Abstract
Fibrosis, characterized by excessive collagen protein deposition, is a progressive disease that can fatally inhibit organ function. Prolonged exposure to pathogens or environmental toxicants such as asbestos can lead to chronic inflammatory responses associated with fibrosis. Significant exposure to amphibole asbestos has been reported in and around Libby, Montana due to local mining of asbestos-contaminated vermiculite. These exposures have been implicated in a unique disease etiology characterized predominantly by pleural disorders, including fibrosis. We recently reported the discovery of mesothelial cell autoantibodies (MCAAs) in the sera of Libby residents and demonstrated a positive and significant correlation with pleural disease; however, a mechanistic link was not determined. Here we demonstrate that MCAAs induce pleural mesothelial cells to produce a collagen matrix but do not affect production of the pro-inflammatory cytokine tumor growth factor-β. While autoantibodies commonly induce a pro-fibrotic state by inducing epithelial–mesenchymal transition (EMT) of target cells, we found no evidence supporting EMT in cells exposed to MCAA positive human sera. Although implicated in other models of pulmonary fibrosis, activity of the protein SPARC (secreted protein, acidic and rich in cysteine) did not affect MCAA-induced collagen deposition. However, matrix formation was dependent on matrix metalloproteinase (MMP) activity, and we noted increased expression of MMP-8 and -9 in supernatants of mesothelial cells incubated with MCAA positive sera compared to control. These data suggest a mechanism by which MCAA binding leads to increased collagen deposition through altering MMP expression and provides an important mechanistic link between MCAAs and asbestos-related, autoimmune-induced pleural fibrosis.
Keywords: Autoantibodies, Libby amphibole, pleural fibrosis
Introduction
Asbestos exposure has long been linked with increased incidence of lung abnormalities including fibrosis, pleural plaques and respiratory tract cancers (Kamp, 2009). However, evidence suggests that in addition to these traditional asbestos-related diseases (ARDs), asbestos may also trigger autoimmune responses, as evidenced by circulating auto-antibodies and immune complexes in asbestos workers (Lange, 1980; Nigam et al., 1993). A high prevalence of systemic autoimmune disease has also been reported in Libby, Montana (Noonan et al., 2006; Pfau et al., 2005) where local mining activities resulted in wide-spread occupational and environmental exposure to amphibole asbestos between 1924 and 1990 (Bandli & Gunter, 2006; Whitehouse et al., 2008). These exposures have also been associated with high incidences of pleural changes and non-malignant respiratory disease morbidity, coupled with decreased pulmonary function due to progressive fibrotic disease (Larson et al., 2010; Peipins et al., 2003; Rohs et al., 2008; Sullivan, 2007; Whitehouse, 2004).
Recent studies support a role for pathogenic autoantibodies in a number of fibrotic and vascular disorders, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and systemic scleroderma (SSc) (Chizzolini et al., 2002; Del Papa et al., 1994; Ihn et al., 2000; Renaudineau et al., 1999). Interestingly, exposure to Libby amphibole (LA) has been linked to increased risk of developing one of these three systemic autoimmune disorders (Noonan et al., 2006), and area clinicians caring for Libby residents have noted an increased disease progression in patients presenting with both pulmonary fibrosis and autoimmune symptoms [manuscript in preparation]. Thus, the evidence suggests an autoimmune component to Libby ARD, as similarly noted in studies of other asbestos-exposed groups (Tamura et al., 1993, 1996).
We recently reported on the presence of mesothelial cell autoantibodies (MCAA) in the serum of LA-exposed subjects in which MCAA presence was positively and significantly (p =0.044) correlated with radiographic changes indicative of pleural, but not interstitial, disease (Marchand et al., 2012). Thus, we suspected a pathogenic role of these MCAAs in development of asbestos-related pleural fibrosis. Discovery of a pathogenic role for these autoantibodies may potentially provide a therapeutic target for pleural fibrosis in individuals where these autoantibodies are present.
Mesothelial cells have long been reported to deposit extracellular matrices in vitro (Harvey & Amlot, 1983) and in vivo (Davila & Crouch, 1993; Nasreen et al., 2009; Wynn, 2008) following pleural injury and exposure to pro-fibrotic and inflammatory cytokines. Such cytokines may induce pleural cell differentiation to a myofibroblast-like phenotype (Guarino et al., 2009; Parsons et al., 2007; Zavadil & Bottinger, 2005) characterized by smooth muscle α-actin (SMA) expression and increased secretion of collagen proteins type I and III (Hinz et al., 2007; Phan, 2002; Zhang et al., 1994). While these processes are part of the normal response to tissue injury and wound-healing, they may become pathogenic upon disruption of collagen metabolism homeostasis. Autoantibodies to fibroblast cells have been shown to increase collagen accumulation by inducing cell differentiation to a myofibroblast cell (Pfau et al., 2011) or by directly stimulating signaling cascades leading to an up-regulation of collagen gene expression (Baroni et al., 2006). We hypothesized that MCAA binding might similarly drive mesothelial cell differentiation and collagen protein synthesis.
Alternatively, decreased collagen degradation and turnover can result in a net increase in extracellular protein accumulation. Following collagen synthesis and secretion, proteins are cleaved by collagenolytic enzymes to produce mature proteins. Multiple types of collagen proteinases have been implicated in the development of pulmonary fibrosis. We examined the potential contribution of such proteinases to MCAA-associated fibrosis. The matricellular glycoprotein SPARC (secreted protein acidic and rich in cysteine) is expressed during development and tissue remodeling and repair (Sage et al., 1989a,b) and mediates pro-collagen processing and assembly into fibrils (Harris et al., 2011; Rentz et al., 2007). Additionally, SPARC has been implicated in collagen protein expression and accumulation in bleomycin-induced pulmonary fibrosis (Strandjord et al., 1999; Wang et al., 2010) and following asbestos exposure (Pershouse et al., 2009; Wang et al., 2010). It is also suspected that SPARC plays a role as a scavenger chaperone protein responsible for collagen turn-over (Chlenski et al., 2011; Martinek et al., 2007). Thus, we considered the possibility that MCAA binding affects endogenous SPARC expression, potentially affecting collagen accumulation.
Additionally, we considered that alterations in expression of matrix metalloproteinases (MMPs) could contribute to MCAA-associated fibrosis development. Several members of the MMP zinc-dependent endoproteinase family display activity toward collagen type I, including MMP 1, 2, 8, 9 and 13. Multiple MMPs have been implicated in pulmonary and interstitial lung fibrosis, including idiopathic pulmonary fibrosis and silicosis (Dancer et al., 2011; Scabilloni et al., 2005), as well as in asbestos-associated inflammation and fibrosis (Tan et al., 2006). Exposure to chrysotile asbestos was shown to increase MMP-8 release from neutrophils in vitro (Hedenborg et al., 1990) while exposure to the more fibrogenic asbestos crocidolite increased MMP-2 and -9 expression in vivo (Tan et al., 2006). MMP-8 deficiency was shown to be protective in a bleomycin model of pulmonary injury, potentially through decreased processing of the anti-fibrotic cytokine IL-10 (Garcia-Prieto et al., 2010). Based on this evidence, we examined the effect of MCAA binding on MMP activity and expression in mesothelial cell supernatants.
Here, we report findings indicating that MCAAs induce pleural mesothelial cells to deposit extracellular collagen type I proteins in an MMP-dependent fashion, potentially by modulating MMP expression. Additionally, we demonstrate that MCAAs do not elicit mesothelial cell differentiation to a myofibroblast-like cell, as has been extensively reported for fibroblast-mediated collagen deposition. Further, this collagen deposition was not dependent on TGF-β or SPARC activity, as reported for other models of asbestos-associated fibrosis. Thus, the MCAAs seem to promote collagen deposition by unique mechanisms, which likely work in parallel or conjunction with other reported mechanisms of fibrosis, including asbestos-induced cell damage and chronic inflammation. The ability of an autoimmune and inflammatory response to cooperate in exacerbating fibrotic processes is a novel and important consideration for fibrotic disorders with unexplained etiology.
Methods
Cell culture
Non-malignant, transformed human mesothelial cells, MeT-5A (ATCC, Manassas, VA) were grown in RPMI medium (CellGro Mediatec, Manassas, VA) supplemented with 5% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA) and antibiotics. Cells were maintained at 37 °C and 5% CO2.
Human serum samples and IgG clearance
Serum samples were collected by the Center for Asbestos Related Disease (CARD) in Libby, Montana in accordance with Idaho State University IRB project approval #3292MOD and was stored at −80 °C until needed. Samples previously identified as MCAA positive (+ive) or negative (−ive) were pooled (Marchand et al., 2012) and small aliquots stored at −20 °C. Sera cleared of IgG antibodies were used as negative controls. Sera were cleared using Protein A Agarose Beads (Thermo Scientific, Rockford, IL) according to manufacturer’s instructions. IgG removal was confirmed by running sera on a 12% Bis–Tris gel (Novex, Life Technologies, Carlsbad, CA) and staining with GelCode Blue Stain Reagent (Thermo Scientific) and checking for lack of bands corresponding to the molecular weight of IgG heavy and light chains (50 and 25 kDa).
MCAA cell binding assay
A cell-based ELISA was performed as previously described (Marchand et al., 2012) to compare the binding efficacy of pooled sera to individual samples. Briefly, MeT-5A cells were seeded at confluency on 96 well plates, attached overnight and fixed in 1% paraformaldehyde. Following washing with PBS-Tween (0.05%), cells were blocked with 5% dried milk/ PBS and then exposed to pooled sera previously identified as MCAA positive or negative or cleared sera in 3% BSA/PBS (1:100). Following a 2-h incubation with primary antibody, cells were washed and blocked a second time. The secondary antibody HRP-conjugated goat anti-human IgG (Jackson ImmunoResearch, West Grove, PA) was applied at a dilution of 1:1000 in 3% BSA/PBS and incubated for 1 h. Excess antibody was removed and plates developed using TMB reagent (Thermo Scientific) followed by 30 μl 1 M HCl. Plates were analyzed at 450 nm on a microtiter plate reader (BioTek Instruments, Winooski, VT). Non-specific secondary antibody binding was corrected for on a plate-to-plate basis by subtracting the mean optical density (OD) for the secondary antibody-only control wells from the mean OD of each sample.
Cell-based ELISA for detecting collagen and SPARC
Collagen and SPARC proteins were detected using cell-based ELISA. Cells were seeded as above and then exposed to sera, diluted 1:50 in FBS-free RPMI. Following a 4-d incubation at 37 °C, supernatant was removed and cells were fixed, washed and blocked as described earlier. Primary antibody diluted in 3% BSA/PBS was applied for 2 h and consisted of mouse monoclonal anti-collagen I (AbCam), 1:2000, or rabbit polyclonal IgG anti-SPARC, H-90 (Santa Cruz Biotechnology, Santa Cruz, CA), 1:200. Following another round of washing and blocking, the HRP-conjugated secondary antibodies (1:1000) were applied for 1 h. Plates were then washed, developed and analyzed as described earlier.
A cell-based ELISA was also used to assess the contribution of endogenous SPARC and MMPs to collagen matrix formation. Cells were treated with sera as above, with or without the addition of SPARC inhibitor (anti-SPARC antibody, 1 μg/mL) on day 1 or the MMP inhibitor, EDTA (5 mM), on day 3. Collagen protein deposition was determined using the ELISA protocol above.
Soluble collagen assay
The concentrations of soluble collagen proteins in supernatant of cells incubated for 4 days with MCAA +ive or −ive sera (1:50 in FBS-free RPMI) were determined using a Sircol Collagen Assay (Biocolor, Carrickfergus, North Ireland) according to the manufacturer’s instructions. Following collagen extraction from supernatants, samples were analyzed using a microtiter plate reader (BioTek Instruments) with absorbances measured at 562 nm. Mean OD of samples were normalized to reagent blank, and collagen concentrations (μg/ mL) were determined by comparing sample absorbance against the standard curve. All samples were read in duplicate.
Additionally, detergent soluble fractions of collagen were collected by lysing cells in buffer containing 0.2% deoxycholic acid and incubated on a rotor at 4 °C overnight. Cell debris was removed by centrifugation and the supernatants assayed for soluble collagen proteins as above.
Smooth muscle α-actin expression
Cells were plated at near confluency and attached overnight. Cells were then treated with MCAA +ive or −ive sera (1:50), normal human sera (1:50), or TGF-β (2 ng/mL) as a positive control, in FBS-free RPMI, 100 μl final volume. After 4 d, medium was removed and cells were released from plates by 5-min treatment with trypsin/EDTA (Mediatech, Inc.). Cells were pelleted by centrifugation, washed once with 3% BSA/ PBS, fixed in Fixation Buffer (BD Pharmingen, BD Biosciences, San Jose, CA) for 20 min and then washed twice in 1 × Perm/Wash buffer (BD Pharmingen). The cells were stained for smooth muscle α-actin (SMA) by adding 1 μg of mouse monoclonal antibody (Thermo Scientific) per 1 × 106 cells. Following 15-min incubation, cells were washed twice and incubated for 15 min with the secondary FITC-conjugated goat anti-mouse antibody (1:1000). Excess antibody was removed by washing and cells were resuspended in PBS and analyzed on a FACS Calibur flow cytometer (BD Biosciences).
Samples were analyzed by gating on live cells and mean fluorescence intensity measured against background fluorescence of control cells stained with secondary Ab only.
Cytokine bead flex set analysis of TGF-β
TGF-β1 concentrations in human sera samples and cell supernatants were determined using a human TGF-β1 single plex cytokine bead flex set kit (BD Biosciences, San Diego, CA). Supernatants were collected from cells treated for 4 d with MCAA +ive or −ive sera, normal human serum, or media only. Latent TGF-β was activated in supernatants and serum samples by acidification (pH<3.0) for 10 min followed by neutralization (pH 7.2–7.6) prior to testing for cytokine concentrations. Activated samples were then incubated with prepared flex set capture beads then incubated with prepared PE detection reagent according to manufacturer’s instructions. Samples were analyzed on a FACS Calibur flow cytometer and analyzed using CellQuest software (BD Biosciences, San Jose, CA). Total TGF-β1 concentrations were determined by comparing geometric means of samples to a standard curve.
Immunoblots
Cells were seeded at near confluency and attached overnight. MCAA +ive or −ive sera or cleared serum was then added at 1:50 dilution in FBS-free RPMI and incubated for 4 d at 37 °C. Supernatants were collected and stored at −20 °C until use. Cells were collected via scraping and lysed in buffer (1% Triton, 0.2% Deoxycholic Acid, 50 mM Tris, 150 mM NaCl), centrifuged to remove cell debris and stored at −20 °C until use.
Total protein concentrations were determined using a BCA Protein Assay Kit (Thermo Scientific, Waltham, MA). Supernatants or lysates were then were mixed with 5 × SDS loading buffer containing β-mercaptoethanol and heated to 100 °C for 5–10 min. Proteins were separated on a 12% Bis–Tris gel (Novex, Life Technologies, Carlsbad, CA) in 1 × NuPAGE MOPS SDS Running Buffer (Invitrogen, Carlsbad, CA) for 1.5 h at 150 V and transferred to a PVDF membrane at 30 V for 45 min in 1×NuPAGE Transfer Buffer (Invitrogen). Membranes were air dried, briefly re-wetted in methanol, washed twice with PBS and blocked in 5% milk/ PBS for 1 h at room temperature.
The primary antibody diluted 1:200 in 3% BSA/PBS was applied overnight at room temperature and consisted of rabbit polyclonal IgG antibody anti-SPARC or anti-MMP-8 (Santa Cruz Biotechnology). Membranes were washed twice in PBS-T (0.05%), blocked and incubated for 1 h at room temperature with secondary antibody, HRP conjugated goat anti-rabbit (1:1000). Excess antibody was removed by washing and the blot developed with TMB reagent (Thermo Scientific). Bands were visualized on a VersDoc Imaging System with Quantity One software (version 4.6.5, BioRad). Densitometry was determined using ImageJ software.
Collagenase zymography
Zymogen assay was used to assess the activity of collagenases in supernatants of cells exposed to MCAAs. Supernatants were collected from cells incubated for 4 days with MCAA +ive or −ive sera, normal human serum, or medium only. About 10 μg total protein was loaded onto a 10% acrylamide gel impregnated with type I collagen (Sigma Aldrich) and run under non-reducing conditions. Proteins were separated at 65 V for 1 h. Gels were washed for 1 h at room temperature with shaking in activation solution (50 mM Tris, pH 7.4, 5 mM CaCl2, 1 μM ZnCl2) containing 2.5% Triton-X. Gels were then rinsed briefly in DI H2O and incubated for 18 h at 37 °C in activation buffer. Gels were then stained in Coomassie Blue stain for 30 min and destained in 30% EtOH/10% AcOH overnight. Staining was stopped in 2% AcOH and bands visualized on a VersDoc Imaging System with Quantity One software (version 4.6.5, BioRad). Collagenase from Clostridium histolyticum, 200 pg (Sigma-Aldrich) was used as a positive control.
Statistical analyses
One-way or two-way ANOVAs were performed using SigmaPlot version 11.0 (Systat Software, San Jose, CA). Statistical significance was defined as a p values ≤ 0.05. Data are graphed with error bars indicating either SEM or SD.
Results
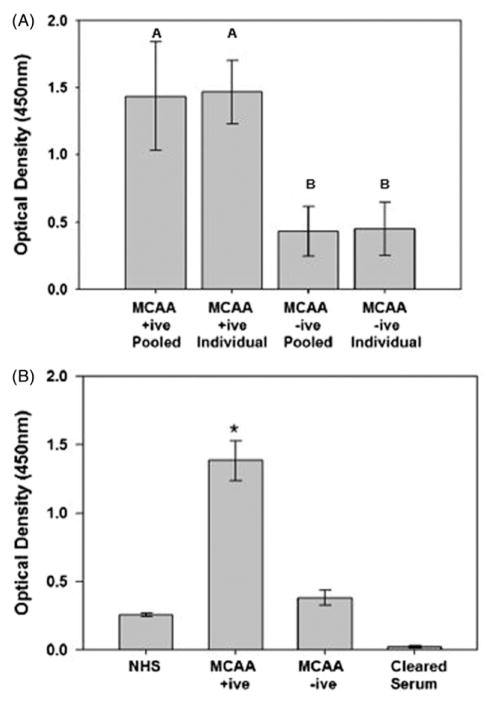
Sera of Libby residents were previously identified as MCAA positive (+ive) or negative (−ive) using a cell-based ELISA to assess binding to cultured pleural human mesothelial cells (Marchand et al., 2012). We randomly pooled MCAA +ive and −ive samples and compared the binding ability between pooled and individual samples. Additionally, binding of pooled MCAA +ive and −ive samples were compared to binding ability of normal human sera (NHS) obtained from a donor with no known asbestos exposure and to sera from which IgG was removed (Cleared Sera). We determined that individual and pooled samples did not significantly vary in their binding ability for either MCAA +ive (p =0.427) or −ive sera (p =0.422), indicating that pooled samples are appropriate for use in our experimental model (Figure 1A). However, MCAA +ive sera demonstrated significantly higher binding (p<0.001) compared with MCAA −ive sera and NHS, suggesting a lack of antibody against mesothelial cells in the latter samples (Figure 1B). Binding was lost upon clearance of IgG from samples, indicating that MCAAs are IgG type antibodies.
Figure 1.
Binding of serum antibodies to human mesothelial cells indicates the presence of mesothelial cell autoantibodies (MCAAs) as determined by cell-based ELISA as previously described (Marchand et al., 2012). (A) Binding by MCCA +ive samples was significantly higher compared to the MCAA −ive samples, though no differences were seen in MCAA binding of individual serum samples compared pooled sera. Mean ±SE, n =at least 6, p =0.002. (B) Binding in pooled sera samples previously identified as MCAA +ive was significantly higher than pooled samples of MCAA −ive sera. MCAAs were not detected in normal human sera (NHS) obtained from subjects with no known asbestos exposure. Removal of IgG by Protein G precipitation (cleared serum) resulted in loss of MCAA binding compared to NHS, p =0.001. Mean ±SE, n =6, *p<0.001 by one-way ANOVA.
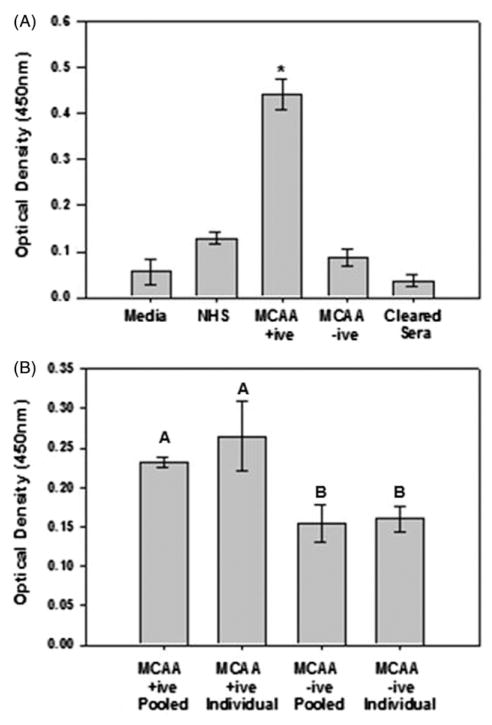
To determine if MCAA binding induces collagen deposition, cells were incubated with pooled sera for 4 d and collagen I measured using a cell-based ELISA. Cells exposed to MCAA +ive sera demonstrated a significant increase in collagen detection compared to those incubated with MCAA −ive or NHS (p<0.001). Clearing sera of IgG antibodies reduced collagen presence to background levels (Figure 2A), suggesting that the MCAAs specifically increased collagen detection. Additionally, collagen deposition did not significantly differ between cells treated with pooled sera or individual serum samples (p =0.277), thus further validating the use of pooled sera for MCAA analyses (Figure 2B).
Figure 2.
MCAAs induced deposition of extracellular type I collagen proteins. (A) Collagen detection significantly increased following exposure to MCAA +ive sera compared to MCAA −ive sera or NHS. Clearance of IgG reduced collagen detection to background levels. Mean ±SE, n =8, *p<0.001 by one-way ANOVA. Collagen deposition by MeT5A cells was determined using cell-based ELISA following a 4-day incubation of cells with human sera. (B) No significant differences in collagen detection were observed when cells were exposed to pooled MCAA +ive sera compared to individual samples, but cell exposure to both pooled and individual MCAA +ive samples significantly increased collagen compared to respective MCAA −ive samples. Mean ± SD, n =3, p<0.05 by one-way ANOVA with Tukey post hoc test.
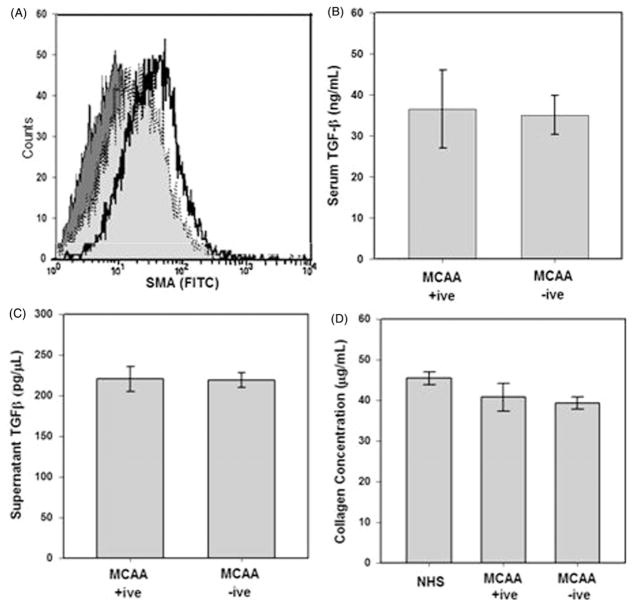
Extracellular collagen deposition is carefully regulated by a balance of collagen synthesis and catabolism. Disruption of these processes may result in a net accumulation of collagen proteins and progression of fibrosis (Wynn, 2007). Cellular activation to express a myofibroblast-like phenotype has been reported following cell exposure to pro-fibrotic cytokines (Hinz et al., 2007) or autoantibody binding (Pfau et al., 2011). We hypothesized that MCAA binding would promote mesothelial cell differentiation by activating an intracellular signaling cascade leading to expression of myofibroblast genes following cell binding (Pfau et al., 2011). We previously showed that MCAAs bind to the cell surface of cultured mesothelial cells (Marchand et al., 2012) and that asbestos-associated auto-antibody binding activates signal transduction pathways and mesenchymal transition in cultured fibroblast cells (Pfau et al., 2011). Mesothelial cell differentiation was assessed by using flow cytometry to measure the intracellular expression of smooth muscle α-actin (SMA) in the mesothelial cell. Since SMA is a myofibroblast marker, increased expression indicates cellular differentiation. However, flow cytometric analysis of SMA expression revealed no significant differences between cells incubated with MCAA +ive or −ive sera, indicated by overlapping plots, although SMA expression did increase in cells exposed directly to the potent pro-fibrogenic cytokine TGF-β (5 ng/μL) used as a positive control (Figure 3A). Interestingly, exposure to sera of asbestos-exposed subjects did increase SMA expression compared to NHS, just not to the extent observed in positive control cells. We suspected that this increase might be a result of TGF-β present in the sera. Using a cytokine bead array (BD Biosciences), we measured TGF-β concentrations in the pooled MCAA +ive and −ive sera samples. Approximately 35 ng/μl of TGF-β was detected in these samples, with no statistical difference between them (Figure 3B). Additionally, we examined the cell supernatants to ensure that exposure to sera of asbestos-exposed subjects did not induce mesothelial cell production of TGF-β. We detected ~0.7 ng/μl of TGF-β in the supernatants (Figure 3C). Since sera was added to cells at a 1:50 dilution, the TGF-β detected in supernatants is likely solely from the sera and not produced from the mesothelial cells. Additionally, even at this low TGF-β concentration, we would expect to see a slight effect on SMA expression (May et al., 1988).
Figure 3.
MCAA exposure does not induce a mesenchymal transition in cultured human pleural mesothelial cells. (A) SMA expression was measured in cells exposed to human sera or TGF-β (5 ng) as a positive control for 4 days. Cells were fixed and stained with primary anti-SMA and secondary FITC conjugated antibodies and mean fluorescence measured by flow cytometry. TGF-β (
 ) induced SMA expression compared with exposure to secondary antibody control (
) induced SMA expression compared with exposure to secondary antibody control (
 ,
,
 ), MCAA +ive (
), MCAA +ive (
 ,
,
 ), or MCAA −ive (
), or MCAA −ive (
 ) sera. Exposure to MCAA +ive or −ive sera resulted in increased SMA expression over secondary control (x-axis) but expression did not increase to the same degree as TGF-β exposure. TGF-β was detected in MCAA +ive and −ive sera (B) and supernatants (C) of cells exposed to these sera samples. TGF-β was detected using a cytokine bead array analysis and concentrations suggest that the TGF-β detected in supernatants was added upon addition of sera and not produced by the mesothelial cells, mean ± SD, n =3). (D) Total soluble collagen concentrations in cell supernatants were determined using a Sircol Soluble Collagen Assay. Incubation with sera from asbestos-exposed subjects did not significantly affect soluble collagen compared to NHS.
) sera. Exposure to MCAA +ive or −ive sera resulted in increased SMA expression over secondary control (x-axis) but expression did not increase to the same degree as TGF-β exposure. TGF-β was detected in MCAA +ive and −ive sera (B) and supernatants (C) of cells exposed to these sera samples. TGF-β was detected using a cytokine bead array analysis and concentrations suggest that the TGF-β detected in supernatants was added upon addition of sera and not produced by the mesothelial cells, mean ± SD, n =3). (D) Total soluble collagen concentrations in cell supernatants were determined using a Sircol Soluble Collagen Assay. Incubation with sera from asbestos-exposed subjects did not significantly affect soluble collagen compared to NHS.
Since mesenchymal cells are characterized by increased collagen synthesis, we further established the lack of mesenchymal transition by comparing concentrations of newly synthesized collagens in supernatants of cells exposed to MCAA +ive or −ive sera. No differences in collagen concentrations were detected following exposure to any human sera as determined by Sircol Soluble Collagen Assay (Figure 3D). Taken together, these data confirmed that exposure to MCAA +ive sera did not increase collagen deposition by inducing a phenotypic change in these cells.
Since MCAA binding does not induce collagen synthesis or a mesenchymal transition, we hypothesized that MCAA binding might affect the expression or activity of proteolytic enzymes that function in collagen catabolism. Several such enzymes are associated with fibrosis and altered extracellular collagen metabolism, including SPARC and the MMP family of enzymes.
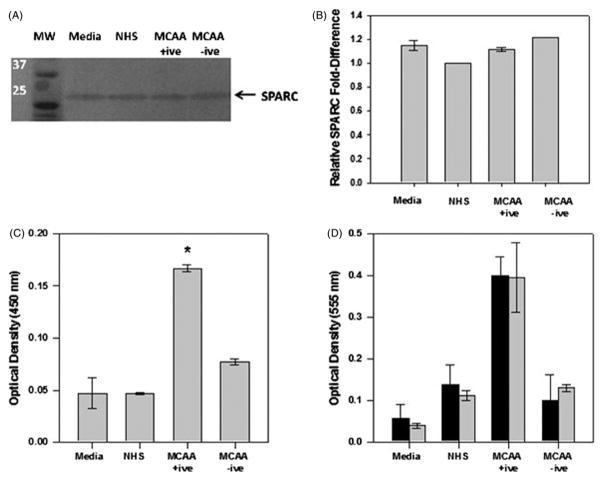
Endogenous SPARC expression was detected in supernatants of pleural mesothelial cells, but protein levels did not change following cell exposure to MCAA +ive or −ive sera (Figure 4A and B). Using a cell-based ELISA, SPARC detection significantly increased in cells exposed to MCAA +ive sera (Figure 4C). To determine if SPARC affects MCAA-induced collagen production or merely associates with collagen following its deposition, we used a neutralizing antibody to inhibit SPARC activity (Goldblum et al., 1994) and then examined collagen I deposition. Inhibition of SPARC did not significantly affect collagen deposition (Figure 4D), indicating that SPARC does not significantly contribute to MCAA-induced collagen deposition in vitro.
Figure 4.
SPARC expressed by human mesothelial cells associates with extracellular matrix but does not contribute to matrix formation. (A) Immunoblot analysis was used to detect endogenous SPARC in lysates of MeT5A cells incubated with human sera. (B) Densitometry analysis using Image J software revealed no differences in expression levels between cells exposed to MCAA +ive sera or controls, mean ±SD, n =2. (C) Using a cell-based ELISA, detection of SPARC increased significantly in MeT5A cells exposed to MCAA +ive sera compared to controls, mean ±SE, n =4, *p<0.001 as determined by one-way ANOVA. Cells were incubated with human sera for 4 days and SPARC detected using a rabbit polyclonal IgG anti-SPARC, H-90 antibody. (D) Addition of anti-SPARC antibody (
 ) did not significantly alter detection of collagen proteins compared to no treatment (■), indicating that SPARC does not directly affect MCAA-induced collagen deposition, as determined by two-way ANOVA, mean ±SE, n =4.
) did not significantly alter detection of collagen proteins compared to no treatment (■), indicating that SPARC does not directly affect MCAA-induced collagen deposition, as determined by two-way ANOVA, mean ±SE, n =4.
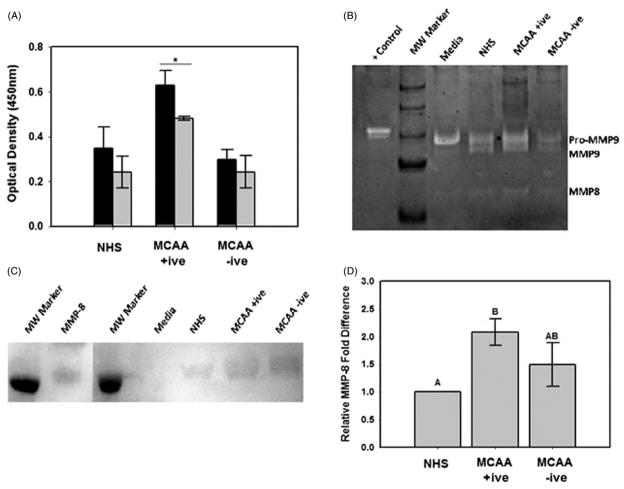
In order to determine if MMP activity contributes to MCAA-induced collagen deposition, we detected collagen I as described earlier with or without the addition of the non-specific MMP inhibitor EDTA (5 mM). EDTA significantly reduced collagen deposition in cells incubated with MCAA +ive sera but not in MCAA −ive sera or NHS (Figure 5A). Collagen zymography was used to determine which MMPs were present in supernatants of cells incubated with human sera and active toward type I collagen. The zymogen assay revealed double band at ~98 and 92 kDa corresponding to reported molecular weights of pro and active MMP-9, respectively (Figure 5B). A single band was also detected at ~58 kDa, which corresponded to the active form of MMP-8 (Figure 5B). Taken together, these data suggest a role of MMPs in MCAA-induced collagen matrix deposition in vitro, potentially through activity of MMP-8 or MMP-9. Immunoblot analysis confirmed the presence of MMP-8 (Figure 5C) and densitometric analysis (Figure 5D) revealed a significant increase in MMP-8 expression by cells exposed to MCAA +ive sera compared to NHS exposure (p ≤ 0.01). Cell exposure to MCAA −ive sera also resulted in increased MMP-8 expression, although this increase was not significant (p =0.085). Attempts to detect MMP-9 via immunoblot analysis were unsuccessful.
Figure 5.
MCAA-induced collagen deposition is dependent on MMP activity. (A) Cell-based ELISA was used to detect extracellular collagen deposited by MeT5A cells exposed to human sera for 4 days in the absence (■) or presence (
 ) of the non-specific MMP inhibitor EDTA (5 mM). Collagen deposition was significantly inhibited by EDTA in cells exposed to MCAA +ive sera (*p =0.018) but not to NHS (p =0.28) or MCAA −ive sera (p =0.32) as determined by two-way ANOVA. (B) Collagen zymography revealed the presence of MMP-8 and MMP-9 in supernatants of cells exposed to human sera for 4 days. MMPs were identified by their reported molecular weights and bacterial collagenase (200 pg) was used as a positive control. (C) Immunoblot analysis of MMP-8 confirmed its identity. (D) Densitometry analysis of the immunoblot revealed significantly more MMP-8 present in supernatants of cells exposed to MCAA +ive sera compared to NHS. However, exposure to MCAA −ive sera also resulted in increased MMP-8, although this increase was not significant compared to NHS. Statistical significance is indicated by different letters at p<0.01 by one-way ANOVA, mean ±SD, n =3.
) of the non-specific MMP inhibitor EDTA (5 mM). Collagen deposition was significantly inhibited by EDTA in cells exposed to MCAA +ive sera (*p =0.018) but not to NHS (p =0.28) or MCAA −ive sera (p =0.32) as determined by two-way ANOVA. (B) Collagen zymography revealed the presence of MMP-8 and MMP-9 in supernatants of cells exposed to human sera for 4 days. MMPs were identified by their reported molecular weights and bacterial collagenase (200 pg) was used as a positive control. (C) Immunoblot analysis of MMP-8 confirmed its identity. (D) Densitometry analysis of the immunoblot revealed significantly more MMP-8 present in supernatants of cells exposed to MCAA +ive sera compared to NHS. However, exposure to MCAA −ive sera also resulted in increased MMP-8, although this increase was not significant compared to NHS. Statistical significance is indicated by different letters at p<0.01 by one-way ANOVA, mean ±SD, n =3.
Discussion
Fibrotic diseases are characterized by excessive collagen deposition resulting in decreased organ function and eventual organ failure. Increasing evidence implicates a role for autoantibodies in driving collagen accumulation (Chizzolini et al., 2002; Ihn et al., 2000; Renaudineau et al., 1999), often by inducing cells to undergo a phenotypic change to a myofibroblast-like cell. Such a mesenchymal transition is has been described for epithelial (Willis et al., 2005), endothelial (Hashimoto et al., 2010; Piera-Velazquez et al., 2011), fibroblastic (Pfau et al., 2011; Zhang et al., 1996) and mesothelial cells (Liu et al., 2008; Nasreen et al., 2009). Alternatively, autoantibody binding stimulates production of pro-fibrogenic or inflammatory cytokines (Guo et al., 2004) which may in turn act in autocrine or paracrine fashions to further exacerbate chronic inflammatory conditions attributed to fibrosis progression.
Pleural fibrosis arising from prolonged exposure to LA asbestos is a relentlessly progressive disease (Whitehouse, 2004) associated with chronic inflammation and increased serum autoantibodies to fibroblast and mesothelial cells (Marchand et al., 2012; Pfau et al., 2011). While the role of fibroblasts in regulating pleural fibrosis is better characterized than that of mesothelial cells (Chizzolini et al., 2002; Fineschi et al., 2008; Gabrielli et al., 2007; Kuwahara et al., 1994; Pfau et al., 2011; Phan, 2008), evidence suggests that mesothelial cells are also crucial mediators of pleural disease progression (DavilaandCrouch, 1993; HarveyandAmlot, 1983; Nasreen et al., 2009). Harvey and Amlot demonstrated in 1983 that mesothelial cells obtained from pleural effusions were capable of depositing type I and III collagens in vitro, implicating a role for mesothelial cells in pleural fibrosis (Harvey & Amlot, 1983). Additionally, several groups have demonstrated that mesothelial cells are important sources of cytokines and chemokines within the pleura, thus providing a means of recruiting fibrogenic and immunogenic cells (Antony et al., 1995; Boylan et al., 1992; Griffith et al., 1994; Kuwahara et al., 1994). It was also recently suggested that mesothelial cells themselves are potentially fibrogenic following exposure to the pro-inflammatory growth factor TGF-β1 (Nasreen et al., 2009). Thus, our investigations focused on the role of mesothelial cell autoantibodies (MCAAs) in inducing fibrogenic changes within cultured human pleural mesothelial cells.
Our results demonstrated increased detection of extracellular collagen I proteins in cells exposed to MCAA +ive sera compared to MCAA −ive sera or NHS. Clearance of IgG from the sera eliminated MCAA binding as well as collagen detection, thus implicating a role for MCAA binding in driving the observed collagen production. We additionally screened the sera of LA-exposed subjects for anti-fibroblast antibodies, but no cross-reactivity was detected, indicating that MCAA binding was specific to mesothelial cells.
Interestingly, our results suggest that MCAA binding induced collagen production in the absence of a mesothelial-mesenchymal transition. This finding counters the currently accepted model of fibrosis development in which collagen-producing cells are either myofibroblasts recruited to the area via chemokine release or are local cells that have undergone a mesenchymal transition. Here, we suggest an alternative in which autoantibodies themselves induce target cells to deposit extracellular matrix proteins.
Since MCAAs do not affect mesothelial cell phenotypes, we considered the possibility that binding directly stimulates collagen I synthesis or processing. Collagen I is a hetero-trimer composed of two α1(I) and one α2(I) chains. These proteins are encoded by the genes, COL1A1 and COL1A2, respectively, and are translated into immature pro-collagen peptides. Collagenolytic proteins cleave pro-peptides from both the N- and C-termini, leaving the processed proteins to arrange themselves into long, thin fibrils. Fibril maturation into strong collagen I matrices occurs upon fibril cross-linking (Cooper, 1970; Siegel, 1976). Since the Sircol Soluble Collagen Assay does not detect cross-linked collagen fibers, we used this assay to compare the concentrations of newly synthesized collagen in supernatants of cells exposed to MCAA +ive or −ive sera. No differences were detected between exposure groups, suggesting that MCAAs likely do not directly stimulate collagen synthesis and confirming the lack of MCAA-associated mesothelial–mesenchymal transition.
A key regulator of collagen production is TGF-β, which was detected in sera of the asbestos-exposed subjects. However, detection of TGF-β does not preclude the possibility that another serum component was responsible for the slight increase in collagen synthesis detected. Since asbestos fibers themselves are potent inducers of inflammation and fibrogenic responses, it is likely that these responses would work synergistically with MCAAs to further promote fibrosis development in vivo. Additionally, no increases in the fibrogenic cytokine TGF-β were detected in supernatants of cells exposed to MCAA +ive sera. Thus, MCAA binding does not induce secretion of this cytokine. While TGF-β clearly plays a crucial role in driving fibrosis development in vivo, this cytokine was not responsible for driving collagen production by mesothelial cells in vitro. However, since asbestos exposure increases TGF-β production (Dai et al., 1998; Liu & Brody, 2001; Liu et al., 1996), it is likely that following asbestos inhalation, MCAAs and TGF-β work synergistically to further enhance fibrosis development beyond the effects of either protein alone.
Since collagen processing is a key regulatory event leading to production of mature collagen fibers and formation of the collagen matrices observed in fibrotic diseases, we examined the effect of MCAA binding on the expression and activity of several collagenolytic proteins. SPARC is a matricellular protein associated with exacerbation of fibrosis in asbestos and bleomycin exposure models (Pershouse et al., 2009; Strandjord et al., 1999; Wang et al., 2010). While SPARC detection increased in cells exposed to MCAA +ive sera compared to controls, MCAA exposure did not affect protein expression by mesothelial cells.
Since SPARC associates with extracellular collagen matrices (Iruela-Arispe et al., 1996), it is likely that the increased detection of SPARC merely confirms its association with the collagen produced by MCAA-exposed cells. The pattern of SPARC detection in our assays suggests that the collagen I proteins detected by ELISA are mature fibers and not pro-collagen proteins. It has been demonstrated that collagen I precursors are bound to cell surfaces and released, and subsequently incorporated into a collagen matrix, following cleavage by SPARC (Harris et al., 2011; Rentz et al., 2007). Thus, if the ELISA analyses were merely detecting collagen precursors bound to cell surfaces, we would expect that SPARC inhibition would also inhibit release of pro-collagen, thus increasing collagen detection via ELISA. Conversely, SPARC inhibition would decrease the amount of soluble collagen detected in cell supernatants. However, SPARC inhibition did not significantly alter collagen detection by either ELISA or Sircol assay. Thus, we suspect that we are not merely detecting cell-bound collagen precursor proteins but mature collagen fibrils following cell stimulation with MCAA +ive sera. Additionally, the collagen fractions we detect via ELISA were detergent insoluble, thus indicating a mature matrix structure.
To further examine the possibility that MCAA binding alters expression or activity of collagenolytic proteins, thereby increasing collagen processing and maturation events, we examined the effect of MMP activity on MCAA-induced collagen deposition. Addition of EDTA (5 mM) to our cell culture model significantly decreased collagen deposition by cells exposed to MCAA +ive sera but did not significantly affect the low levels of collagen detected in cells exposed to MCAA −ive sera or NHS. However, MMP inhibition did not reduce collagen levels to background, suggesting MMPs act with another collagenolytic protein or mechanism to contribute to MCAA-induced collagen deposition. Zymography revealed that MMP-8 and MMP-9 are produced by the cultured mesothelial cells and are active toward collagen I; however, MMP-9 demonstrated greater activity toward collagen compared MMP-8. Reports on the role of MMP-9 in fibrosis development are often contradictory, with some evidence supporting a pathogenic role of MMP-9 (Sung et al., 2005) and others showing that the absence of this protein increases ECM deposition (Oh et al., 2002). In our model, MMP-9 is secreted as a zymogen by the cultured mesothelial cell, but is activated upon addition of human sera. MMP-9 demonstrated activity toward type I collagen, suggesting that it may play a role in ECM remodeling. However, it is unlikely that this is a MCAA-specific mechanism of collagen deposition.
Immunoblot analysis of MMPs revealed significantly more MMP-8 protein in supernatants of cells exposed to MCAA +ive sera compared to NHS. While incubation with MCAA −ive sera also resulted in a non-significant increase in MMP-8, it suggests that another serum component may contribute to MMP expression following asbestos exposure. The potential involvement of MMP-8 in MCAA-induced collagen deposition is especially intriguing given a recent study demonstrating that MMP-8 inactivates the fibrotic cytokine IL-10 via cleavage, thus promoting fibrosis development (Garcia-Prieto et al., 2010). A potential in vivo role of the observed MMP-8 increase following MCAA exposure could include deactivation of local IL-10, thus promoting fibrosis development within the pleural cavity. Future studies are planned to more closely examine the potential role of MMPs in MCAA-induced collagen deposition.
Conclusions
The ability of MCAA binding to induce extracellular collagen I deposition suggests a potential role of these autoantibodies in contributing to the development of asbestos-associated pleural fibrosis. Interestingly, mesothelial cells are not often reported as key cellular contributors to fibrosis, but our results presented here suggest that they may play a more important role than has previously been considered. Additionally, our results suggest that MCAAs induce collagen production in the absence of a mesenchymal transition, thus suggesting a novel mechanism by which autoantibodies may augment fibrotic disease development or progression. Correlative data further support the role for MCAAs in pleural disease development or progression (Marchand et al., 2012) with these data supported by clinical observations that patients presenting with symptoms of both pulmonary and autoimmune disease often progress rapidly to severe pleural and/or interstitial lung disease [personal communication, Dr Brad Black, CARD clinic, Libby, Montana]. One goal of on-going research centered on an LA-exposed cohort is to examine the association of autoimmune disease and apparent “rapid progression” of pulmonary disease. MCAA presence will be included in this study in order to better understand how MCAA presence affects disease progression or, alternatively, if MCAA presence might be a useful diagnostic marker of impending pleural fibrosis. By perpetuating pro-fibrosis behavior beyond any scarring initially caused by the asbestos itself, it is expected that these autoantibodies could promote the non-resolution and active progression of scarring observed in pleural fibrosis.
Acknowledgments
In memoriam, we gratefully acknowledge Dr Stephen Levin’s intellectual contributions to the conceptual development of this research. We also acknowledge Dr Elizabeth Putnam, University of Montana, for her invaluable help with examining SPARC.
Footnotes
Declaration of interest
All the authors report no declaration of interest. This work was supported by CDC/ATSDR R01 Grant TS000099-01, the Libby Epidemiology Research Program (LERP) and Idaho State University’s University Research Council Graduate Student grant to K.M.S. Core facilities that supported this work were funded in part by NIH grant P20 RR016454 (INBRE). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Toxic Substances and Disease Registry.
References
- Antony VB, Hott JW, Kunkel SL, et al. Pleural mesothelial cell expression of C-C (monocyte chemotactic peptide) and C-X-C (interleukin 8) chemokines. Am J Respir Cell Mol Biol. 1995;12:581–8. doi: 10.1165/ajrcmb.12.6.7766422. [DOI] [PubMed] [Google Scholar]
- Bandli BR, Gunter ME. A review of scientific literature examining the mining history, geology, mineralogy, and amphibole asbestos health effects of the Rainy Creek igneous complex, Libby, Montana, USA. Inhal Toxicol. 2006;18:949–62. doi: 10.1080/08958370600834982. [DOI] [PubMed] [Google Scholar]
- Baroni SS, Santillo M, Bevilacqua F, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–76. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- Boylan AM, Ruegg C, Kim KJ, et al. Evidence of a role for mesothelial cell-derived interleukin 8 in the pathogenesis of asbestos-induced pleurisy in rabbits. J Clin Invest. 1992;89:1257–67. doi: 10.1172/JCI115710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzolini C, Raschi E, Rezzonico R, et al. Autoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosis. Arthritis Rheum. 2002;46:1602–13. doi: 10.1002/art.10361. [DOI] [PubMed] [Google Scholar]
- Chlenski A, Guerrero LJ, Salwen HR, et al. Secreted protein acidic and rich in cysteine is a matrix scavenger chaperone. PLoS One. 2011;6:e23880. doi: 10.1371/journal.pone.0023880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. Thermodynamic studies of the assembly in vitro of native collagen fibrils. Biochem J. 1970;118:355–65. doi: 10.1042/bj1180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Gilks B, Price K, Churg A. Mineral dusts directly induce epithelial and interstitial fibrogenic mediators and matrix components in the airway wall. Am J Respir Crit Care Med. 1998;158:1907–13. doi: 10.1164/ajrccm.158.6.9805010. [DOI] [PubMed] [Google Scholar]
- Dancer RC, Wood AM, Thickett DR. Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J. 2011;38:1461–7. doi: 10.1183/09031936.00024711. [DOI] [PubMed] [Google Scholar]
- Davila RM, Crouch EC. Role of mesothelial and submesothelial stromal cells in matrix remodeling following pleural injury. Am J Pathol. 1993;142:547–55. [PMC free article] [PubMed] [Google Scholar]
- Del Papa N, Conforti G, Gambini D, et al. Characterization of the endothelial surface proteins recognized by anti-endothelial antibodies in primary and secondary autoimmune vasculitis. Clin Immunol Immunopathol. 1994;70:211–16. doi: 10.1006/clin.1994.1031. [DOI] [PubMed] [Google Scholar]
- Fineschi S, Goffin L, Rezzonico R, et al. Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of toll-like receptor 4. Arthritis Rheum. 2008;58:3913–23. doi: 10.1002/art.24049. [DOI] [PubMed] [Google Scholar]
- Gabrielli A, Svegliati S, Moroncini G, et al. Stimulatory autoantibodies to the PDGF receptor: a link to fibrosis in scleroderma and a pathway for novel therapeutic targets. Autoimmun Rev. 2007;7:121–6. doi: 10.1016/j.autrev.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Garcia-Prieto E, Gonzalez-Lopez A, Cabrera S, et al. Resistance to bleomycin-induced lung fibrosis in MMP-8 deficient mice is mediated by interleukin-10. PLoS One. 2010;5:e13242. doi: 10.1371/journal.pone.0013242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum SE, Ding X, Funk SE, Sage EH. SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sci U S A. 1994;91:3448–52. doi: 10.1073/pnas.91.8.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith DE, Miller EJ, Gray LD, et al. Interleukin-1-mediated release of interleukin-8 by asbestos-stimulated human pleural mesothelial cells. Am J Respir Cell Mol Biol. 1994;10:245–52. doi: 10.1165/ajrcmb.10.3.8117443. [DOI] [PubMed] [Google Scholar]
- Guarino M, Tosoni A, Nebuloni M. Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition. Hum Pathol. 2009;40:1365–76. doi: 10.1016/j.humpath.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Guo H, Leung JC, Chan LY, et al. The pathogenetic role of immunoglobulin G from patients with systemic lupus erythematosus in the development of lupus pleuritis. Rheumatology (Oxford) 2004;43:286–93. doi: 10.1093/rheumatology/keh054. [DOI] [PubMed] [Google Scholar]
- Harris BS, Zhang Y, Card L, et al. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am J Physiol Heart Circ Physiol. 2011;301:H841–7. doi: 10.1152/ajpheart.01247.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W, Amlot PL. Collagen production by human mesothelial cells in vitro. J Pathol. 1983;139:337–47. doi: 10.1002/path.1711390309. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Phan SH, Imaizumi K, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–72. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenborg M, Sorsa T, Lauhio A, Klockars M. Asbestos fibers induce release of collagenase by human polymorphonuclear leukocytes. Immunol Lett. 1990;26:25–9. doi: 10.1016/0165-2478(90)90171-l. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihn H, Sato S, Fujimoto M, et al. Characterization of autoantibodies to endothelial cells in systemic sclerosis (SSc): association with pulmonary fibrosis. Clin Exp Immunol. 2000;119:203–9. doi: 10.1046/j.1365-2249.2000.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Vernon RB, Wu H, et al. Type I collagen-deficient Mov-13 mice do not retain SPARC in the extracellular matrix: implications for fibroblast function. Dev Dyn. 1996;207:171–83. doi: 10.1002/(SICI)1097-0177(199610)207:2<171::AID-AJA5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kamp DW. Asbestos-induced lung diseases: an update. Transl Res. 2009;153:143–52. doi: 10.1016/j.trsl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara M, Kuwahara M, Verma K, et al. Asbestos exposure stimulates pleural mesothelial cells to secrete the fibroblast chemo-attractant, fibronectin. Am J Respir Cell Mol Biol. 1994;10:167–76. doi: 10.1165/ajrcmb.10.2.8110473. [DOI] [PubMed] [Google Scholar]
- Lange A. An epidemiological survey of immunological abnormalities in asbestos workers. II. Serum immunoglobulin levels. Environ Res. 1980;22:176–83. doi: 10.1016/0013-9351(80)90129-2. [DOI] [PubMed] [Google Scholar]
- Larson TC, Meyer CA, Kapil V, et al. Workers with Libby amphibole exposure: retrospective identification and progression of radiographic changes. Radiology. 2010;255:924–33. doi: 10.1148/radiol.10091447. [DOI] [PubMed] [Google Scholar]
- Liu JY, Brody AR. Increased TGF-beta1 in the lungs of asbestos-exposed rats and mice: reduced expression in TNF-alpha receptor knockout mice. J Environ Pathol Toxicol Oncol. 2001;20:97–108. [PubMed] [Google Scholar]
- Liu JY, Morris GF, Lei WH, et al. Up-regulated expression of transforming growth factor-alpha in the bronchiolar-alveolar duct regions of asbestos-exposed rats. Am J Pathol. 1996;149:205–17. [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Mao H, Nie J, et al. Transforming growth factor {beta}1 induces epithelial-mesenchymal transition by activating the JNK-Smad3 pathway in rat peritoneal mesothelial cells. Perit Dial Int. 2008;28:S88–95. [PubMed] [Google Scholar]
- Marchand LS, St-Hilaire S, Putnam EA, et al. Mesothelial cell and anti-nuclear autoantibodies associated with pleural abnormalities in an asbestos exposed population of Libby MT. Toxicol Lett. 2012;208:168–73. doi: 10.1016/j.toxlet.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek N, Shahab J, Sodek J, Ringuette M. Is SPARC an evolutionarily conserved collagen chaperone? J Dent Res. 2007;86:296–305. doi: 10.1177/154405910708600402. [DOI] [PubMed] [Google Scholar]
- May JV, Frost JP, Schomberg DW. Differential effects of epidermal growth factor, somatomedin-C/insulin-like growth factor I, and transforming growth factor-beta on porcine granulosa cell deoxyribonucleic acid synthesis and cell proliferation. Endocrinology. 1988;123:168–79. doi: 10.1210/endo-123-1-168. [DOI] [PubMed] [Google Scholar]
- Nasreen N, Mohammed KA, Mubarak KK, et al. Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-beta1 in vitro. Am J Physiol Lung Cell Mol Physiol. 2009;297:L115–24. doi: 10.1152/ajplung.90587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK, Suthar AM, Patel MM, et al. Humoral immunological profile of workers exposed to asbestos in asbestos mines. Indian J Med Res. 1993;98:274–7. [PubMed] [Google Scholar]
- Noonan CW, Pfau JC, Larson TC, Spence MR. Nested case-control study of autoimmune disease in an asbestos-exposed population. Environ Health Perspect. 2006;114:1243–7. doi: 10.1289/ehp.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh CK, Ariue B, Alban RF, et al. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002;294:1155–60. doi: 10.1016/S0006-291X(02)00577-6. [DOI] [PubMed] [Google Scholar]
- Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S79–84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- Peipins LA, Lewin M, Campolucci S, et al. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environ Health Perspect. 2003;111:1753–9. doi: 10.1289/ehp.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershouse MA, Smartt AM, Schwanke C, Putnam EA. Differences in gene expression profiles from asbestos-treated SPARC-null and wild-type mouse lungs. Genomics. 2009;94:101–9. doi: 10.1016/j.ygeno.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau JC, Li S, Holland S, Sentissi JJ. Alteration of fibroblast phenotype by asbestos-induced autoantibodies. J Immunotoxicol. 2011;8:159–69. doi: 10.3109/1547691X.2011.562257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau JC, Sentissi JJ, Weller G, Putnam EA. Assessment of autoimmune responses associated with asbestos exposure in Libby, Montana, USA. Environ Health Perspect. 2005;113:25–30. doi: 10.1289/ehp.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–9S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–7. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–80. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudineau Y, Revelen R, Levy Y, et al. Anti-endothelial cell antibodies in systemic sclerosis. Clin Diagn Lab Immunol. 1999;6:156–60. doi: 10.1128/cdli.6.2.156-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz TJ, Poobalarahi F, Bornstein P, et al. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282:22062–71. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- Rohs AM, Lockey JE, Dunning KK, et al. Low-level fiber-induced radiographic changes caused by Libby vermiculite: a 25-year follow-up study. Am J Respir Crit Care Med. 2008;177:630–7. doi: 10.1164/rccm.200706-841OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage H, Decker J, Funk S, Chow M. SPARC: a Ca2+-binding extracellular protein associated with endothelial cell injury and proliferation. J Mol Cell Cardiol. 1989a;21:13–22. doi: 10.1016/0022-2828(89)90833-x. [DOI] [PubMed] [Google Scholar]
- Sage H, Vernon RB, Decker J, et al. Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem. 1989b;37:819–29. doi: 10.1177/37.6.2723400. [DOI] [PubMed] [Google Scholar]
- Scabilloni JF, Wang L, Antonini JM, et al. Matrix metalloproteinase induction in fibrosis and fibrotic nodule formation due to silica inhalation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L709–17. doi: 10.1152/ajplung.00034.2004. [DOI] [PubMed] [Google Scholar]
- Siegel RC. Collagen cross-linking. Synthesis of collagen cross-links in vitro with highly purified lysyl oxidase. J Biol Chem. 1976;251:5786–92. [PubMed] [Google Scholar]
- Strandjord TP, Madtes DK, Weiss DJ, Sage EH. Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. Am J Physiol. 1999;277:L628–35. doi: 10.1152/ajplung.1999.277.3.L628. [DOI] [PubMed] [Google Scholar]
- Sullivan PA. Vermiculite, respiratory disease, and asbestos exposure in Libby, Montana: update of a cohort mortality study. Environ Health Perspect. 2007;115:579–85. doi: 10.1289/ehp.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HJ, Johnson CE, Lessner SM, et al. Matrix metalloproteinase 9 facilitates collagen remodeling and angiogenesis for vascular constructs. Tissue Eng. 2005;11:267–76. doi: 10.1089/ten.2005.11.267. [DOI] [PubMed] [Google Scholar]
- Tamura M, Liang D, Tokuyama T, et al. Study on the relationship between appearance of autoantibodies and chest X-ray findings of asbestos plant employees. Sangyo Igaku. 1993;35:406–12. doi: 10.1539/joh1959.35.406. [DOI] [PubMed] [Google Scholar]
- Tamura M, Tokuyama T, Kasuga H, et al. Study on correlation between chest X-P course findings and change in antinuclear antibody in asbestos plant employees. Sangyo Eiseigaku Zasshi. 1996;38:138–41. [PubMed] [Google Scholar]
- Tan RJ, Fattman CL, Niehouse LM, et al. Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice. Am J Respir Cell Mol Biol. 2006;35:289–97. doi: 10.1165/rcmb.2005-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Lai S, Guo X, et al. Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res Ther. 2010;12:R60. doi: 10.1186/ar2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AC. Asbestos-related pleural disease due to tremolite associated with progressive loss of lung function: serial observations in 123 miners, family members, and residents of Libby, Montana. Am J Ind Med. 2004;46:219–25. doi: 10.1002/ajim.20053. [DOI] [PubMed] [Google Scholar]
- Whitehouse AC, Black CB, Heppe MS, et al. Environmental exposure to Libby Asbestos and mesotheliomas. Am J Ind Med. 2008;51:877–80. doi: 10.1002/ajim.20620. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–32. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Gharaee-Kermani M, Zhang K, et al. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148:527–37. [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–25. [PMC free article] [PubMed] [Google Scholar]