Abstract
OBJECTIVES
The Society for Vascular Surgery (SVS) Lower Extremity Guidelines Committee has composed a new threatened lower extremity classification system that reflects the three major factors that impact amputation risk and clinical management: wound, ischemia, and foot infection (WIfI). Our goal was to evaluate the predictive ability of this scale following any infrapopliteal endovascular intervention for critical limb ischemia (CLI).
METHODS
From 2004 to 2014, a single institution, retrospective chart review was performed at the Beth Israel Deaconess Medical Center for all patients undergoing an infrapopliteal angioplasty for CLI. Throughout these years, 673 limbs underwent an infrapopliteal endovascular intervention for tissue loss (77%), rest pain (13%), stenosis of a previously treated vessel (5%), acute limb ischemia (3%), or claudication (2%). Limbs missing a grade in any WIfI component were excluded. Limbs were stratified into clinical stages 1 to 4 based on the SVS WIfI classification for 1-year amputation risk, as well as a novel WIfI composite score from 0 to 9. Outcomes included patient functional capacity, living status, wound healing, major amputation, major adverse limb events (MALE), RAS events (reintervention, major amputation, or stenosis [>3.5x step-up by duplex]), amputation-free survival (AFS), and mortality. Predictors were identified using Kaplan-Meier survival estimates and Cox regression models.
RESULTS
Of the 596 limbs with CLI, 551 were classified in all three WIfI domains on a scale of 0 (least severe) to 3 (most severe). Of these 551, 84% were treated for tissue loss and 16% for rest pain. A Cox regression model illustrated that an increase in clinical stage increases the rate of major amputation (Hazard Ratio (HR), 1.6; 95% Confidence Interval [CI], 1.1–2.3). Separate regression models showed that a one-unit increase in the WIfI composite score is associated with a decrease in wound healing (1.2 [1.1–1.4]) and an increase in the rate of RAS events (1.2 [1.1–1.4]) and major amputations (1.4 [1.2–1.8]).
CONCLUSIONS
This study supports the ability of the SVS WIfI classification system to predict 1-year amputation, RAS events, and wound healing in patients with CLI undergoing endovascular infrapopliteal revascularization procedures.
INTRODUCTION
Since the definition of critical limb ischemia (CLI) in 1982, many classification systems have been developed in an attempt to assist with clinical decision-making and the objective stratification of patients being treated.1 With the rapidly increasing prevalence of diabetes and peripheral artery disease (PAD), however, use of the term “CLI” has been applied to a far wider spectrum of patients than was initially anticipated.2 Advances in the treatment of CLI, such as percutaneous transluminal angioplasty with or without stenting (PTA/S) and other revascularization strategies, have rendered many of the current classification systems inadequate. Furthermore, although early success with infrapopliteal PTA/S has been reported, evidence has emerged to suggest that primary treatment with PTA/S may threaten the success of subsequent bypass, further increasing the need to better understand the most effective and efficient use of this treatment strategy.3–8
To address the need for a more applicable classification arrangement that encompasses not only the changing patient demographics of CLI, but also the expanded use of revascularization interventions, the Society for Vascular Surgery Lower Extremity Guidelines Committee recently created the Lower Extremity Threatened Limb (Wound, Ischemia, foot Infection [WIfI]) Classification System (SVS WIfI).9 The SVS WIfI classification system was developed by merging the existing CLI and diabetic foot ulcer (DFU) classification systems and stratifies risk based on three major categories: wound, ischemia, and foot infections.10–16
There have been few comprehensive evaluations of the SVS WIfI as a classification system concerning its relevance to endovascular-specific procedures. In this retrospective cohort study, we sought to evaluate the predictive ability of the SVS WIfI classification system following infrapopliteal endovascular interventions for CLI.
METHODS
We performed a retrospective chart review of all Beth Israel Deaconess Medical Center patients undergoing an infrapopliteal angioplasty for CLI between 2004 and 2014 by vascular surgeons. Current Procedural Terminology (CPT) codes were used to identify all patients undergoing infrapopliteal endovascular revascularization (angioplasty and atherectomy, with or without stenting). Patients undergoing endovascular procedures that were aborted due to failure to cross the lesion and patients undergoing a diagnostic angiogram only were excluded from the study group. The follow-up interval and modality were at the discretion of the primary vascular attending, with typical practice being every 4 months for 1–2 years and every six months thereafter with arterial duplex ultrasound imaging, ankle-brachial indices, and toe pressures.
A retrospective study was performed to examine the predictive ability of the newly proposed WIfI classification system. Developed in 2013, the SVS WIfI system provides an objective classification for wound healing and limb amputation based on three independent risk factors: wound extent (e.g., size, depth, presence of gangrene), degree of ischemia, and extent of foot infection. All three factors are individually graded on a scale of 0 to 3.9 A detailed description of the SVS WIfI grading is presented in Table I.
Table I.
Detailed description of study data categories with the SVS Wound, Ischemia, and foot Infection (WIfI) grades9,18
| Wound Gradea | Ischemia Gradeb | Infection Grade |
|---|---|---|
|
|
|
ABI, ankle-brachial index; ASP, ankle systolic pressure; SIRS, systemic inflammatory response syndrome; SQ, subcutaneous tissue; TP, toe pressure.
WIfI classification system suggests that depth of wound take priority over size of wound.
If ABI and TP result in different grades, TP is recommended to determine grade.
After a limb has been graded on each of the three WIfI components, the grades are combined to create a WIfI clinical stage as a means to predict the risk of limb amputation at 1 year and, in a separate analysis, the likelihood that the limb would benefit from limb revascularization. A rubric of the WIfI clinical stages, including the consensus panel predictions regarding the risk of limb amputation at 1 year (very low risk, low risk, moderate risk, high risk) for each score, is provided in Table II. WIfI clinical stages estimated to be “very low risk” for limb amputation at 1 year are categorized as clinical stage 1. Scores deemed low risk, moderate risk, and high risk for limb amputation at 1 year are categorized as clinical stage 2, stage 3, and stage 4, respectively. Due to the potential variability in the expert consensus of the clinical stages constructed by Mills et al., we intended to generate a novel scoring system with the ability to weigh all individual components of the WIfI classification system equally. Preliminary analysis on the individual WIfI components demonstrated similar predictive ability for each, allowing us to implement a novel WIfI composite score (graded from 0–9), calculated by summing the three individual WIfI components together (e.g., a patient with a wound score of 3, an ischemia score of 2, and an infection score of 1 would have a WIfI composite score of 6). Additionally, for statistical purposes, a further stratification was created within these WIfI composite scores by separating limbs into low-risk and high-risk groups (WIfI composite 1–4 and 5–9, respectively).
Table II.
Consensus expert estimates of 1-year amputation risk based on the collaboration of Wound characteristic, Ischemia, and foot Infection (WIfI) grades9
| Ischemia-0 | Ischemia-1 | Ischemia-2 | Ischemia-3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W-0 | VL | VL | L | M | VL | L | M | H | L | L | M | H | L | M | M | H |
| W-1 | VL | VL | L | M | VL | L | M | H | L | M | H | H | M | M | H | H |
| W-2 | L | L | M | H | M | M | H | H | M | H | H | H | H | H | H | H |
| W-3 | M | M | H | H | H | H | H | H | H | H | H | H | H | H | H | H |
| fI-0 | fI-1 | fI-2 | fI-3 | fI-0 | fI-1 | fI-2 | fI-3 | fI-0 | fI-1 | fI-2 | fI-3 | fI-0 | fI-1 | fI-2 | fI-3 | |
W, Wound grade; fI , Foot infection grade; VL, very low 1-year amputation risk; L, low 1-year amputation risk; M, moderate 1-year amputation risk; H, high 1-year amputation risk.
Limbs missing an initial grade in any of the three WIfI categories were excluded from the analysis. Indications for intervention included tissue loss (i.e., ulcer or gangrene) or rest pain, where patients presenting with more than one indication for intervention were assigned as having only the most severe symptom according to the following hierarchy: gangrene, ulcer, and rest pain. As of September 2007, routine ultrasound guidance has been used by the vascular surgeons at our institution to gain percutaneous access for catheter-based interventions. Stents were placed based on the clinical judgment of the attending physician at the time of the procedure and stent type varied based on availability and surgeon preference.
All analyses were performed on a per-limb basis. Pearson chi-square and Fisher exact tests were used for comparisons of categorical variables. Primary outcomes included patient functional capacity, living status, wound healing, major amputation, major adverse limb events (reintervention or major amputation; MALE), RAS events (revascularization, major amputation, or stenosis [>3.5x step-up by duplex]), amputation-free survival (AFS), and mortality. When relevant, patients undergoing subsequent minor (toe or foot) amputations as well as wound debridements for infected or ischemic lesions were included in wound healing analyses. Both bivariate and multivariable predictors of these outcomes were identified using the log-rank test and Cox proportional hazards regression modeling. All statistical analyses were performed using STATA 12 (StataCorp, College Station, Tex). The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived the need for patient consent due to the retrospective design.
RESULTS
Of the 596 limbs receiving an infrapopliteal intervention between 2004 and 2014, 551 limbs, in 475 patients, were classified in all three WIfI domains on a scale from 0 (least severe) to 3 (most severe). Of these 551, 84% were treated for tissue loss and 16% were treated for rest pain. Mean follow-up was 15.5 ± 11 months. Kaplan-Meier analysis illustrated that 22% (N = 89) underwent a reintervention within 1 year of the index procedure, while 15% (N = 61) underwent an ipsilateral major amputation. By 3 years, 37% (N = 109) received a reintervention and 21% (N = 70) underwent an ipsilateral major amputation. Among limbs with tissue loss, complete wound healing at 1 year occurred in 37% (N = 140), with a median of 4 months (range, 1–130).
Demographics with comorbidities of patients graded in all three WIfI components are provided in Table III. Patients within this study were most often male (53%), and had an average age of approximately 71 years. Patients commonly suffered from hypertension (84%), diabetes (77%), coronary artery disease (CAD; 49%), congestive heart failure (CHF; 26%), and chronic renal insufficiency (CRI; 23%). Over half of the patients in the study (54%) had a history of smoking. Of note, pre-operative dependent living status (i.e., dependent with activities of daily living) was significantly more common throughout increasing clinical stage (stages 1–4; 40%, 20%, 26%, 42%; P = .001), WIfI composite score (scores 2–9; 20%, 23%, 29%, 33%, 41%, 50%, 85%, 100%; P = .002), and WIfI composite sub-grouping (low-risk [WIfI composite scores 1–4] vs. high-risk [WIfI composite 5–9]; 26% vs. 39%, respectively; P = .002). After adjusting for baseline characteristics, multivariable regression demonstrated that, among pre-operative ambulatory patients (independent or with assistance; 84%), the only predictor of 6-month postoperative wheel chair dependence was the increasing WIfI composite score (Hazard Ratio (HR), 1.6; 95% Confidence Interval [CI], 1.1–2.5). Death and major amputation were independently associated with dialysis dependence (2.5 [1.8–3.6] and 2.1 [1.2–3.8], respectively). Additionally, separate regression models illustrated that major amputation and RAS events were independently associated with worsening tibial Trans-Atlantic Inter-Society Consensus (TASC) classification (1.6 [1.2–2.0] and 1.2 [1.1–1.4], respectively).17 Finally, future RAS events were independently associated with limbs that had any prior lower extremity ipsilateral intervention (1.4 [1.1–1.9]). Between the clinical stages (i.e., clinical stages 1, 2, 3, and 4), there were significant differences in the proportion of patients suffering from diabetes (P = .001), dialysis dependence (P = .001), chronic renal insufficiency (P < .001), COPD (P = .03), and a history of smoking (P = .03).
Table III.
Demographics and Comorbidities of patients graded in all three WIfI components
| Variables | No. (%) or mean +/− SD (N = 475) |
|---|---|
| Age, years | 71.2 +/− 12 |
| Male gender | 251 (53) |
| Hypertension | 396 (84) |
| Diabetes | 365 (77) |
| Coronary artery disease | 232 (49) |
| Dialysis dependence | 84 (18) |
| Chronic renal insufficiency | 110 (23) |
| Congestive heart failure | 122 (26) |
| History of myocardial infarction | 94 (20) |
| COPD | 36 (8) |
| Smoking history | 213 (54) |
| Current smoker | 100 (23) |
COPD, chronic obstructive pulmonary disease
After converting the initial wound, ischemia, and infection data of the study population into WIfI grades and, further, into a specific WIfI clinical stage (1–4), we were able to parallel our observed 1-year limb amputation rates with Mills’ WIfI clinical stage rubric, illustrating that, as hypothesized, the rates of 1-year major amputation increase as clinical stage increases (Table IV). It is important to note that, within Table IV, many individual WIfI component combinations did not have patients available for analysis (denoted with “-“). A similar trend is seen among clinical stage stratification and 1-year wound healing rates (100% in clinical stage 1, 42% in clinical stage 2, 39% in clinical stage 3, and 30% of clinical stage 4); however, this trend cannot be seen in freedom from RAS events within 1 year (25%, 49%, 57%, and 40%, respectively) or in the rates of 1-year survival (60%, 80%, 83%, 71%, respectively) (Table V). Cox regression models showed that a one-unit increase in clinical stage increases the rate of major amputation (1.7 [1.2–2.5]), which is the outcome that Mills et al. originally intended clinical stage to predict (Table VI). Further, additional regression models showed that a one-unit increase in clinical stage is also associated with an increased risk of incomplete wound healing (1.5 [1.2–2.0]), MALE (1.4 [1.1–1.7]), RAS events (1.3 [1.1–1.6]), and lower AFS (1.2 [1.1–1.5]).
Table IV.
One-year institutional amputation rate based on Clinical Stage as per the SVS WIfI Classification System9
| 1-Year Amputation (N = 61) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ischemia-0 | Ischemia-1 | Ischemia-2 | Ischemia-3 | |||||||||||||
| W-0 | - | - | - | - | - | - | - | - | - | - | - | - | 15% | - | - | - |
| W-1 | - | - | - | - | 0% | 0% | 0% | - | 7% | 20% | 0% | - | 10% | 25% | 17% | 0% |
| W-2 | - | - | - | - | 10% | 27% | 20% | - | 18% | 11% | 30% | - | 34% | 40% | 0% | 0% |
| W-3 | - | - | - | - | 0% | - | 67% | - | 0% | 0% | 33% | 100% | 0% | 100% | 50% | 0% |
| fI-0 | fI-1 | fI-2 | fI-3 | fI-0 | fI-1 | fI-2 | fI-3 | fI-0 | fI-1 | fI-2 | fI-3 | fI-0 | fI-1 | fI-2 | fI-3 | |
W, Wound; fI, Foot infection grade “-“ denotes no patients available for analysis within this WIfI component combination
Table V.
Observed 1-year outcomes by Wound, Ischemia, and foot Infection (WIfI) clinical stage
| WIfI Clinical Stage | Patient No. | Outcome
|
|||
|---|---|---|---|---|---|
| Complete Healing, No. (%) | Limb Salvage, No. (%) | Freedom from RAS, No (%) | Survival, No3 (%) | ||
| Clinical Stage 1 | 5 | 100% | 100% | 25% | 60% |
| Clinical Stage 2 | 111 | 42% | 90% | 49% | 80% |
| Clinical Stage 3 | 222 | 39% | 89% | 57% | 83% |
| Clinical Stage 4 | 213 | 30% | 76% | 40% | 71% |
|
| |||||
| WIfI Comp 1–4 | 313 | 38% | 90% | 55% | 57% |
| WIfI Comp 5–9 | 238 | 32% | 77% | 41% | 45% |
RAS, reintervention, amputation, or stenosis
Table VI.
Multivariable analysis of Wound, Ischemia, and foot Infection (WIfI) classification types
|
|
||||
|---|---|---|---|---|
| WIfI Classification | Major Amputation, HR (95% CI) | Wound Healing, HR (95% CI) | RAS Events, HR (95% CI) | Mortality, HR (95% CI) |
| Wound (0–3) | 1.5 (1.1 – 2.0)* | 1.4 (1.1 – 1.7) * | 1.1 (0.9 – 1.3) | 1.1 (0.9 – 1.3) |
| Ischemia (1–3) | 1.3 (0.9 – 1.8) | 0.7 (0.6 – 1.0) | 1.1 (0.9 – 1.4) | 0.9 (0.8 – 1.1) |
| Infection (0–3) | 1.5 (1.1 – 2.0) * | 1.4 (1.1 – 1.8) * | 1.3 (1.1 – 1.6) * | 1.1 (0.9 – 1.3) |
| Clinical Stage (1–4) | 1.7 (1.2 – 2.5) * | 1.5 (1.2 – 2.0) * | 1.3 (1.1 – 1.6) * | 1.2 (0.9 – 1.4) |
| Composite (1–9) | 1.4 (1.2 – 1.8) * | 1.2 (1.1 – 1.4) * | 1.2 (1.1 – 1.4) * | 1.1 (0.9 – 1.2) |
| High-risk Composite (5–9)a | 2.2 (1.3 – 3.7) * | 1.5 (1.1 – 2.0) * | 1.7 (1.2 – 2.2) * | 1.2 (0.9 – 1.6) |
P < .05; RAS, reintervention, amputation, or stenosis; Adjusted for age, gender, dialysis dependence, prior ipsilateral intervention, current smoking, and tibial TASC Classification
Analysis performed exclusively on the high-risk WIfI composite sub-group (i.e., low-risk [Composite 1–4] vs. high-risk [Composite 5–9])
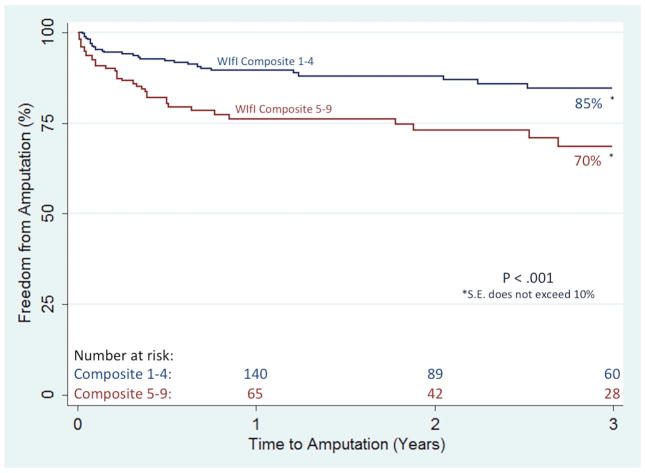
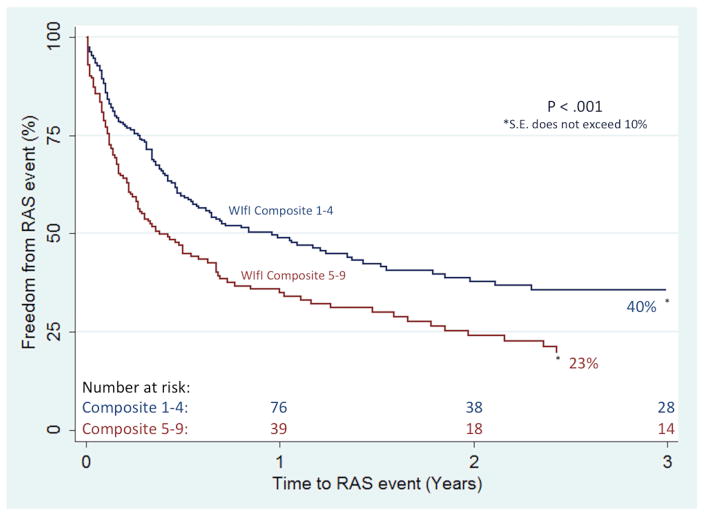
Separate multivariable models showed that, individually, one-unit increases in the wound and infection components were independently associated with incomplete wound healing (1.4 [1.1–1.7] and 1.4 [1.2–1.8], respectively). An increasing wound component was also independently associated with increasing rates of major amputation (1.4 [1.1–1.9]), while an increasing infection component was independently predictive of both major amputation (1.5 [1.1–2.0]) and RAS events (1.3 [1.1–1.6]). Further, a one-unit increase in the novel WIfI composite score was associated with incomplete wound healing (1.2 [1.1–1.4]) and increasing rates of major amputation (1.4 [1.2–1.8], MALE (1.3 [1.1–1.5]), and RAS events (1.2 [1.1–1.4]). Importantly, as compared to the WIfI clinical stage, the individual WIfI components, and the WIfI composite stratification, the high-risk (WIfI composite 5–9) sub-group proved most predictive of incomplete wound healing (1.5 [1.1–2.0]), major amputation (2.2 [1.3–3.7]), MALE (1.7 [1.2–2.4]), RAS events (1.7 [1.2–2.2]), and AFS (1.3 [1.1–1.8]). Kaplan-Meier estimates reflect these findings, as freedom from major amputation was significantly lower in the high-risk WIfI composite group at 3 years (70% vs. 85%; P < .01), as was freedom from RAS events (23% vs. 40%, P < .01) (Figures I and II, respectively). Ultimately, the WIfI clinical stage, the WIfI composite score (including the sub-grouping), and the individual WIfI components were unable to predict mortality.
Figure 1.
Freedom from major amputation based on the WIfI composite score sub-grouping
S.E., standard error
Figure 2.
Freedom from RAS events based on the WIfI composite score sub-grouping
RAS, reintervention, major amputation, or stenosis; S.E., standard error
Importantly, in order to eliminate any potential concern regarding lack of independence of observations within this cohort, a separate sensitivity analysis limited to the first limb in patients who had bilateral limb interventions was performed, and illustrated no appreciable changes in any of our findings.
DISCUSSION
Our data illustrate that, for patients undergoing an infrapopliteal angioplasty for CLI, an increasing WIfI wound component, an increasing WIfI infection component, and an increasing WIfI clinical stage are independently associated with incomplete wound healing and an increased rate of major amputations. Additionally, an increase in the WIfI composite score independently predicted incomplete wound healing, an increased rate of major amputations, and an increased rate of RAS events. Further, our 1-year major amputation rate mimics the expected progression outlined by the Mills et al. clinical stage delineations.9 Importantly, within this cohort, the high-risk (WIfI composite 5–9) sub-group proved most predictive of incomplete wound healing, amputation, and RAS events. Ultimately, although prediction of wound healing and RAS events were not the primary outcomes of the original Mills et al. study, an increase in the WIfI scoring methodologies – especially the clinical stage and composite score – were able to significantly predict these additional endpoints, as well as the initially-sought major amputation endpoint.
Since the initial WIfI publication, several important studies have been published to validate the WIfI classification system. In 2014, Cull et al. examined and graded 139 foot wound patients undergoing any lower extremity revascularization, concluding that the WIfI clinical stages correlate with wound healing and 1-year limb salvage.18 Additionally in 2014, Zhan et al. evaluated 201 patients with threatened limbs undergoing any lower extremity revascularization, concluding that as the clinical stage progresses, the risk of major amputation increases, 1-year amputation-free survival decreases, and time to wound healing is prolonged.19 Our data corroborate these claims, validating the WIfI classification for endovascular revascularizations of infrapopliteal lesions.
Although the expert consensus on the 1-year amputation risk proposes the potential for some of these patient limbs to, in fact, be high risk, it is important to note that none of the limbs in our cohort exhibited an Ischemia grade of 0. Due to this lack of data, we cannot properly validate the risk of amputation in this specific cohort of patients. Further, although we expected those with a greater burden of disease to more commonly exhibit undesired outcomes, severity of ischemia was not predictive of any endpoints within this analysis. Similarly, we were unable to address the Mills et al. staging of the proposed benefit from revascularization, as we had no patients who did not undergo a revascularization.
There are some additional limitations to this study. Although the inclusion of multiple limbs and multiple procedures from a single patient allowed us to utilize a larger analytic sample, the potential lack of independence of observations may be of concern. Additionally, the small number of limbs classified as exhibiting WIfI clinical stage 1 disease may provide statistical shortcomings. Ultimately, as a retrospective study, the potential for selection and information bias exists: Since our data represent the experience of one group of surgeons at a single institution, our results are subject to the influence of specific referral patterns, surgeon experience, and patient selection preferences. Moreover, our cohort may represent a lower proportion of high risk (i.e., clinical stage 4) limbs, as we did not capture patients that either underwent a primary amputation or patients who did not undergo any intervention. Additionally, it is important to note the apparent heterogeneity in the clinical stage stratification, as illustrated by the non-linear trends within our study’s outcomes: Although further stratification of the novel WIfI composite score was not statistically viable within this cohort, we hope that the future projects can further validate this novel scoring system as a means to better identify the heterogeneity within the clinical staging system.
CONCLUSION
We believe that this study has proven the value and utility of the WIfI classification system and its related components in regards to patients undergoing a tibial angioplasty for CLI. In particular, prospectively, the WIfI classification system can be used to stratify these patients’ risk of amputation at presentation and during treatment. We found the WIfI composite score to be most useful in consistently predicting outcomes – including risk for amputation – where it’s easy-to-conceptualize nature allows for quick incorporation into clinical practice. Additionally, we believe that, retrospectively, the WIfI system – and the WIfI composite score, specifically – can be useful for comparative effectiveness analysis, as it is successful in predicting wound healing, risk of amputation, MALE, RAS events, and AFS, gives equal weight to each of the WIfI variables, and provides clinicians easier comparisons in outcomes between groups. In conjunction with patient risk factors and comborbidites, clinical incorporation of the WIfI classification system and our novel WIfI composite score may play an important role in selecting the most efficacious therapy for select patients. This study investigates the largest cohort in support of the WIfI classification system and its promise as both a counseling tool for patients as well as a step towards better standardization for inter-study comparative analysis in patients with CLI; however, multicenter studies comprising more patients are justified to fully validate this newly proposed system.
Footnotes
Presented at the 43rd annual symposium of the Society for Clinical Vascular Surgery, Miami, Florida, March 29, 2015 (Plenary)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell P, RFCD, DePalma RG, Eastcott HHG, Eklöf B, Jamieson CW, et al. The definition of critical ischemia of a limb. Working Party of the International Vascular Symposium. Br J Surg. 1982;69:S2. [Google Scholar]
- 2.Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. J Diabetes Sci Technol. 2011;5(6):1591–5. doi: 10.1177/193229681100500636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad MF, Kang J, Cambria RP, Brewster DC, Watkins MT, Kwolek CJ, et al. Infrapopliteal balloon angioplasty for the treatment of chronic occlusive disease. J Vasc Surg. 2009;50(4):799–805. e4. doi: 10.1016/j.jvs.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Darling JD, McCallum JC, Curran T, Buck DB, Guzman R, Wyers M, et al. Consequences of Failed Tibial Endovascular Intervention. J Vasc Surg. 2014;59(6):102S–3S. [Google Scholar]
- 5.Lo RC, Darling J, Bensley RP, Giles KA, Dahlberg SE, Hamdan AD, et al. Outcomes following infrapopliteal angioplasty for critical limb ischemia. J Vasc Surg. 2013;57(6):1455–63. doi: 10.1016/j.jvs.2012.10.109. discussion 63–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosiers M, Hart JP, Deloose K, Verbist J, Peeters P. Endovascular therapy as the primary approach for limb salvage in patients with critical limb ischemia: experience with 443 infrapopliteal procedures. Vascular. 2006;14(2):63–9. doi: 10.2310/6670.2006.00014. [DOI] [PubMed] [Google Scholar]
- 7.Nolan BW, De Martino RR, Stone DH, Schanzer A, Goodney PP, Walsh DW, et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg. 2011;54(3):730–5. doi: 10.1016/j.jvs.2011.03.236. discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinelli F, Stilo F, Benedetto F, De Caridi G, La Spada M. Early and one-year results of infrainguinal bypass after failure of endovascular therapy. Int Angiol. 2011;30(2):156–63. [PubMed] [Google Scholar]
- 9.Mills JL, Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59(1):220–34. e1–2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 11.Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helv Chir Acta. 1954;21(5–6):499–533. [PubMed] [Google Scholar]
- 12.Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev. 2004;20(Suppl 1):S90–5. doi: 10.1002/dmrr.464. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–9. doi: 10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 14.Treece KA, Macfarlane RM, Pound N, Game FL, Jeffcoate WJ. Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med. 2004;21(9):987–91. doi: 10.1111/j.1464-5491.2004.01275.x. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-De Jesus FR. A checklist system to score healing progress of diabetic foot ulcers. Int J Low Extrem Wounds. 2010;9(2):74–83. doi: 10.1177/1534734610371594. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. J Am Podiatr Med Assoc. 2013;103(1):2–7. doi: 10.7547/1030002. [DOI] [PubMed] [Google Scholar]
- 17.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Cull DL, Manos G, Hartley MC, Taylor SM, Langan EM, Eidt JF, et al. An early validation of the Society for Vascular Surgery lower extremity threatened limb classification system. J Vasc Surg. 2014;60(6):1535–41. doi: 10.1016/j.jvs.2014.08.107. [DOI] [PubMed] [Google Scholar]
- 19.Zhan LX, Branco BC, Armstrong DG, Mills JL., Sr The Society for Vascular Surgery lower extremity threatened limb classification system based on Wound, Ischemia, and foot Infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg. 2015;61(4):939–44. doi: 10.1016/j.jvs.2014.11.045. [DOI] [PubMed] [Google Scholar]