Abstract
Memantine, a partial antagonist of N-methyl-D-aspartate receptor (NMDAR), approved for moderate to severe Alzheimer’s disease (AD) treatment within the US and Europe under brand name Namenda (Forest), Axura and Akatinol (Merz), and Ebixa and Abixa (Lundbeck), may have potential in alleviating additional neurological conditions, such as vascular dementia (VD) and Parkinson’s disease (PD). In various animal models, memantine has been reported to be a neuroprotective agent that positively impacts both neurodegenerative and vascular processes. While excessive levels of glutamate result in neurotoxicity, in part through the over-activation of NMDARs, memantine—as a partial NMDAR antagonist, blocks the NMDA glutamate receptors to normalize the glutamatergic system and ameliorate cognitive and memory deficits. The key to memantine’s therapeutic action lies in its uncompetitive binding to the NMDAR through which low affinity and rapid off-rate kinetics of memantine at the level of the NMDAR-channel preserves the physiological function of the receptor, underpinning memantine’s tolerability and low adverse event profile. As the biochemical pathways evoked by NMDAR antagonism also play a role in PD and since no other drug is sufficiently effective to substitute for the first-line treatment of L-dopa despite its side effects, memantine may be useful in PD treatment with possibly fewer side effects. In spite of the relative modest nature of its adverse effects, memantine has been shown to provide only a moderate decrease in clinical deterioration in AD and VD, and hence efforts are being undertaken in the design of new and more potent memantine-based drugs to hopefully provide greater efficacy.
Keywords: Alzheimer’s disease, Parkinson’s disease, vascular dementia, memantine, NMDAR, amantadine
INTRODUCTION
Alzheimer’s disease (AD), a neurodegenerative disorder characterized by irreversible, progressive loss of memory followed by complete dementia, is marked by cognitive decline accompanied by impaired performance of daily activities, behavior, speech and visual-spatial perception. It is the most common type of dementia among people older than 65, accounting for about 60%-70% of cases [1], associated with heterogeneous risks including genetic, epigenetic, dietary, and lifestyle factors [2].
The most striking and early symptom of AD is a loss of short-term memory (amnesia). When AD is suspected, the diagnosis is usually confirmed with behavioral assessments and cognitive tests, often followed by a brain scan. As the disorder progresses, cognitive impairment includes difficulty in producing or comprehending spoken or written language (aphasia), difficulty of execution of movements (apraxia), loss of perception (agnosia) [3], and disorientation [4]. AD may also involve behavioral changes, such as outbursts of violence or excessive passivity in people who have no previous history of such behavior [5, 6]. Gradually, basic physiological functions are lost, ultimately leading to death.
AD afflicts at least 26 million people throughout the world [7] of which 5.4 million are Americans [8]. In the US, in 2011, it is estimated that at least $183 billion will be spent on direct AD care [8] and these costs will rise as the population ages. Several FDA-approved drugs are currently in use for the treatment of AD, however they mostly bring symptomatic relief and do not cure AD. Such an absence of treatment options sets the stage for the present review, which is primarily focused on the physiological role and utility of NMDAR antagonists, especially memantine, and its treatment not only for AD but other neurodegenerative disorders also, such as Vascular Dementia (VD) and Parkinson’s Disease (PD). The present review summarizes the recent advances in pathogenic processes underlying these diseases, including the amyloid pathway, pharmacology of NMDARs, glutamatergic transmission and the utility of NMDAR antagonists for therapy. Keeping the current interest of the field in mind, we will place a special emphasis on memantine treatment. It is worth mentioning that several aspects of memantine, such as its molecular basis [9], pharmacology [10] and clinical trials [11] have been previously discussed in this journal. However, the present review brings forward distinctly the unique role of memantine in treating AD, VD and PD in a comprehensive manner.
ALZHEIMER’S DISEASE PATHOGENESIS, NMDARS AND MEMANTINE TREATMENT
Alzheimer’s Disease Pathology and NMDARs
Cognitive impairment in AD is caused, in large part, by the death of cholinergic neurons in the basal forebrain area [12]. Therefore, well characterized in the AD brain are a deficit in acetylcholine (ACh) and classical cholinergic markers, epitomized by choline acetyltransferase and acetylcholineseterase [2, 13].
AD neuropathology is routinely characterized by the accumulation of insoluble amyloid protein that originates from the amyloidogenic processing of a much larger metalloprotein – amyloid precursor protein (APP) [14] that leads to the formation of extracellular neuritic amyloid plaques containing the peptide- beta amyloid peptide (Aβ, see Fig. 1). The other major pathological hallmarks include neurofibrillary tangles (NFTs) that are comprised of misfolded, abnormally phosphorylated microtubule-associated tau protein [15], inflammatory changes with astrocytosis and microgliosis [16], oxidative stress [17, 18] and neuropil threads that have been found in the post-mortem AD brain [19], and in addition, a variety of other neurochemical and cellular alterations that result in anatomic as well as functional impairment of the neurotransmitter systems.
Fig. (1).
Schematic illustration of APP protein and its Aβ product after cleavage by α-, β- and γ-secretases. β- and γ-secretase cleaves on the N- and C-terminal ends of the Aβ region respectively. γ-Secretase cleavage yields a 39–43 amino acid product. Long and more fiblillogenic 42–43 amino acid Aβ species are implicated in AD pathogenesis and may seed the formation of Aβ40 fibrils. Mutations in the APP gene and in genes encoding proteins known as presenilins increase the production of long Aβ. Presenilins-1 and −2 is thought function as γ-secretases (for a review, see [142]).
Although genetic, biochemical and neuropathological data strongly point to Aβ and amyloid plaque formation as a central event in AD pathogenesis [20], the etiopathology of AD remains unclear. A considerable weight of data suggests that it is polygenic and multifactorial [21] and that, likely, Aβ metabolism is sensitive to a range of influences and multiple mechanisms that can cause a shift towards the pathogenic pathways that lead to AD [22]. A small fraction of patients develop AD before the age of 65 known as early onset familial AD (FAD) that is believed to be caused by ~200 mutations in one of three genes: APP (on chromosome 21), and presenilin-1 and −2 (PS1, PS2) (31, 177 and 14 mutations, respectively) [http://www.molgen.ua.ac.be/ADMutations]. A commonality of these numerous mutations is that they, albeit via different routes, increase generation of Aβ and, in particular, the ratio of Aβ42: Aβ40 [23]. Major evidence indicates that soluble aggregates of Aβ and Aβ-derived diffusible ligands (ADDLs) target synapses and impair memory and also can induce cellular dysfunction. Recently, it has been suggested that APP proteolysis generates additional fragments that contribute to neuronal dysfunction [24].
Aβ40 (40 amino acid residues) is the main soluble Aβ species that is found in the cerebrospinal fluid at low nanomolar concentrations [25]. Aβ42 (42 residues) is a minor Aβ species that is more fibrillogenic than Aβ40 and heavily enriched in interstitial plaque amyloid [26]. It is generally agreed that Aβ peptide neurotoxicity is dependent on its conformational state [27]. The in vitro solubility of synthetic Aβ42, in neutral aqueous solutions is lower than Aβ40, consequent to the hydophobicity of the additional carboxylterminal amino acids. Also, it has been demonstrated that soluble Aβ40 can be destabilized through seeding with Aβ42 fibrils [28]. However, the presence or overproduction of Aβ42 alone appears to be insufficient to initiate Aβ amyloid deposition. Overexpression of APP and consequential Aβ overproduction in transgenic mice models rarely results in mice bearing full-blown Alzheimer’s-like neuropathology [29]. Rather, it appears more likely that additional neurochemical factors are required for Aβ amyloidosis.
Some of the potential disease-modifying treatments for AD include NMDAR blockade, use of P-sheet breakers, antioxidant strategies, Aβ-peptide vaccination, secretase inhibitors, APP synthesis inhibitors, cholesterol-lowering drugs, metal chelators and anti-inflammatory agents. Strategies targeting the Aβ protein directly include anti-Aβ immunization, γ- and P-secretase inhibitors, aggregation inhibitors and copper/zinc chelators. Interest in the use of metal chelator drugs stems from recent research suggesting that Aβ plaque formation relies upon the binding of metal ions [22]. Cholinergic drugs such as donepezil, rivastigmine and galantamine represent primary treatments for AD and are based on increasing available levels of ACh to surviving neurons. However, they have not been shown to prevent neuronal death [30] or disease progression [31]. Therefore, the evaluation of potential AD treatments that target other mechanisms is a major focus of current investigation and offers the greatest potential to enhance clinical management.
Considerable evidence supports the role of dysregulated glutamate in the pathophysiology of neurodegenerative disorders and excitotoxicity [32]. Therefore, glutamate NMDARs have emerged as key therapeutic targets for AD.
Glutamate is the main excitant neurotransmitter in the mammalian brain, implicated in the excitatory postsynaptic transmission through several ionotropic and metabotropic glutamate receptors. There are three classes of glutamategated channels and a group of G-protein coupled glutamate receptors (which cause mobilization of Ca2+ from internal stores) [33, 34] named according to their activating synthetic agonist: the α-amino 3-hydroxy 5-methyl 4-isoxazole-propionic acid (AMPA) activated receptors, kainate activated receptors, and the N-methyl D-aspartate (NMDA) receptors, have great importance in long-term adaptive processes [35]. Among these, the ion channels coupled to classical NMDARs are generally the most permeable to Ca2+ [36], that can in turn function as a second messenger in various signaling pathways.
NMDA glutamate receptors are abundant and ubiquitously distributed throughout the central nervous system (CNS), playing a critical role in synaptic plasticity and the cellular processes that underlie learning and memory [37]. Long-term potentiation (LTP) is a representation of neuronal synaptic plasticity that consists of a brief induction phase that elicits a long-lasting enhancement in signal transmission between two neurons. A stimulus into a presynaptic cell releases neurotransmitters, mostly glutamate, onto the postsynaptic cell membrane. There, glutamate binds to AMPA receptors in the postsynaptic membrane and triggers the influx of positively charged Na+ ions into the postsynaptic cell, causing a short-lived depolarization called excitatory postsynaptic potential. In synapses that exhibit NMDAR-dependent LTP, sufficient depolarization plus binding of glutamate can unblock NMDARs and relieve the Mg2+ blockade of the NMDAR [38] allowing Ca2+ to flow into the cell.
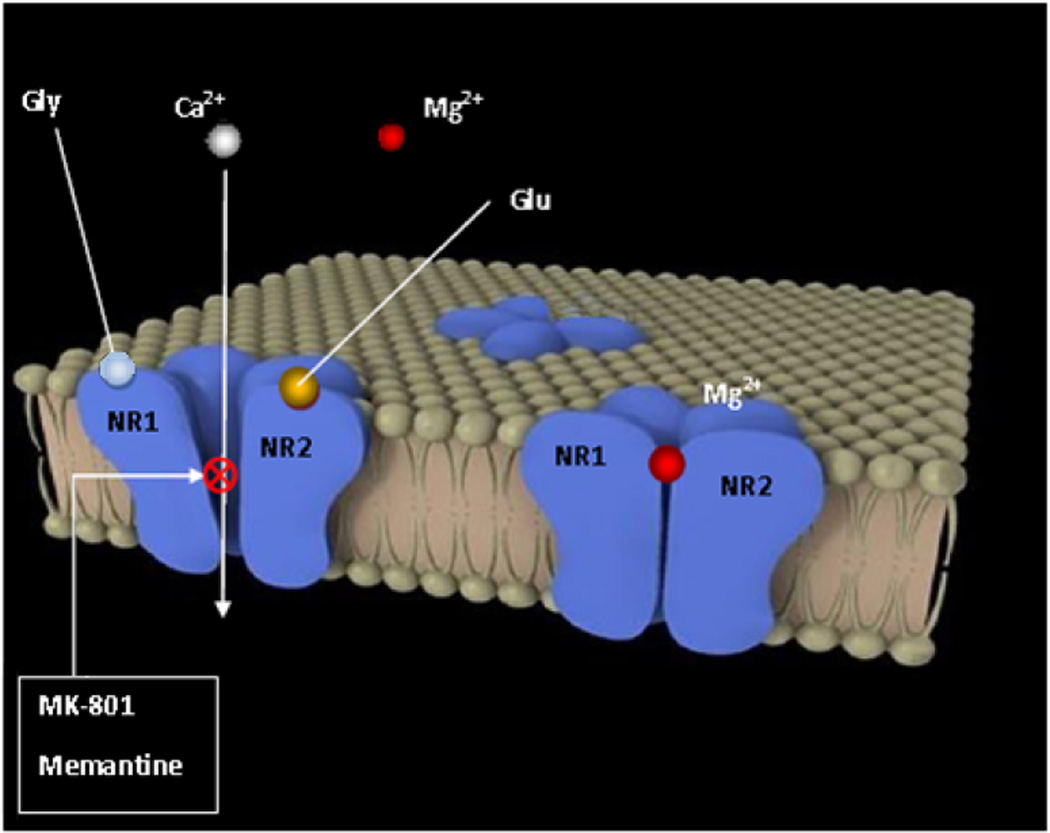
NMDARs are tetrameric complexes (see Fig. 2) composed by two NR1 subunits (eight splice isoforms, see Fig. 3) that form the channel itself, and two NR2A, NR2B, NR2C or NR2D subunits (derived from four independent genes) [39]. NR2 subunits modulate the characteristics of the NR1 channel; therefore each combination shows different physiological and pharmacological properties [34]. For example, in vitro recombinant NMDARs composed by NR1 and NR2B subunits result in much more sensitivity to the non-competitive antagonist ifenprodil than the NR1/NR2A combination [40]. Recently, NR3 subunits have been found [41], although NR1/NR3A or NR1/NR3B complexes are not activated by glutamate but rather elicit an excitatory response through glycine activation that is independent of Ca2+ influx. Glycine is a mandatory co-factor with glutamate for NMDA channel opening [42] for binding sites onto NR1 and NR2 subunits, respectively, although a more potent inducer than glycine, an uncommon amino acid, D-serine, has been found [43].
Fig. (2).
Depiction of the tetrameric NMDAR at rest (right) and activated after depolarization and binding of agonists glycine and glutamate, suppressing the magnesium channel blockade (left), where antagonists MK-801 and memantine have their allosteric binding site.
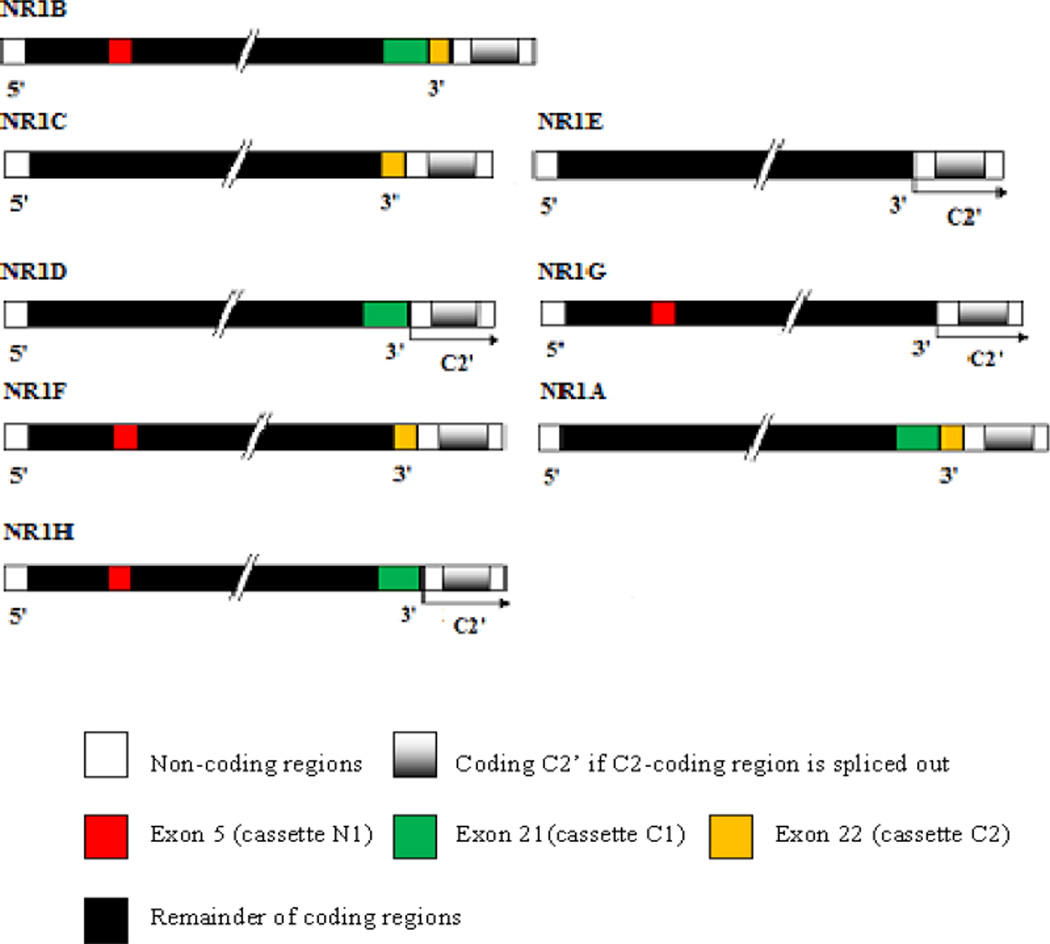
Fig. (3).
Schematic structure of eight NR1 receptor isoforms (NR1A–H). Exons 5, 21 and 22 encode three splice cassettes named N1, C1 and C2. Carboxy-terminals variants are generated by splicing out of cassettes C1 and/or C2; and amino-terminal variant, by splicing out of N1. If C2 is excised, the first stop codon is suppressed, resulting in a new open reading frame that encodes the sequence named C2’ [143].
NMDARs have been implicated as a mediator of neuronal injury associated with many neurological disorders including ischemia, epilepsy, brain trauma, dementia, and neurodegenerative disorders, such as PD [44]. Pathological elevations of glutamate levels and possibly other disturbances that alter resting membrane potential (e.g. impaired metabolism) can cause over-stimulation of the NMDAR that can lead to cellular dysfunction and death [45]. Under normal conditions of synaptic transmission, the NMDAR channel is blocked by Mg2+ sitting within the channel and only activated for brief periods of time. However, under pathological conditions, the normal block of the ion channel by Mg2+ is removed and abnormally enhances NMDAR activity [46]. Over-activation of the receptor causes an excessive amount of Ca2+ influx into a neuron to then trigger a variety of processes that can lead to necrosis or apoptosis [47]. The latter process includes Ca2+ overload of mitochondria and the activation of caspases and Ca2+-dependent activation of neuronal nitric oxide synthase (nNOS), leading to increased nitric oxide (NO) production [48]. Mitochondrial Ca2+ overload disables the membrane electrochemical gradient and, therefore, the ATP synthesis [49]. In addition, as consequence of electron chain failure, there is an excessive reactive oxygen species (ROS) production, such as the superoxide anion (O2−) that reacts with NO producing peroxynitrite (ONOO−) that can in turn oxidize lipids, proteins and DNA [50]. Interestingly, Ca2+-triggered neurotoxicity depends on the activation of a determinate pathway, since Ca2+ influx through L-type voltage-gated channels or non-NMDA receptors was not toxic to cells, while a similar Ca2+ load via NMDRs was neurotoxic [51]. Increased activity of nNOS is also associated with excitotoxic cell death [52]. The nNOS is physically tethered to NMDAR through the postsynaptic density protein of 95 kDa (PSD-95) [53] and is activated by Ca2+ entry via calmodulin [41]. In fact, increased levels of NO have been detected in animal models of stroke and neurodegenerative diseases [54, 55]. It has been shown that in PSD-95 mutant mice, the production of NO induced by Ca2+ entry via NMDR is blocked without affecting nNOS expression, indicating the specificity of NMDAR in neurotoxicity [53].
Importantly, elevations in extracellular glutamate are not necessary to invoke an excitotoxic mechanism. Excitotoxicity can come into play even with normal levels of glutamate if NMDAR activity is increased, e.g. when neurons are injured and, thus, become depolarized [56]. Much evidence shows that the AD pathogenic cascade includes an excitotoxic component. Application of Aβ has been shown to promote endocytosis of NMDARs in cortical neurons [57]. However, the specific effects of Aβ on excitotoxicity are not yet fully understood, and the exact role of NMDAR activation in AD remains unclear, although several studies have evidenced that Aβ could bind to NMDAR and increase Ca2+ influx into the cell [58].
Many potential neuroprotective agents block virtually all NMDAR activity and therefore, have unacceptable adverse effects, such as psychosis, nausea, vomiting, and a state called dissociative anesthesia, marked by catalepsy, amnesia, and analgesia. Neuronal cell death may accompany complete NMDAR blockade that may occur with high binding affinity of some drugs towards NMDARs [59]. Such possibilities dramatize the crucial role of the NMDAR in normal neuronal processes and explain why many NMDAR antagonists have disappointingly failed to advance in clinical trials for a number of neurodegenerative diseases. To be clinically acceptable, an anti-excitotoxic therapy must block excessive activation of the NMDAR while leaving normal function relatively intact to also avoid side effects. Drugs that simply compete with glutamate for the agonist binding site and block normal physiological functions do not meet this requirement. Both competitive glutamate and glycine antagonists, even although effective in preventing glutamate -mediated neurotoxicity, cause generalized inhibition of NMDAR activities [60]. Non-competitive antagonists, like MK-801, is an effective suppressor of excitotoxicity that acts allosterically (i.e. its binding site is other than the agonist’s) in the ion channel, but due to its high affinity, slow off-rate kinetics and a lesser voltage-dependent activity, blocks the channel for a clinically unacceptable period of time [60]. Such drugs have thus failed in clinical trials to date because of the development of adverse events occurring when the drugs are administered in their therapeutic range [59].
One mechanistic type of drug that can preferentially block higher, pathological levels of glutamate is an ‘uncompetitive’ antagonist that differs from non-competitive antagonists in that it requires receptor activation by an agonist before it can bind to a separate allosteric binding site. This uncompetitive mechanism of action, unlike competitive or non-competitive antagonists, yields a drug that blocks NMDAR channels preferentially when it is excessively open and prevents an excessive flux of calcium inside the cell [61]. Most importantly, it does not substantially accumulate in the channel to interfere with normal synaptic transmission [60]. Evidence suggests that memantine acts by such a mechanism, given that it is a low, moderate affinity, uncompetitive NMDAR antagonist.
MEMANTINE TREATMENT FOR ALZHEIMER’S DISEASE
Memantine (1-amino-3,5-dimethyladamantane), an amino-alkyl cyclohexane derivative was first synthesized by Eli Lilly and Company (Indianapolis, IN) and patented in 1968, as documented in the Merck Index, as a derivative of adamantine, an anti-influenza agent. It possesses a three-ring (adamantane) structure with a bridgehead amine (−NH2) that, under physiological conditions, carries a positive charge that binds at or near the Mg2+ site within the NMDAR-associated channel.
Memantine was relatively ineffective at blocking the low levels of receptor activity associated with normal neurological function but becomes increasingly effective at higher concentrations of glutamate associated with over-activation of NMDARs [60]. During normal synaptic activity, NMDA channels are open on average for only several milliseconds, and memantine is unable to act or accumulate within the channels; accordingly, synaptic activity continues largely unabated [56]. During prolonged activation of the receptor, however, as occurs under excitotoxic conditions, memantine becomes a highly effective blocker.
In addition to its low to moderate affinity, memantine blocks/unblocks the NMDAR ion channel with rapid kinetics and high voltage dependency [62]. These properties are thought to underlie the apparent ability of memantine to allow normal physiological function of the receptor while impairing pathological activation. Blocking NMDARs has also been reported to mitigate Aβ-induced degeneration of cholinergic neurons in the rat magnocellular nuclear basalis and in rat hippocampal neurons [63–65]. Preclinical data suggest that NMDAR-mediated excitotoxicity may be linked to the effects of abnormal Aβ deposition in AD. More recently, memantine has been found to lower levels of secreted APP and Aβ peptide levels in neuronal cultures and in APP-Swe+PS1 AD transgenic mice [66, 67]. This is important as Aβ accumulation is the precipitating event in AD that leads to synaptic loss among other pathological features. Aβ is known to alter neuronal structure by mechanisms that involve the NMDARand inhibition of the NMDAR can reduce the toxic effects of Aβ.
As a currently approved drug, memantine is indicated for the symptomatic treatment of moderate to severe AD, and has been associated with a moderate decrease in clinical deterioration in AD [68]. Its usefulness has been proved in several clinical trials in which it has shown little but statistically significant improvements [69–72], also assessed by brain imaging [73]. Several systematic reviews of randomized controlled trials have established that memantine has small but helpful actions on cognition, mood, behavior, and the ability to perform daily activities in moderate to severe AD [74–76] nevertheless, the action of the drug in mild to moderate AD remains largely unknown. Importantly, memantine appears capable of achieving its pharmacological actions in a clinically well-tolerated manner and does not show the adverse effects typically associated with high-affinity NMDA-blockers. In trials reporting adverse effects, the primary memantine-induced adverse actions found were infrequent and comparable to placebo such as dizziness, occasional restlessness/agitation, constipation, ocular effects (cataracts, conjunctivitis), nausea, dyspnea, confusion, headache, fatigue, rash, diarrhea and urinary incontinence [77, 78]. On the basis of successful clinical trials, the use of memantine in the modulation of glutamatergic function may therefore represent a useful strategy for the treatment of AD. Furthermore, in vitro and clinical data indicate no adverse interactions between the approved cholinesterase inhibitors and memantine [79, 80].
Because memantine has exhibited efficacy and safety in placebo-controlled trials in patients with moderate to severe AD, the combination of memantine and various cholinesterase inhibitors appears well tolerated and they seem act synergistically due to their distinct mechanisms of action [81–85].
At present, a series of second generation memantine derivatives are currently in development and may have even greater neuroprotective properties than memantine [9, 86]. Whether and how these drugs translate to clinical medicine are awaited with interest.
MEMANTINE TREATMENT FOR VASCULAR DEMENTIA
Memantine has also been described as effective and well tolerated for the treatment of mild to moderate VD in several randomized clinical trials [87–89]. VD ranks second in prevalence as a form of dementia after AD [1], and they often co-exist. It is not infrequent that confusion can occur between both disorders, due to the similarity of their clinical symptoms. VD is a degenerative cerebrovascular disease that leads to a progressive decline in memory and cognitive functioning. The root of this issue relates to a chronic reduced cerebral blood flow (vascular insufficiency) carrying oxygen and nutrients to the brain that may be impaired by a strategic infarct (ischemia) or small (silent) multi-infarcts, hemorrhagic cerebrovascular disease, or can derive from small vessel disease (including lacunar lesions and Binswanger’s disease) [90]. Apart from acute ischemia from embolic or atherothrombotic large vessels occlusion, ischemic-hypoxic brain lesions also can be originated by cardiovascular diseases, such as hypertension or diabetes, which cause a narrowing of the lumen of small vessels [91]. In addition, senile arteriolosclerosis produces tortuosity and elongation of arterioles [92].
Since it may be partly preventable, VD needs to be differentiated from other causes of dementia such as AD, Lewy body-type dementia and PD. Special attention has to be given when attempting to make a differential diagnosis, to the following steps that may lead to a diagnosis of VD [93]:
Detection of vascular risk factors, including hypertension, diabetes, orthostatic hypotension, smoking, cardiac arrhythmias and heart failure.
Examination of the cardiovascular system that may be a cause of thromboembolism that results in transient ischemic episodes and a history of strokes.
Neurological and psychometric assessments to evidence particular neurological deficits.
A search for treatable factors that might lead to VD, such as hypothyroidism, neurosyphilis, vitamin B12 deficiency, cerebral vasculitis or frontal lobe tumors.
Oxygen and glucose deprivation are followed by an elevation of extracellular glutamate both in ischemic brain damage and traumatic brain injury [50] that results in consequent NMDR overactivation and massive Ca2+ influx. Failure of astrocyte functions also has been reported [94] such as maintenance of blood-brain barrier (BBB) cells in cerebral microvasculature and endothelial permeability [95]. Disruption of tight junctions among endothelial cells, degradation of the basal lamina and extracellular matrix by metalloproteinases-2 and −9 are involved in BBB breakdown and cerebral hemorrhagia [96–98]. In addition, adhesion and transmigration of leukocyte occurs, leading to activation of an inflammatory response [99]. For a review of downstream processes after calcium entry in ischemia, see Ref. [48].
In general, cerebral lesions after ischemic injury begin with an initial reversible stage where neurons finally become necrotic [100]. The cells of the ischemic core undergo anoxic depolarizations that expand to the penumbral region and a lack of energy that leads to necrosis [101]. Since the ischemic area grows as the number of peri-infarct depolarizations increases, the extension of severe damaged area can be attenuated by blocking NMDAR-mediated depolarizations [102].
PARKINSON’S DISEASE PATHOGENESIS, NMDARS, GLUTAMATERGIC TRANSMISSION AND MEMANTINE TREATMENT
Parkinson’s Disease Pathology, NMDARs and Glutamatergic Transmission
PD is the second most frequent progressive-type neurodegenerative disorder after AD [103] and represents, like AD, a large health burden to society. Approximately 1% of the population over 60 years of age is affected [104]. Classical clinical symptoms include tremors; bradykinesia, or slowness of movement; and rigidity, or akinesia.
The primary underlying pathology of PD is the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) that innervates the striatum. At post-mortem examination, the depletion frequently exceeds 90% [105] with consequent loss of neuronal systems responsible for motor functions. Besides, cell death is not limited to dopaminergic neurons in SNpc and can expand to other areas of the brain, leading to extensive neuronal death. It is not infrequent to find dementia in patients afflicted by PD. A further archetypal hallmark is the formation of intracytoplasmic inclusions, termed Lewy bodies, in remaining neurons [106].
To date, no curative treatment for PD exists but symptomic control can be achieved. The most effective treatments are based on the replacement of dopamine (DA) loss using either the precursor of DA, L-dihydroxyphenylalanine (L-dopa), or agonist-mediated stimulators of DA receptors, epitomized by pramipexole or ropinirole. Essentially, all patients require L-dopa at some stage of disease progression, in spite of its adverse effects, such as the “wearing-off” phenomenon – relating to the shortening of sustainable pharmacological activity [107] as occurs when the symptoms of PD, attenuated by the treatment, become more intense prior to the next expected dose. Albeit that L-dopa is associated with dyskinesia or diminished voluntary movements and the presence of involuntary movements, it must be recognized that, since L-dopa’s clinical introduction, survival with PD has been considerably prolonged [108].
NMDARs are very abundant in the striatum [109], comprised by putamen and caudate nucleus, where they regulate the release of neurotransmitters like γ-aminobutyric acid (GABA) and ACh [110]. NMDARs downstream of the striatum also participate in modulating the activities of basal ganglia circuit and are present in the subthalamic nucleus (STN), globus pallidus internus (GPi) [111], and cerebral cortex [112]. The activity of NMDARs is largely regulated by dopaminergic afferents. D1 receptor agonists elicit a fast enhancement of NMDA-induced depolarization of striatal cells, whereas D2 receptor agonists attenuate this [113].
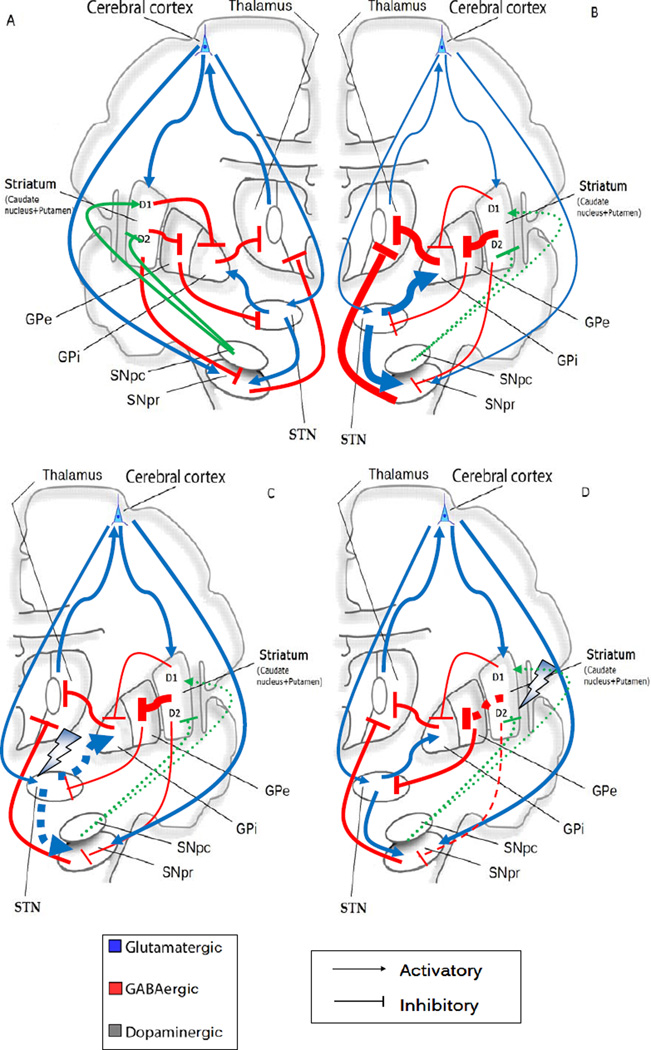
Execution of voluntary movement starts with the cortex (executive) signaling the caudate and putamen via glutamatergic projections or from the SNpc via dopamine. The Striatum then sends inhibitory GABAergic signals to GPi leads to disinhibition of the thalamic ventral anterior (VA). This leads to stimulatory signaling of the motor cortex by the thalamic VA and initiation of movement. As described, PD is characterized by depletion of dopaminergic neurons, leading to a disinhibition of striatal neurons that have inhibitory D2 dopaminergic receptors and project to the globus pallidus externus (GPe), and to a decrease of striatal neuron activity projecting to the GPi and the substantia nigra pars reticulata (SNpr) that have D1 excitatory dopaminergic receptors [114]. All these striatal efferent pathways are mediated by GABA and are inhibitory so that GPe projection to the STN (also inhibitory) is reduced. Therefore, the suppression of dopaminergic modulation disinhibits STN neurons, rendering them overactive [115]. The enhanced activity of excitatory glutamatergic STN projections to GPi and SNpr (besides lack of inhibitory input from the GPe stated above) further enhance the activity of their GABAergic neurons [116]. Spontaneous movement is reduced by inhibiting the activity of thalamic glutamatergic and excitatory VA projections VA to the motor frontal cortex (see Fig. 4A and B). Acetylcholinergic neurons in the brainstem and basal forebrain areas, are regulated by prefrontal cortex glutamatergic projections, and seem to be of special relevance in modulating motor, emotional and mnemonic functions [117], therefore a decrease of thalamocortical input entails a deficit of ACh, leading to a decline in voluntary movements. Likewise, the striatum and SNpc can receive glutamatergic excitatory input from the neocortex [118].
Fig. (4).
Classical model of normal activity of the basal ganglia and malfunctioning in PD (reviewed in Ref. [114]). Thin arrows indicate downregulation and thick arrows upregulation. Blue arrows show glutamatergic activatory efferents, red arrows indicate inhibitory GABAergic efferents and green arrows, activatory/inhibitory projections. (A) The ‘direct pathway’ is comprised of striatal neurons with D1 activatory dopaminergic receptors and their GABAergic efferent projections to the globus pallidus internus (GPi) which together the SNpr transmit inhibitory signals via GABAergic output to the thalamic ventral anterior (VA) nucleus. The ‘indirect pathway’ is comprised of striatal neurons with D2 inhibitory dopaminergic receptors. The striatum (composed of caudate and putamen) projects GABAergic output to the globus pallidus externus (GPe). From this point, they synapse to the nucleus subthalamicus (STN), from which glutamatergic activatory projections reach the GPi and the substantia nigra (SNpc/SNpr). The subthalamic nucleus also gets excitatory input directly from the cortex and induces the GPi to increase GABA release in the VA. While GPe keeps it in check, the SNpc dopamine binds D2 receptors to inhibit this pathway, blocking the inhibition of the subthalamic circuit by GPe. Additionally, the striatum and the SNpc receive glutamatergic efferents from the neocortex.
(B) Loss of dopaminergic afferents (broken green arrows) entails a dis-repression of striatal D2 neurons leads to over-activity of their GABAergic projections to the GPe, and this in turn decreases its GABAergic efferent activity to the STN. As a consequence, STN glutamatergic projections to the GPi render over-active, increasing GABAergic output from the GPi to the VA. On the contrary, the D1 striatal neurons are underactive, therefore the GABAergic output to the GPi and to the SNpr are reduced and consequently the GABAergic output from both to the VA is increased. As a consequence in both cases, the loss of dopaminergic projections causes a failure to desinhibit the thalamocortical output, leading to bradykinesia, a typical symptom in PD. (C) (D) Possible targets at the basal ganglia level of NMDAR antagonists amantadine and memantine. Broken arrows mean suppression of output projections. A putative target is the STN (C), overactive in PD by blocking the subthalamic stimulation of GPi and a normalization of downstream thalamocortical connections. Other target could be the indirect pathway from the striatum (D), triggering a dis-repression of SNpr and leading to a normalization of STN activity and therefore a thalamic-cortical neuron well-functioning.
Accordingly, increased activity of STN neurons has been described in monkeys treated with 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine (MPTP), a neurotoxin that induces parkinsonism in animal models [119], and NMDAR antagonists have been reported to provide potent neuroprotective action [120]. NMDARs localized to the STN also play a role in sustaining pathological hyperactivity observed in a 6-hydroxydopamine rat model of parkinsonism, and the infusion of a NMDAR antagonist into the STN normalized the activity of the basal ganglia [121].
NMDARs are also found on dopaminergic neurons in the SNpc [122]. Increased glutamatergic input to dopaminergic neurons through NMDARs might accelerate the degenerative process [118]. It has been reported that NMDAR antagonists elevate striatal DA release in vivo [123–125] and, given this, would likely be beneficial in PD. In testing this conjecture, the initial class of selective antagonists’ studies in parkinsonism were the phenylethanolamines. One of them, ifenprodil, shows anti-parkinsonian activity in reserpine-treated rats and MPTP-treated monkeys [126].
The effect of an anti-PD medication can be enhanced by NMDAR antagonists. In animal models of PD, NMDAR antagonists have shown a potentiation of the anti-parkinsonian effect of L-dopa and locomotion [109]. Some anticholinergic drugs are also non-competitive antagonists of the NMDAR and, at therapeutic concentrations, may interact with NMDARs and palliate PD [127]. Just as anticholinergics are able to work as NMDA antagonists, NMDA antagonists also can function as anticholinergic agents [128] and normalize the glutamatergic control of corticostriatal ACh release. In the striatum, NMDA stimulation enhances the release of ACh, and antagonists can effectively inhibit this release [110].
Since oxidative products of DA play a role in dopaminergic cell death [129], the use of NMDA antagonists could additionally allow for a decrease in L-dopa dosage and, thereby, diminish any potential oxidative damage. Other NMDA antagonists, such as dextromethorphan, have been reported to suppress dyskinesia in PD patients, but adverse effects (primarily drowsiness) at higher doses would likely limit such a treatment strategy [130]. On the other hand, amantadine (1-amino adamantine) a non-selective NMDA antagonist used to treat PD, provides mild L-dopa-induced anti-dyskinetic benefit with a moderate degree of NMDA antagonist activity [131]. Furthermore, in a large retrospective study, amantadine was associated with an increased lifespan in patients with PD, suggesting that it may have neuroprotective properties [132].
Memantine Treatment for Parkinson’s Disease
Given that memantine carries out its therapeutic action by targeting the glutamatergic transmission, and the role of NMDARs in basal ganglia for the development of PD symptoms, memantine has also been tested in parkinsonian patients with a degree of moderate success.
Although memantine’s chemical structure is related to amantadine’s, and they act in a somewhat similar pharmacological fashion, memantine does not appear to share the anti-dyskinetic activity of amantadine [133]. However, like DA agonists and other NMDA antagonists, memantine is able to reverse neuroleptic-induced catalepsy [134].
Using patch-clamp techniques, it has been reported that memantine blocks the NMDA ion channel [135], binds to the MK-801 binding site of the NMDAR [136], and decreases NMDA-induced membrane currents. The mechanism of action postulated is a normalization of the corticostriatal glutamatergic activity and/or the subthalamopallidal pathways that are overactive in PD (see Fig. 4C and D). Contrasting with amantadine, in which anti-dyskinetic activity could be rationalized by blocking subthalamic activity, the anti-parkinsonian and synergistic effect of memantine could be due to the inhibition of glutamatergic transmission in the striatum, whereby striatonigral neurons are GABAergic and inhibitory, causing a decreased inhibition of nigrostriatal dopaminergic neurons in SNpc and thereby an elevation in DA release [137].
To investigate the primary efficacy of memantine, a double-blind crossover exploratory trial was designed [133], in which 12 patients with idiopathic PD were randomized to memantine or placebo during two weeks in an escalated dosage from 10 mg/day to 30 mg/day at day 7, and after this time, a single dose of L-dopa was administrated to each arm. Five patients were taking concomitant PD medication (but not amantadine). As expected, a clear anti-parkinsonian activity was observed in terms of counteracting bradykinesia and resting tremor. A synergistic enhancement of L-dopa and memantine seemed evident with motor function. Side effects, mainly drowsiness and nausea, occurred with a similar frequency in both groups.
To further assess the efficacy of memantine, a randomized controlled study was performed with patients suffering from dementia with Lewy bodies or PD dementia that resulted in an improvement in the majority of variables stated in the clinical global impression of change test for the memantine group compared to placebo [138, 139] and a better response assessed in other useful rating scales [140], while the proportion of adverse events was similar to placebo and improved L-dopa-related dyskinesia and the “off” effect [141].
CONCLUDING REMARKS
Several studies show how memantine positively impacts cognition and, hence, they lend credence to the hypothesis that neurotoxicity of glutamatergic overstimulation is involved in dementia. The neuroprotective properties of memantine have also been demonstrated in several in vitro experimental settings, albeit further studies are needed to examine whether memantine treatment and cholinergic treatments could ultimately prove to be complementary or even synergistic. Memantine has also shown efficacy in PD, revealing itself as a potentially promising new therapeutic option. Importantly, as memantine treatment is generally well tolerated, combining it with other therapies is a valuable and feasible option both with AD and PD. However, although memantine was approved for treating mild to moderate AD, its results are modest; therefore, a second generation of adamantane-based drugs are being designed in the hope of improving its clinical efficacy. In conclusion, given the wealth of data on NMDAR activity in AD, VD, and PD, memantine and other drugs that emerge in the NMDAR antagonist class are likely to have an increasingly significant role to play in the future treatment of these diseases.
Acknowledgments
This work was supported in part by Radiology Department of Brigham and Women’s Hospital (BWH) and the Intramural Research Program, National Institute on Aging (NIA). Jack T. Rogers is a recipient of Zenith Fellows award of Alzheimer’s Association. We want to thank Ms. Kim Lawson at BWH Radiology Department for her editing of our manuscript. This work was also supported by Grants from Alzheimer’s Association and NIH to DKL.
LIST OF ABBREVATIONS
- Ach
Acetylcholine
- AD
Alzheimer’s disease
- ADDLs
Aβ-derived diffusible ligands
- Amantadine
1-Amino adamantine
- AMPA
α-Amino 3-hydroxy 5-methyl 4-isoxazolepropionic acid
- APP
Amyloid precursor protein
- Aβ
Beta amyloid peptide
- BBB
Blood-brain barrier
- CNS
Central nervous system
- DA
Dopamine
- FAD
Familial Alzheimer’s disease
- GABA
γ-Aminobutyric acid
- GPe
Globus pallidus externus
- GPi
Globus pallidus internus
- L-dopa
L-dihydroxyphenylalanine
- LTP
Long-term potentiation
- Memantine
1-Amino-3,5-dimethyladamantane
- MPTP
1-Methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine
- NFTs
Neurofibrillary tangles
- NMDA
N-Methyl D-Aspartate
- NOS and NO
Nitric oxide synthase and nitric oxide
- PCP
Phencyclidine
- PD
Parkinson’s disease
- PS1 and PS2
Presenilin-1 and Presenilin-2 protein
- SNpc
Substantia nigra pars compacta
- SNpr
Substantia nigra pars reticulate
- STN
Subthalamic nucleus
- VD
Vascular dementia
Footnotes
CONFLICT OF INTEREST
The authors declare that they do not have any conflicts of interest.
REFERENCES
- 1.Care TSCoTAiH. Dementia - Etiology and Epidemiology. A Systematic Review. 2008;1:755. [Google Scholar]
- 2.Lahiri DK, Rogers JT, Greig NH, Sambamurti K. Rationale for the development of cholinesterase inhibitors as anti-Alzheimer agents. Curr Pharm Des. 2004;10(25):3111–3119. doi: 10.2174/1381612043383331. [DOI] [PubMed] [Google Scholar]
- 3.Delacourte A. [From physiopathology to treatment of Alzheimer’s disease] Rev Neurol (Paris) 2006;162(10):909–912. doi: 10.1016/s0035-3787(06)75099-8. [DOI] [PubMed] [Google Scholar]
- 4.Joray S, Herrmann F, Mulligan R, Schnider A. Mechanism of disorientation in Alzheimer’s disease. Eur Neurol. 2004;52(4):193–197. doi: 10.1159/000082034. [DOI] [PubMed] [Google Scholar]
- 5.Esposito F, Rochat L, Van der Linden AC, Lekeu F, Quittre A, Charnallet A, et al. Apathy and executive dysfunction in Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(2):131–137. doi: 10.1097/WAD.0b013e3181c9c168. [DOI] [PubMed] [Google Scholar]
- 6.Scarmeas N, Brandt J, Blacker D, Albert M, Hadjigeorgiou G, Dubois B, et al. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol. 2007;64(12):1755–1761. doi: 10.1001/archneur.64.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–1891. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 8.Alzheimer’s Association. 2011 Alzheimer’s Disease Facts and Figures. 2011 doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Lipton SA. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res. 2005;2(2):155–165. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Eu J, Washburn M, Gong T, Chen HS, James WL, et al. The pharmacology of aminoadamantane nitrates. Curr Alzheimer Res. 2006;3(3):201–204. doi: 10.2174/156720506777632808. [DOI] [PubMed] [Google Scholar]
- 11.Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5(1):83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 12.Stahl SM. The new cholinesterase inhibitors for Alzheimer’s disease, Part 2: illustrating their mechanisms of action. J Clin Psychiatry. 2000;61(11):813–814. doi: 10.4088/jcp.v61n1101. [DOI] [PubMed] [Google Scholar]
- 13.Herholz K. Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2008;35(1):S25–S29. doi: 10.1007/s00259-007-0699-4. [DOI] [PubMed] [Google Scholar]
- 14.Randall AD, Witton J, Booth C, Hynes-Allen A, Brown JT. The functional neurophysiology of the amyloid precursor protein (APP) processing pathway. Neuropharmacology. 2010;59(4–5):243–267. doi: 10.1016/j.neuropharm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62(11):1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 16.Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008;13(4):359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- 17.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9(10):1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 18.Lee HP, Zhu X, Casadesus G, Castellani RJ, Nunomura A, Smith MA, et al. Antioxidant approaches for the treatment of Alzheimer’s disease. Expert Rev Neurother. 2010;10(7):1201–1208. doi: 10.1586/ern.10.74. [DOI] [PubMed] [Google Scholar]
- 19.Arima K. Ultrastructural characteristics of tau filaments in tauopathies: immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology. 2006;26(5):475–483. doi: 10.1111/j.1440-1789.2006.00669.x. [DOI] [PubMed] [Google Scholar]
- 20.Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3(1):75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 21.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT. Redox-active metals, oxidative stress, and Alzheimer’s disease pathology. Ann NY Acad Sci. 2004;1012:153–163. doi: 10.1196/annals.1306.012. [DOI] [PubMed] [Google Scholar]
- 23.Yin YI, Bassit B, Zhu L, Yang X, Wang C, Li YM. {gamma}-Secretase Substrate Concentration Modulates the Abeta42/Abeta40 Ratio: IMPLICATIONS FOR ALZHEIMER DISEASE. J Biol Chem. 2007;282(32):23639–23644. doi: 10.1074/jbc.M704601200. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien RJ, Wong PC. Amyloid Precursor Protein Processing and Alzheimers Disease. Annu Rev Neurosci. 2010 Jul 21; doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao CM, Yam AY, Wang X, Magdangal E, Salisbury C, Peretz D, et al. Abeta40 oligomers identified as a potential biomarker for the diagnosis of Alzheimer’s disease. PLoS One. 2010;5(12):e15725. doi: 10.1371/journal.pone.0015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kung MP, Skovronsky DM, Hou C, Zhuang ZP, Gur TL, Zhang B, et al. Detection of amyloid plaques by radioligands for Abeta40 and Abeta42: potential imaging agents in Alzheimer’s patients. J Mol Neurosci. 2003;20(1):15–24. doi: 10.1385/JMN:20:1:15. [DOI] [PubMed] [Google Scholar]
- 27.Kirkitadze MD, Kowalska A. Molecular mechanisms initiating amyloid beta-fibril formation in Alzheimer’s disease. Acta Biochim Pol. 2005;52(2):417–423. [PubMed] [Google Scholar]
- 28.Huang X, Atwood CS, Moir RD, Hartshorn MA, Tanzi RE, Bush AI. Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer’s Abeta peptides. J Biol Inorg Chem. 2004;9(8):954–960. doi: 10.1007/s00775-004-0602-8. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 30.Forette F, Hauw JJ. [Alzheimer’s disease: from brain lesions to new drugs] Bull Acad Natl Med. 2008;192(2):363–378. discussion 78–80. [PubMed] [Google Scholar]
- 31.Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med. 2007;4(11):e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45(5):583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 34.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 35.D’Angelo E, Rossi P. Integrated regulation of signal coding and plasticity by NMDA receptors at a central synapse. Neural Plast. 1998;6(3):8–16. doi: 10.1155/NP.1998.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozawa s. Permeation of calcium through glutamate receptor channels. Semin Neurosci. 1996;8(5):261–269. [Google Scholar]
- 37.Castellano C, Cestari V, Ciamei A. NMDA receptors and learning and memory processes. Curr Drug Targets. 2001;2(3):273–283. doi: 10.2174/1389450013348515. [DOI] [PubMed] [Google Scholar]
- 38.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 39.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 40.Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48(5):841–848. [PubMed] [Google Scholar]
- 41.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460(2):525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 43.Wolosker H. D-serine regulation of NMDA receptor activity. Sci STKE. 2006;2006(356):pe41. doi: 10.1126/stke.3562006pe41. [DOI] [PubMed] [Google Scholar]
- 44.Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5:1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- 45.Greene JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Prog Neurobiol. 1996;48(6):613–634. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 46.Zeevalk GD, Nicklas WJ. Evidence that the loss of the voltage-dependent Mg2+ block at the N-methyl-D-aspartate receptor underlies receptor activation during inhibition of neuronal metabolism. J Neurochem. 1992;59(4):1211–1220. doi: 10.1111/j.1471-4159.1992.tb08430.x. [DOI] [PubMed] [Google Scholar]
- 47.Zipfel GJ, Babcock DJ, Lee JM, Choi DW. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J Neurotrauma. 2000;17(10):857–869. doi: 10.1089/neu.2000.17.857. [DOI] [PubMed] [Google Scholar]
- 48.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34(4–5):325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 50.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61(6):657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sattler R, Charlton MP, Hafner M, Tymianski M. Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J Neurochem. 1998;71(6):2349–2364. doi: 10.1046/j.1471-4159.1998.71062349.x. [DOI] [PubMed] [Google Scholar]
- 52.Brown GC. Nitric oxide and neuronal death. Nitric Oxide. 2010;23(3):153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284(5421):1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 54.Chung KK, David KK. Emerging roles of nitric oxide in neurodegeneration. Nitric Oxide. 2010;22(4):290–295. doi: 10.1016/j.niox.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Taffi R, Nanetti L, Mazzanti L, Bartolini M, Vignini A, Raffaelli F, et al. Plasma levels of nitric oxide and stroke outcome. J Neurol. 2008;255(1):94–98. doi: 10.1007/s00415-007-0700-y. [DOI] [PubMed] [Google Scholar]
- 56.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1(1):101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, et al. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30(17):5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molnar Z, Soos K, Lengyel I, Penke B, Szegedi V, Budai D. Enhancement of NMDA responses by beta-amyloid peptides in the hippocampus in vivo. Neuroreport. 2004;15(10):1649–1652. doi: 10.1097/01.wnr.0000134471.06244.d2. [DOI] [PubMed] [Google Scholar]
- 59.Wood PL. NMDA antagonists for stroke and head trauma: current status. Expert Opin Investig Drugs. 1998;7(9):1505–1508. doi: 10.1517/13543784.7.9.1505. [DOI] [PubMed] [Google Scholar]
- 60.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97(6):1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 61.Robinson DM, Keating GM. Memantine: a review of its use in Alzheimer’s disease. Drugs. 2006;66(11):1515–1534. doi: 10.2165/00003495-200666110-00015. [DOI] [PubMed] [Google Scholar]
- 62.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38(6):735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 63.Wenk GL, Danysz W, Mobley SL. MK-801, memantine and amantadine show neuroprotective activity in the nucleus basalis magnocellularis. Eur J Pharmacol. 1995;293(3):267–270. doi: 10.1016/0926-6917(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 64.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by beta-amyloid(1–40) Brain Res. 2002;958(1):210–221. doi: 10.1016/s0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- 65.Nyakas C, Granic I, Halmy LG, Banerjee P, Luiten PG. The basal forebrain cholinergic system in aging and dementia. Rescuing cholinergic neurons from neurotoxic amyloid-beta42 with memantine. Behav Brain Res. 2011;221(2):594–603. doi: 10.1016/j.bbr.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 66.Ray B, Banerjee PK, Greig NH, Lahiri DK. Memantine treatment decreases levels of secreted Alzheimer’s amyloid precursor protein (APP) and amyloid beta (A beta) peptide in the human neuroblastoma cells. Neurosci Lett. 2010;470(1):1–5. doi: 10.1016/j.neulet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alley GM, Bailey JA, Chen D, Ray B, Puli LK, Tanila H, et al. Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice. J Neurosci Res. 2010;88(1):143–154. doi: 10.1002/jnr.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossi S. Australian medicines handbook 2006. 2006 [Google Scholar]
- 69.Aguera-Ortiz LF. [Memantine in the pharmacologic treatment of moderately severe to severe Alzheimer’s disease in Spain (MEMORY study)] Rev Neurol. 2010;51(9):525–534. [PubMed] [Google Scholar]
- 70.Grossberg GT, Pejovic V, Miller ML, Graham SM. Memantine therapy of behavioral symptoms in community-dwelling patients with moderate to severe Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;27(2):164–172. doi: 10.1159/000200013. [DOI] [PubMed] [Google Scholar]
- 71.Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14(8):704–715. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 72.van Dyck CH, Tariot PN, Meyers B, Malca Resnick E. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21(2):136–143. doi: 10.1097/WAD.0b013e318065c495. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt R, Ropele S, Pendl B, Ofner P, Enzinger C, Schmidt H, et al. Longitudinal multimodal imaging in mild to moderate Alzheimer disease: a pilot study with memantine. J Neurol Neurosurg Psychiatry. 2008;79(12):1312–1317. doi: 10.1136/jnnp.2007.141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev. 2005;(3):CD003154. doi: 10.1002/14651858.CD003154.pub3. [DOI] [PubMed] [Google Scholar]
- 75.Emre M, Mecocci P, Stender K. Pooled analyses on cognitive effects of memantine in patients with moderate to severe Alzheimer’s disease. J Alzheimers Dis. 2008;14(2):193–199. doi: 10.3233/jad-2008-14207. [DOI] [PubMed] [Google Scholar]
- 76.Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry. 2008;69(3):341–348. doi: 10.4088/jcp.v69n0302. [DOI] [PubMed] [Google Scholar]
- 77.Farlow MR, Graham SM, Alva G. Memantine for the treatment of Alzheimer’s disease: tolerability and safety data from clinical trials. Drug Saf. 2008;31(7):577–585. doi: 10.2165/00002018-200831070-00003. [DOI] [PubMed] [Google Scholar]
- 78.Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother. 2004;2(4):303–312. doi: 10.1016/j.amjopharm.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Periclou AP, Ventura D, Sherman T, Rao N, Abramowitz WT. Lack of pharmacokinetic or pharmacodynamic interaction between memantine and donepezil. Ann Pharmacother. 2004;38(9):1389–1394. doi: 10.1345/aph.1D638. [DOI] [PubMed] [Google Scholar]
- 80.Wenk GL, Quack G, Moebius HJ, Danysz W. No interaction of memantine with acetylcholinesterase inhibitors approved for clinical use. Life Sci. 2000;66(12):1079–1083. doi: 10.1016/s0024-3205(00)00411-2. [DOI] [PubMed] [Google Scholar]
- 81.Grossberg GT. Rationalizing therapeutic approaches in Alzheimer’s disease. CNS Spectr. 2005;10(11 Suppl 18):17–21. doi: 10.1017/s109285290001419x. [DOI] [PubMed] [Google Scholar]
- 82.Rountree SD, Chan W, Pavlik VN, Darby EJ, Siddiqui S, Doody RS. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res Ther. 2009;1(2):7. doi: 10.1186/alzrt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao X, Marszalec W, Toth PT, Huang J, Yeh JZ, Narahashi T. In vitro galantamine-memantine co-application: mechanism of beneficial action. Neuropharmacology. 2006;51(7–8):1181–1191. doi: 10.1016/j.neuropharm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 84.Feldman HH, Schmitt FA, Olin JT. Activities of daily living in moderate-to-severe Alzheimer disease: an analysis of the treatment effects of memantine in patients receiving stable donepezil treatment. Alzheimer Dis Assoc Disord. 2006;20(4):263–268. doi: 10.1097/01.wad.0000213859.35355.59. [DOI] [PubMed] [Google Scholar]
- 85.Schmitt FA, van Dyck CH, Wichems CH, Olin JT. Cognitive response to memantine in moderate to severe Alzheimer disease patients already receiving donepezil: an exploratory reanalysis. Alzheimer Dis Assoc Disord. 2006;20(4):255–262. doi: 10.1097/01.wad.0000213860.35355.d4. [DOI] [PubMed] [Google Scholar]
- 86.Albensi BC, Ilkanich E. Open-channel blockers of the NMDA receptor complex. Drug News Perspect. 2004;17(9):557–562. doi: 10.1358/dnp.2004.17.9.872569. [DOI] [PubMed] [Google Scholar]
- 87.Orgogozo JM, Rigaud AS, Stoffler A, Mobius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke. 2002;33(7):1834–1839. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 88.Wilcock G, Mobius HJ, Stoffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500) Int Clin Psychopharmacol. 2002;17(6):297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Mobius HJ, Stoffler A. Memantine in vascular dementia. Int Psychogeriatr. 2003;15(1):207–213. doi: 10.1017/S1041610203009219. [DOI] [PubMed] [Google Scholar]
- 90.Vicenzini E, Ricciardi MC, Altieri M, Puccinelli F, Bonaffini N, Di Piero V, et al. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial Doppler study. Eur Neurol. 2007;58(2):84–89. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- 91.Roman GC. Vascular dementia revisited: diagnosis, pathogenesis, treatment, and prevention. Med Clin North Am. 2002;86(3):477–499. doi: 10.1016/s0025-7125(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 92.van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Brain. Pt 2. 114: 1991. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces; pp. 761–774. [DOI] [PubMed] [Google Scholar]
- 93.Amar K, Wilcock G. Vascular dementia. BMJ. 1996;312(7025):227–231. doi: 10.1136/bmj.312.7025.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mori T, Tateishi N, Kagamiishi Y, Shimoda T, Satoh S, Ono S, et al. Attenuation of a delayed increase in the extracellular glutamate level in the peri-infarct area following focal cerebral ischemia by a novel agent ONO-2506. Neurochem Int. 2004;45(2–3):381–387. doi: 10.1016/j.neuint.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200(6):629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282(4):H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289(2):H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 98.Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35(4):998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66(3):232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 100.Garcia JH, Liu KF, Ho KL. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke. 1995;26(4):636–642. doi: 10.1161/01.str.26.4.636. discussion 43. [DOI] [PubMed] [Google Scholar]
- 101.Ohta K, Graf R, Rosner G, Heiss WD. Calcium ion transients in peri-infarct depolarizations may deteriorate ion homeostasis and expand infarction in focal cerebral ischemia in cats. Stroke. 2001;32(2):535–543. doi: 10.1161/01.str.32.2.535. [DOI] [PubMed] [Google Scholar]
- 102.Lu XC, Williams AJ, Wagstaff JD, Tortella FC, Hartings JA. Effects of delayed intrathecal infusion of an NMDA receptor antagonist on ischemic injury and peri-infarct depolarizations. Brain Res. 2005;1056(2):200–208. doi: 10.1016/j.brainres.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 103.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 104.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 105.Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci. 2001;21(17):6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Olivares D, Huang X, Branden L, Greig NH, Rogers JT. Physiological and Pathological Role of Alpha-synuclein in Parkinson’s Disease Through Iron Mediated Oxidative Stress; The Role of a Putative Iron-responsive Element. Int J Mol Sci. 2009;10(3):1226–1260. doi: 10.3390/ijms10031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guttman M, Kish SJ, Furukawa Y. Current concepts in the diagnosis and management of Parkinson’s disease. CMAJ. 2003;168(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- 108.Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD. Parkinsonism in Ontario: increased mortality compared with controls in a large cohort study. Neurology. 2001;57(12):2278–2282. doi: 10.1212/wnl.57.12.2278. [DOI] [PubMed] [Google Scholar]
- 109.Kuppenbender KD, Standaert DG, Feuerstein TJ, Penney JB, Jr, Young AB, Landwehrmeyer GB. Expression of NMDA receptor subunit mRNAs in neurochemically identified projection and interneurons in the human striatum. J Comp Neurol. 2000;419(4):407–421. doi: 10.1002/(sici)1096-9861(20000417)419:4<407::aid-cne1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 110.Marti M, Paganini F, Stocchi S, Mela F, Beani L, Bianchi C, et al. Plasticity of glutamatergic control of striatal acetylcholine release in experimental parkinsonism: opposite changes at group-II metabotropic and NMDA receptors. J Neurochem. 2003;84(4):792–802. doi: 10.1046/j.1471-4159.2003.01569.x. [DOI] [PubMed] [Google Scholar]
- 111.Gotz T, Kraushaar U, Geiger J, Lubke J, Berger T, Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci. 1997;17(1):204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conti F, Minelli A, DeBiasi S, Melone M. Neuronal and glial localization of NMDA receptors in the cerebral cortex. Mol Neurobiol. 1997;14(1–2):1–18. doi: 10.1007/BF02740618. [DOI] [PubMed] [Google Scholar]
- 113.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90(20):9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9(9):665–677. doi: 10.1038/nrn2471. [DOI] [PubMed] [Google Scholar]
- 115.Koutsilieri E, Riederer P. Excitotoxicity and new antiglutamatergic strategies in Parkinson’s disease and Alzheimer’s disease. Parkinsonism Relat Disord. 2007;13(3):S329–S331. doi: 10.1016/S1353-8020(08)70025-7. [DOI] [PubMed] [Google Scholar]
- 116.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 117.Del Arco A, Mora F. Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm. 2009;116(8):941–952. doi: 10.1007/s00702-009-0243-8. [DOI] [PubMed] [Google Scholar]
- 118.Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2004;102(2):155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 119.Heikkila RE, Sonsalla PK. The use of the MPTP-treated mouse as an animal model of parkinsonism. Can J Neurol Sci. 1987;14(3 Suppl):436–440. doi: 10.1017/s0317167100037860. [DOI] [PubMed] [Google Scholar]
- 120.Nash JE, Fox SH, Henry B, Hill MP, Peggs D, McGuire S, et al. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp Neurol. 2000;165(1):136–142. doi: 10.1006/exnr.2000.7444. [DOI] [PubMed] [Google Scholar]
- 121.Blandini F, Greenamyre JT, Fancellu R, Nappi G. Blockade of subthalamic glutamatergic activity corrects changes in neuronal metabolism and motor behavior in rats with nigrostriatal lesions. Neurol Sci. 2001;22(1):49–50. doi: 10.1007/s100720170041. [DOI] [PubMed] [Google Scholar]
- 122.Albin RL, Makowiec RL, Hollingsworth ZR, Dure L, St Penney JB, Young AB. Excitatory amino acid binding sites in the basal ganglia of the rat: a quantitative autoradiographic study. Neuroscience. 1992;46(1):35–48. doi: 10.1016/0306-4522(92)90006-n. [DOI] [PubMed] [Google Scholar]
- 123.Biggs CS, Fowler LJ, Whitton PS, Starr MS. NMDA receptor antagonists increase the release of dopamine in the substantia nigra of reserpine-treated rats. Eur J Pharmacol. 1996;299(1–3):83–91. doi: 10.1016/0014-2999(95)00837-3. [DOI] [PubMed] [Google Scholar]
- 124.Rabiner EA. Imaging of striatal dopamine release elicited with NMDA antagonists: is there anything there to be seen? J Psychopharmacol. 2007;21(3):253–258. doi: 10.1177/0269881107077767. [DOI] [PubMed] [Google Scholar]
- 125.Del Arco A, Segovia G, Mora F. Blockade of NMDA receptors in the prefrontal cortex increases dopamine and acetylcholine release in the nucleus accumbens and motor activity. Psychopharmacology (Berl) 2008;201(3):325–338. doi: 10.1007/s00213-008-1288-3. [DOI] [PubMed] [Google Scholar]
- 126.Nash JE, Hill MP, Brotchie JM. Antiparkinsonian actions of blockade of NR2B–containing NMDA receptors in the reserpine-treated rat. Exp Neurol. 1999;155(1):42–48. doi: 10.1006/exnr.1998.6963. [DOI] [PubMed] [Google Scholar]
- 127.McDonough JH, Jr, Shih TM. A study of the N-methyl-D-aspartate antagonistic properties of anticholinergic drugs. Pharmacol Biochem Behav. 1995;51(2–3):249–253. doi: 10.1016/0091-3057(94)00372-p. [DOI] [PubMed] [Google Scholar]
- 128.Greenamyre JT, O’Brien CF. N-methyl-D-aspartate antagonists in the treatment of Parkinson’s disease. Arch Neurol. 1991;48(9):977–981. doi: 10.1001/archneur.1991.00530210109030. [DOI] [PubMed] [Google Scholar]
- 129.Jones DC, Gunasekar PG, Borowitz JL, Isom GE. Dopamine-induced apoptosis is mediated by oxidative stress and Is enhanced by cyanide in differentiated PC12 cells. J Neurochem. 2000;74(6):2296–2304. doi: 10.1046/j.1471-4159.2000.0742296.x. [DOI] [PubMed] [Google Scholar]
- 130.Verhagen Metman L, Blanchet PJ, van den Munckhof P, Del Dotto P, Natte R, Chase TN. A trial of dextromethorphan in parkinsonian patients with motor response complications. Mov Disord. 1998;13(3):414–417. doi: 10.1002/mds.870130307. [DOI] [PubMed] [Google Scholar]
- 131.Crosby NJ, Deane KH, Clarke CE. Amantadine for dyskinesia in Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CtD003467. doi: 10.1002/14651858.CD003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Uitti RJ, Rajput AH, Ahlskog JE, Offord KP, Schroeder DR, Ho MM, et al. Amantadine treatment is an independent predictor of improved survival in Parkinson’s disease. Neurology. 1996;46(6):1551–1556. doi: 10.1212/wnl.46.6.1551. [DOI] [PubMed] [Google Scholar]
- 133.Merello M, Nouzeilles MI, Cammarota A, Leiguarda R. Effect of memantine (NMDA antagonist) on Parkinson’s disease: a double-blind crossover randomized study. Clin Neuropharmacol. 1999;22(5):273–276. [PubMed] [Google Scholar]
- 134.Rukoyatkina NI, Gorbunova LV, Gmiro VE, Lukomskaya NY. The ability of new non-competitive glutamate receptor blockers to weaken motor disorders in animals. Neurosci Behav Physiol. 2003;33(3):273–278. doi: 10.1023/a:1022107516333. [DOI] [PubMed] [Google Scholar]
- 135.Sobolevsky AI, Koshelev SG, Khodorov BI. Interaction of memantine and amantadine with agonist-unbound NMDA-receptor channels in acutely isolated rat hippocampal neurons. J Physiol. 1998;512(Pt 1):47–60. doi: 10.1111/j.1469-7793.1998.047bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kato T. [Memantine: a therapeutic drug for Alzheimer’s disease and the comparison with MK-801] Nippon Yakurigaku Zasshi. 2004;124(3):145–151. doi: 10.1254/fpj.124.145. [DOI] [PubMed] [Google Scholar]
- 137.Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990;13(7):272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 138.Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 139.Emre M, Tsolaki M, Bonuccelli U, Destee A, Tolosa E, Kutzelnigg A, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969–977. doi: 10.1016/S1474-4422(10)70194-0. [DOI] [PubMed] [Google Scholar]
- 140.Litvinenko IV, Odinak MM, Mogil’naya VI, Perstnev SV. Use of memantine (akatinol) for the correction of cognitive impairments in Parkinson’s disease complicated by dementia. Neurosci Behav Physiol. 2010;40(2):149–155. doi: 10.1007/s11055-009-9244-1. [DOI] [PubMed] [Google Scholar]
- 141.Varanese S, Howard J, Di Rocco A. NMDA antagonist memantine improves levodopa-induced dyskinesias and “on-off” phenomena in Parkinson’s disease. Mov Disord. 2010;25(4):508–510. doi: 10.1002/mds.22917. [DOI] [PubMed] [Google Scholar]
- 142.Nunan J, Small DH. Regulation of APP cleavage by alpha-, beta-and gamma-secretases. FEBS Lett. 2000;483(1):6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- 143.Zukin RS, Bennett MVL. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci. 1995;18(7):306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]