difference in duration of chemotherapy and its independent impact on survival. Treatment duration was 27 weeks or longer in all COG patients but shorter than 27 weeks in 90% of the European patients. The impact of treatment duration has not been previously reported as prognostic in studies of RMS, but the value of prolonged additional treatment with “maintenance” chemotherapy is now under exploration in current European studies.
As with all meta-analyses, the interpretation of our results comes with inherent limitations. For example, there was significant evolution in imaging modalities that could influence stage, changes in the histological definition of ARMS, and refinements in radiation therapy technologies during the review period. Comparisons between patients enrolled on differing protocols are non-randomized and uncontrolled. Nonetheless, our large study size and common elements of data collection allow us to make comparison that would not be possible within a single, smaller clinical study.
CONCLUSIONS
The value of pooled analysis of data from parallel international studies of RMS has been demonstrated in several previous reports. [14–16,19,28–30] Optimizing therapy for children with RMS at extremity sites remains a challenge for all studies. Data from this analysis, and from other current literature, suggest that efforts should be focused on: better recognition of lymph node status at diagnosis and improving methods for treating those with nodal involvement, including the management of in-transit nodes; improving primary surgical resection, whilst retaining limb function; and optimizing chemotherapy, targeting in particular better treatment for ARMS and the place of longer duration of therapy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Angela Scagnellato from University Hospital of Padova, Italy and Christoph Int-Veen from Olga Hospital of Stuttgart, Germany for assembling and sending the data; V eronique Minard from Gustave Roussy, France, for her helpful review, and Lorna Saint Ange from Gustave Roussy, France, for editing the manuscript.
Grant sponsor: COG clinical trials were supported by the National Cancer Institute/National Institutes of Health (U10 CA98543-08, U10 CA98413-08). - SIOP clinical trials were partly supported by Cancer Research UK, Gustave Roussy, and Association Enfant et Santé, France - AIEOP-STSC studies were supported by Fondazione Città della Speranzah; Grant numbers: U10 CA98543-08; U10 CA98413-08; Grant sponsor: Cancer Research UK, Gustave Roussy, Association Enfant et Santé, France; Grant sponsor: Fondazione Città della Speranza
Abbreviations
- ARMS
alveolar rhabdomyosarcoma
- COG
Children's Oncology Group
- CWS
Cooperative Weichteilsarkom Studiengruppe
- ERMS
embryonal rhabdomyosarcoma
- ICG
Italian Cooperative Group
- IRS
Intergroup Rhabdomyosarcoma Study
- MMT
malignant mesenchymal tumors
- N ?stage
nodal involvment
- OS
overall survival
- PFS
progression free survival
- RMS
rhabdomyosarcoma
- RT
radiotherapy
- SIOP
International Society of Paediatric Oncology
- T stage
invasiveness
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Bisogno G, Pastore G, Perilongo G, Sotti G, Cecchetto G, Dallorso S, Carli M. Long-term results in childhood rhabdomyosarcoma: A report from the Italian cooperative study RMS 79. Pediatr Blood Cancer. 2012;58:872–876. doi: 10.1002/pbc.23292. [DOI] [PubMed] [Google Scholar]
- 2.Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, Breneman J, Qualman SJ, Wiener E, Wharam M, Lobe T, Webber B, Maurer HM, Donaldson SS. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 3.Dantonello TM, Int-Veen C, Harms D, Dantonello TM, Int-Veen C, Harms D, Leuschner I, Schmidt BF, Herbst M, Juergens H, Scheel-Walter HG, Bielack SS, Klingebiel T, Dickerhoff R, Kirsch S, Brecht I, Schmelzle R, Greulich M, Gadner H, Greiner J, Marky I, Treuner J, Koscielniak E. Cooperative trial CWS-91 for localized soft tissue sarcoma in children, adolescents, and young adults. J Clin Oncol. 2009;27:1446–1455. doi: 10.1200/JCO.2007.15.0466. [DOI] [PubMed] [Google Scholar]
- 4.Oberlin O, Rey A, Sanchez de Toledo J, Martelli H, Jenney ME, Scopinaro M, Bergeron C, Merks JH, Bouvet N, Ellershaw C, Kelsey A, Spooner D, Stevens MC. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: Long-term results from the International Society of Pediatric Oncology MMT95 study. J Clin Oncol. 2012;30:2457–2465. doi: 10.1200/JCO.2011.40.3287. [DOI] [PubMed] [Google Scholar]
- 5.Stevens MC, Rey A, Bouvet N, Ellershaw C, Flamant F, Habrand JL, Marsden HB, Martelli H, Sanchez de Toledo J, Spicer RD, Spooner D, Terrier-Lacombe MJ, van Unnik A, Oberlin O. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: Third study of the International Society of Paediatric Oncology—SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23:2618–2628. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 6.Meza JL, Anderson J, Pappo AS, Meyer WH. Children's Oncology Group. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: The Children's Oncology Group. J Clin Oncol. 2006;24:3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 7.Neville HL, Andrassy RJ, Lobe TE, Bagwell CE, Anderson JR, Womer RB, Crist WM, Wiener ES. Preoperative staging, prognostic factors, and outcome for extremity rhabdomyosarcoma: A preliminary report from the Intergroup Rhabdomyosarcoma Study IV (1991-1997). J Pediatr Surg. 2000;35:317–321. doi: 10.1016/s0022-3468(00)90031-9. [DOI] [PubMed] [Google Scholar]
- 8.Arndt CA, Stoner JA, Hawkins DS, Rodeberg DA, Hayes-Jordan AA, Paidas CN, Parham DM, Teot LA, Wharam MD, Breneman JC, Donaldson SS, Anderson JR, Meyer WH. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's oncology group study D 9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crist W, Gehan EA, Ragab AH, Dickman PS, Donaldson SS, Fryer C, Hammond D, Hays DM, Herrmann J, Heyn R, Herrmann J, Heyn R, Jones Pat Morris, Lawrence W, Newton W, Ortega J, Raney RB, Ruymann FB, Tefft M, Webber B, Wiener E, MWharam M, Vietti TJ, Maurer HM. The third intergroup rhabdomyosarcoma study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson SS, Meza J, Breneman JC, Crist WM, Laurie F, Qualman SJ, Wharam M. Children's Oncology Group Soft Tissue Sarcoma Committee. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma-a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 11.Koscielniak E, Jürgens H, Winkler K, Bürger D, Herbst M, Keim M, Bernhard G, Treuner J. Treatment of soft tissue sarcoma in childhood and adolescence. A report of the German Cooperative Soft Tissue Sarcoma Study. Cancer. 1992;70:2557–2567. doi: 10.1002/1097-0142(19921115)70:10<2557::aid-cncr2820701027>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Koscielniak E, Treuner J, Jürgens H, Winkler K, Bürger D, Herbst M, Ritter J, Niethammer D, Müller-Weihrich S, Bernhard G. Results of treatment for soft tissue sarcoma in childhood and adolescence: a final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol. 1999;17:3706–3719. doi: 10.1200/JCO.1999.17.12.3706. [DOI] [PubMed] [Google Scholar]
- 13.Flamant F, Rodary C, Rey A, Praquin MT, Sommelet D, Quintana E, Theobald S, Brunat-Mentigny M, Otten J, VoÛte PA, Habrand JL, Martelli H, Barrett A, Terrier-Lacombe MJ, Oberlin O. Treatment of non-metastatic rhabdomyosarcomas in childhood and adolescence. Results of the second study of the International Society of Paediatric Oncology: MMT84. Eur J Cancer. 1998;34:1050–1062. doi: 10.1016/s0959-8049(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 14.Merks JH, De Salvo GL, Bergeron C, Bisogno G, De Paoli A, Ferrari A, Rey A, Oberlin O, Stevens MC, Kelsey A, Michalski J, Hawkins DS, Anderson JR. Parameningeal rhabdomyosarcoma in pediatric age: Results of a pooled analysis from North American and European cooperative groups. Ann Oncol. 2014;25:231–236. doi: 10.1093/annonc/mdt426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberlin O, Rey A, Anderson J, Carli M, Raney RB, Treuner J, Stevens MC. International Society of Paediatric Oncology Sarcoma Committee, Intergroup Rhabdomyosarcoma Study Group, Italian Cooperative Soft Tissue Sarcoma Group, German Collaborative Soft Tissue Sarcoma Group. Treatment of orbital rhabdomyosarcoma: Survival and late effects of treatment-results of an International Workshop. J Clin Oncol. 2001;19:197–204. doi: 10.1200/JCO.2001.19.1.197. [DOI] [PubMed] [Google Scholar]
- 16.Rodeberg DA, Anderson JR, Arndt CA, Ferrer FA, Raney RB, Jenney ME, Brecht IB, Koscielniak E, Carli M, Bisogno G, Oberlin O, Rey A, Ullrich F, Stevens MC, Meyer WH. Comparison of outcomes based on treatment algorithms for rhabdomyosarcoma of the bladder/prostate: Combined results from the Children's Oncology Group, German Cooperative Soft Tissue Sarcoma Study, Italian Cooperative Group, and International Society of Pediatric Oncology Malignant Mesenchymal Tumors Committee. Int J Cancer. 2011;128:1232–1239. doi: 10.1002/ijc.25444. [DOI] [PubMed] [Google Scholar]
- 17.Joshi D, Anderson JR, Paidas C, Breneman J, Parham DM, Crist W. Soft Tissue Sarcoma Committee of the Children's Oncology Group. Age is an independent prognostic factor in rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Pediatr Blood Cancer. 2004;42:64–73. doi: 10.1002/pbc.10441. [DOI] [PubMed] [Google Scholar]
- 18.LaQuaglia MP, Ghavimi F, Penenberg D, Mandell LR, Healey JH, Hadju SI, Exelby PR. Factors predictive of mortality in pediatric extremity rhabdomyosarcoma. J Pediatr Surg. 1990;25:238–243. doi: 10.1016/0022-3468(90)90409-3. [DOI] [PubMed] [Google Scholar]
- 19.Oberlin O, Rey A, Lyden E, Bisogno G, Stevens MC, Meyer WH, Carli M, Anderson JR. Prognostic factors in metastatic rhabdomyosarcomas: Results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dantonello TM, Int-Veen C, Winkler P, Leuschner I, Schuck A, Schmidt BF, Lochbuehler H, Kirsch S, Hallmen E, Veit-Friedrich I, Bielack SS, Niggli F, Kazanowska B, Ladenstein R, Wiebe T, Klingebiel T, Treuner J, Koscielniak E. Initial patient characteristics can predict pattern and risk of relapse in localized rhabdomyosarcoma. J Clin Oncol. 2008;26:406–413. doi: 10.1200/JCO.2007.12.2382. [DOI] [PubMed] [Google Scholar]
- 21.La TH, Wolden SL, Rodeberg DA, Hawkins DS, Brown KL, Anderson JR, Donaldson SS. Regional nodal involvement and patterns of spread along in-transit pathways in children with rhabdomyosarcoma of the extremity: A report from the Children's Oncology Group. Int J Radiat Oncol Biol Phys. 2011;80:1151–1157. doi: 10.1016/j.ijrobp.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulino AC, Pappo A. Alveolar rhabdomyosarcoma of the extremity and nodal metastasis: Is the in-transit lymphatic system at risk? Pediatr Blood Cancer. 2009;53:1332–1333. doi: 10.1002/pbc.22252. [DOI] [PubMed] [Google Scholar]
- 23.Alcorn KM, Deans KJ, Congeni A, Sulkowski JP, Bagatell R, Mattei P, Minneci PC. Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: Utility and concordance with imaging. J Pediatr Surg. 2013;48:1903–1906. doi: 10.1016/j.jpedsurg.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Ben Arush MW, Bar Shalom R, Postovsky S, Militianu D, Haimi M, Zaidman I, Israel O. Assessing the use of FDG-PET in the detection of regional and metastatic nodes in alveolar rhabdomyosarcoma of extremities. J Pediatr Hematol Oncol. 2006;28:440–445. doi: 10.1097/01.mph.0000212949.12856.02. [DOI] [PubMed] [Google Scholar]
- 25.Federico SM, Spunt SL, Krasin MJ, Billup CA, Wu J, Shulkin B, Mandell G, McCarville MB. Comparison of PET-CT and conventional imaging in staging pediatric rhabdomyosarcoma. Pediatr Blood Cancer. 2013;60:1128–1134. doi: 10.1002/pbc.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parham DM, Barr FG. Classification of rhabdomyosarcoma and its molecular basis. Adv Anat Pathol. 2013;20:387–397. doi: 10.1097/PAP.0b013e3182a92d0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens MC. Treatment for childhood rhabdomyosarcoma: The cost of cure. LancetOncol. 2005;6:77–84. doi: 10.1016/S1470-2045(05)01733-X. [DOI] [PubMed] [Google Scholar]
- 28.Williamson D, Missiaglia E, de Reyni es A, Pierron G, Thuille B, Palenzuela G, Thway K, Orbach D, La e M, Fr eneaux P, Pritchard-Jones K, Oberlin O, Shipley J, Delattre O. Fusion gene-negative alveolar rhabdomyosarcoma is clinically and molecularly indistinguishable from embryonal rhabdomyosarcoma. J Clin Oncol. 2010;28:2151–2158. doi: 10.1200/JCO.2009.26.3814. [DOI] [PubMed] [Google Scholar]
- 29.Raney B, Parham D, Sommelet-Olive D, Stevens MC, Treuner J, Carli M. Summary of the International Symposium on Childhood Non-Rhabdomyosarcoma Soft-Tissue Sarcomas, Padua, Italy, February 10-12, 1994. Med Pediatr Oncol. 1996;26:425–430. doi: 10.1002/(SICI)1096-911X(199606)26:6<425::AID-MPO11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Rodary C, Gehan EA, Flamant F, Treuner J, Carli M, Auquier A, Maurer H. Prognostic factors in 951 nonmetastatic rhabdomyosarcoma in children: A report from the International Rhabdomyosarcoma Workshop. Med Pediatr Oncol. 1991;19:89–95. doi: 10.1002/mpo.2950190204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.