Circadian clocks are present in most organisms and mediate the interplay between the environment and physiologic processes1. Normally, clocks adjust physiologic responses to anticipated stimuli times. Disruption of circadian clocks/rhythms exacerbates several chronic diseases. The “central” circadian pacemaker is in the suprachiasmatic nucleus (SCN) in the hypothalamus and is responsible for biological rhythms regulated by the light/dark cycle (LDC). Peripheral tissues show circadian oscillations that are coordinated by the central pacemaker2. The LDC entrains circadian rhythms over a 24-hour cycle, enabling organisms to adapt to environmental changes. Peripheral tissues also possess self-sustaining, entrainable circadian timers not regulated by the LDC. In particular, the gastrointestinal (GI) tract and liver, which mediate food processing, are entrained by food and eating times3.
Circadian rhythms regulate a variety of GI processes including cell proliferation, immune homeostasis, gut permeability and microbial balance4 and metabolism. In May 2016, with NIH R13 support, we held the first symposium on “Circadian Rhythms in GI Health and Disease” in Chicago, in recognition of the emerging role of circadian disruption in diseases of modern life style (obesity; metabolic syndrome5) and cancer6, as well as alcohol’s effects on gut and liver7. This summary reviews the topics covered in this meeting (Figure 1).
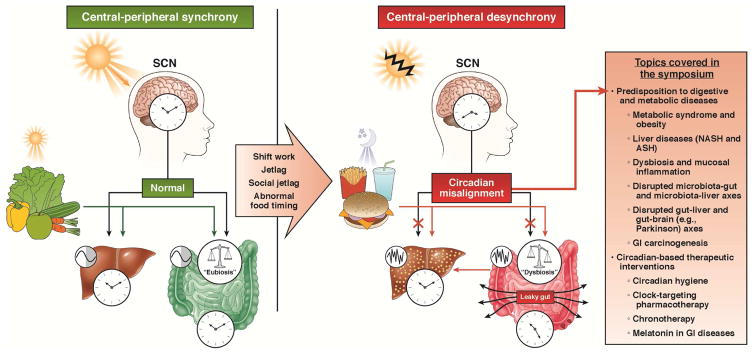
Figure 1.
Under physiologic conditions, there is a synchrony between the central circadian clock in the suprachiasmatic nucleus (SCN) and the peripheral (hepato-intestinal) circadian clock (left panel). While SCN is entrained by light, the latter, in addition to SCN signals, could respond to food. Disrupted light exposures (e.g., shift work, jet lag) or dyssynchrony between food and light signals (e.g., abnormal eating pattern) could cause central-peripheral misalignment, predisposing to a variety of digestive and metabolic pathologies (right panel).
Clocks and the Digestive System
In this section, Dr. Turek covered the role of circadian clocks in the digestive tract. Clocks encompass Transcriptional, Translational Feedback Loops (TTFL) that keep time through several oscillating proteins in interwoven feedback loops. These clocks regulate up to 30% of genes expressed in a given tissue including gut and liver4. In modern societies, humans – when they are active, or when they sleep or eat out of synchrony with their SCN clock – frequently experience disruption of the normal phase relationship between the central pacemaker and peripheral clocks. This circadian misalignment can contribute to GI and metabolic diseases.
Dr. Brown reviewed how metabolism is governed by the clock, both systemically and locally via cell-autonomous circadian clocks5. Clock control of metabolism can be exerted via chromatin-level modifications of nuclear hormone receptors. Recent data suggest the clock can directly control, by post-translational mechanisms, cellular respiration and other mitochondrial functions, particularly in frequently-dividing cells such as GI epithelial cells5. This metabolic adaptation to circadian rhythms allows coordination of digestive-system physiology with the environment (e.g., food acquisition and consumption) 3.
Dr. Tischkau covered how this coordination is then orchestrated by sensors linking the environment to circadian machinery. One of these environmental sensors is PAS-domain-containing proteins. The aryl hydrocarbon receptor (AhR), a member of the PAS family, can directly affect TTFL8. AhR, upon activation, forms a heterodimer with the circadian clock protein BMAL1, inhibiting CLOCK/BMAL1 activity. Multiple ligands in food can bind AhRs, which could mediate the effects of eating habits on circadian rhythms, and circadian-controlled genes. AhRs are intriguing targets for circadian-based interventions that strengthen intestinal-central circadian synchrony and control metabolism.
Circadian Dysrhythmia in GI Diseases
Intestinal barrier integrity is critical to health; barrier dysfunction contributes to development/progression of inflammation-mediated diseases both in and outside of the GI tract. Dr. Keshavarzian showed that circadian disruption, in environmental models (shifting the daily LDC) or genetic models (ClockΔ19) induced intestinal hyperpermeability (IHP), which was exaggerated by alcohol. Barrier dysfunction was associated with shifts toward pro-inflammatory profiles in intestinal microbiota and intestinal gene expression7. This suggests that circadian disruption contributes to IHP and a pro-inflammatory state, due, at least in part, to perturbed relationships between intestinal microbiota and intestinal gene expression. Dr. Swanson presented a recent human study on the effects of circadian disruption on alcohol-induced IHP (AIHP) comparing night-shift to day-shift workers for response to small doses of alcohol (0.5g/kg=2 glasses of wine/day for 7 days) 9. Night-workers had increased AIHP, which was correlated with 24-hour area under the curve (AUC) of serum melatonin, a measure of central circadian alignment. Alcohol, even in small amounts, can disrupt central circadian rhythms if combined with circadian misalignment (night-shift workers). Also, alcohol disrupted peripheral circadian rhythms (expression of circadian genes in peripheral blood mononuclear cells). The interaction of alcohol and circadian disruption in disease states was confirmed by looking at serum inflammatory markers. Therefore, circadian disruption is a key cofactor in AIHP and vulnerable individuals (night workers) should be monitored more closely for conditions associated with AIHP (e.g., liver injury).
Circadian disruption was recently implicated in pathologies associated with Westernized diets (e.g., high fat). Dr. Leone discussed the mediating role of microbiota in regulating circadian homeostasis and high-fat-induced obesity10. A high fat diet could alter diurnal patterns of gut microbiota and microbial metabolites in mice. Butyrate modulated circadian clock gene expression (e.g., in hepatic organoids) in vitro, suggesting that microbe-derived metabolites directly affect circadian clocks within peripheral tissues involved in metabolic outcomes. Therefore, Westernized diets can alter diurnal patterns of gut microbiota and their metabolites, re-setting peripheral circadian clocks and host metabolic responses.
Another new question rose at the meeting concerned how do eating habits (e.g., type and time of food) control and shift microbiome rhythms? A potential role for gut-secreted melatonin in passing circadian timing cues from host to commensal bacteria was presented by Jiffin Paulose from Dr. Cassone’s laboratory. Melatonin increased motility and swarming in Enterobacter aerogenes, an effect that is at least partially mediated by melatonin receptors11.
Circadian Rhythms, Obesity, and Metabolic Syndrome
Metabolism is closely-regulated by circadian rhythms; rhythm disruption is increasingly recognized in pathologies related to metabolism (obesity; metabolic syndrome).
Dr. Bass discussed the link between the clock and glucose control. Blood glucose levels are tightly controlled by insulin, which is released by pancreatic β-cells in response to a meal. How does the intrinsic circadian clock of β-cells synchronize with cycling food intake and the body’s demands? Oscillations of insulin secretion occur in synchrony with the expression of insulin regulatory genes. The cycling transcription of this metabolic gene network is regulated by CLOCK/BMAL1, co-localizing with the pancreatic transcription factor PDX1 within the active-enhancers region. Clock disruption (i.e., via Bmal1 ablation) causes diabetes in mice. This highlights transcriptional mechanisms by which the circadian machinery controls peripheral metabolism12.
Circadian clocks control metabolism also through post-transcriptional effects as reviewed by Dr. Green. Nocturnin, is an output of the clock and a circadian-controlled gene that affects metabolism by removing the poly(A) tails from mRNAs, modulating mRNA stability or translatability. Nocturnin-deficient mice are resistant to obesity due to inefficient utilization of dietary lipids in the intestine13. Nocturnin also affects lipid metabolism and mitochondrial functions outside the intestine, and links the clock to metabolism. Thus, circadian clocks control metabolism by modulating the expression of genes involved in metabolism, using both transcriptional and post-transcriptional mechanisms.
Our understanding of metabolic regulation by circadian clocks can affect the management of metabolic diseases. Alterations in time of food intake via restricted feeding, enhances weight loss in animals independent of calorie-restriction. In a recently conducted one-year randomized controlled trial conducted by Dr. Varady, effects of alternate-day fasting vs. daily calorie restriction on 100 obese individuals was compared. Alternate day fasting was as effective as calorie restriction (which has a lower adherence) for losing weight and improving metabolic markers14.
Clock and Liver
In this section, Dr. Bailey presented that chronic alcohol consumption induces central-peripheral circadian desynchronization by altering the timing of the liver clock. This desynchronization can mediate alcohol’s effects on hepatic lipid, glycogen, and mitochondrial metabolism. Her findings suggest a regulatory role for the liver clock on alcohol-induced liver injury and glycogen depletion 15. In a hepatocyte-specific BMAL1 knockout mouse model for alcoholic fatty liver disease, failures in clock-driven metabolic adaptive processes in the alcohol-exposed liver mediated alcohol-induced hepatic bioenergetic stress and liver injury. Circadian machinery disruption can also synergize with alcohol in promoting liver disease. Dr. Forsyth discussed the data that circadian disorganization enhances alcohol-induced steatohepatitis, correlating with IHP and endotoxemia. These findings suggest that circadian clock is critical for maintaining intestinal barrier integrity and homeostasis in the gut/liver axis7.
Circadian disruption also promotes non-alcoholic liver injury. Given the regulatory effects of the peripheral liver clock on metabolism, it is not surprising that circadian disruption increases risks of metabolic syndrome and non-alcoholic fatty liver disease. Here, Dr. Chiang covered the role of circadian rhythms in bile acid metabolism. Bile acid signaling affects metabolic homeostasis by regulating insulin secretion in pancreatic β-cells. Bile acids control their own synthesis through a feedback mechanism by inhibiting transcription of the rate-limiting enzyme cholesterol-7α-hydroxylase (CYP7A1). Bile acid synthesis exhibits a strong circadian rhythm via (a) transcriptional regulation of Cyp7a1, regulated by the clock protein Rev-erbα and the clock-controlled transcription factor D-site binding protein (DBP) and (b) regulation of the cholic acid synthesis enzyme sterol-12α-hydroxylase (CYP8B), another key enzyme in bile acid biosynthesis, controlled by the clock-protein RORα. Diurnal expression of Cyp7a1 and Cyp8b1 show reciprocal patterns. Eating patterns can affect bile acid signaling through the Cyp7a1/Cyp8b1 axis, affecting postprandial nutrient absorption. Involvement of circadian clocks in bile acid and liver metabolism is supported by abnormal bile acids, fatty acid synthesis, and liver damage observed in the genetic (e.g., Per1/Per2 double knockout mice) as well as environmentally-induced (e.g., short-term sleep disruption) animal models of circadian disruption16.
Circadian Dysrhythmia in Brain-gut axis
Evidence suggests a bidirectional neuro-humoral communication between the gut and brain. Dr. Mattson showed that timed feeding (intermittent fasting) increases numbers and strengths of synapses, enhancing brain function. Intermittent fasting activates brain-derived neurotrophic factor, which is involved in mitochondrial biogenesis, DNA repair and removal of oxidative stress products and organelles, hence increasing neuronal activation. This can be enhanced by metabolic benefits of fasting; production of fatty acids and ketone bodies can promote neuronal health and neuronal coping with stress by providing alternative energy sources. Animal-based studies also indicate the neuroprotective and neurorestorative effects of intermittent fasting in chronic neurodegenerative disorders and acute brain injury. This protection occurs via enhanced antioxidant defenses and decreased inflammation17.
Other presentations in this section support a role for the gut-brain axis, and microbiota in mediating circadian effects on neurologic disorders. Dr. Voigt, in an animal model of Parkinson’s disease, linked the disease with altered intestinal microbiota, and increased intestinal inflammation. Thus, circadian desynchronization is a potential enhancer of neurologic disorders. These findings need to be verified in humans.
However, circadian measurements in humans remain technically and practically challenging. Dr. Burgess discussed several methods for assessing central-clock timing including chronotype questionnaires, or melatonin measurements in urine or saliva, as well as with rest-activity or skin surface temperature monitoring. The challenges and practical issues surrounding these measurements and shifting of central circadian rhythms were addressed18.
Circadian Dysrhythmia and Cancer
An increased incidence of colorectal cancer (CRC) and pancreatic cancer in shift workers suggests interplay between circadian rhythms and GI cancer. Cell-cycling is tightly coupled to circadian clocks; circadian clocks are implicated in cancer survival and proliferation pathways. Moreover, the epigenetic landscape, which is altered during carcinogenesis, heavily regulates transcriptional activity of the circadian clock as addressed by Dr. Sassone-Corsi. Therefore, clocks and cancer could be connected at different molecular levels. Drs. Khazaie and Bishehsari showed a potential role of circadian disruption in alcohol-induced CRC. Chronic intestinal inflammation promotes carcinogenesis. Circadian disruption can accelerate alcohol-induced intestinal inflammation, which could be associated with accelerated polyposis.
Not only can circadian disruption predispose tissues to undergo neoplastic transformation, but cancer itself could exploit the circadian clock for its metabolic needs and growth. Dr. Masri presented on reprogramming of hepatic metabolism in a mouse model of extra-hepatic (lung) cancer. Cancer operated as an endogenous reorganizer of circadian metabolism by affecting transcripts and metabolites cycling in the liver, while having no effect on the core clock. Inflammatory signaling, the STAT3-Socs3 pathway, downstream disruption of AKT, and AMPK were shown to mediate cancer rewiring of circadian hepatic metabolism19.
Therapeutic Implications of Circadian Science
How can increased understanding of circadian rhythms be translated into management of GI diseases? Cancer-promoting signals that are affected by circadian clocks are potential therapeutic targets. Another therapeutic implication of circadian-based approaches is chronotherapy, which was covered by Dr. Levi. Chronotherapy aims to optimize efficiency and decrease toxicity of a drug through delivery of medicines according to the Circadian Timing System (CTS). The CTS is a dynamic network that controls most metabolic pathways and cellular proliferation. In cancer, where the side effects of chemotherapy could be limiting, chrono-chemo-therapeutic approaches can cause up to a 10-fold improvement in tolerability to chemotherapeutic drugs.
Chronotherapy improves patient outcomes for several chemotherapies in GI cancers. Molecular clock profiling is predictive in CRC chronotherapy. Circadian transcription patterns of Bmal1 and Rev-erbα in mouse liver or colon can predict optimal circadian timing of Irinotecan. Bmal1 silencing in CRC cells ablates Irinotecan chronopharmacology. Liver-directed chronotherapy into the hepatic artery can eradicate metastatic disease in patients despite prior failure of conventional chemotherapy. In this era of personalized medicine, chronotherapy is a promising approach to treating chronic GI diseases in which tailoring drug delivery to the CTS increases drug activity and safety7.
Finally, Dr. Reiter discussed the potential role of melatonin in treating GI diseases20. Melatonin helps synchronize circadian rhythmicity between central and peripheral clocks. The pineal gland synthesizes and secrets melatonin primarily at night, hence, the high nocturnal levels of circulating melatonin. Several GI diseases have been linked to perturbations in melatonin cycling. Besides secretion by the pineal glands, melatonin is found in the GI tract, at levels 400 times that in the pineal gland. Enterochromaffin cells convert serotonin to melatonin and are thought to be a major source of GI melatonin. Unlike pineal melatonin, the dynamics of GI melatonin are unclear. Melatonin signaling is mediated by membrane melatonin receptors.
Melatonin may also play a role in cross-talk between host and microbiome as discussed earlier. Not only may the gut microbiome contribute to the GI pool of melatonin, the microbiome responds to melatonin, which may help synchronize host and bacterial clocks. Given the growing role of the microbiome in health and disease, implications for fine-tuning of the GI circadian system via melatonin therapy for GI as well as non-GI diseases could be broad. However, we first need to address many as yet unanswered questions on: 1) different sources of melatonin in the GI tract; 2) the role of melatonin in normal and pathophysiology of the GI tract; 3) the dynamic, rhythm and downstream signaling of melatonin in the GI tract.
Consensus and Conclusion
The biological clock plays a crucial role in maintaining GI metabolic homeostasis; circadian disruption is implicated in various GI and liver pathologies, metabolic diseases, alcohol-related injuries and cancers. In light of the growing understanding of circadian regulation in GI health and disease, preventive and therapeutic implications from a chronobiological perspective are anticipated. Identifying factors that can cause central-peripheral circadian misalignment could help us risk-stratify at-risk individuals. Improved understanding of the mechanisms by which circadian rhythm disruption accelerates pathologies allow for discovery of diagnostic biomarkers and future targets for drug development. In the era of personalized medicine, the dimension of time needs to be brought into the equation within translational research and clinical medicine. To reach these goals, it is crucial to develop multi-center, multi-discipline collaborations between basic and translational scientists in the fields of circadian biology, GI/liver, and metabolic disorders.
Acknowledgments
The authors acknowledge the support of the NIH-NIAAA and NIDDK R13DK108293-01, and Rush University Medical Center. We thank the members of scientific committee (Drs. Faraz Bishehsari, Francis Levi, Fred Turek, Eugene Chang and, Ali Keshavarzian), speakers, local organizers, and Denise Labedz for her administrative assistance.
Funding
FB is supported by Rush Translational Sciences Consortium/Swim Across America and Rush University, Department of Medicine, Academic Mentoring Program Research Track Award; AK is supported by NIH-NIAAA R01AA023417 and R01AA020216.
Appendix
List of Faculty Speakers
Shannon Bailey, University of Alabama at Birmingham; Joe Bass, Northwestern University; Faraz Bishehsari, Rush University Medical Center; Steven Brown, University of Zurich; Helen Burgess, Rush University Medical Center; John Y. L. Chiang, Northeast Ohio Medical University; Christopher B. Forsyth, Rush University Medical Center; Carla Green, UT Southwestern Dallas; Khashayar Khazaie, Mayo clinic; Ali Keshavarzian, Rush University Medical Center; Vanessa Leone, University of Chicago; Francis Levi, Warwick University; Mark Mattson, National Institute of Aging; Russel Reiter, University of Texas; Paolo Sassone-Corsi, University of California; Garth Swanson, Rush University Medical Center; Shelley A. Tischkau, Southern Illinois University; Fred W. Turek, Northwestern University; Krista Varady, UIC; Robin M. Voigt, Rush University Medical Center;
Footnotes
Conflicts of interest
No author received any additional compensation related to this meeting summary.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hogenesch JB, Herzog ED. Intracellular and intercellular processes determine robustness of the circadian clock. FEBS Lett. 2011;585(10):1427–34. doi: 10.1016/j.febslet.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenvers DJ, et al. Nutrition and the circadian timing system. Prog Brain Res. 2012;199:359–76. doi: 10.1016/B978-0-444-59427-3.00020-4. [DOI] [PubMed] [Google Scholar]
- 4.Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol. 2011;62(2):139–50. [PubMed] [Google Scholar]
- 5.Brown SA. Circadian Metabolism: From Mechanisms to Metabolomics and Medicine. Trends Endocrinol Metab. 2016 doi: 10.1016/j.tem.2016.03.015. S1043-2760(16)30001-7. [DOI] [PubMed] [Google Scholar]
- 6.Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, Lévi FA. The circadian timing system in clinical oncology. Ann Med. 2014;46(4):191–207. doi: 10.3109/07853890.2014.916990. [DOI] [PubMed] [Google Scholar]
- 7.Voigt RM, Forsyth CB, Keshavarzian A. Circadian disruption: potential implications in inflammatory and metabolic diseases associated with alcohol. Alcohol Res. 2013;35(1):87–96. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Zhang ZM, Xu CX, Tischkau SA. Interplay between Dioxin-mediated signaling and circadian clock: a possible determinant in metabolic homeostasis. Int J Mol Sci. 2014;15(7):11700–12. doi: 10.3390/ijms150711700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson GR, Gorenz A, Shaikh M, Desai V, Kaminsky T, Van Den Berg J, Murphy T, Raeisi S, Fogg LF, Vitaterna MH, Forsyth C, Turek FW, Burgess HJ, Keshavarzian A. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Physiol Gastrointest Liver Physiol. 2016;19:ajpgi.00087. doi: 10.1152/ajpgi.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17(5):681–9. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulose JK, Wright JM, Patel AG, Cassone VM. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS One. 2016;11(1):e0146643. doi: 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350(6261):aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stubblefield JJ, Terrien J, Green CB. Nocturnin: at the crossroads of clocks and metabolism. Trends Endocrinol Metab. 2012;23(7):326–33. doi: 10.1016/j.tem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoddy KK, Gibbons C, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Gabel K, Finlayson G, Varady KA. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin Nutr. 2016;30 doi: 10.1016/j.clnu.2016.03.011. pii: S0261-5614(16)00102-3. [DOI] [PubMed] [Google Scholar]
- 15.Udoh US, Swain TM, Filiano AN, Gamble KL, Young ME, Bailey SM. Chronic ethanol consumption disrupts rhythms of hepatic glycogen metabolism in mice. Am J Physiol Gastrointest Liver Physiol. 2015;308(11):G964–G74. doi: 10.1152/ajpgi.00081.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrell JM, Chiang JY. Short-term circadian disruption impairs bile acid and lipid homeostasis in mice. Cell Mol Gastroenterol Hepatol. 2015;1(6):664–677. doi: 10.1016/j.jcmgh.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Praag H, Fleshner M, Schwartz MW, Mattson MP. Exercise, energy intake, glucose homeostasis, and the brain. J Neurosci. 2014;34(46):15139–49. doi: 10.1523/JNEUROSCI.2814-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int. 2011;28(8):714–8. doi: 10.3109/07420528.2011.597531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, Jacks T, Sassone-Corsi P. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell. 2016;165(4):896–909. doi: 10.1016/j.cell.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Barceló EJ, Mediavilla MD, Tan DX, Reiter RJ. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17(19):2070–95. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]