Abstract
Signing differs from typical non-linguistic hand actions because movements are not visually guided, finger movements are complex (particularly for fingerspelling), and signs are not produced as holistic gestures. We used positron emission tomography to investigate the neural circuits involved in the production of American Sign Language (ASL). Different types of signs (one-handed (articulated in neutral space), two-handed (neutral space), and one-handed body-anchored signs) were elicited by asking deaf native signers to produce sign translations of English words. Participants also fingerspelled (one-handed) printed English words. For the baseline task, participants indicated whether a word contained a descending letter. Fingerspelling engaged ipsilateral motor cortex and cerebellar cortex in contrast to both one-handed signs and the descender baseline task, which may reflect greater timing demands and complexity of handshape sequences required for fingerspelling. Greater activation in the visual word form area was also observed for fingerspelled words compared to one-handed signs. Body-anchored signs engaged bilateral superior parietal cortex to a greater extent than the descender baseline task and neutral space signs, reflecting the motor control and proprioceptive monitoring required to direct the hand toward a specific location on the body. Less activation in parts of the motor circuit was observed for two-handed signs compared to one-handed signs, possibly because, for half of the signs, handshape and movement goals were spread across the two limbs. Finally, the conjunction analysis comparing each sign type with the descender baseline task revealed common activation in the supramarginal gyrus bilaterally, which we interpret as reflecting phonological retrieval and encoding processes.
Keywords: sign language, fingerspelling, positron emission tomography, lexical production
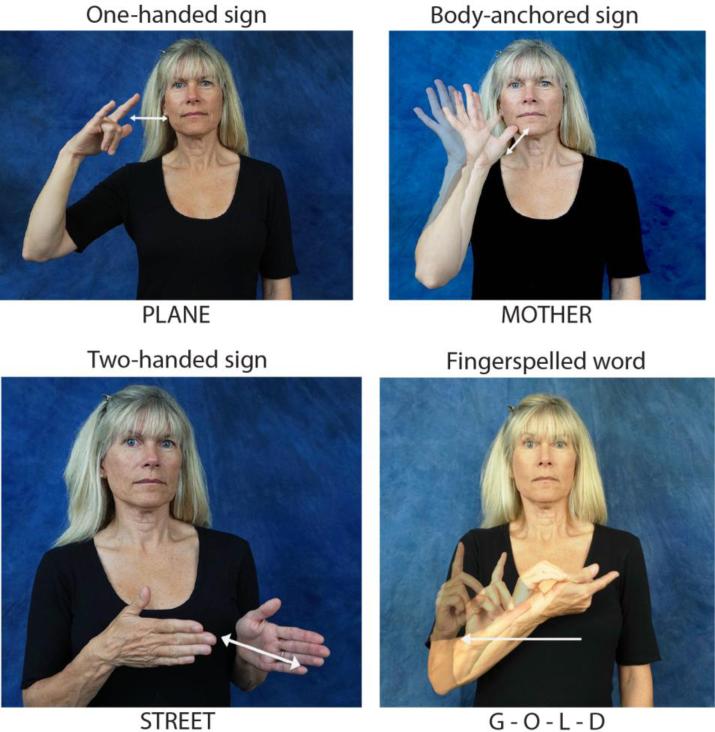
Although much is known about the neural systems involved in the production of speech sounds (e.g., Guenther, 2006), we know very little about the neural circuits that are recruited during the production of manual signs. Speech involves coordination of the larynx and vocal tract which are located along the midline of the body; in contrast, the primary linguistic articulators for sign language are the hands and arms which are independent, symmetrical articulators controlled by contralateral motor cortex and ipsilateral cerebellar cortex. Direct contrasts between sign and speech production in hearing bilinguals fluent in American Sign Language (ASL) and English have revealed greater activation for signing than speaking in the superior parietal lobule (SPL), as well as in the supramarginal gyrus (SMG) (Braun, Guillemin, Hosey, & Varga, 2001; Emmorey, McCullough, Mehta, & Grabowski, 2014; Zou et al., 2012; see also Emmorey, Mehta, & Grabowski, 2007, for evidence from deaf signers). A primary goal of the present study was to investigate the role of such sign-specific production regions by examining the neural substrates that support the articulation of different types of signs that vary in their phonological features: one-handed signs produced in “neutral space” (no body contact), “body-anchored” signs produced at specific locations on or very near the body, two-handed signs produced in neutral space, and one-handed fingerspelled words that involve the production of complex sequences of handshapes in neutral space. Examples of these types of signs are provided in Figure 1. This is the first study to examine how several sign-specific phonological features (i.e., number of hands, body contact, and handshape complexity) affect the neural substrates involved in lexical production.
Figure 1.
Examples of the three different sign types and fingerspelling.
Previously, Corina, San Jose-Robertson, Guillemin, High, and Braun (2003) investigated the production of one-handed ASL signs in a positron emission tomography (PET) study in which right-handed deaf signers were asked to repeat one-handed signs (all nouns) and to produce verbs associated with the nouns using only their right or left hand. Generating verbs with either hand (vs. noun repetition) produced very similar activation patterns in left lateral frontal regions associated with lexical-semantic processing (BA 44/45, 47) and with working memory and selection demands (BA 46). In addition, the conjunction analysis revealed that generating verbs with either hand produced activation in the right lateral cerebellum and along the cerebellar midline. Given that right-lateralized cerebellar activation was observed for left-handed sign production, Corina et al. (2003) suggested this cerebellar activation was associated with cognitive or linguistic processes involved in the verb generation task. The English-to-ASL translation task used in the present study (which is also a PET investigation) does not require the same cognitive or linguistic demands as verb generation, and thus we can investigate whether right lateral cerebellar activation is present under an easier task condition.
Further, Corina et al. (2003) reported that direct contrasts between left- and right-handed verb-generation versus their respective noun-repetition baselines revealed that right-handed signing was associated with greater activity in the precentral gyrus (BA 4/6) bilaterally, while left-handed signing was associated with greater activity in left parietal cortex (SPL and SMG). Corina et al. speculated that the surprising activation in right precentral gyrus for right-handed signing reflected active suppression of the left hand. The left hand is normally involved in sign production because many signs are two-handed with the non-dominant hand serving either as a mirror articulator or as a place of articulation (Sandler, 1993). When signers reverse dominance and sign with their left hand, they typically do not utilize the right hand because this hand may be occupied (thus forcing left-handed signing) and because bimanual co-ordination with reversed dominance is difficult. Here we investigate whether activation within the right precentral gyrus is observed when the left hand does not need to be suppressed because the targeted signs are all one-handed (some verbs produced in the Corina et al. study would normally have been articulated with both hands, thus requiring left-hand suppression). Corina et al. attributed the greater left parietal activation for left-handed than right-handed signing to the increased motor control demands when signing with the non-dominant hand. Here we investigate the specific role of left parietal regions in motor control during sign production by contrasting lexical signs and fingerspelled words, which differ in their motoric demands.
Specifically, ASL uses a one-handed fingerspelling system in which distinct handshapes represent letters of the English alphabet. This system is not universal - other signed languages use a two-handed system (e.g., British Sign Language; Sutton-Spence & Woll, 1999) or a different set of handshapes (and movements) to represent the orthography of the surrounding spoken language (see papers in Brentari, 2001). Fingerspelled words in ASL differ from ASL signs because lexical (monomorphemic) signs are limited to a sequence of at most two handshapes, with severe restrictions on the type of handshapes that can occur in the sequence (e.g., Brentari, 1998). In contrast, fingerspelled words are composed of strings of handshapes that are sequenced to spell out an English word. Signers fingerspell English words in a variety of contexts, such as expressing proper names, indicating specific English terms (e.g., technical jargon), or specifying concepts that do not have a corresponding lexical sign. A PET study by Emmorey et al. (2003) investigated the neural underpinnings for the production of fingerspelled words versus ASL signs by asking deaf signers to name pictures of animals that could be named with ASL signs (e.g., ROOSTER) and animals that had to be named by fingerspelling because no standard ASL sign exists (e.g., P-A-N-D-A). In contrast to the production of lexical signs, fingerspelled words engaged a region in superomesial frontal cortex extending into the supplementary motor area (SMA), which Emmorey et al. (2003) suggested reflected the sequencing demands of fingerspelling. However, this superomesial region also encompassed the cingulate gyrus, which may have been more engaged in the fingerspelling condition because the animals that did not have lexical signs were less common and were more difficult to name. The anterior cingulate is known to be involved in error detection and conflict monitoring (e.g., Fiehler, Ullsperger, & von Cramon, 2004). The fingerspelling task used in the study presented here did not involve difficult lexical retrieval, and thus we can investigate whether the SMA is recruited more for fingerspelling than for sign production when task demands are light. In the current study, participants either fingerspelled a printed English word (lexical retrieval not required) or produced the ASL sign translation of an English word.
Emmorey et al. (2003) also found that lexical signs engaged left SMG and SPL to a greater extent than fingerspelled words, and suggested that SMG activation reflected the phonological complexity of lexical signs compared to fingerspelled words, while SPL activation reflected self-monitoring of sign production via proprioceptive feedback. In contrast to fingerspelled words, signs are often specified for contrastive locations on the body (e.g., face, head, neck, torso), and contact with body locations was particularly common for the animal signs in the Emmorey et al. (2003) study due to their iconic origins, e.g., depicting visual features of an animal, such as the trunk of an elephant which is shown in ASL by tracing the slope of the trunk outward from the nose. To produce such body-anchored signs, the hand must be targeted to reach a specific location on the body. Here, we investigate the possible role of either SMG or SPL in targeting body locations by comparing body-anchored signs with both fingerspelled words (no body contact) and one-handed signs that are produced in “neutral space” in front of the body.
Recently, Emmorey, McCullough, and Weisberg (2015) contrasted the brain regions engaged during the comprehension of fingerspelled words and ASL signs when deaf participants made concreteness judgments. Emmorey et al. (2015) found greater activation for ASL signs than fingerspelled words in left SMG, but not in left SPL. Differential engagement of SMG for ASL signs was not found for the hearing (sign-naïve) control group, who alternated button presses while viewing the signs and fingerspelled words. These results lend support to the hypothesis that left SMG is involved in representing and processing phonological features that are specific to ASL signs (e.g., distinct places of articulation on the body, varied path movements, use of the two hands). The fact that SPL was not differentially engaged during the perception of ASL signs (relative to fingerspelled words) is consistent with the possible role of SPL in the monitoring and control of overt sign production. Unlike non-linguistic reaching and grasping actions, sign language production is not visually guided – signers do not visually track their hands. Rather, signers rely on internal somatosensory feedback to catch errors and control sign production (Emmorey, Bosworth, & Kraljic, 2009; Emmorey, Korpics, & Petronio, 2009).
The study by Emmorey et al. (2015) also replicated Waters et al. (2007) who found that comprehension of fingerspelled words in British Sign Language (BSL) activated the putative visual word form area (VWFA; Cohen et al., 2002) to a greater extent than lexical signs. Emmorey et al. (2015) found that one-handed ASL fingerspelled words, like two-handed fingerspelled words in BSL, engaged the VWFA, providing further evidence that this region plays a general role in mapping orthographically structured input onto lexical representations. In the current study, we investigate whether the VWFA is also differentially engaged during the production of fingerspelled words (relative to lexical signs). If so, it will suggest that the VWFA is also involved in mapping English orthographic representations to fingerspelled representations.
Finally, in the current study, we also investigated neural regions involved in bimanual vs. unimanual articulation by targeting the production of two-handed and one-handed signs without face or body contact. For linguistic expression, the two hands are not independent in their articulation, and the form of two-handed signs is highly restricted in ASL (as in many sign languages) by the Symmetry and Dominance conditions (Battison, 1978). Essentially, these phonological constraints require that a) the non-dominant hand copy the handshape and movement of the dominant hand if both hands move and b) the non-dominant hand can serve as a passive (non-moving) base of articulation but is restricted to a small set of unmarked, simple handshapes. Although much is known about bimanual articulation processes for non-linguistic hand and finger movements (see Cardoso de Oliveira (2002) and Swinnen and Wenderoth (2004) for reviews), almost nothing is known about the nature of bimanual movements when they are internally generated and bound to a linguistic representation. Studies of non-linguistic movement have indicated that activation in the SMA (and the cingulate motor cortex) is more pronounced during bimanual than unimanual articulation, suggesting this region is involved in coordinating actions produced with two effectors (i.e., arms, hands, or fingers; e.g, Jäncke et al., 2000; Toyokuro, Muro, Komiya, & Obara, 2002). However, Koeneke, Lutz, Wüstenberg, and Jäncke (2004) found that SMA activation was not greater for bimanual than unimanual finger movements when the unimanual movement condition involved coordination of fingers on the same hand (rather than moving just one finger). Koeneke et al. (2004) found no regions that were more active for bimanual articulation; rather, they observed stronger activations for unimanual movements in core parts of the motor network, including premotor cortex and the intraparietal sulcus. They attributed this finding to the fact that controlling two finger movements on one hand is more demanding than when the finger movements are spread across the two hands (e.g., when typing, cross-hand keystrokes are shorter than within-hand keystrokes; Rosenbaum, Kenny, & Derr, 1983). Here, we explore whether the SMA is involved in coordinating bimanual sign production (indicated by more activation in this region during two-handed than one-handed sign production) and whether adding the left hand as a second articulator might actually reduce neural activity within the motor network (indicated by greater activation in motor regions for one-handed than two-handed sign production).
In sum, we used PET imaging to clarify the role of different brain regions previously implicated in ASL production by systematically varying the type or presence of phonological features within a sign. Specifically, we asked the following questions:
-
a)
What is the role of left parietal cortices in sign production? For example, do body-anchored signs activate parietal regions more than neutral space signs because they must target locations on the body?
-
b)
What neural regions are differentially engaged for the production of fingerspelled words compared to lexical signs (and vice versa)? For example, does fingerspelling activate the SMA or VWFA more than lexical signs?
-
c)
How does the neural control of two-handed signs (bimanual movements) differ from one-handed signs (unimanual movements)?
We also conducted a conjunction analysis (excluding fingerspelled words) to identify regions of common activation during the production of these different types of lexical signs.
Methods
Participants
Eleven right-handed deaf signers aged 21-35 years (mean age = 27 years) participated in the study (5 females). All had deaf parents and acquired ASL as their first language from birth. All participants were congenitally deaf, and all but one participant had severe or profound hearing loss (one participant had a mild-to-moderate hearing loss). All deaf participants used ASL as their primary and preferred language. All participants had 12 or more years of formal education, and all gave informed consent in accordance with Federal and institutional guidelines.
Materials and Task
Participants were asked to fingerspell printed English words presented on the computer screen or to produce the ASL sign translation for English words that corresponded to a) one-handed (neutral space) signs, b) body-anchored signs (all one-handed), or d) two-handed signs in neutral space. For the baseline task, participants indicated whether a printed word contained a descending letter (e.g., p, j, y) using the one-handed fingerspelled loan signs #YES or #NO. These are fingerspelled words that have been borrowed into the ASL lexicon and conform to phonological constraints on signs in the core lexicon (Brentari & Padden, 2001). The descender baseline task controls for visual orthographic and basic motoric processing demands. There were 40 words in each condition: descender baseline task (D), fingerspelled words (FS), one-handed (OH), body-anchored (BA), and two-handed (TH) signs. English words in each condition were matched for log10 word frequency per million (D mean = 2.94, SD = 0.78; FS mean = 2.74, SD = 0.75; OH mean = 3.22, SD = 0.84; BA mean = 3.47; SD = 0.87; TH mean = 3.29, SD = 0.86; from SUBTLEXUS: http://expsy.ugent.be/subtlexus/), letter length (D mean = 5.75, SD = 1.33; FS mean = 5.73, SD = 1.32; OH mean = 5.75, SD = 1.39; BA mean = 5.75; SD = 1.71; TH mean = 5.75, SD = 1.39), and imageability (rating data from the MRC psycholinguistics database for how easy it is to create a sensory image of a word: D mean = 499, SD = 89; FS mean = 498, SD = 79; OH mean = 497, SD = 92; BA mean = 497; SD = 118; TH mean = 495, SD = 107). The signs produced in the three translation conditions were matched for subjective frequency based on our database of frequency ratings of ASL signs rated on a scale of 1 (very infrequent) to 7 (very frequent) (Caselli, Sevcikova Sehyr, Cohen-Goldberg, & Emmorey, 2016): one-handed signs (mean = 4.65; SD = 0.92); two-handed signs (mean = 4.44; SD = 1.34); body-anchored signs (mean = 4.68; SD = 1.16). The English words in the translation task consistently elicited the expected ASL sign – a different sign translation was produced by participants for only 2% of the data.
The one-handed signs were all produced in neutral space without any body contact, and the majority of signs (all but 3) had no change in handshape. Body-anchored signs were all one-handed, and the majority (80%) were articulated on or near the head/neck. The two-handed signs were all produced in neutral space without contact on the head or torso. For half of the two-handed signs, the hands moved together or alternated movement (same handshapes), and for the other half, the left hand served as a passive place of articulation for the right hand.
For all tasks (fingerspelling, sign translation, descender baseline), each word was presented for 1500ms followed by a 500 ms inter-stimulus interval. Words were presented to participants using I-glasses SVGA Pro goggles (I-O Display Systems; Sacramento, CA), and all words were printed in lower case in Myriad Pro font and appeared as black text on a white background. For each condition, there were two separate runs (scans) of 20 words, one with high frequency words and the other low frequency words. However, the frequency manipulation yielded inconclusive results, and results were collapsed across runs.
For each run, the stimuli were presented from 5 seconds after the injection (approximately 7–10 seconds before the bolus arrived in the brain) until 35 seconds after injection (one injection per run). Approximately 7-10 minutes separated each run, allowing the 15O to decay. There were seven practice items for each task, and the presentation order of conditions was randomized across participants.
Procedure
Image Acquisition
All participants underwent MR scanning in a 3.0T TIM Trio Siemens scanner to obtain a 3D T1-weighted structural scan with isotropic 1 mm resolution using the following protocol: MP-RAGE, TR 2530, TE 3.09,TI 800, FOV 25.6cm, matrix 256 × 256 × 208. The MR scans were used to confirm the absence of structural abnormalities, aid in anatomical interpretation of results, and facilitate registration of PET data to a Talairach-compatible atlas.
Positron emission tomography (PET) data were acquired with a Siemens ECAT EXACT HR+ PET system using the following protocol: 3D, 63 image planes, 15 cm axial FOV, 4.6mm transaxial and 3.5mm axial FWHM resolution. Participants performed the experimental tasks during the intravenous bolus injection of 15 mCi of [15O]water. Arterial blood sampling was not performed.
Images of rCBF were computed using the [15O]water autoradiographic method (Herscovitch, Markham, & Raichle, 1983, Hichwa, Ponto, & Watkins, 1995) as follows. Dynamic scans were initiated with each injection and continued for 100 seconds, during which 20 five-second frames were acquired. To determine the time course of bolus transit from the cerebral arteries, time-activity curves were generated for the whole brain over major vessels at the base of the brain. The eight frames representing the first 40 seconds immediately after transit of the bolus from the arterial pool were summed to make an integrated 40-second count image. These summed images were reconstructed into 2mm pixels in a 128×128 matrix.
Spatial normalization
PET data were spatially normalized to a Talairach-compatible atlas through a series of coregistration steps (see Damasio, H., Tranel, Grabowski, Adolphs, & Damasio, A.R., 2004; Grabowski et al., 1995; Emmorey et al., 2011 for details). Prior to registration, the MR data were manually traced to remove extracerebral voxels. Talairach space was constructed directly for each participant via user-identification of the anterior and posterior commissures and the midsagittal plane on the 3D MRI data set in Brainvox. An automated planar search routine defined the bounding box and piecewise linear transformation was used (Frank, Damasio, & Grabowski, 1997), as defined in the Talairach atlas (Talairach & Tournoux, 1988). After Talairach transformation, the MR data sets were warped (AIR 5th order nonlinear algorithm) to an atlas space constructed by averaging 50 normal Talairach-transformed brains, rewarping each brain to the average, and finally averaging them again, analogous to the procedure described in Woods et al. (1999).
For each participant, PET data from each injection were coregistered to each other using Automated Image Registration (AIR 5.25, Roger Woods, UCLA). The coregistered PET data were averaged to produce a mean PET image. Additionally, the participants’ MR images were segmented using a validated tissue segmentation algorithm (Grabowski et al., 2000), and the gray matter partition images were smoothed with a 10 mm kernel. These smoothed gray matter images served as the target for registering participants’ mean PET data to their MR images, with the registration step performed using FSL's linear registration tool (Jenkinson & Smith, 2001; Jenkinson et al., 2002). The deformation fields computed for the MR images were then applied to the PET data to bring them into register with the Talairach-compatible atlas.
After spatial normalization, the PET data were smoothed with a 16 mm FWHM Gaussian kernel using complex multiplication in the frequency domain. The final calculated voxel resolution was 17.9 × 17.9 × 18.9 mm. PET data from each injection were normalized to a global mean of 1000 counts per voxel.
Regression Analyses
PET data were analyzed with a pixelwise general linear model (Friston et al. 1995). Regression analyses were performed using tal_regress, a customized software module based on Gentleman's least squares routines (Miller, 1991) and cross-validated against SAS (Grabowski et al, 1996). The regression model included covariables for the task conditions and subject effects. We contrasted the following conditions: a) fingerspelling and ASL signs each vs. the descender baseline task (Table 1); b) fingerspelling and one-handed signs (Table 2); c) body-anchored and one-handed signs (Table 3); d) two-handed signs and one-handed signs (Table 4); and e) two-handed signs and body-anchored signs (Table 5). Contrasts were tested with t-tests (familywise error rate p < 0.05), using random field theory (RFT) to correct for multiple spatial comparisons across the whole brain (Worsley 1994; Worsley et al., 1992).
Table 1.
Local maxima of areas with increased activation for producing fingerspelled words and signs vs. the descender baseline task (Talairach coordinates). Results are from the whole brain analysis (critical t(94) = ±4.59, p < 0.05).
| Region | Brodmann Area | Side | X | Y | Z | t |
|---|---|---|---|---|---|---|
| Fingerspelling | ||||||
| Central sulcus | BA 4 | R | 30 | –19 | 51 | 11.82 |
| Postcentral gyrus | BA 2 | L | –53 | –20 | 32 | 4.59 |
| BA 4 | L | –15 | –35 | 62 | 4.62 | |
| Intraparietal sulcus | L | –45 | –42 | 54 | 4.70 | |
| Cerebellum | B | –2 | –55 | –12 | 6.04 | |
| One Handed Signs | ||||||
| Precentral gyrus | BA 6 | L | –21 | –4 | 67 | 6.34 |
| Precentral gyrus | BA 4 | L | –22 | –20 | 69 | 6.46 |
| BA 4 | L | –17 | –29 | 56 | 6.71 | |
| Paracentral lobule | BA 6 | L | –12 | –10 | 50 | 6.33 |
| Inferior frontal gyrus | BA 44 | L | –46 | 11 | 13 | 7.62 |
| BA 47 | L | –25 | 26 | –5 | 5.30 | |
| Parietal operculum | L | –43 | –37 | 21 | 8.44 | |
| R | 41 | –39 | 24 | 5.62 | ||
| Superior parietal lobule | BA 7 | L | –11 | –64 | 60 | 5.11 |
| Superior temporal gyrus | BA 42 | R | 64 | –33 | 16 | 4.97 |
| Parahippocampal gyrus | BA 28 | L | –17 | –16 | –13 | 5.67 |
| BA 35 | L | –13 | –29 | –11 | 5.89 | |
| Anterior fusiform gyrus | BA 20 | L | –37 | –18 | –18 | 6.02 |
| BA 20 | L | –29 | –11 | –38 | 6.04 | |
| Temporal pole | BA 20 | R | 27 | –10 | –39 | 7.11 |
| Lateral occipital cortex | BA 19 | L | –33 | –82 | 38 | 5.86 |
| Occipital pole | BA 18 | R | 19 | –99 | 15 | 4.63 |
| Caudate head | L | –12 | 5 | 14 | 8.85 | |
| Putamen | R | 28 | –9 | 11 | 5.29 | |
| Cerebellum | R | 11 | –71 | –12 | 5.45 | |
| Body-Anchored Signs | ||||||
| Paracentral lobule | BA 6 | L | –25 | –9 | 62 | 8.17 |
| Inferior frontal gyrus | BA 44 | L | –50 | 5 | 11 | 5.52 |
| Superior parietal lobule | BA 7 | L | –15 | –55 | 66 | 12.65 |
| Parietal operculum | L | –43 | –35 | 23 | 8.14 | |
| Inferior parietal lobule | BA 40 | R | 48 | –28 | 27 | 7.52 |
| Cingulate sulcus | BA 31 | L | –8 | –10 | 44 | 6.37 |
| Temporal pole | BA 36 | L | –33 | –1 | –41 | 6.29 |
| R | 24 | –5 | –40 | 6.85 | ||
| Posterior parahippocampal gyrus | BA 30 | L | –11 | –40 | –2 | 5.01 |
| Calcarine sulcus | BA 17 | L | –20 | –59 | 20 | 4.84 |
| Lateral occipital cortex | BA 19 | L | –18 | –84 | 40 | 9.92 |
| Cerebellum | R | 20 | –48 | –16 | 9.44 | |
| R | 16 | –88 | –27 | 5.42 | ||
| Two Handed Signs | ||||||
| Paracentral lobule | BA 6 | L | –27 | –6 | 65 | 6.63 |
| BA 4 | R | 35 | –25 | 65 | 14.67 | |
| Postcentral gyrus | BA 5 | L | –21 | –40 | 69 | 8.51 |
| Superior frontal gyrus | BA 32 | R | 10 | –16 | 47 | 11.72 |
| Parietal operculum | L | –48 | –35 | 23 | 7.21 | |
| Inferior parietal lobule | BA 40 | R | 47 | –25 | 29 | 8.04 |
| Posterior Insula | R | 34 | –8 | 9 | 7.40 | |
| Cerebellum | L | –3 | –57 | –13 | 12.84 | |
| L | –22 | –43 | –20 | 11.87 | ||
| L | –22 | –49 | –39 | 8.06 | ||
| R | 12 | –28 | –13 | 4.76 |
Table 2.
Local maxima of areas with increased activation for producing fingerspelled words vs. one-handed signs (Talairach coordinates). Results are from a whole brain analysis (critical t(94) = ±4.59, p < 0.05).
| Region | Brodmann Area | Side | X | Y | Z | t |
|---|---|---|---|---|---|---|
| Fingerspelling > One-handed signs | ||||||
| Frontal pole | BA 10 | R | 26 | 64 | 16 | 7.41 |
| Middle frontal gyrus | BA 8 | R | 32 | 37 | 38 | 7.87 |
| Frontal pole | BA 10 | R | 14 | 62 | 0 | 6.70 |
| Anterior fusiform gyrus | BA 20 | R | 45 | –16 | –23 | 6.08 |
| Posterior fusiform gyrus | BA 19 | L | –25 | –68 | –6 | 8.97 |
| Lateral occipital cortex | BA 18 | L | –38 | –90 | 10 | 5.48 |
| Anterior cingulate | BA 32 | L | –5 | 41 | 2 | 5.68 |
| Subgenual cingulate | BA 25 | R | 5 | 14 | –6 | 4.81 |
| Cerebellum | R | 14 | –57 | –17 | 6.59 | |
| L | –6 | –73 | –33 | 7.72 | ||
| One-handed signs > Fingerspelling | ||||||
| Precentral gyrus | BA 4 | L | –16 | –23 | 56 | –4.70 |
| Postcentral gyrus | BA 3 | R | 22 | –30 | 56 | –5.21 |
| Superior frontal gyrus | BA 8 | L | –8 | 29 | 57 | –5.32 |
| BA 8 | L | –19 | 19 | 45 | –6.04 | |
| Medial frontal motor cortex | BA 6 | R | 14 | –15 | 49 | –5.02 |
| Inferior frontal gyrus | BA 46 | L | –44 | 27 | 13 | –9.17 |
| Anterior insula | L | –25 | 26 | 1 | –8.55 | |
| Orbital frontal cortex | BA 11 | L | –31 | 28 | –17 | –7.48 |
| Middle and inferior temporal gyrus | BA 37 | L | –55 | –53 | –2 | –10.00 |
| Inferior temporal gyrus | R | 63 | –47 | –10 | –5.66 | |
| Anterior fusiform gyrus | BA 20 | L | –30 | –13 | –36 | –4.78 |
| Cuneus/occipital pole | BA 18 | R | 13 | –90 | 14 | –4.73 |
Table 3.
Local maxima of areas with increased activation for producing one-handed body anchored vs. one-handed neutral space signs (Talairach coordinates). Results are from a whole brain analysis (critical t(94) = ±4.59, p < 0.05).
| Region | Brodmann Area | Side | X | Y | Z | t |
|---|---|---|---|---|---|---|
| Body Anchored > One Handed | ||||||
| Postcentral gyrus | BA 3 | R | 63 | –14 | 36 | 4.75 |
| Superior parietal lobule | BA 7 | L | –20 | –53 | 65 | 8.93 |
| BA 7 | R | 28 | –48 | 61 | 5.82 | |
| Supramarginal gyrus | BA 40 | R | 50 | –25 | 31 | 4.74 |
| Inferior parietal lobule | BA 39 | R | 50 | –71 | 30 | 5.00 |
| Fusiform gyrus | BA 19 | R | 18 | –53 | –9 | 6.59 |
| Parieto-occipital fissure | L | –11 | –67 | 19 | 4.95 | |
| BA 19 | L | –11 | –82 | 43 | 6.52 | |
| R | 24 | –81 | 42 | 8.27 | ||
| Lateral occipital cortex | BA 19 | R | 52 | –73 | 2 | 4.62 |
| BA 19 | L | –18 | –66 | 9 | 4.67 | |
| Cerebellum | B | –2 | –75 | –37 | 7.22 | |
| R | 43 | –40 | –39 | 7.92 | ||
| One Handed > Body Anchored | ||||||
| Frontal pole | BA 10 | L | –11 | 65 | 20 | –7.03 |
| Inferior frontal gyrus | BA 45 | L | –43 | 18 | 10 | –4.72 |
| Middle temporal gyrus | BA 21 | L | –50 | –47 | –1 | –9.04 |
| Parahippocampal gyrus | BA 36 | L | –32 | –17 | –22 | –6.58 |
| Lingual gyrus | BA 18 | L | –9 | –86 | –2 | –5.40 |
| BA 19 | R | 8 | –77 | –6 | –6.24 | |
| Posterior cingulate | BA 31 | R | 13 | –45 | 27 | –5.90 |
| Cuneus | BA 17 | R | 16 | –85 | 11 | –6.53 |
| Caudate | L | –13 | 3 | 14 | –9.82 |
Table 4.
Local maxima of areas with increased activation for producing two-handed vs. one-handed neutral space signs (Talairach coordinates). Results are from a whole brain analysis (critical t(94) = ±4.59, p < 0.05).
| Region | Brodmann Area | Side | X | Y | Z | t |
|---|---|---|---|---|---|---|
| Two Handed > One Handed | ||||||
| Paracentral/superior parietal lobule | BA 4 | R | 41 | –23 | 62 | 22.29 |
| Paracentral lobule | BA 6 | R | 14 | –16 | 48 | 10.85 |
| Insula | R | 37 | –6 | 3 | 4.92 | |
| Supramarginal gyrus | BA 40 | L | –65 | –34 | 33 | 5.31 |
| Lateral occipital cortex | BA 19 | R | 52 | –73 | 10 | 5.46 |
| Cerebellum | L | –5 | –56 | –14 | 15.58 | |
| One Handed > Two Handed | ||||||
| Superior frontal gyrus | BA 8 | R | 13 | 47 | 44 | –6.05 |
| Middle frontal gyrus | BA 8 | L | –35 | 18 | 53 | –7.81 |
| BA 9 | L | –28 | 14 | 35 | –8.56 | |
| BA 9 | R | 52 | 24 | 32 | –6.51 | |
| Frontal pole | BA 10 | L | –11 | 64 | 23 | –6.23 |
| Gyrus rectus | BA 11 | L | –6 | 48 | –22 | –4.69 |
| Inferior frontal gyrus | BA 47 | L | –28 | 27 | –10 | –6.71 |
| BA 47 | R | 47 | 46 | –8 | –5.37 | |
| Intraparietal sulcus | BA 7 | L | –30 | –73 | 45 | –8.40 |
| BA 39 | R | 25 | –58 | 27 | –6.07 | |
| Supramarginal gyrus | BA 40 | R | 50 | –48 | 41 | –5.36 |
| Middle temporal gyrus | BA 21 | L | –50 | –49 | –1 | –6.30 |
| L | –34 | –48 | 6 | –6.77 | ||
| Inferior, middle temporal gyri | BA 37 | R | 58 | –44 | –7 | –7.09 |
| Lateral occipital cortex | BA 19 | L | –47 | –83 | 5 | –4.91 |
| Occipital pole | BA 18 | R | 22 | –88 | 13 | –8.49 |
| Lingual gyrus | BA 18 | R | 14 | –77 | –9 | –7.83 |
| Cuneus | BA 18 | L | –9 | –83 | 21 | –6.28 |
| Uncus | BA 20 | R | 25 | –8 | –39 | –5.49 |
| Caudate | L | –12 | 5 | 14 | –8.80 | |
| Putamen | L | –18 | 7 | –4 | –8.46 | |
| Cerebellum | R | 29 | –71 | –20 | –6.71 |
Table 5.
Local maxima of areas with increased activation for producing two-handed vs. body anchored signs (Talairach coordinates). Results are from a whole brain analysis (critical t(94) = ±4.59, p < 0.05).
| Region | Brodmann Area | Side | X | Y | Z | t |
|---|---|---|---|---|---|---|
| Two Handed > Body Anchored | ||||||
| Sensorimotor cortex | BA 4 | R | 41 | –22 | 62 | 20.80 |
| Insula | R | 45 | –13 | 15 | 5.48 | |
| L | 36 | –11 | 4 | 5.29 | ||
| Cerebellum | L | –5 | –57 | –15 | 12.99 | |
| L | –24 | –42 | –21 | 9.57 | ||
| Body Anchored > Two Handed | ||||||
| Superior frontal gyrus | BA 8 | R | 13 | 50 | 42 | –5.49 |
| Middle frontal gyrus | BA 6 | R | 39 | 19 | 51 | –5.16 |
| Middle frontal gyrus | BA 46 | L | –37 | 25 | 24 | –5.59 |
| BA 6 | L | –37 | 12 | 55 | –5.34 | |
| BA 8 | L | –27 | 10 | 39 | –5.07 | |
| R | 37 | 38 | 38 | –5.25 | ||
| Orbitofrontal area | BA 11 | L | –28 | 46 | –13 | –5.28 |
| Posterior insula | L | –36 | –7 | –1 | –5.35 | |
| Superior parietal lobule | BA 7 | L | –14 | –61 | 62 | –8.43 |
| BA 7 | L | –17 | –71 | 36 | –11.66 | |
| Intraparietal sulcus | BA 7 | R | 30 | –71 | 45 | –7.54 |
| Inferior parietal lobule | BA 39 | R | 48 | –70 | 36 | –7.00 |
| Posterior angular gyrus | BA 39 | R | 38 | –85 | 24 | –5.84 |
| Fusiform gyrus | BA 37 | R | 26 | –51 | –8 | –6.44 |
| R | 48 | –60 | –18 | –6.86 | ||
| Ventral striatum | L | –20 | 5 | –9 | –4.95 | |
| R | 16 | 5 | –5 | –4.69 | ||
| Cerebellum | R | 28 | –81 | –24 | –7.01 |
Results
Participants performed the production tasks with very few errors. Non-responses, incorrectly fingerspelled words (e.g., C-H-O-R-M-E for chrome), and erroneous sign translations (e.g., EXPERIMENT for experience) were scored as incorrect. Mean accuracy for all conditions ranged from 98-100% correct.
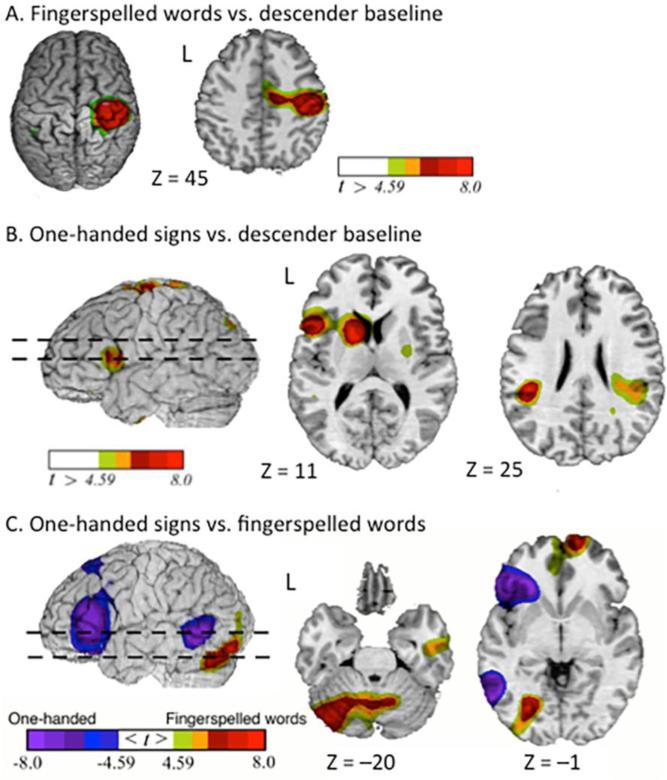
Fingerspelling – one-handed signs
The contrast between fingerspelling and the descender baseline task revealed greater activation for fingerspelling in sensory-motor cortices bilaterally, left inferior parietal lobule, and in the cerebellum (Table 1; Figure 2A). The contrast between one-handed sign production and the baseline revealed greater activation for one-handed signs in several regions, including left inferior frontal gyrus, left superior parietal lobule, and right cerebellum (Table 1; Figure 2B). The direct contrast between the production of fingerspelling and one-handed signs (Table 2; Figure 2C) revealed greater activation for fingerspelling in the right frontal pole, left fusiform gyrus, left occipital cortex, and bilateral cerebellum. Greater activation for one-handed signs was observed in left inferior frontal cortex, left insula, and bilateral middle temporal cortex.
Figure 2.
One-handed production conditions. Greater activity for (A) fingerspelled words and (B) one-handed signs compared to the descender baseline task is indicated by warm colors (greater activation for the descender task is not shown). For the contrast between one-handed signs and fingerspelled words (C), greater activation for one-handed signs is indicated by cool colors and greater activation for fingerspelled words is indicated by warm colors. The images have been thresholded using RFT (p < 0.05, corrected for a whole-brain search).
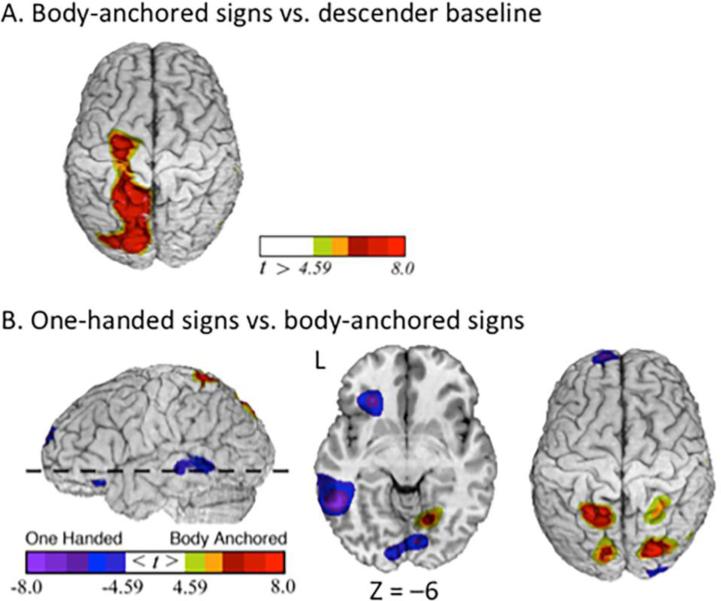
Body-anchored signs – one-handed signs
The contrast between the production of body-anchored signs and the descender baseline task (Table 1; Figure 3A) revealed greater activation for body-anchored signs in several regions including left inferior frontal gyrus, left superior parietal lobule, right inferior parietal lobule, and right cerebellum. The direct contrast between body-anchored and one-handed signs (Table 3; Figure 3B) revealed increased activation for body-anchored signs in bilateral parietal cortex and bilateral cerebellar cortex. Increased activation for one-handed signs relative to body-anchored signs was observed in left inferior frontal gyrus, left middle temporal gyrus, bilateral lingual gyrus, and the left caudate.
Figure 3.
Production of body-anchored signs. Greater activity for (A) body-anchored signs compared to the descender baseline task is indicated by warm colors (greater activation for the descender task is not shown). For the contrast between one-handed and body-anchored signs (C), greater activation for one-handed signs is indicated by cool colors and greater activation for body-anchored signs is indicated by warm colors. The images have been thresholded using RFT (p < 0.05, corrected for a whole-brain search).
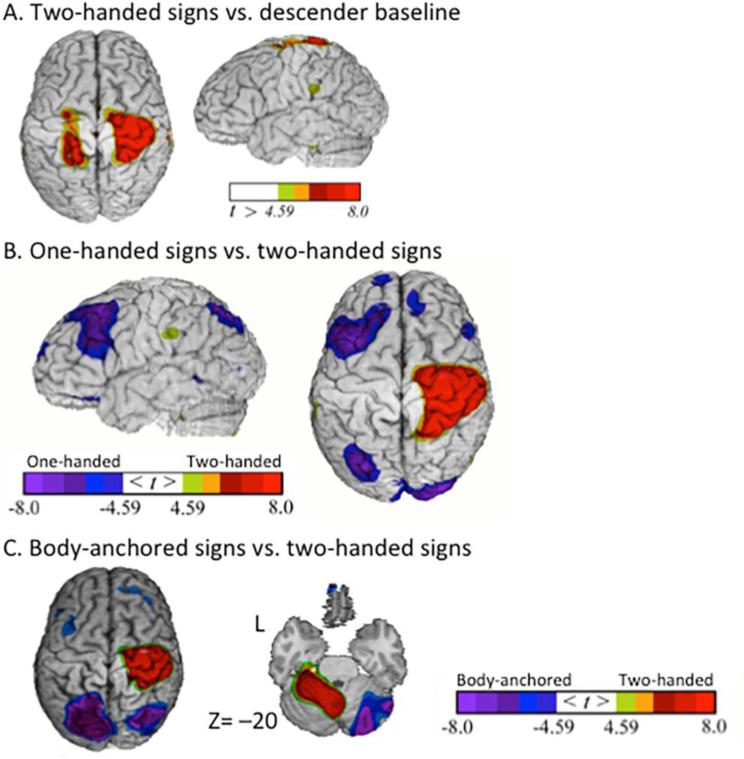
Two-handed signs – one-handed signs, body-anchored signs
The contrast between the production of two-handed signs and the descender baseline task (Table 1; Figure 4A) revealed greater activation for two-handed signs in several regions including right parietal cortex, right insula, and the left cerebellum. The direct contrast between two-handed and one-handed signs (Table 4; Figure 4B) revealed increased activation for two-handed signs in right sensorimotor cortices, left cerebellum, and right insula. Increased activation for one-handed signs relative to two-handed signs was observed in several regions, including bilateral inferior frontal cortex, left partial cortex, and bilateral occipital cortex. The direct contrast between two-handed and body-anchored signs (Table 5; Figure 4C) revealed increased activation for two-handed signs in right sensorimotor cortex, right insula, and left cerebellum. Increased activation for body-anchored signs relative to two-handed signs was observed in several regions, including left middle frontal cortex, bilateral parietal cortex, and right cerebellum.
Figure 4.
Production of two-handed signs. Greater activity for (A) two-handed signs compared to the descender baseline task is indicated by warm colors (greater activation for the descender task is not shown). For the contrasts between two-handed signs and (B) one-handed signs and (C) body-anchored signs, greater activity for two-handed signs is indicated by warm colors. The images have been thresholded using RFT (p < 0.05, corrected for a whole-brain search).
Conjunction analysis
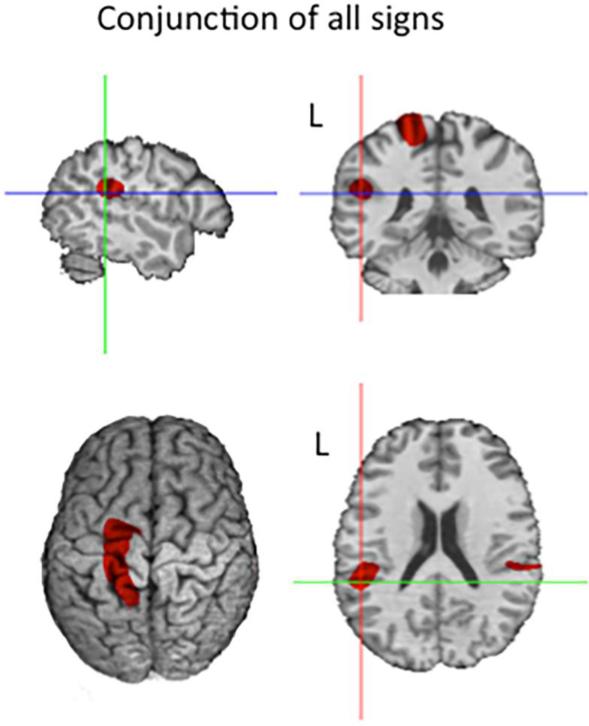
We performed a conjunction analysis of the contrasts of each signing condition (two-handed, one-handed, and body-anchored signs) with the descender baseline task using the minimum statistic approach of Nichols, Brett, Andersson, Wager, and Poline (2005). In this approach, a voxel is considered significant if it is independently significant in each of the component contrasts. The component contrast images were thresholded for significance with random field theory, using an appropriately more lenient threshold (t = 4.02) over an a priori search volume comprising both parietal lobes, the left inferior and middle frontal gyri and the left extrasylvian temporal lobe (cf. Emmorey et al., 2003), and a more stringent threshold (t = 4.59) outside this search region. The results are shown in Figure 5. We observed common activation for all signs in the following regions: left sensorimotor cortex (at the level of the hand area) (−15, −22, +58); bilateral supramarginal gyri (−46, −35, +22; +48, −30, +24); right cerebellum (+10, −68, −12), and the left inferior frontal gyrus (−56, +6, +10). All regions were significant with the more stringent threshold, except for left IFG which was only significant with the a priori search volume threshold.
Figure 5.
Spatial conjunction of independently significant results for the three signing conditions (two-handed, one-handed, body-anchored) relative to the baseline condition. All voxels in color mark more activity in signing relative to baseline. The three-plane crosshairs mark the left supramarginal gyrus locus (−46, −35, +22).
Discussion
Signing differs from speaking with respect to the motor systems required to control the linguistic articulators. Signing also differs from non-linguistic hand and arm actions because movements are not visually guided (unlike most reaching and grasping actions), finger movements are much more complex (particularly for fingerspelling), and signed “actions” are not produced as holistic gestures – rather they involve the assembly of phonological units, as evidenced by slips of the hand (Newkirk, Klima, Pedersen, & Bellugi, 1980) and by partial retrieval of phonological information during tip-of-the-finger states (Thompson, Emmorey, & Gollan, 2005). In this study, we used a fingerspelling task and a translation task to investigate the neural networks that are engaged during sign production. We specifically targeted different sign types in order to clarify the role of parietal cortex (e.g., SMG and SPL) and other brain regions (e.g., SMA, cerebellar cortex) in sign production, to identify neural regions that support the sequencing and production of complex handshapes in fingerspelling, and to examine the neural control of bimanual sign production.
When fingerspelling was contrasted with the descender baseline task (which required repeated production of the one-handed loan signs #YES and #NO), we observed increased activation for fingerspelling in ipsilateral motor cortex (including the “hand knob” area). Greater activation in right motor cortex is somewhat surprising given that fingerspelled words were produced with the dominant right hand. However, Verstynen et al. (2005) found that ipsilateral motor responses increased with the complexity of movements for both the left and the right hand. In addition, Chen et al. (1997) found that rTMS to ipsilateral motor cortex in either hemisphere resulted in timing errors when participants produced a piano sequence (with greater effects for more complex sequences) and that ipsilateral stimulation during right hand movements was disruptive only during execution (not during planning). We suggest that it is the complexity of handshape sequences involved in the execution of fingerspelled words that leads to recruitment of ipsilateral motor cortex. Right motor cortex may actively contribute to fine motor control of right-handed fingerspelling via uncrossed descending projections or via callosal projections to the left hemisphere (or both). Given the role of the cerebellum in the timing of motor movements (e.g., Cheron et al., 2015), we suggest that increased cerebellar activation for fingerspelled words reflects the precise timing required to rapidly articulate a long sequence of complex handshapes (the mean length of fingerspelled words was six handshapes).
Fingerspelled words did not recruit superomesial frontal cortex (supplementary motor area) more than one-handed signs, suggesting that the previous finding by Emmorey et al. (2003) may have been related to task difficulty when naming less common animals. Contrary to our predictions, we did not observe any differences in parietal activation for the contrast between fingerspelled words and one-handed signs. The results with body-anchored signs suggest that this null result may be due to the fact that both fingerspelled words and the one-handed signs were produced in neutral space and not directed toward the body. The contrasts between body-anchored and one-handed signs and between body-anchored and two-handed (neutral space) signs both revealed greater activation in parietal cortex bilaterally for body-anchored signs. In a follow-up analysis, we also contrasted body-anchored signs with fingerspelled words and again observed greater activation in bilateral parietal cortex for body-anchored signs (peak coordinates: −18, −51, +64, t = 10.53; +26, −43, +63, t = 8.82). These findings suggest that the increase in parietal activation reported by Emmorey et al. (2003) when signers named animals with signs compared to fingerspelled words may have been due to the fact that the majority of the animal signs were produced with movement toward the body.
Greater activation for fingerspelled words compared to one-handed signs was also found along the left fusiform gyrus encompassing the visual word form area (VWFA; Cohen et al., 2002). We hypothesize that activation in this region of left ventral occipitotemporal cortex reflects task differences and the role of the VWFA in processing orthographic representations, whether print or fingerspelling (Emmorey et al. 2015; Waters et al., 2007). Compared to the translation task used to elicit sign production, the fingerspelling task demanded more thorough orthographic analysis in order to map each printed letter to the correct fingerspelled handshape. Supporting this hypothesis, a follow-up analysis contrasting body-anchored signs with fingerspelling also revealed greater activation in ventral occipitotemporal cortex for fingerspelling (peak coordinates: −59, −62, −3, t = 5.55).
We observed greater activation in left inferior frontal gyrus (IFG) for one-handed signs in comparison to both the fingerspelling and descender baseline tasks. We hypothesize that activation in left IFG reflects lexical and cognitive operations involved in translating from English to ASL, such as lexical selection and recall processes. Supporting this hypothesis, the contrast between body-anchored signs and the descender baseline task also revealed greater activation in left IFG for body-anchored signs (also elicited via translation), while the contrast between fingerspelling and the descender baseline task revealed no difference in activation in left IFG (neither task required lexical processing). Overall, this pattern of results implies a role for left IFG in lexical retrieval and translation processes and is consistent with previous studies that reported left IFG activation when sign production was elicited by picture naming or verb generation tasks (e.g., Emmorey et al., 2007; Corina et al. 2003). In addition, the contrast between one-handed signs and fingerspelled words revealed greater activation in left middle temporal cortex for one-handed signs (Figure 2C). We suggest that this left temporal activation reflects greater lexical-semantic processing when translating English words into ASL than when mapping letters to handshapes for the fingerspelling task.
As noted above, body-anchored signs engaged the superior parietal cortex to a greater extent than one-handed signs, fingerspelled words, and two-handed signs, which were all produced in neutral space. In addition, these contrasts revealed greater activation for body-anchored signs in the right cerebellum. We hypothesize that increased activation in parietal and cerebellar cortices reflects the increased motor control and proprioceptive monitoring that are required to direct the hand toward a specific location on the head, face, or torso. The superior parietal lobule (SPL) is known to play an important role in the planning and control of reaching movements (e.g., Striemer, Chouinard, & Goodale, 2011), and medial SPL appears to play a specific role in updating postural representations of the arm and hand when movements are not visually guided (e.g., Parkinson, Condon, & Jackson, 2010). Increased activation in right cerebellar cortex for body-anchored signs compared to signs produced in neutral space may reflect the cerebellum's role in predicting proprioceptive information about limb position (Boisgonier & Swinnen, 2014; Bhanpuri, Okamura, & Bastian, 2013). Body-anchored signs are not rare – in a large sample of ASL signs (~1000), 65% were specified for locations on the body (trunk, head, arm, or hand) (Caselli et al., 2016). Our results suggest that the activation that has been observed in SPL during the production of ASL may be due in part to the articulatory requirements of body-anchored signs.
Not surprisingly, the contrasts between two-handed signs and both one-handed signs and (one-handed) body-anchored signs revealed greater activation in right sensorimotor and left cerebellar cortices involved in the control of the left hand. We did not observe increased activation in the SMA for two-handed signs, in contrast to studies investigating the control of non-linguistic bimanual movements. In addition, the contrast between two-handed signs and both one-handed and body-anchored signs revealed less activation for the two-handed signs in frontal and parietal cortices, as well as in the right cerebellum. This finding is consistent with the results of Koeneke et al. (2004) who found decreased activation in the motor circuit for bimanual versus unimanual movements when the goal of the finger movements (to direct a cursor) was spread across the two hands, rather than controlled by one hand. Post et al. (2007) also found that cortical activity in premotor regions was reduced during bimanual compared to unimanual muscle contractions, reflecting the “bilateral deficit” (the same muscles produce less force when contracting simultaneously than when contracting individually). We hypothesize that the reduced cortical and cerebellar activation found for two-handed signs may have been observed here because for half of the signs in this condition the hand configurations and movement patterns were the same across the two hands. For such signs, the movement goal can be spread across the two hands, and linguistic analyses propose that the phonological specifications of the dominant hand are not specified separately but are spread to the non-dominant hand (e.g., Sandler, 1989). Further, in contrast to two-handed signs, one-handed signs may require active suppression of mirror movements of the non-dominant hand (cf. Cincotta & Ziemann, 2008). In sum, our results suggest that the production of two-handed signs may lead to reduced activation within the neural system that supports sign articulation.
The conjunction analysis for all sign types (excluding fingerspelled words) revealed the expected overlap in left sensorimotor cortex and right cerebellar motor cortex, reflecting right-hand articulation. Activation in lateral cerebellar cortex was not observed for our relatively easy translation task, supporting the hypothesis that the right posterolateral cerebellar activation observed by Corina et al. (2003) during sign production was due to the linguistic and cognitive demands of the verb generation task. A small region of common activation was also observed in the left inferior frontal gyrus (BA 44), which may reflect phonological articulatory processes (see Horwitz et al., 2003) or possibly processes associated with lexical translation. The most intriguing finding was that the production of all sign types engaged the supramarginal gyrus (SMG) bilaterally. Most studies have implicated left SMG in sign production (e.g., Corina et al., 1999; Emmorey et al., 2003; Hu et al., 2011), but some studies have reported bilateral SMG activation for signing (e.g., San José-Roberson et al., 2004; Emmorey et al., 2014). The neural overlap occurred in an anterior and inferior region of SMG (see Figure 5) that has also been implicated in speech production (e.g., Sollmann et al., 2014 (right SMG); Hartwigsen et al., 2010 (bilateral SMG); Schwartz, Faseyitan, Kim, & Coslett, 2012). Given that participants were asked to translate written English words into ASL signs, it is possible that they may have covertly articulated the English words, leading to activation within SMG. Although possible, we suggest that this is unlikely for two reasons. One is that when deaf individuals read English words, they do not appear to strongly activate speech-based phonological codes (e.g., Bélanger, Mayberry, & Rayner, 2013). Second, when deaf readers are asked to make phonological decisions about English words, evidence of covert articulation is argued to be found in the left inferior frontal gyrus and left precentral gyrus, rather than SMG (Emmorey, Weisberg, McCullough, & Petrich, 2013; MacSweeney, Waters, Brammer, Woll, & Goswami, 2008).
For spoken language, this anterior region of SMG has been argued to be critically involved in the auditory guidance of speech production and in the motor planning and programming of the phonological units of speech (e.g., Guenther, 2006; Hickok, 2012). For sign production, we suggest that SMG is probably not involved in the visual guidance of signing because visual feedback is not used to fine-tune manual articulations (Emmorey, Bosworth, & Kraljic, 2009), and signers do not look at their hands while signing. Rather, SMG may be involved in the assembly and programming of the phonological units of sign language (see Corina et al., 1999). Data from sign errors and sign language aphasia indicate that signs are assembled from phonological units (i.e., locations, hand configurations, movements) that can be transposed or mis-selected during production (Corina, 2000; Newkirk et al., 1980; Hohenberger, Happ, & Leuniger, 2002). Our findings suggest that SMG may play a role in the retrieval and encoding of phonological units during language production that is independent of the sensory-motoric nature of phonological features.
Finally, a critical drawback of the PET technique is that, because only one measurement of blood flow is made per injection, it is not possible to examine neural responses to different stimuli or types of stimuli within a run, and this would have been quite useful for our study. For example, it is possible that asymmetric two-handed signs (i.e., the non-dominant hand serves as a place of articulation) differ from symmetrical two-handed signs (both hands have the same handshape and movement), with the former engaging SPL to some degree because the dominant hand must contact a location on the non-dominant hand. However, these two sign types were mixed within each run, and with PET we can only examine the neural response across the entire run (or possibly at the beginning or end of a run, but not neural responses to individual items within a run). Similarly, it would be useful to know whether SPL is differentially engaged for body-anchored signs that actually contact the body compared to those without body contact or whether signs that target locations on the face and head (involving finely contrasting locations) differ from those that are articulated on the torso (a much larger target). Unfortunately, we must leave these questions for future research.
In sum, we are beginning to identify the neural circuit that supports sign language production. Evidence from the articulation of different types of ASL signs revealed distinct roles for the superior parietal lobule and the supramarginal gyrus. The production of body anchored signs (those that require the hand to move to a specific location on the face or torso) differentially engaged SPL in contrast to signs produced in neutral space, indicating a specific role for SPL in controlling movements toward the body. Two-handed signs did not preferentially engage the supplementary motor area, which has been hypothesized to be involved in the coordination of non-linguistic bimanual movements. Interestingly, the production of two-handed signs resulted in reduced neural activation compared to one-handed signs in frontal and parietal cortices, as well as in the cerebellum. We hypothesize that this result in part reflects the fact that articulatory goals are spread across the two hands for symmetrical signs, thus reducing demand within motor cortices. The articulation of fingerspelled words engaged iplsilateral motor cortex and cerebellar cortex in comparison to one-handed signs and the descender baseline task. We suggest that these additional motor regions are recruited due to the timing and articulatory demands of rapidly producing sequences of complex hand configurations. Finally, the conjunction analysis indicated that all signs engaged the supramarginal gyrus, suggesting a general role for this region in sign production. We suggest that SMG is involved in the retrieval and encoding of phonological units for both spoken and signed language. Overall, the results revealed a network of brain regions (including SPL, SMG, IFG, VWFA, and cerebellar cortices) that are recruited during the production of manual signs and/or fingerspelling, reflecting phonological, orthographic, motoric, and proprioceptive processing demands. As we learn more about how this network functions, we will gain further insight into how the human motor system achieves internally-generated, linguistically-specified action goals.
Highlights.
We used PET to identify neural circuits that support signing and fingerspelling
Fingerspelled words engaged ipsilateral motor cortex reflecting motor complexity
Signs targeting body locations engaged SPL more than signs produced in neutral space
Two-handed signs activated motor cortex less than one-handed signs
A conjunction of sign types showed overlap in SMG reflecting phonological coding
Acknowledgments
This research was supported by NIH grants RO1 DC006708 and R01 DC010997 awarded to Karen Emmorey and San Diego State University. We would like to thank Joel Bruss, Jocelyn Cole, Lucinda Farnady, and the University of Iowa PET Center nurses and staff for their assistance with the study. We also thank two anonymous reviewers for helpful comments on an earlier version of this manuscript and all of the Deaf participants who made this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karen Emmorey, San Diego State University.
Sonya Mehta, University of Washington.
Stephen McCullough, San Diego State University.
Thomas J. Grabowski, University of Washington
References
- Allen JS, Emmorey K, Bruss J, Damasio H. Morphology of the insula in relation to hearing status and sign language. Journal of Neuroscience. 2008;28(46):11900–11905. doi: 10.1523/JNEUROSCI.3141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battison R. Lexical borrowing in American Sign Language. Linstok Press; Silver Spring, MD: 1978. [Google Scholar]
- Bélanger NN, Mayberry RI, Rayner K. Orthographic and phonological preview benefits: Parafoveal processing in skilled and less-skilled deaf readers. Quarterly Journal of Experimental Psychology. 2013;66(11):2237–2252. doi: 10.1080/17470218.2013.780085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. Journal of Neuroscience. 2013;33(36):14301–14306. doi: 10.1523/JNEUROSCI.0784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier M, Swinnen SP. Proprioception in the cerebellum. Frontiers in Human Neuroscience. 2014;8:212. doi: 10.3389/fnhum.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AR, Guillemin A, Hosey L, Varga M. The neural organization of discourse - An (H2O)-O-15-PET study of narrative production in English and American sign language. Brain. 2001;124:2028–2044. doi: 10.1093/brain/124.10.2028. [DOI] [PubMed] [Google Scholar]
- Brentari D. A prosodic model of sign language phonology. The MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Brentari D. Foreign vocabulary in sign languages: A cross-linguistic investigation of word formation. Psychology Press; New York: 2001. [Google Scholar]
- Brentari D, Padden C. Native and foreign vocabulary in American Sign Language: A lexicon with multiple origins. In: Brentari D, editor. Foreign vocabulary in sign languages: A cross-linguistic investigation of word formation. Psychology Press; New York: 2001. pp. 87–120. [Google Scholar]
- Caselli N, Sevcikova Sehyr Z, Cohen-Goldberg A, Emmorey K. ASL-LEX: A lexical database of American Sign Language. Behavior Research Methods. 2016 doi: 10.3758/s13428-016-0742-0. DOI 10.3758/s13428-016-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso de Oliveira S. The neuronal basis of bimanual coordination: recent neurophysiological evidence and functional models. Acta Psychologica. 2002;110:139–159. doi: 10.1016/s0001-6918(02)00031-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen L. Involvement of the ipsilateral motor cortex in finger movements of different complexities. American Neurological Association. 1997;41:274–254. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- Cheron G, Márquez-Ruiz J, Dan B. oscillations, timing, plasticity, and learning in the cerebellum. Cerebellum. 2015 doi: 10.1007/s12311-015-0665-9. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Zeimann U. Neurophysiology of unimanual motor control and mirror movements. Clinical Neurophysiology. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Corina DP. Some observations on paraphasia in American Sign Language. In: Emmorey K, Lane H, editors. The signs of language revisited. LEA; Hillsdale, NJ: 2000. [Google Scholar]
- Corina DP, McBurney SL, Dodrill C, Hinshaw K, Brinkley J, Ojemann G. Functional roles of Broca's area and supramarginal gyrus: Evidence from cortical stimulation mapping in a deaf signer. NeuroImage. 1999;10:570–581. doi: 10.1006/nimg.1999.0499. [DOI] [PubMed] [Google Scholar]
- Corina DP, San Jose-Robertson L, Guillemin A, High J, Braun AR. Language lateralization in a bimanual language. Journal of Cognitive Neuroscience. 2003;15(5):718–730. doi: 10.1162/089892903322307438. [DOI] [PubMed] [Google Scholar]
- Emmorey K, Bosworth R, Kraljic T. Visual feedback and self-monitoring of sign language. Journal of Memory and Language. 2009;61:398–411. doi: 10.1016/j.jml.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Korpics F, Petronio K. The use of visual feedback during signing: Evidence from signers with impaired vision. Journal of Deaf Studies and Deaf Education. 2009;14(1):99–104. doi: 10.1093/deafed/enn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Grabowski T, McCullough S, Damasio H, Ponto L, Hichwa R, Bellugi U. Neural systems underlying lexical retrieval for sign language. Neuropsychologia. 2003;41(1):85–95. doi: 10.1016/s0028-3932(02)00089-1. [DOI] [PubMed] [Google Scholar]
- Emmorey K, McCullough S, Mehta S, Grabowski TJ. How sensory-motor systems impact the neural organization for language: Direct contrasts between spoken and signed language. Frontiers in Psychology. 2014;5(484) doi: 10.3389/fpsyg.2014.00484. doi: 10.3389/fpsyg.2014.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Mehta S, Grabowski TJ. The neural correlates of sign and word production. NeuroImage. 2007;36:202–208. doi: 10.1016/j.neuroimage.2007.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Weisberg J, McCullough S, Petrich JAF. Mapping the reading circuitry for skilled deaf readers: An fMRI study of semantic and phonological processing. Brain and Language. 2013;126:169–180. doi: 10.1016/j.bandl.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon Y. Neural correlates of error detection and error correction: is there a common neuroanatomical substrate? European Journal of Neuorscience. 2004;19:3081–3087. doi: 10.1111/j.0953-816X.2004.03414.x. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Grabowski TJ, Damasio H, Frank R, Hichwa RD, Ponto LL, Watkins GL. A new technique for PET slice orientation and MRI-PET coregistration. Human Brain Mapping. 1995;2:123–133. [Google Scholar]
- Grabowski TJ, Frank R, Brown CK, Damasio H, Boles Ponto LL, Watkins GL, et al. Reliability of PET activation across statistical methods, subject groups, and sample sizes. Human Brain Mapping. 1996;4:23–46. doi: 10.1002/(SICI)1097-0193(1996)4:1<23::AID-HBM2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Frank RJ, Szumski NR, Brown CK, Damasio H. Validation of partial tissue segmentation of single-channel magnetic resonance images. NeuroImage. 2000;12:640–656. doi: 10.1006/nimg.2000.0649. [DOI] [PubMed] [Google Scholar]
- Guenther F. Cortical interactions underlying the production of speech sounds. Journal of Communication Disorders. 2006;39:350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hichwa RD, Ponto LL, Watkins GL. Clinical blood flow measurement with [150]water and positron emission tomography (PET). In: Emran AM, editor. Chemists' views of imaging centers, symposium proceedings of the International Symposium on “Chemists' Views of Imaging Centers”. Plenum Publishing; New York: 1995. [Google Scholar]
- Horwitz B, Amunts K, Bhattacharyya R, Patkin D, Jeffries K, Zilles K, Braun A. Activation of Broca's area during the production of spoken and signed language: a combined cytoarchitectonic mapping and PET analysis. Neuropsychologia. 2003;41:1868–1876. doi: 10.1016/s0028-3932(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Peters M, Himmelbach M, Nösselt T, Shah J, Steinmetz H. fMRI study of bimanual coordination. Neuropsychologia. 2000;38:165–174. doi: 10.1016/s0028-3932(99)00062-7. [DOI] [PubMed] [Google Scholar]
- MacSweeney M, Waters D, Brammer MJ, Woll B, Goswami U. Phonological processing in deaf signers and the impact of age of first language acquisition. NeuroImage. 2008;40:1369–1379. doi: 10.1016/j.neuroimage.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ. Least Squares Routines to Supplement those of Gentleman. Applied Statistics Algorithm. 1991;274:AS274. [Google Scholar]
- Newkirk D, Klima E, Pederson C, Bellugi U. Linguistic evidence from slips of the hand. In: Fromkin V, editor. Errors in Linguistic Performance: Slips of the Tongue, Ear, Pen, and Hand. Academic Press; New York: 1980. pp. 165–197. [Google Scholar]
- Nichols T1, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Koeneke A, Lutz K, Wüstenberg T, Jäncke L. Bimanual versus unimanual coordination: what makes the difference? NeuroImage. 2004;22:1336–1350. doi: 10.1016/j.neuroimage.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Post M, van Duinen H, Steens A, Renken R, Kuipers B, Maurits N, Zijdewind I. Reduced cortical activity during maximal bilateral contractions of the index finger. NeuroImage. 2007;35:16–27. doi: 10.1016/j.neuroimage.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Sandier W. Hand in hand: The role of the nondominant band in ASL phonology. The Linguistic Review. 1993:337–390. [Google Scholar]
- Sutton-Spence R, Woll B. The Linguistics of British Sign Language. Cambridge University Press; Cambridge, UK: 1999. [Google Scholar]
- Swinnen SP, Wenderoth N. Two hands, one brain: cognitive neuroscience of bimanual skill. Trends in Cognitive Sciences. 2004;8(1):18–25. doi: 10.1016/j.tics.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Toyokura M, Muro I, Komiya T, Obara M. Activation of pre-supplementary motor area (SMA) and SMA proper during unimanual and bimanual complex sequences: An analysis using functional magnetic resonance imaging. Journal of Neuroimaging. 2002;12(2):172–178. doi: 10.1111/j.1552-6569.2002.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Waters D, Campbell R, Capek CM, Woll B, David AS, McGuire PK, et al. Fingerspelling, signed language, text and picture processing in deaf native signers: the role of the mid-fusiform gyrus. Neuroimage. 2007;35(3):1287–1302. doi: 10.1016/j.neuroimage.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Dapretto M, Sicotte NL, Toga AW, Mazziotta JC. Creation and use of a Talairach-compatible atlas for accurate, automated, nonlinear intersubject registration, and analysis of functional imaging data. Human Brain Mapping. 1999;8(2-3):73–79. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<73::AID-HBM1>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ. Local maxima and the expected Euler characteristic of excursion sets of chi-squared, F and t fields. Advanced Applied Probability. 1994;26:13–42. [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992 Nov;12(6):900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zou L, Abutalebi J, Zinszer B, Yan X, Shu H, Peng D, Ding G. Second language experience modulates functional brain network for native language production in bimodal bilinguals. NeuroImage. 2012;62:1367–1375. doi: 10.1016/j.neuroimage.2012.05.062. [DOI] [PubMed] [Google Scholar]