Abstract
In humans, passive immunotherapy with anti-CD20 monoclonal antibodies (mAbs) has created immeasurable improvements in outcomes of patients with B-cell malignancies. However, the lack of comparable reagents has precluded development of this approach in dogs. We developed a novel anti-canine CD20 mAb designated as 6C8. 6C8 recognized the extracellular domain of canine CD20 and showed high-affinity binding to canine CD20 in solution, as well as in its native conformation on canine B-cells. The 6C8 target was expressed invariably in B-cell lineage cells, but not in T-cells or in myeloid cells. 6C8 promoted phagocytosis of B-cell lymphoma cells by macrophages, but in its current framework, it did not induce direct cytotoxicity or complement dependent cytotoxicity. In summary, we have established a novel anti-canine CD20 mAb that is useful as a diagnostic tool to phenotype B-cells, and which could be integrated as a tool for passive immunotherapy to treat dogs with B-cell disorders.
Keywords: B-cell lymphoma, CD20, monoclonal antibody, dogs, phagocytosis
INTRODUCTION
Lymphomas are among the most common malignant tumors of dogs. While the term “lymphoma” describes various clinical entities, about 70% of all lymphomas in dogs originate from transformed B cells [1]. Dogs diagnosed with the most common types of B-cell lymphoma (diffuse large B-cell lymphoma and marginal zone lymphoma) survive less than 6 weeks without treatment. The introduction of multi-agent chemotherapy protocols as the standard of care for canine lymphoma in the 80’s and 90’s made this disease treatable, if not curable. Survival increases progressively with chemotherapy: about 50% of dogs with lymphoma treated with multi-agent chemotherapy will survive 10-14 months, and ~10% will survive for two years [2-7]. Even now though, therapy is beyond the reach of many owners and more dogs receive palliative care than standard of care. This is due in part to the potential side effects of chemotherapy, which sometimes makes veterinarians uncomfortable and lead owners to opt out of chemotherapeutic treatments for their dogs.
The introduction of passive immunotherapies changed the landscape of therapy for human B-cell lymphoma: the rate of durable remissions increased to ~60%, a 30% gain over what was achievable with chemotherapy alone, and it continues to show improvement [8]. Even though rituximab (the first FDA-approved anti-CD20 antibody) is not curative, its effect to extend life with minimal toxicity has made it an unqualified medical success. The consistent expression of CD20 has been confirmed in canine B-cell lymphomas by immunohistochemistry using anti-human CD20 polyclonal antibodies that recognize the intracellular domains of CD20 [9]. However, rituximab and other currently available anti-human or anti-mouse CD20 antibodies specific to the extracellular domains do not bind native canine CD20, and thus cannot be used as tools for passive immunotherapy in dogs [9,10]. Therefore, species-specific tools are needed to develop comparable passive immunotherapy approaches to treat canine B-cell lymphoma.
Here, we report a newly established anti-canine CD20 monoclonal antibody that can be used to identify B-cells by flow cytometry and that stably binds an epitope that promotes macrophage-mediated phagocytosis of canine B-cell lymphoma cells in vitro. This antibody thus provides a new diagnostic tool to phenotype canine hematopoietic malignancies and has favorable properties for therapeutic applications.
MATERIALS AND METHODS
Cells
Primary B-cell lymphoma, T-cell lymphoma, and chronic myeloid leukemia (CML) samples, and blood samples were obtained from pet dogs with owner consent with IACUC approval (protocols 0802A27363 and 1101A94713 approved by the University of Minnesota IACUC). Tumor cells were prepared from sterile lymph node biopsy samples from dogs with lymphoma and cryopreserved in liquid nitrogen. The canine B-cell lymphoma cell line CLBL1 was obtained from Dr. Barbara Rütgen (University of Vienna, Austria) [11] and cultured in complete Iscove’s Modified Dulbecco's medium (IMDM) (Gibco/BRL, Grand Island, NY) containing 20% fetal bovine serum (FBS; Atlas Biologicals, Fort Collins, CO), L-glutamine (Mediatech Inc., Manassas, VA), and Primocin (Invivogen, San Diego, CA). A mouse macrophage cell line Raw264.7 was obtained form Dr. Kaylee Schwertfeger (University of Minnesota); COS7 (an African green monkey fibroblastic-like kidney cell line that is permissive for ectopic expression of mammalian proteins) was from the American Type Culture Collection (ATCC, Manassas, VA). Both of these cell lines were maintained in Dulbecco's Modified Eagle Medium (DMEM) (Gibco/BRL) containing 10% FBS and Primocin. A human diffuse large B-cell lymphoma cell line SU-DHL4 was obtained from Dr. Lee Honigberg (Pharmacyclics, Inc., Sunnyvale, CA) and maintained in RPMI1640 (Gibco/BRL) supplemented with 10% FBS, 2-mercaptoethanol (Gibco/BRL), HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], L-glutamine, sodium pyruvate (Mediatech, Inc.), non-essential amino acid (Sigma-Aldrich, St. Louis, MO) and Primocin. All cells were maintained at 37°C in a humidified 5% CO2 atmosphere.
Preparation of anti-canine CD20 monoclonal antibodies
Anti-canine CD20 mAbs were developed and provided for this study by IDEXX Laboratories, Inc. (Westbrook, ME). The full-length canine CD20 gene sequence was cloned from canine peripheral blood mononuclear cells (PBMCs) cDNA (GenBank Accession: GP432299) and the amino acid sequence of canine CD20 was determined (ACS04289). A polypeptide sequence that represents the major extracellular domain of the canine CD20 protein was identified (ACS04293; CaCD20 ED, Figure 1). The CaCD20 ED peptide was synthesized alone, as well as in conjunction with a H-2b restricted ovalbumin class II epitope (ISQAVHAAHAEINEAGR). The latter peptide was conjugated to keyhole limpet hemocyanin (KLH) and used for immunization to generate monoclonal antibodies against the predominant extracellular domain of canine CD20. Two female Balb/c mice were immunized with 75ug of KLH:CD20 peptide in Freund's complete adjuvant by intraperitoneal injection. The second and third immunizations occurred at 3-week intervals and utilized Freund's incomplete adjuvant. Standard fusion protocols were followed to generate hybridomas [12]. To identify positive clones for CaCD20 ED, a polypeptide enzyme-linked immunosorbent assay (ELISA) was performed. One mg of the CaCD20ED peptide was dissolved in 1 ml of dimethyl sulfoxide (Sigma-Aldrich) and the peptide was coated onto 96-well microtiter plates (Immunlon 4HB, Dynatech) at a concentration of 10 μg/ml in 50 mM carbonate buffer (pH 9.0) overnight at room temperature. Plates were washed four times in phosphate buffered saline (pH 7.2, 0.05% Tween-20 (PBS-T) and blocked with 2% Tween-20 in 100 mM Tris (pH 7.4) for 2 hours at room temperature. Plates were washed four times in PBS-T before hybridoma supernatants or purified antibody were added. Plates were incubated for 1 hour at room temperature, washed four times in PBS-T, and a 1:2500 dilution of horseradish peroxidase conjugated goat anti-mouse IgG antibody (Jackson ImmunoResearch, West Grove, PA) in sample diluent (50 mM Tris (pH 7.2), 0.05% Tween-20, 50% FBS) was added. Following an one-hour incubation at room temperature, plates were washed six times in PBS-T and developed with a 3,3',5,5'-Tetramethylbenzidine (TMB) substrate. ELISAs using fragments of the CaCD20ED peptide were performed in the same manner. Positive clones were propagated using a modified limiting dilution technique. Hybridoma 6C8 was isotyped as an IgG1 using a commercially available kit (Roche Applied Sciences, Indianapolis, IN). Antibody was produced in ascites from 5 Balb/c × ICR F1 mice and purified using protein G sepharose immunochromatography as described [12].
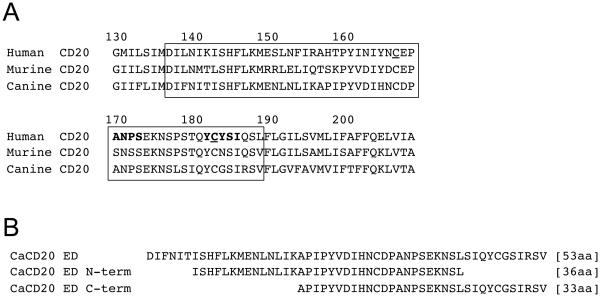
Figure 1.
Amino acid sequence of human, mouse, and canine CD20. (A) Amino acid sequences of human CD20 (AAA35581), murine CD20 (AAA37394), and canine CD20 (ACS04289), are shown. Numbers indicate amino acid positions in human CD20. The box represents the large CD20 extracellular loop between the third and forth transmembrane domains that contains a binding site of rituximab. The bold font represents a proposed primary binding site (170ANPS173) [16-18] and a suggested discontinuous epitope (182YCYSI185) [16] of rituximab. The underline represents Cys167 and Cys183 that are predicted to form a disulfide-bond and have also been implicated to play an important role in the recognition and binding of rituximab [15]. (B) Polypeptide sequences of the extracellular domain of canine CD20 (CaCD20 ED).y
Immunoblotting
COS7 cells were transfected with a plasmid (pCMV4-FLAG, Sigma-Aldrich) containing the canine CD20 sequence (GenBank Accession: GP432299) using Lipofectamine 2000 Reagent (Life Technologies, Carlsbad, CA) according to the manufacture’s protocol (CaCD20 COS7). Control COS7 cells were transfected with a plasmid encoding an unrelated protein (ILTV). Cryopreserved primary canine B-cell chronic lymphoid leukemia (CLL) cells and COS7 cells at 48 hours post-transfection were resuspended in 50-200ul of sample buffer, sonicated, and heated to 80°C for 10 minutes. Cell lysates were analyzed by gel electrophoresis using gradient gels in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (NuPAGE 4-12% Bis-Tris Gels; Novex, Life Technologies). Immunoblots were performed using 4 μg/ml of monoclonal antibody 6C8 or a commercially available rabbit anti-human CD20 polyclonal antibody directed against the intracellular domain of CD20 (Thermo Scientific, Waltham, MA) [9]. HRP conjugated goat anti-mouse or goat anti-rabbit secondary antibodies were used at a 1:5000 dilution. Blots were developed using either a colorometric (Immunoblot Opti 4-CN, Bio-Rad Laboratories Inc., Hercules, CA) or chemiluminescent (ECL, GE Healthcare LifeSciences, Pittsburgh, PA) reagents for 1 minute and visualized using GeneGnome (Syngene, Frederick, MD).
Flow cytometry
We prepared single cell suspension from 29 primary canine lymphoma biopsy samples submitted to our laboratory between 11/20/2009 and 5/25/2011 and analyzed them using flow cytometry as described [13]. At the time of the flow cytometry analysis, the phenotype of the samples was not known. Diagnosis of B-cell and T-cell lymphoma was confirmed by histopathology and immunohistochemistry by board certified pathologists. Cells were incubated with dog immunoglobulin G (IgG; Jackson ImmunoResearch) to prevent non-specific binding of antibodies to Fc receptors and stained using phycoerythrin (PE) or fluorescein isothiocyanate (FITC) conjugated antibodies against dog CD3 (CA17.2A12), dog CD5 (YKIX322.3), dog CD45 (YKIX716.13) (eBioscience, San Diego, CA), human CD14 (TÜK4), dog CD21 (CA2.1D6; AbD Serotec, Raleigh, NC), and human CD22 (RFB4; Abcam, Cambridge, MA) [13]. Antibody 6C8 was labeled using the Zenon anti-mouse IgG1 Alexa-Fluor 647 labeling kit (Invitrogen-Molecular Probes, Carlsbad, CA). 0.5 μg/mL 7-amino-actinomycin D (7-AAD; eBioscience) was added to each tube before flow cytometry analysis. Tumor cells and lymphocytes were gated based on their light scatter properties and dead cells were excluded using 7-AAD staining. Flow cytometry was performed using a LSRII cytometer (BD Immunocytometry Systems, San Jose, CA) and results were analyzed using FlowJo software (Tree Star, Ashland, OR).
In vitro cytotoxicity assay - Direct toxicity, CDC
Cell viability was determined by the MTS (3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay using the CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI). Briefly, 1 × 105 primary B-cell lymphoma cells and SU-DHL4 cells were cultured in 100 μL of complete medium in 96-well plates in the presence of anti-CD20 antibody (6C8 or rituximab) or mouse IgG1 antibody. To ensure the viability of primary B-cell lymphoma cells, we supplemented 100 ng/ml megaCD40L (Enzo Life Sciences, Inc., Farmingdale, NY) in culture as described [14]. After 72 hours, 20 μL of MTS solution was added to each well and cells were incubated for another 4 hours before measuring absorbance at 490 nm using a Wallac Victor2 1420 Multilabel Counter (Perkin Elmer, Waltham, MA). For complement-dependent cytotoxicity (CDC) assays, 3-4 week old baby rabbit complement (1:50 dilution at final, Life Technologies) were added to wells and incubated for 2 hours followed by the MTS assay.
In vitro phagocytosis assay
Two-hundred thousand Raw264.7 cells were plated in each well of a 12-well tissue culture plate in DMEM containing 10% FBS with mouse IFNγ (100 ng/ml). On the next day, the medium was replaced with serum-free IMDM and incubated at 37°C for 2 hrs. Primary B-cell lymphoma cells were labeled with CFSE; CLBL1 cells were genetically modified to stably express GFP (CLBL1-GFP) using the 4D nucleofection method (Lonza, Allendale, NJ). Four-hundred thousand CFSE-labeled primary B-cell lymphoma cells or CLBL1-GFP cells were resuspended in serum-free IMDM and added to the wells containing Raw264.7 cells at a target:effector cell ratio of 2:1. Ten μg/ml of the indicated antibodies were added to each well, centrifuged at 1,000 rpm for 2 minutes, and incubated at 37°C for 2 hours to allow phagocytosis to take place. At the end of this incubation period, cells were harvested using Trypsin-EDTA, stained with anti-mouse CD45 conjugated to PE (BD Biosciences) to label the Raw264.7 cells, and analyzed using flow cytometry.
RESULTS
Anti-canine CD20 mAb 6C8 recognizes the canine CD20 extracellular domain
CD20 is a tetra-spanning membrane protein with a molecular weight of approximately 35-kD. Both termini are in the cytoplasm and there is a large extracellular loop between the third and fourth transmembrane domains (Figure 1A) [15]. It is reported that rituximab primarily recognizes 170ANPS173 [16-18] and the involvement of a discontinuous epitope 182YCYSI185 [16] and a disulfide bond between Cys167 and Cys183 that bridges these epitopes [15] has also been implicated to play an important role in the recognition and binding of rituximab.
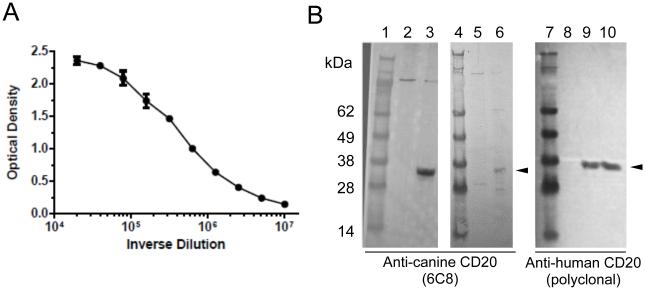
We immunized mice using a peptide containing the extracellular domain of canine CD20 (Figure 1B) and established hybridomas that produce anti-canine CD20 monoclonal antibodies. By screening them using CaCD20 ED, we identified clone 6C8 (IgG1) that recognized the CaCD20 ED peptide with high affinity (Figure 2A). We also confirmed that 6C8 bound to the N-terminal fragment of CaCD20 ED, but not to the C-terminal fragment of CaCD20 ED (Table I and Figure 1B) even though both peptide fragments contain at least one of the two proposed rituximab recognition epitopes [16]. We also demonstrated that 6C8 detected a protein of ~35 kDa in lysates from COS7 cells transfected with canine CD20, as well as from a primary canine B-cell malignancy by immunoblotting (Figure 2B).
Figure 2.
6C8 recognizes the extracellular domain of canine CD20. (A) ELISA for 6C8 using the full-length of CaCD20 ED. (B) Immunoblotting analysis to detect canine CD20 using 6C8 and rabbit anti-human CD20 polyclonal antibody: lane 1, molecular weight marker (MWM); lane 2, Control COS7; lane 3, CaCD20 COS7; lane 4, MWM; lane 5, Control COS7; lane 6, primary canine CLL; lane 7, MWM; lane 8, Control; lane 9, CaCD20 COS 7; lane 10, primary canine CLL.
Table I.
Binding of 6C8 to CaCD20 ED polypeptides
| CaCD20 ED | C-terminal CaCD20 ED |
Cyclic C-terminal CaCD20 ED |
N-terminal CaCD20 ED |
|---|---|---|---|
| + | − | − | + |
Antibody 6C8 uniquely binds to canine B-cells
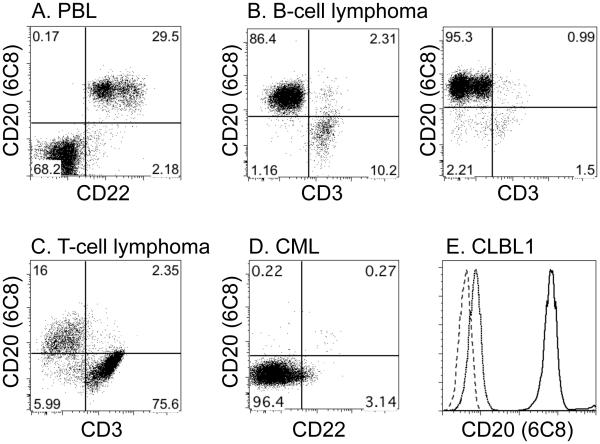
CD20 is initially upregulated in late pro-B-cells and its expression is maintained throughout development in both naïve and memory B-cells [19]. CD20 is not expressed in plasma cells, but its expression has been reported in a small subset of normal human T-cells, and rarely, in human T-cell malignancies [20,21]. We previously showed that CD20 expression, as measured by immunohistochemistry staining of the intracellular domains, was invariably seen in canine B-cell lymphomas, but not in T-cell lymphomas [9], leading to routine use of this method to phenotype canine lymphomas in fixed tissues. Thus, we first examined the performance of antibody 6C8 as a tool to phenotype canine B-cells using flow cytometry. We analyzed the binding of 6C8 to peripheral blood lymphocytes obtained from 2 healthy dogs, 22 primary B-cell lymphoma samples, 7 primary T-cell lymphoma samples, 1 primary CML sample, and the canine B-cell lymphoma cell line CLBL1. 6C8 invariably bound an antigen expressed on the surface of canine B-cells, including peripheral CD22+ B-cells (Figure 3A), CD22+ B-cell lymphoma cells (Figure 3B), and residual CD22+ B-cells in T-cell lymphoma samples (Figure 3C). In contrast, residual CD3+ T-cells in B-cell lymphoma samples (Figure 3B), T-cell lymphoma cells and CML cells did not bind 6C8 (Figure 3C and 3D). CLBL1 was also positively stained by 6C8 (Figure 3E). The phenotyping result of all 29 lymphoma samples was summarized in Supplementary Table I.
Figure 3.
6C8 specifically binds B-cells and B-cell lymphoma cells. (A-D) Representative examples of two-dimensional flow cytometry dot plot analysis of canine peripheral blood lymphocytes (PBL), primary canine B-cell lymphoma, T-cell lymphoma, and CML, using 6C8 and antibodies for lymphocyte markers (CD3 and CD22) are shown. (E) One-dimensional histogram of 6C8 binding to CLBL1 cells where the dashed line, the dotted line, and the solid line represent no antibody, isotype control antibody, and 6C8 staining, respectively. Phenotyping results of all lymphoma samples tested are summarized in Supplementary Table I.
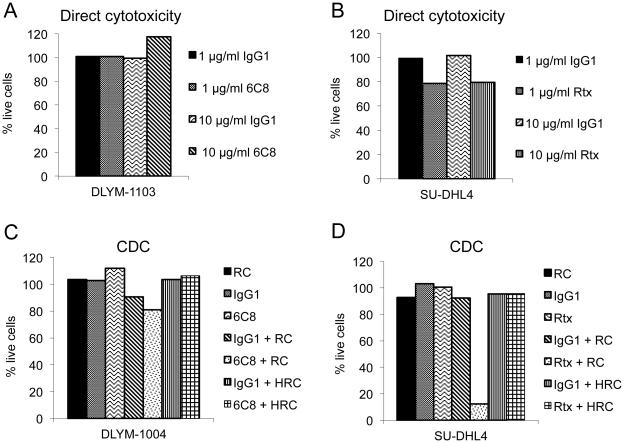
Antibody 6C8 does not mediate direct or complement-dependent cytotoxicity against canine B-cell lymphoma cells
Three biological mechanisms have been recognized for the elimination of B-cells by rituximab and other anti-CD20 mAbs, which include direct cytotoxicity (induction of apoptosis), CDC, and, antibody-dependent cellular cytotoxicity (ADCC) mediated by effector cells [22]. We first examined whether antibody 6C8 had direct cytotoxicity against canine B-cell lymphoma cells. 6C8 showed no direct cytotoxicity against primary canine B-cell lymphoma cells (Figure 4A and Supplementary Table II). In contrast, rituximab exhibited modest cytotoxicity against human malignant B-cells (SU-DHL4) (Figure 4B). Next, the CDC activity of 6C8, the ability to fix complement on the cell surface and lyse target cells, was analyzed by culturing primary canine B-cell lymphoma cells in the presence of 6C8 and rabbit complement. 6C8 did not show CDC activity against primary canine B-cell lymphoma cells (Figure 4C and Supplementary Table III). In contrast, rituximab induced complement-mediated cell lysis against SU-DHL4 (Figure 4D). We also obtained the equivalent results of CDC assay in two additional independent primary dog B-cell lymphoma samples using the KtCD40L feeder cell system as described [14] (data not shown). Intriguingly, antibody 6C8 enhanced ADCC of canine B-cell lymphoma cells when activated macrophages were present in the cultures (Supplementary Figure 1), perhaps by bringing the target and effector cells into close proximity.
Figure 4.
Assessment of direct cytotoxicity and CDC of 6C8 against canine B-cell lymphoma cells. (A) Primary canine B-cell lymphoma cells were cultured with 6C8 or mouse IgG1 in the presence of soluble CD40L for 72 hours. Replicate samples (duplicates or triplicates depending on the number of cells available from each sample tested) were tested in each experiment. The percent of live cells was normalized to the number of cells at the end of experiment in the conditions with no antibody treatment using the MTS assay. One representative example of four independent canine B-cell lymphomas tested is shown. A summary of the results from all four samples is provided in Supplementary Table II. (B) Human B-cell lymphoma cell line SU-DHL4 was cultured with rituximab (Rtx) or mouse IgG1 for 72 hours. The average of duplicate results for each condition was shown. (C) Primary canine B-cell lymphoma cells with soluble CD40L were cultured with 6C8 or mouse IgG1 in the presence of rabbit complement (RC) or heat-inactivated rabbit complement (HRC) for 2 hours. Experiments were performed in duplicate or triplicate and normalized to the number of cells in the RC only treatment condition using the MTS assay. One representative sample of three independent canine B-cell lymphomas tested is shown. A summary of the results from all three samples is provided in Supplementary Table III. (D) SU-DHL4 was cultured with Rtx or mouse IgG1 in the presence of RC or HRC for 2 hours. The average of duplicate results for each condition was shown.
Antibody 6C8 induces macrophage-mediated phagocytosis of canine B-cell lymphoma cells
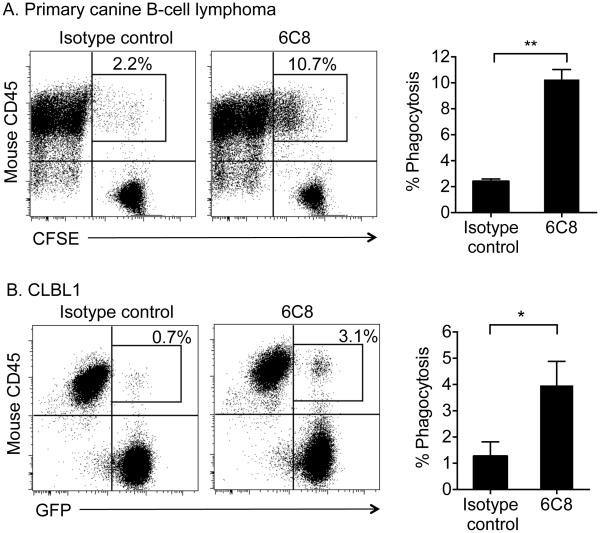
The therapeutic effects of rituximab, as well as some of its side effects, also appear to rely on antibody-dependent B cell phagocytosis. Hence, we investigated whether antibody 6C8 would promote phagocytosis of canine B-cell lymphoma cells by activated macrophages. We pre-activated mouse Raw264.7 cells with IFNγ and then co-cultured them with CFSE-labeled primary B-cell lymphoma cells or GFP expressing CLBL1 cells in the presence of an irrelevant IgG1 isotype control or antibody 6C8 for 2 hours at 37°C. We quantified macrophage-dependent phagocytosis using flow cytometry. Antibody 6C8 enabled macrophages to phagocytize both primary B-cell lymphoma cells and CLBL1 cells as determined by the increase in dually-labeled cells compared to control (Figure 5), indicating that in addition to its ability to promote ADCC by effector cells, the binding of antibody 6C8 to canine CD20 is sufficiently stable to promote tumor cell removal through phagocytosis by the same effector cells.
Figure 5.
Antibody 6C8 induces macrophage-mediated phagocytosis of canine B-cell lymphoma cells. (A) CFSE-labeled primary canine B-cell lymphoma cells and (B) CLBL1-GFP cells were co-cultured with IFNγ-primed RAW-264.7 cells in the presence of 6C8 or isotype control antibody, and phagocytosis was analyzed using flow cytometry. Cell populations after excluding cell debris and 7-AAD+ dead cells are shown. Boxes represent RAW-264.7 cells that engulfed tumor cells, thus emitting fluorescence from both CFSE/GFP and PE. Representative flow cytometry plots with the percent of RAW-264.7 cells that engulfed tumor cells within total RAW-264.7 cells are shown. Summary of phagocytosis assay results using primary B-cell lymphoma (n=2) and CLBL1 (n=3) are also shown with means ± S.E.M. (*P <0.05 and **P <0.01, two-tailed t test).
DISCUSSION
CD20 is expressed consistently on almost all human B-cells, and several mAbs against this molecule are now approved or in advanced clinical trials to treat human B-cell malignancies. Rituximab, the first anti-CD20 mAb approved by the FDA, is recommended as first-line therapy for some subtypes of non-Hodgkin lymphoma (NHL), as adjuvant therapy for other subtypes of NHL, and as rescue therapy for relapsed or refractory NHL [23]. Like most other anti-CD20 mAbs that show clinical benefit, rituximab is a type-I antibody that promotes redistribution of CD20 to detergent-insoluble lipid rafts [22]. Thus, both the CD20 epitope and the consequences of binding such epitope appear to be therapeutically important. Even though rituximab does not bind to canine CD20 [9,10], the proposed epitope(s) of rituximab in human CD20 is(are) relatively conserved in canine CD20 (Figure 1), suggesting there are additional mechanisms, such as the structural formation of the large extracellular loop of canine CD20, which determine the binding avidity of anti-CD20 antibodies to canine CD20 in this region.
Specific and reproducible expression of CD20 in canine B-cells is established [9], but until now, an antibody that would specifically bind to the extracellular domain of canine CD20 had not been reported. This created both diagnostic and therapeutic limitations for dogs with B-cell malignancies and other B-cell dependent conditions, as expression of CD20 could only be measured in fixed or permeabilized cells and anti-CD20-targeted passive immunotherapy was not feasible. Here, we report the development of 6C8, a mAb that binds to the extracellular loop domain of canine CD20 and that shows excellent performance and exquisite specificity to bind canine B-cells as measured by multi-parameter flow cytometry. In addition, antibody 6C8 promotes ADCC and phagocytosis of malignant canine B-cells that is mediated by activated effector macrophages. 6C8 did not show apparent direct cytotoxicity and CDC against canine B-cell tumor cells. This is not entirely surprising because direct toxicity and CDC induced by anti-human CD20 mAbs also varies considerably. The success of anti-CD20 mAbs in mediating CDC against malignant B cells appears to be dependent on multiple factors, including CD20 density in target cells, the ability to concentrate CD20 to detergent-insoluble lipid rafts, slow off-rates of the mAb, close proximity of Fc regions to the target membrane, and the heavy chain isotype of the antibody [24,25]. Overall, the relative importance of Fc-dependent effector functions and antibody mediated cell death during treatment of lymphoma patients is still disputed, and it seems likely that a combination of effector mechanisms underlies the therapeutic success of rituximab and other CD20 mAb [22,26].
The natural ligand for CD20, if one exists, is unknown, although the molecule can initiate signal transduction upon antibody binding [19]. The variance in the sequence of the extracellular loop domain among different species could be explained by the absence of a natural ligand, which would reduce or eliminate evolutionary constraints associated with its signaling function, although we cannot exclude the possibility that this drift is due to co-evolution of a ligand-receptor pair. Nevertheless, this peculiar feature of CD20 has made it challenging to refine mechanisms and pharmacology associated with targeting this antigen in naturally occurring animal models of B-cell lymphoma, instead having to rely on xenotransplant models. The availability of antibody 6C8, which binds the large extracellular loop of canine CD20 will facilitate such studies, including homology of signal transduction and mechanisms of cell death. Antibody 6C8 is well suited to flow-based phenotyping and other diagnostic applications that require enumeration of live B-cells in biological samples. The apparent stability of the interaction between canine CD20 on the cell surface and antibody 6C8 also support the concept to engineer the variable regions of this antibody onto a canine IgG frameworks to enhance its therapeutic potential.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Catherine Smith for antibody development and Regis Krah for assistance with protein expression constructs. This work was supported by MAF First Award Grant D12CA-302 (DI), Morris Animal Foundation D13CA-033 (JFM and DI), Skippy Frank Fund for Life Sciences and Translational Research (DI and JFM), NIH grant P30CA077598 (NCI Core Support Grant for the Masonic Cancer Center), Masonic Cancer Center Hematologic Malignancy Innovations Award, and University of Minnesota Animal Cancer Care and Research Program.
Footnotes
POTENTIAL CONFLICT OF INTEREST
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
REFERENCES
- 1.Modiano JF, Breen M, Burnett RC, et al. Distinct B-Cell and T-Cell Lymphoproliferative Disease Prevalence among Dog Breeds Indicates Heritable Risk. Cancer Res. 2005;65:5654–5661. doi: 10.1158/0008-5472.CAN-04-4613. [DOI] [PubMed] [Google Scholar]
- 2.Chun R. Lymphoma: which chemotherapy protocol and why? Top Companion Anim Med. 2009;24:157–162. doi: 10.1053/j.tcam.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Modiano JF, Breen M, Valli VE, Wojcieszyn JW, Cutter GR. Predictive value of p16 or Rb inactivation in a model of naturally occurring canine non-Hodgkin's lymphoma. Leukemia. 2007;21:184–187. doi: 10.1038/sj.leu.2404392. [DOI] [PubMed] [Google Scholar]
- 4.Frantz AM, Sarver AL, Ito D, et al. Molecular profiling reveals prognostically significant subtypes of canine lymphoma. Vet Pathol. 2013;50:693–703. doi: 10.1177/0300985812465325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrett LD, Thamm DH, Chun R, Dudley R, Vail DM. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med. 2002;16:704–709. doi: 10.1892/0891-6640(2002)016<0704:eoacpw>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald VS, Thamm DH, Kurzman ID, Turek MM, Vail DM. Does L-asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? J Vet Intern Med. 2005;19:732–736. doi: 10.1892/0891-6640(2005)19[732:dlieot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Vail DM, Pinkerton MM, Young KM. Canine Lymphoma and Lymphoid Leukemia. In: Withrow SJ, Vail DM, Page RL, editors. Withrow and MacEwen's Small Animal Clinical Oncology. 5th St. Louis; Saunders: 2012. pp. 608–638. [Google Scholar]
- 8.Traullé C, Coiffier BB. Evolving role of rituximab in the treatment of patients with non-Hodgkin's lymphoma. Future Oncol. 2005;1:297–306. doi: 10.1517/14796694.1.3.297. [DOI] [PubMed] [Google Scholar]
- 9.Jubala CM, Wojcieszyn JW, Valli VEO, et al. CD20 expression in normal canine B cells and in canine non-Hodgkin lymphoma. 2005;42:468–476. doi: 10.1354/vp.42-4-468. [DOI] [PubMed] [Google Scholar]
- 10.Impellizeri JA, Howell K, McKeever KP, Crow SE. The role of rituximab in the treatment of canine lymphoma: an ex vivo evaluation. Vet J. 2006;171:556–558. doi: 10.1016/j.tvjl.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Rütgen BC, Hammer SE, Gerner W, et al. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk Res. 2010;34:932–938. doi: 10.1016/j.leukres.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies : a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. pp. 139–318. [Google Scholar]
- 13.Ito D, Endicott MM, Jubala CM, et al. A tumor-related lymphoid progenitor population supports hierarchical tumor organization in canine B-cell lymphoma. J Vet Intern Med. 2011;25:890–896. doi: 10.1111/j.1939-1676.2011.0756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito D, Frantz AM, Williams C, et al. CD40 ligand is necessary and sufficient to support primary diffuse large B-cell lymphoma cells in culture: a tool for in vitropreclinical studies with primary B-cell malignancies. Leuk Lymphoma. 2012;53:1390–1398. doi: 10.3109/10428194.2011.654337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst JA, Li H, Kim HS, Nakamura GR, Yansura DG, Vandlen RL. Isolation and characterization of the B-cell marker CD20. Biochemistry. 2005;44:15150–15158. doi: 10.1021/bi0511078. [DOI] [PubMed] [Google Scholar]
- 16.Binder M, Otto F, Mertelsmann R, Veelken H, Trepel M. The epitope recognized by rituximab. Blood. 2006;108:1975–1978. doi: 10.1182/blood-2006-04-014639. [DOI] [PubMed] [Google Scholar]
- 17.Perosa F, Favoino E, Caragnano MA, Dammacco F. Generation of biologically active linear and cyclic peptides has revealed a unique fine specificity of rituximab and its possible cross-reactivity with acid sphingomyelinase-like phosphodiesterase 3b precursor. Blood. 2006;107:1070–1077. doi: 10.1182/blood-2005-04-1769. [DOI] [PubMed] [Google Scholar]
- 18.Du J, Wang H, Zhong C, et al. Structural basis for recognition of CD20 by therapeutic antibody Rituximab. J Biol Chem. 2007;282:15073–15080. doi: 10.1074/jbc.M701654200. [DOI] [PubMed] [Google Scholar]
- 19.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 20.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 21.Buckner CL, Christiansen LR, Bourgeois D, Lazarchick JJ, Lazarchick J. CD20 positive T-cell lymphoma/leukemia: a rare entity with potential diagnostic pitfalls. Ann Clin Lab Sci. 2007;37:263–267. [PubMed] [Google Scholar]
- 22.Lim SH, Beers SA, French RR, Johnson PW, Glennie MJ, Cragg MS. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica. 2010;95:135–143. doi: 10.3324/haematol.2008.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dotan E, Aggarwal C, Smith MR. Impact of rituximab (Rituxan) on the treatment of B-cell non-Hodgkin's lymphoma. 2010;35:148–157. [PMC free article] [PubMed] [Google Scholar]
- 24.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 25.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 26.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.