Abstract
Objective
To determine preliminary efficacy and to identify baseline characteristics predicting who would benefit most from fast walking training plus a step activity monitoring program (FAST+SAM) compared to fast walking training alone (FAST) in persons with chronic stroke.
Design
Randomized controlled trial with blinded assessors
Setting
Outpatient clinical research laboratory
Participants
37 individuals greater than 6 months post-stroke.
Interventions
Subjects were assigned to either FAST which was walking training at their fastest possible speed on the treadmill (30 minutes) and over ground 3 times/week for 12 weeks or FAST plus a step activity monitoring program (FAST+SAM). The step activity monitoring program consisted of daily step monitoring with a StepWatch Activity monitor, goal setting, and identification of barriers to activity and strategies to overcome barriers.
Main Outcome Measures
Daily step activity metrics (steps/day, time walking/day), walking speed and six minute walk test distance (6MWT).
Results
There was a significant effect of time for both groups with all outcomes improving from pre to post-training, (all p<0.05). The FAST+SAM was superior to FAST for 6MWT (p=0.018), with a larger increase in the FAST+SAM group. The interventions had differential effectiveness based on baseline step activity. Sequential moderated regression models demonstrated that for subjects with baseline levels of step activity and 6MWT distances that were below the mean, the FAST+SAM intervention was more effective than FAST (1715±1584 vs. 254±933 steps/day, respectively; p<0.05 for overall model and ΔR2 for steps/day and 6MWT).
Conclusions
The addition of a step activity monitoring program to a fast walking training intervention may be most effective in persons with chronic stroke that have initial low levels of walking endurance and activity. Regardless of baseline performance, the FAST + SAM intervention was more effective for improving walking endurance.
Keywords: stroke, physical activity, walking
INTRODUCTION
Approximately 6.8 million adult Americans are living with stroke1 and stroke is a leading cause of serious, long-term disability in the US1. As a group, stroke survivors are more physically inactive than even the most sedentary older adults2–4. Lack of physical activity has serious consequences in persons with stroke, including an increased risk of having a second stroke5, developing other diseases and mortality2, 3, 6. The potential consequences of inactivity are even more alarming considering that inactivity gets worse over the first year after stroke5.
Despite the severe consequences of inactivity after stroke, little attention has been paid to whether activity is influenced by rehabilitation interventions for chronic stroke survivors7–11. The few intervention studies that have examined daily walking activity (steps/day outside of rehabilitation) have generally found no improvement in activity with the intervention7, 9, 11. However, one novel intervention, fast treadmill training, was able to demonstrate improvements in daily walking activity in chronic stroke survivors when compared with traditional physical therapy8. The participants in the fast treadmill training group also demonstrated a significant improvement in walking speed and endurance, which have been suggested to contribute to daily walking activity8, 12–14. Thus, fast treadmill training may improve daily walking activity through improvements in walking capacity. Unfortunately, even with these gains, the stroke survivors’ daily walking activity (~4500 steps/day) after fast training was still well below recommended levels (7,000–10,000 steps/day)15, 16, suggesting that fast walking training alone may not provide an adequate stimulus to change real-world daily walking activity in those with chronic stroke.
A recent meta-analysis found that step activity monitoring is an extremely effective stimulus for increasing daily walking activity17. The key ingredients to the success of these programs are: 1) monitoring step activity with a pedometer or similar device, 2) setting a daily step activity goal and 3) identifying barriers to activity and strategies to overcome those barriers18–21. Recent evidence from our lab suggests that a step activity monitoring program results in improvements in real-world walking activity in persons with chronic stroke22. However, similar to what was observed with the fast walking training, even with the gains, subject’s walking activity was still well below recommended amounts22. Moreover, while the majority of subjects improved, the range of improvement was large and there were several subjects who did not improve with the intervention22. This result is consistent with other rehabilitation clinical trials in chronic stroke showing variable effects of interventions, with some individuals showing significant improvements and others no improvement11, 23, 24. This is also consistent with recent studies showing that baseline factors may play an important role in moderating the effectiveness of different post-stroke walking interventions and that these factors are important for determining for whom the intervention may be most effective25, 26. Together, these results suggest that some individuals do not respond to specific therapies. There is growing recognition in the post-stroke rehabilitation research community that studies should not simply focus on identifying which interventions are efficacious, but rather identify for whom certain interventions are most efficacious11, 27, acknowledging that one intervention is not likely best for all. Therefore, in the present study, we were interested in investigating the relationship between baseline factors and the effectiveness of each intervention.
In the present study we therefore hypothesized that the combination of a fast walking intervention (that improves walking capacity), with a step activity monitoring program (that facilitates translation of gains from the clinic to the “real-world”), would generate greater improvements in real world walking activity than fast walking training alone, but that this effect would depend on baseline walking characteristics. The purpose of this study, therefore, was to determine preliminary efficacy and to identify baseline characteristics predicting who would benefit most from fast walking training plus a step activity monitoring program (FAST+SAM) compared to fast walking training alone (FAST) in persons with chronic stroke.
METHODS
Participants
Participants were recruited from local physical therapy clinics, stroke support groups, and newspaper advertisements. Individuals age 21–85 were included in the study if they had sustained a stroke greater than 6 months prior, were able to walk without assistance (the use of orthotics or assistive devices were allowed), were able to walk 5 minutes at a self-selected pace on the treadmill, were able to walk outside the home prior to stroke, walked less than 10,000 steps per day, and were able to communicate with the investigators. Individuals post stroke were not included in the study if they had experienced more than one stroke, had evidence of a cerebellar stroke, additional neurologic diseases, cardiac event less than 3 months prior, had received Botox in the lower extremities less than 4 months prior, pain that limited walking, unexplained dizziness in the past 6 months, or were participating in skilled physical therapy services. All participants post stroke received medical clearance prior to beginning the study and signed an informed consent for the study. The study was approved by the Human Subjects Review Board at University of Delaware. Due the preliminary nature of the study, formal sample size calculation was not completed.
Study Design
Subjects completed laboratory performance-based and walking activity evaluations at baseline (pre) and after 12 weeks of training (post) in a University-based laboratory. Random assignment without replacement was completed following treadmill acclimatization (see below) and subjects were assigned to either the Fast Walking alone (FAST) group or the Fast Walking plus Step Activity Monitoring Program (FAST+SAM) group. Prior to the start of training, participants underwent a submaximal stress test and secured cardiac clearance. Subjects then completed training 3 times per week for 12 weeks (36 sessions) followed by the post-training evaluation. Adverse events were monitored throughout the study.
Intervention
Prior to the start of training, subjects participated in a treadmill acclimatization session. In this session, the subject’s self-selected and fastest walking speeds on the treadmill were determined based on over ground walking speed, patient comfort and safety. The fastest walking speed on the treadmill, as determined in this session, was used as the initial treadmill training speed.
Treadmill and over ground walking training
Both groups completed a fast walking treadmill training program followed directly by 10 minutes of over-ground walking activities. At the beginning of the session, all subjects donned a subject-specific calibrated StepWatch Activity Monitor (SAM). This was done so that in-session walking activity was recorded, monitored, and accounted for when monitoring steps taken in training vs. steps taken out of training for those in the FAST+SAM group. All treadmill walking was completed while subjects were in an overhead chest-harness system; no body weight support was provided. Heart rate was measured continuously using a Polar™ heart rate monitor worn around the subject’s chest. Subjects walked for 30 minutes with the goal of walking at the fast training speed, one at which Target Heart Rate (THR) = ((220-age) - Resting heart rate) × 80%)+ Resting heart rate, was achieved. As stated above, this speed was initially determined at the acclimatization session and progressed based on heart rate response throughout the intervention. The fast training speed was reduced if the subject requested to walk slower, the fast speed was no longer safe (i.e. increased toe scuffing or tripping, inability to stay toward the front of the treadmill), the subject reported a rating of perceived exertion of ≥17 on the 6–20 Borg Rate of Perceived Exertion Scale28 and/if THR was exceeded. If this occurred, the treadmill speed was lowered to allow the heart rate to return to, ((220-age) – Resting heart rate) × 50–60%)+ Resting heart rate, or to a rate of perceived exertion ≤13. If the recovery criteria were not achieved by walking slower, the treadmill was stopped and the subject took a standing or seated rest break to achieve recovery. Once recovery was reached, the subject was transitioned into the fast training speed again. If a subject was unable to achieve his/her THR through speed increases alone, an incline was added. If a subject was unable to walk on an incline, resistance was added by placing a resistance band around the subject’s torso and providing a posterior pull that the subject had to overcome while fast walking on the treadmill. Following treadmill walking, 10 minutes of over-ground walking activities were performed with the same THR and rate of perceived exertion criteria as on the treadmill. Subjects were guarded and activities were progressed by a physical therapist during all sessions. The purpose of this over ground walking was for the participant to practice walking activities experienced during everyday activities (e.g. turning, backward stepping, walking while carrying objects) to gain both skill and confidence with these routine walking activities that are important in real-world walking.
Step activity monitoring program
Subjects in the FAST+SAM group wore the SAM during the 12 weeks of training so that steps/day could be tracked and used for goal setting. Baseline step activity data for the FAST+SAM group was used to categorize16 and assign step activity goals29. Subjects that completed an average of < 5,000 steps per day (SPD) were given a goal to increase walking activity by 8%. Subjects that averaged between 5,000–7,499 SPD were given a goal of increasing walking activity by 5%. Subjects that averaged between 7,500–9,999 SPD were given a goal to increase walking activity by 3%. Subjects were expected to achieve their daily step goal on the days that they did not attend treadmill training sessions. In order to advance the goal, subjects needed to attain 6 days of goal achievement over a 2 week period of time. For those who were able to accomplish 6 days of elevated activity, a new activity goal was calculated based on the average SPD completed in the 2nd week of the 2 week monitoring period.
Step activity data were reviewed at each treadmill training session and used to determine and promote goal achievement. Particularly, subjects were told the number of steps they had taken in the interim days between training sessions. Since there was no immediate feedback on the SAM unit, subjects used this information to help them understand how much walking activity they were performing during certain daily activities, like walking to the mailbox or walking laps around their home, and how that added to their total steps per day. Subjects were also advised of how their steps per day related to goal achievement. An individualized discussion of barriers to increased activity and how to overcome those barriers occurred at each session22. Subjects were encouraged to use these strategies to increase steps during routine daily life around their home and community. Examples include getting up to change the television station instead of using the remote, walking to get the mail, and walking from the car parked further from the store22. Like another successful activity monitoring program30, specific topics related to changing activity levels were discussed between the physical therapist and those in the FAST+SAM group. Topics were addressed during the 2 week goal advancement session and included: education on the benefits of activity and risks of inactivity, monitoring a sedentary lifestyle and substituting activity for inactivity, identifying self-motivators and personal benefits to increasing activity, recognizing and resolving inconsistencies of being active daily, using a social support system to achieve goals, improving the ability to differentiate between habits and goals; and throughout the last week of training, relapse prevention.
Outcome Measurement
All outcome measurement testing was done by an investigator blinded to group assignment. Our primary outcomes of interest were measures that capture real world walking activity, including number of steps per day and total time spent walking22, 35. Because previous studies have shown a relationship between walking endurance, speed and walking activity3, 13, 14, distance covered during the 6MWT and self-selected and maximal walking speeds were also outcomes of interest.
For step activity monitoring, data was collected while subjects wore a calibrated StepWatch Activity Monitor (SAM). The SAM has been shown to be an accurate and reliable device for measuring walking activity post-stroke31–33. The SAM was placed above the ankle on the non-paretic lower extremity and calibrated to the participants’ height and walking characteristics per manufacturer’s instructions. To calibrate the SAM, participants walked 30 strides at their self-selected pace and 10 strides at a slightly faster pace. If the number of steps differed from manual counting by ± 2 strides, the sensitivity of the SAM was adjusted until accuracy was obtained. The number of strides was counted in each consecutive 10 second interval (changed from the SAM default interval of 60 seconds) to ensure the most accurate representation of continuous stepping in individuals across a wide range of walking speeds22, 34, 35. At the pre-testing session participants were educated on proper wear and care of the SAM unit and demonstrated appropriate donning and doffing techniques. Participants in both groups wore the SAM for all waking hours, except during bathing and swimming activities for seven days at pre and post-testing.
For laboratory performance-based walking testing, subjects completed the 10 Meter Walk Test to measure short distance walking speed36 and the 6 Minute Walk Test (6MWT) as a measure of long distance walking37. These measures have been shown to be reliable and valid in individuals after stroke37,38,39. During this testing, subjects were permitted to use devices typically used in their everyday life. To assess walking speed (self-selected and maximal), subjects were instructed to walk 10 meters while the time was measured for the intermediate 6 meters, to allow for acceleration and deceleration. To complete the 6MWT, subjects walked a 53.5 meter tiled pathway and were instructed to cover as much ground as possible within the 6 minutes. No verbal encouragement was given and subjects were not permitted to talk during the test.
Data and Statistical Analysis
Data from the SAM for at least 4 complete days of walking pre and post-training was analyzed using a custom MATLAB program (MathWorks, Natick, MA) to obtain information on participants’ walking activity. Days with less than 10 hours of recorded data were examined to determine whether the number of hours recorded were consistent with previous days. To be conservative, if the number of hours was substantially less than other days, the day was not included in the analysis. We determined the start and end of a walking bout based on methods from previous studies40, 41. The start of a walking bout was operationally defined as 2 strides in a 10 second interval and the end of a walking bout was defined as zero strides in a 10 second interval. These algorithms prevented leg movements during rest and standing from being counted as strides. Once walking activity was sorted into bouts, the number of strides from the SAM output was doubled to obtain the total number of steps per day. Total time walking for each day was calculated as the sum of the time spent walking in all bouts for the day.
To determine self-selected and maximal walking speed, time to cover the middle 6 meters of the 10 meter walk test was converted to meters per second and an average of 3 trials was used in the analysis. For the 6MWT, the total distance covered during the test was used in the analysis. All statistical analyses were conducted in SPSS version 22. 2 × 2 mixed design ANOVA’s and linear regression models were used to examine differences across groups and time for the primary outcomes. Assumptions for the statistical tests were evaluated; outliers were identified through residual analysis. One outlier was removed from the analyses of change in self-selected and maximal walking speed and 2 outliers were removed from the analysis of change in 6MWT distance. In addition to examining changes from pre- to post-training, changes relative to baseline values [(pre-training-post-training)/pre-training] were tested for each outcome.
As discussed in the Introduction, we hypothesized that the interventions would be most effective for those with lower initial activity. In order to test this research question, sequential moderated regression models were run to determine how Group and pre-training values were related to changes in outcomes of interest from pre to post-training. A separate model was used for each outcome (e.g.- dependent variables of change in steps/day, change in total time walking/day, change in walking speed, change in endurance). Each model had two blocks (independent variables), the first contained the main effects, Group and the pre-training values (for the outcome being tested), and the second block included the Group × pre-training interaction. For each model, we evaluated the overall model significance along with the ΔR2 with the addition of the interaction term. An α level of .05 was set as the threshold for statistical significance.
RESULTS
Information regarding subject screening, enrollment and drop-out can be found in the CONSORT diagram. No serious adverse events occurred. Two subjects chose to discontinue the intervention due to exacerbation of previous orthopedic pain. Table 1 presents the basic demographic information on the participants. There were no statistically significant differences in age or time since stroke between groups. Participants in the FAST+SAM group attended 29±3 training sessions and participants in the FAST group attended 27±2 training sessions. Participants in the FAST+SAM group met their daily step activity goals on 42% of the non-training days. Training days were not included in this calculation because we were interested in real-world activity outside of the supervised intervention.
Table 1.
Subject characteristics at the pre-training evaluation.
| Group | Gender | Age | Side of Stroke | Time since Stroke | Lower Extremity Fugl-Meyer score |
|---|---|---|---|---|---|
| Female(F) Male(M) |
(years) | Left(L) Right(R) |
(months) | (out of 34) | |
| FAST+SAM | 6 F, 7 M | 59.1±8.7 | 7 L, 6 R | 29.4±21.4 | 16.8±7.1 |
|
| |||||
| FAST | 6 F, 8 M | 58.2±12.4 | 9 L, 5 R | 50.8±44.1 | 18.6±4.6 |
Table 2 shows the means and standard deviations for the walking activity and walking speed and distance variables prior to and following training. There were no significant differences between the groups on any of the outcome measures at baseline, with the exception of total time walking, which was greater in the FAST group at baseline (p=0.025). In the ANOVA’s, a significant effect of time was observed for steps/day, total time walking, self-selected walking speed, maximal walking speed and distance covered on the 6MWT (all p<0.05, Table 2). There was a group × time interaction observed for the 6MWT (p=0.018) with a larger increase in 6MWT distance in the FAST+SAM group (Table 2).
Table 2.
Step Activity and Clinical Data
| Steps per Day [95% CI] | Total Time Walking (hours) [95% CI] | SSWS (m/s) [95% CI] | MWS (m/s) [95% CI] | 6MWT (m) [95% CI] | |
|---|---|---|---|---|---|
| FAST+SAM | |||||
| Pre Mean±std | 4146±2550* | 1.39±.62* | 0.57±0.36* | 0.67±.50* | 221±153*† |
| Pre 95% CI | 2545–5746 | 0.96–1.8 | 0.35–0.79 | 0.37–0.97 | 128–314 |
| Post Mean±std | 5160±2504 | 1.70±.79 | 0.68±0.37 | 0.88±.54 | 281±168 |
| Post 95% CI | 3250–7068 | 1.1–2.3 | 0.45–0.91 | 0.55–1.2 | 182–381 |
| FAST | |||||
| Pre Mean±std | 6080±3015* | 2.09±.88* | 0.68±0.41* | 0.84±.54* | 257±171* |
| Pre 95% CI | 4537–7622 | 1.7–2.5 | 0.46–0.89 | 0.55–1.1 | 168–347 |
| Post Mean±std | 7087±3962 | 2.35±1.17 | 0.76±0.44 | 0.94±0.60 | 281±178 |
| Post 95% CI | 5247–8926 | 1.8–2.9 | 0.54–0.99 | 0.62–1.3 | 185–376 |
Average ±1 standard deviation and 95% confidence intervals for each group pre- and post-training. SSWS = self-selected walking speed; MWS=maximal walking speed; 6MWT=Six minute walk test; m/s= meters/second; m=meters.
indicates a significant change from pre- to post-training and
indicates a significant difference between groups from pre- to post-training.
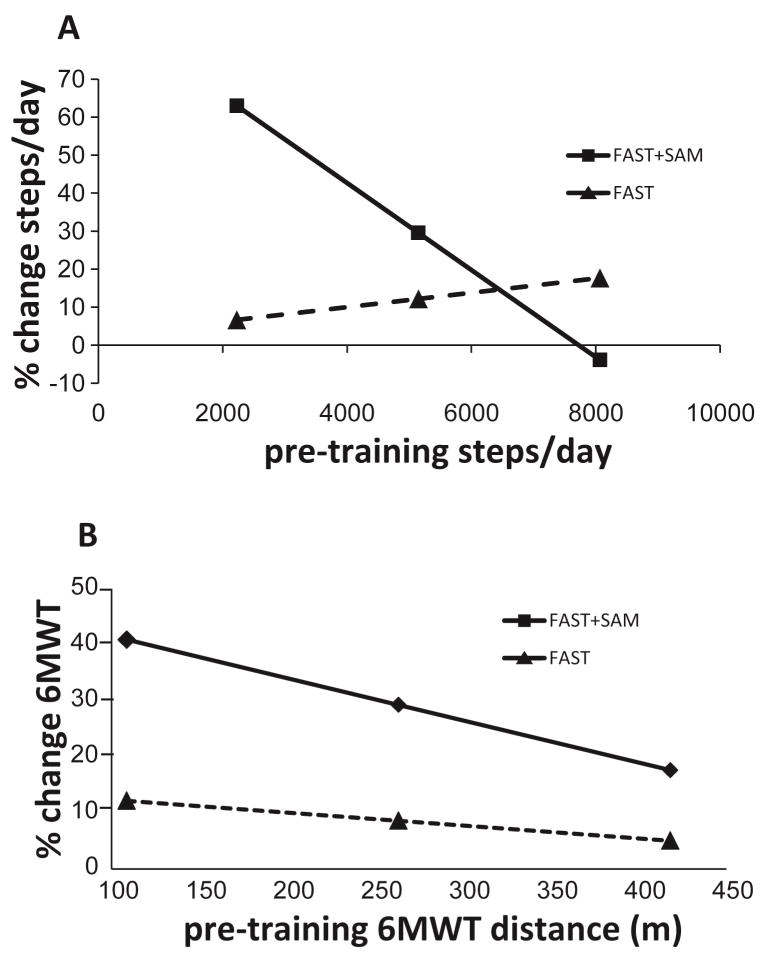
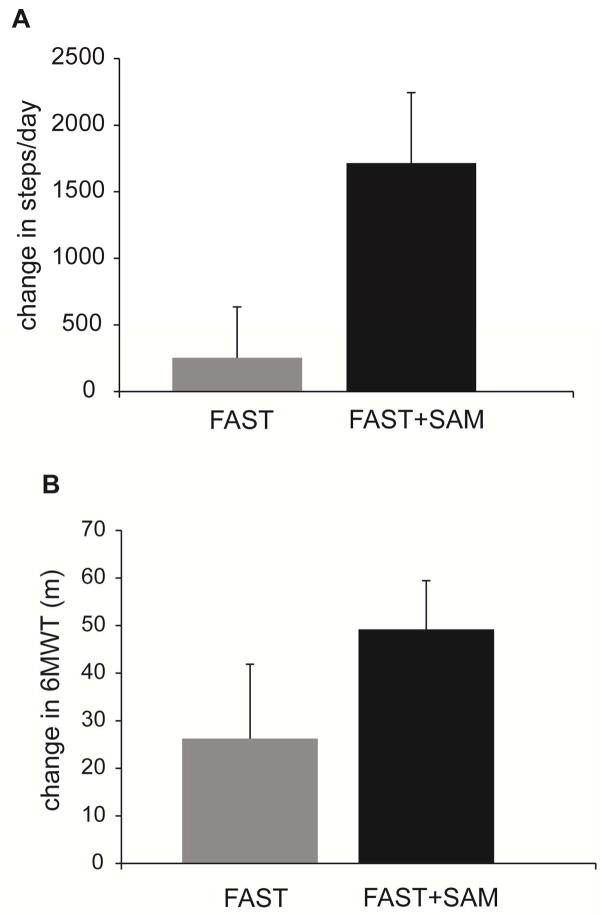
To test the hypothesis that baseline walking activity would moderate the changes in walking activity with each intervention, sequential moderated regressions for the walking activity variables were completed. For the walking activity data, the overall moderated regression model was significant for steps/day (p=0.021, overall model R2= 0.341, Table 3). In the first block, Group and pre-training steps/day did not predict the change in steps/day with the intervention (R2 = 0.158; P = .127); adding the (Group × pre-training steps/day) interaction term significantly improved the model (ΔR2 = 0.183; P = .019; Table 3, Figure 1a). The results of the regression analysis were reinforced by comparing the average change in steps/day across subjects between the two groups for those with low initial steps/day (below the mean at baseline). The average change in steps/day from pre- to post-training in the FAST+SAM group was 1715±1584 steps, compared to only 254±933 for those in the FAST group (Figure 2a).
Table 3.
Sequential regression model predicting change in steps/day from pre- to post-training.
| Model # | Predictors | Model p | ΔR2 | ΔR2 p |
|---|---|---|---|---|
| 1 | Group Pre-training steps/day |
.127 | .158 | .127 |
| 2 | Group Pre-training steps/day Group × pre-training steps/day |
.021 | .183 | .019 |
Figure 1.
Results of the moderated regression analysis for (A) Steps/day and (B) 6MWT. Solid lines represent data from the FAST+SAM group and dotted lines represent data from the FAST group. Each symbol represents the mean (middle symbol) ± 1 standard deviation (first and third symbol) of the group data presented on the x-axis (baseline steps day in (A), and baseline 6MWT distance in (B)).
Figure 2.
Change in steps per day (A) and 6MWT distance (B) from pre- to post-training in the subjects who were below the mean at baseline for the FAST (grey bar) and FAST+SAM (black bar) groups. Error bars represent 1 SE.
To test the hypothesis that baseline walking speed and endurance would moderate the changes in speed and endurance with each intervention, sequential moderated regressions for these variables were completed. The overall moderated regression model was significant for the 6MWT (p=0.010, overall model R2=0.353, Table 4). In the first block, Group and pre-training 6MWT did not predict the change in 6MWT (R2 = 0.102; P = .129); adding the (Group × pre-training 6MWT) interaction term significantly improved the model (ΔR2 = 0.252; P = .009; Table 4, Figure 1b). The results of the regression analysis were reinforced by the result that for subjects with 6MWT distances below the mean value at baseline, the average change in 6MWT distance from pre- to post-training in the FAST+SAM group was 49±28 meters, whereas it was only 26±41 meters for those in the FAST group (Figure 2b).
Table 4.
Sequential regression model predicting change in 6MWT distance from pre- to post-training.
| Model # | Predictors | Model p | ΔR2 | ΔR2 p |
|---|---|---|---|---|
| 1 | Group Pre-training 6MWT |
.129 | .102 | .129 |
| 2 | Group Pre-training 6MWT Group × Pre-training 6MWT |
.010 | .252 | .009 |
DISCUSSION
The results of this study demonstrate that combining a fast walking intervention that improves walking capacity, with a step activity monitoring program that facilitates translation of gains from the clinic to the “real-world”, is effective for increasing real-world walking activity after stroke. The benefits of adding the step activity monitoring program are however, preferentially observed in those with low levels of baseline walking activity and long distance walking. These results suggest that future work studying how participants’ baseline characteristics interact to influence the effects of interventions to improve physical activity may be critical to the advancement of individualized, evidence-based intervention efforts in this heterogeneous population.
Previous studies have shown that a step activity monitoring program can be quite effective for improving daily walking activity in persons with and without a variety of medical diagnoses17 and recent evidence from our lab provides support for such programs in persons with chronic stroke22. In addition to the actual monitoring of real-world walking, critical aspects of such programs include goal-setting, identification of barriers and the development of strategies to overcome those barriers. A recent meta-analysis found that interventions that include such tailored counseling improve long-term physical activity participation and functional exercise capacity after stroke20 more so than supervised exercise alone. The results of the present study extend these findings and suggest that for those with low levels of walking activity at baseline, a supervised walking training program in conjunction with a step activity monitoring program was more effective for increasing real world walking activity after stroke than a fast walking training intervention alone. The present study design, however, cannot address whether step monitoring alone would be equally as effective as the complete step activity monitoring program that was utilized in this study.
Both interventions (FAST and FAST+SAM) were effective for improving standard laboratory based performance measures of walking function after stroke. However the FAST+SAM intervention was superior to FAST for improving 6MWT distance. The average change on the 6MWT in the FAST+SAM group was 61 meters, which exceeds the minimal detectable change for this test in those with chronic stroke (34m42, 52m43). Similarly, both interventions were effective for increasing measures of daily, real world walking activity and the changes were comparable to results from our previous study of a step activity monitoring program alone in those with chronic stroke22. What was most interesting from these results, however, was that baseline walking capacity and activity moderated the effectiveness of each intervention.
The results from the moderated regression analysis suggest that the FAST+SAM intervention is substantially more effective in subjects with limited baseline walking activity and long distance walking. For subjects with steps/day below the mean of all subjects, the FAST+SAM intervention resulted in a more than 60% improvement in steps/days (1715 steps/day on average). Similar results were found for improvements in the distance on the 6MWT; those with baseline levels below the mean had substantially greater improvements with FAST+SAM. This is in contrast to the results from the FAST group, where improvements in walking activity were not moderated by baseline activity or walking distance. Regardless of baseline level, subjects in the FAST group had a less than 10% improvement in measures of daily walking activity. Together these results suggest that subjects with low baseline levels of walking activity and long distance walking will show greater benefit when a step activity monitoring program is used in conjunction with an intervention designed to increase walking capacity.
Why would the addition of the step activity monitoring program be preferentially beneficial for those with low baseline levels of walking activity and long distance walking? One explanation could lie in the design of our step activity monitoring program. The focus of this program was to work with participants to identify barriers to increasing their walking activity and develop strategies to overcome those barriers. To the extent that participants with low levels of real-world walking activity at baseline may have more perceived barriers to walking or more difficulty developing strategies to overcome these barriers44, then it would be likely that a program aimed at addressing these areas would be more beneficial for these participants. In addition, the greater increase in the 6MWT distance in the FAST+SAM group compared to the FAST group would suggest that there is some positive summary effect of the fast walking training and the step activity monitoring program. This effect appears to have the greatest benefit for those who were most impaired at baseline. Previous cross-sectional studies have found a significant relationship between 6MWT distance and real-world step activity in persons with chronic stroke13, 45, 46, thus, it is possible that improvements in one benefit the other.
Study Limitations
This study was limited by a small sample size. Future studies should examine whether the pattern of results observed here are found with a larger sample, based on a priori sample size calculations. Participants and PT’s providing the training were not blinded to group assignment, however, all outcome assessments were completed by a PT blinded to group assignment. Participants in the FAST group were asked not to start using a pedometer-type device during the intervention. To our knowledge, participants honored this request however this was not regularly monitored. Another limitation is that step activity goals were determined using a specific algorithm and participants did not select their step activity goal. Future studies should use such algorithms to assist participants in actively engaging in the goal setting process. Finally, the StepWatch Activity monitor does not provide real-time step activity feedback to the participant. Future studies should utilize accurate and reliable activity monitors that provide real-time feedback which may further enhance participant motivation and engagement.
Conclusions
The results of this study suggest that the addition of a step activity monitoring program to a fast walking training intervention may be most effective in persons with chronic stroke that have initial low levels of walking endurance and activity. Regardless of baseline performance, the FAST + SAM intervention was more effective for improving walking endurance.
Acknowledgments
Funding Source: NIH grant R21HD07142 provided financial support for the conduct of the research and preparation of the article.
Abbreviations
- FAST
Fast Walking alone group
- FAST+SAM
Fast Walking plus Step Activity Monitoring Program group
- SAM
StepWatch Activity Monitor
- 6MWT
6 Minute Walk Test
- THR
Target Heart Rate
- SPD
Steps per day
- ANOVA
Analysis of Variance
Footnotes
Conflict of Interest: NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michael K, Macko RF. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil. 2007;14(2):5–12. doi: 10.1310/tsr1402-5. [DOI] [PubMed] [Google Scholar]
- 3.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86(8):1552–6. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Rand D, Eng JJ, Tang PF, Hung C, Jeng JS. Daily physical activity and its contribution to the health-related quality of life of ambulatory individuals with chronic stroke. Health Qual Life Outcomes. 2010;8:80. doi: 10.1186/1477-7525-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornnes N, Larsen K, Boysen G. Little change of modifiable risk factors 1 year after stroke: a pilot study. Int J Stroke. 2010;5(3):157–62. doi: 10.1111/j.1747-4949.2010.00424.x. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 7.Michael K, Goldberg AP, Treuth MS, Beans J, Normandt P, Macko RF. Progressive adaptive physical activity in stroke improves balance, gait, and fitness: preliminary results. Top Stroke Rehabil. 2009;16(2):133–9. doi: 10.1310/tsr1602-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor Training Improves Daily Stepping Activity and Gait Efficiency in Individuals Poststroke Who Have Reached a “Plateau” in Recovery. Stroke. 2010;41:129–35. doi: 10.1161/STROKEAHA.109.563247. [DOI] [PubMed] [Google Scholar]
- 9.Mudge S, Barber PA, Stott NS. Circuit-based rehabilitation improves gait endurance but not usual walking activity in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(12):1989–96. doi: 10.1016/j.apmr.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot-virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke. 2009;40(1):169–74. doi: 10.1161/STROKEAHA.108.516328. [DOI] [PubMed] [Google Scholar]
- 11.Bowden MG, Behrman AL, Neptune RR, Gregory CM, Kautz SA. Locomotor rehabilitation of individuals with chronic stroke: difference between responders and nonresponders. Arch Phys Med Rehabil. 2013;94(5):856–62. doi: 10.1016/j.apmr.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 12.VanSwearingen JM, Perera S, Brach JS, Cham R, Rosano C, Studenski SA. A randomized trial of two forms of therapeutic activity to improve walking: effect on the energy cost of walking. J Gerontol A Biol Sci Med Sci. 2009;64(11):1190–8. doi: 10.1093/gerona/glp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulk GD, Reynolds C, Mondal S, Deutsch JE. Predicting home and community walking activity in people with stroke. Arch Phys Med Rehabil. 2010;91(10):1582–6. doi: 10.1016/j.apmr.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Mudge S, Stott NS. Timed walking tests correlate with daily step activity in persons with stroke. Arch Phys Med Rehabil. 2009;90(2):296–301. doi: 10.1016/j.apmr.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 16.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 18.Sidman CL, Corbin CB, Le Masurier G. Promoting physical activity among sedentary women using pedometers. Res Q Exerc Sport. 2004;75(2):122–9. doi: 10.1080/02701367.2004.10609143. [DOI] [PubMed] [Google Scholar]
- 19.Tudor-Locke C, Lutes L. Why do pedometers work?: a reflection upon the factors related to successfully increasing physical activity. Sports Med. 2009;39(12):981–93. doi: 10.2165/11319600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Morris JH, Macgillivray S, McFarlane S. Interventions to promote long-term participation in physical activity after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2014;95(5):956–67. doi: 10.1016/j.apmr.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Butler L, Furber S, Phongsavan P, Mark A, Bauman A. Effects of a pedometer-based intervention on physical activity levels after cardiac rehabilitation: a randomized controlled trial. J Cardiopulm Rehabil Prev. 2009;29(2):105–14. doi: 10.1097/HCR.0b013e31819a01ff. [DOI] [PubMed] [Google Scholar]
- 22.Danks KA, Roos MA, McCoy D, Reisman DS. A step activity monitoring program improves real world walking activity post stroke. Disabil Rehabil. 2014;36(26):2233–6. doi: 10.3109/09638288.2014.903303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam JM, Globas C, Cerny J, Hertler B, Uludag K, Forrester LW, et al. Predictors of response to treadmill exercise in stroke survivors. Neurorehabil Neural Repair. 2010;24(6):567–74. doi: 10.1177/1545968310364059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan KJ, Brown DA, Klassen T, Mulroy S, Ge T, Azen SP, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther. 2007;87(12):1580–602. doi: 10.2522/ptj.20060310. discussion 603–7. [DOI] [PubMed] [Google Scholar]
- 25.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking Speed and Step Length Asymmetry Modify the Energy Cost of Walking After Stroke. Neurorehabil Neural Repair. 2015;29(5):416–23. doi: 10.1177/1545968314552528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS. Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke. Neurorehabil Neural Repair. 2015;29(6):499–508. doi: 10.1177/1545968314554625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadeau S, Duclos C, Bouyer L, Richards CL. Guiding task-oriented gait training after stroke or spinal cord injury by means of a biomechanical gait analysis. Prog Brain Res. 2011;192:161–80. doi: 10.1016/B978-0-444-53355-5.00011-7. [DOI] [PubMed] [Google Scholar]
- 28.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–8. [PubMed] [Google Scholar]
- 29.Croteau KA. Strategies used to increase lifestyle physical activity in a pedometer-based intervention. J Allied Health. 2004;33(4):278–81. [PubMed] [Google Scholar]
- 30.De Greef K, Deforche B, Tudor-Locke C, De Bourdeaudhuij I. Increasing physical activity in Belgian type 2 diabetes patients: a three-arm randomized controlled trial. Int J Behav Med. 2011;18(3):188–98. doi: 10.1007/s12529-010-9124-7. [DOI] [PubMed] [Google Scholar]
- 31.Macko RF, Haeuber E, Shaughnessy M, Coleman KL, Boone DA, Smith GV, et al. Microprocessor-based ambulatory activity monitoring in stroke patients. Med Sci Sports Exerc. 2002;34(3):394–9. doi: 10.1097/00005768-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Arch Phys Med Rehabil. 2004;85(12):1997–2001. doi: 10.1016/j.apmr.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 33.Fulk GD, Combs SA, Danks KA, Nirider CD, Raja B, Reisman DS. Accuracy of Two Activity Monitors in Detecting Steps in People With Stroke and Traumatic Brain Injury. Phys Ther. 2014;94(2):222–9. doi: 10.2522/ptj.20120525. [DOI] [PubMed] [Google Scholar]
- 34.Knarr B, Roos MA, Reisman DS. Sampling frequency impacts measurement of walking activity after stroke. J Rehabil Res Dev. 2014;50(8):1107–12. doi: 10.1682/JRRD.2012.12.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roos MA, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: a cross-sectional study. Phys Ther. 2012;92(9):1141–7. doi: 10.2522/ptj.20120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plummer P, Behrman AL, Duncan PW, Spigel P, Saracino D, Martin J, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21(2):137–51. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 37.Fulk GD, Echternach JL. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J Neurol Phys Ther. 2008;32(1):8–13. doi: 10.1097/NPT0b013e31816593c0. [DOI] [PubMed] [Google Scholar]
- 38.Lewek MD, Randall EP. Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. J Neurol Phys Ther. 2011;35(3):116–21. doi: 10.1097/NPT.0b013e318227fe70. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Drutz C, Kumar R, McVicar L, Weinberger R, Brooks D, et al. Use of the six-minute walk test poststroke: is there a practice effect? Arch Phys Med Rehabil. 2008;89(9):1686–92. doi: 10.1016/j.apmr.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Roos M, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: a cross-sectional study. Physical Therapy. 2012;92(9):1141–7. doi: 10.2522/ptj.20120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orendurff MS, Schoen JA, Bernatz GC, Segal AD, Klute GK. How humans walk: bout duration, steps per bout, and rest duration. J Rehabil Res Dev. 2008;45(7):1077–89. doi: 10.1682/jrrd.2007.11.0197. [DOI] [PubMed] [Google Scholar]
- 42.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85(1):113–8. doi: 10.1016/s0003-9993(03)00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 44.Robinson CA, Matsuda PN, Ciol MA, Shumway-Cook A. Participation in community walking following stroke: the influence of self-perceived environmental barriers. Phys Ther. 2013;93(5):620–7. doi: 10.2522/ptj.20110217. [DOI] [PubMed] [Google Scholar]
- 45.Rand D, Eng JJ, Tang PF, Jeng JS, Hung C. How active are people with stroke?: use of accelerometers to assess physical activity. Stroke. 2009;40(1):163–8. doi: 10.1161/STROKEAHA.108.523621. [DOI] [PubMed] [Google Scholar]
- 46.Tiedemann A, Sherrington C, Dean CM, Rissel C, Lord SR, Kirkham C, et al. Predictors of adherence to a structured exercise program and physical activity participation in community dwellers after stroke. Stroke Res Treat. 2012;2012:136525. doi: 10.1155/2012/136525. [DOI] [PMC free article] [PubMed] [Google Scholar]