Abstract
Modulation of the endocannabinoid system has been shown to have a significant impact on outcomes in animal models of stroke. We have previously reported a protective effect of the CB1 antagonist, SR141716A, in a transient reperfusion mouse model of cerebral ischemia. This protective effect was in part mediated by activation of the 5HT1A receptor. Here we have examined its effect in a mouse model of permanent ischemia induced by photoinjury. The CB1 antagonist was found to be protective in this model. As was the case following transient ischemia reperfusion, SR141716A (5 mg/kg) resulted in smaller infarct fractions and stroke volumes when utilized both as a pretreatment and as a post-treatment. In contrast to the effect in a transient ischemia model, the pretreatment effect did not depend on the 5HT1A receptor. Neurological function correlated favorably to the reduction in stroke size when SR141716A was given as a pretreatment. With the incidence of stroke predicted to rise in parallel with an ever aging population, understanding mechanisms underlying ischemia and therapeutics remains a paramount goal of research.
Keywords: Stroke, Cerebral Ischemia, CB1 Antagonist, Cannabinoid Receptor, Photochemical Injury, Permanent Occlusion
1. Introduction
In the US, a stroke occurs every 40 seconds and unfortunately a death from this injury results every 4 minutes [1]. Anywhere from 15-30% of survivors remain permanently disabled and 20% require institutionalized care [1-4]. Predictions suggest that by 2030, an increase of 21.9% in stroke incidence will occur [1]. Therefore, enhancing our understanding of stroke pathophysiology and improving treatments remains an imperative goal in caring for an ever aging population.
The endogenous cannabinoid system has long been shown to serve a role in neuroprotection following ischemic insults [5-10]. There has been much debate regarding the role of the CB1 receptor as to whether its activation or antagonism would serve to protect neuronal tissue. Many reports have shown that antagonizing the CB1 receptor produced favorable outcomes with improved behavioral presentations and reduced stroke volumes [6-11]. In contrast, reports of CB1 activation improving stroke outcome, as well as studies illustrating larger stroke volumes in CB1R −/− mice, gave support to the notion that CB1 receptor agonists are neuroprotective. However, many of these studies utilized mixed CB1/CB2 agonists and therefore did not rule out a role for CB2 receptor agonism in this neuroprotection [11-16]. Many studies regarding protection conferred from CB1 agonists have focused on excitotoxic injury alone [17-19]. In particular, researchers showed that SR141716A reduced stroke volume when administered after permanent infarction by filament method and investigated its role in glutamate and NMDA signaling [20,21]. Therefore, discrepancies among the mechanisms responsible for injury among these different models likely contributes to the seemingly contradictory results. More uniform results have been obtained with studies of the role of the CB2 receptor and the protective effects of CB2 agonism [7-10].
Our laboratory has recently reported that both a CB1 antagonist (SR141716A) and a CB2 agonist (O-1966) decreased stroke volume, and the combination of these two drugs yielded smaller stroke volumes compared to either agent alone [7]. These findings correlated to increased regional cerebral blood flow and increased arteriolar diameter during the occlusion, and these effects were dependent on both the CB2 and 5HT1A receptor [7]. To our knowledge, the exploration of a CB1 antagonist in the outcome of permanent ischemia induced by a photochemical method has not been studied. The photochemically-induced permanent ischemia model utilizes a systemic injection of the photo-sensitive dye, Rose Bengal, followed by cerebral exposure to a light source to induce injury. Rose Bengal has been shown to penetrate into the cytoplasm as well as bind cell membranes and after absorbing photons induces oxidation of membrane components [22,23]. The oxidation leads to abnormal endothelial function, platelet activation, platelet aggregation, and thrombus formation [24,25]. In the present study, we hypothesized that the CB1 antagonist would be protective in the photochemically-induced stroke model and generate much smaller infarct volumes. In parallel to our previously published study, we also sought to explore the role of the 5HT1A receptor prior to the onset of injury.
2. Materials and Methods
2.1 Animals
This study was carried out in accordance with the National Institutes of Health guidelines for the treatment of animals and was approved by the Animal Care and Use Committee at Temple University. Male (n = 35) C57Bl/6 (Jackson Laboratories) mice, 7-8 weeks old, and weighing 20-25g were housed under a 12-h light/dark cycle with access to water and food ad libitum. The animals were anesthetized with a mixture of ketamine (100 mg/mL) and xylazine (20 mg/mL) mixed (1:1 by volume) at a dose of 1 mL/kg. Body temperature during all surgical procedures was maintained with a heating pad and lamp.
2.2 Preparation and Administration of SR14176A and WAY-100635
SR141716A was prepared to 0.5 mg/mL in Ethanol:Cremaphor:Saline (1:1:18) and injected at 5 mg/kg. Injections were intraperitoneal and given 1 hour prior to the photothrombotic injury or 1 hour after the onset [7,10]. WAY-100635 (Sigma Aldrich), a 5HT1A antagonist, was prepared 0.3 mg/mL in 0.9% saline and injected at 3 mg/kg [26-28]. WAY-100635, utilized prior to induction of ischemia, was injected 15 minutes prior to injection of SR141716A. Dosing and timing followed our previous studies with 5HT1A antagonists [7]. Vehicle was administered at the same time for each respective group (vehicle pretreatment, vehicle post-treatment). Vehicle solution contained Ethanol:Cremaphor:Saline (1:1:18) as in the experimental groups.
2.3 Photothrombotic Cerebral Ischemia
After adequate anesthesia, mice were maintained at 36.5° to 37.5° C throughout the procedure. The technique was carried out with some modifications from those described in Kleinschnitz et al [29]. Briefly, 0.1 mL of Rose Bengal (Sigma Aldrich), 10 mg/mL dissolved in 0.9% saline, was injected intraperitoneally. The fur on the head was clipped and skin and periosteum over the right parietal bone removed. The head was secured in place and a cold light source was positioned over the exposed area. Five minutes after the injection of dye, the light source was activated for 20 minutes and temperature monitored. After illumination the mouse was returned to its home cage for recovery.
2.4 Neurological Evaluation
The severity of neurological deficits was evaluated 24 hours after the ischemic insult using a five point deficit score modified from Hata et al [30]. The scale utilized the following criteria adapted from Hata: 0 = normal motor function, 1 = flexion of torso and of contralateral forelimb on lifting of the animal by the tail, 2 = circling but normal posture at rest, 3 = leaning while at rest, 4 = no spontaneous motor activity or lateral rolling.
2.5 Infarct Volume and Fraction Assessment
Animals were euthanized with an overdose of pentobarbital (200 mg/kg interaperitoneal) 24 hr after cerebral ischemia and then the brains were removed. Brains were submerged in cold PBS briefly and then cut into six 2 mm coronal sections using a mouse brain matrix (Zivic Lab, Pittsburgh, PA, USA). The brain sections were placed in 2% triphenyltetrazolium chloride (Sigma, Inc) dissolved in saline and stained for 5 minutes at 37°C in the dark. The brain sections were fixed in 4% paraformaldehyde at 4°C for 24 hr. Next, the anterior and posterior face of each section was scanned by a flatbed color scanner (Microtek Inc., Carson, CA USA). Images were saved as JPEG files and analyzed with Image-J Software (NIH). The infarct volumes were expressed as mm3.
Infarct fraction was also measured to compensate for changes in infarct volume due to swelling as described in the literature [8-10,31-33].
The infarct fraction was expressed as the percentage of total brain volume.
2.6 Statistical Analysis
Pretreatment photothrombosis cerebral ischemia data were analyzed with one way analysis of variance with Bonferroni correction for multiple comparisons. Post-treatment data were analyzed by a student's t-test. Data is reported as the mean ± the standard error of the mean. A p-value < 0.05 was used for statistical significance. Asterisks over bars indicate significance compared with vehicle control.
3. Results
3.1 Effect of SR141716A and WAY-100635 on Infarct Size, Figures 1-3
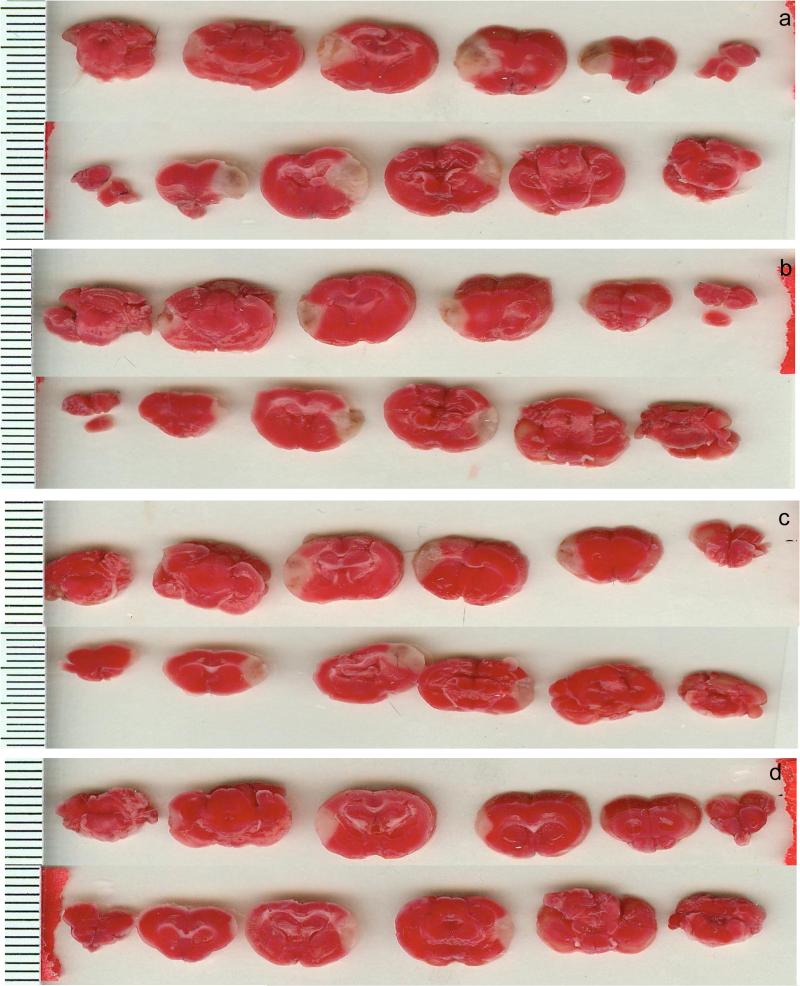
Figure 1. TTC Staining in Photothrombotic Studies.
TTC staining following photothrombotic stroke (24 hours). a: Vehicle Mice. b: SR141716A pretreatment. c: SR141716A post-treatment. d: SR141716A pretreatment with with WAY-100635 pretreatment. The bars on the left are mm. Anterior faces of slices from a single animal from each group are shown. More rostral portions are on the left and progress caudally moving to the right side of the panel.
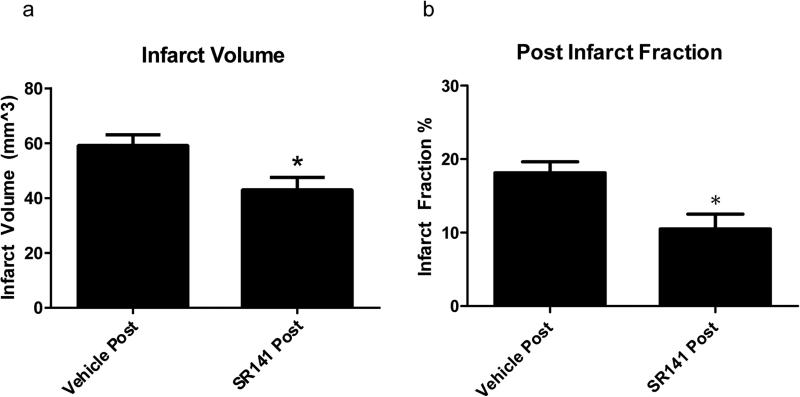
Figure 3. Stroke Size with SR141716A Post-treatment.
Post-treatment of SR141716A. a: Clinical Score among groups. b: Weight loss as a percent of pre-operative body weight. Data expressed as Mean ± SEM. p < 0.05. n = 8 mice in Vehicle Post-Treatment, 12 in SR141716A Post-Treatment
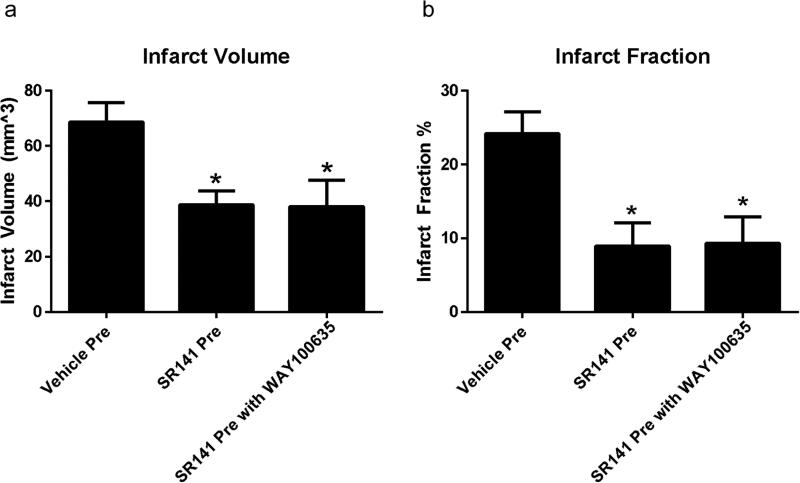
Administration of SR141716A either before or after the induction of ischemia decreased both infarct volume and infarct fraction, Figures 1-3. In contrast to our pervious publication with a reperfusion model, the effect of pretreatment with SR141716A in a permanent ischemic model was not dependent on the 5HT1A receptor, as the addition of the 5HT1A antagonist WAY-100635 did not attenuate the protective effect observed. Mice that had received vehicle pretreatment had an overall infarct volume of 68.63 ± 7.16 mm3 compared to SR141716A pretreatment at 38.67 ± 5.16 mm3 (p < 0.05). The mean infarct volume in animals pretreated with SR141716A and WAY-100635 together was 38.20 ± 9.46 mm3 (p < 0.05, Figure 2). One way ANOVA revealed an effect of SR141716A [F(2,12) = 5.45, p < 0.05, significant]. Infarct fraction similarly decreased in the SR141716A pretreated group, demonstrating that the protective effect was not simply the result of attenuation of edema. Vehicle pretreated mice had an infarct fraction of 24.15 ± 2.30 % whereas SR141716A given as a pretreatment decreased this to 8.95 ± 3.14 % (p < 0.05). The addition of WAY-100635 with SR141716A as a pretreatment resulted in a mean infarct fraction of 9.34 ± 3.57 % (p < 0.05). One way ANOVA analysis showed an effect of SR141716A [F(2,12) = 7.18, p < 0.05, significant]. Vehicle post-treatment yielded an infarct volume of 59.23 ± 3.86 mm3 while the volume following SR141716A post-treatment was 43.01 ± 4.55 mm3 (p < 0.05). Vehicle post-treatment had an infarct fraction of 18.13 ± 1.50% compared to SR141716A post-treatment with a fraction of 10.50 ± 2.01 % (p < 0.05).
Figure 2. Stroke Size with SR141716A Pre-treatment.
Pretreatment with SR141716A. a: Stroke volume among groups. b: Infarct fraction among groups. Data expressed as Mean ± SEM. p < 0.05. n = 5 mice in each group
3.2 Effect of SR141716A and WAY-100635 on Neurological Performance, Figures 4 and 5
Figure 4. Clinical Parameters with SR141716A Pre-treatment.
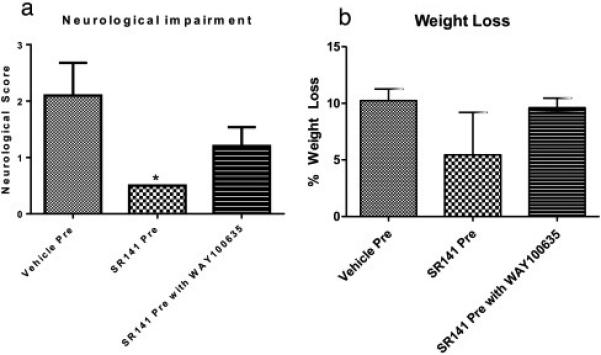
Pretreatment with SR141716A. a: Clinical Score among groups. b: Weight loss as a percent of pre-operative body weight. Data expressed as Mean ± SEM. p < 0.05. n = 5 mice in each group
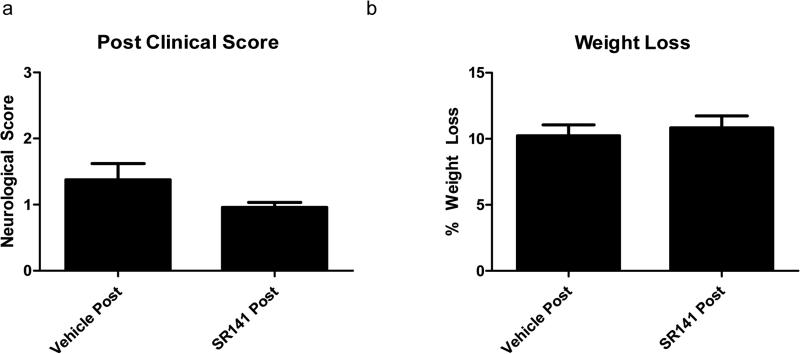
Figure 5. Clinical Parameters with SR141716A Post-treatment.
Post-treatment of SR141716A. a: Clinical Score among groups. b: Weight loss as a percent of pre-operative body weight. Data expressed as Mean ± SEM. p < 0.05. n = 8 mice in Vehicle Post-Treatment, 12 in SR141716A Post-Treatment
Neurological impairment in the SR141716A pretreatment group was significantly less than the Vehicle pretreatment group, Figure 4. The Neurological Score for the Vehicle pretreatment group was 2.1 ± 0.58 and 0.5 ± 0 for the SR141716A pretreatment group (p < 0.05). Additional treatment with WAY-100635 produced a non-significant attenuation of the protective effect of SR141716A, with a clinical score of 1.2 ± 0.34. One way ANOVA showed a significant effect of SR141716A F(2,12) = 4.29, p < 0.05, significant]. SR141716A post-treatment produced a non-significant improvement of clinical score, with mean clinical scores of 0.96 ± 0.07 compared to Vehicle post-treatment with scores of 1.38 ± 0.25, Figure 5, p = 0.07.
Overall, there was no statistical difference among weight loss in the experimental groups although SR141716A pretreatment tended to trend towards a conservation of more body weight, Figure 4 and 5. Vehicle pretreatment mice lost 10.23 ± 1.03 % of their original body weight compared to 5.44 ± 3.75 % in SR141716A pretreatment group and 9.61 ± 0.86 % with SR141716A and WAY-100635 pretreatment. One way ANOVA analysis yielded F(2,12) = 1.28, p = 0.31, non-significant]. Vehicle post-treatment animals lost on average 10.23 ± 0.83 % versus 10.83 ± 0.90 % with SR141716A post-treatment, (p = 0.65).
4. Discussion
The goal of this investigation was to examine the effect of a CB1 receptor antagonist in a model of photochemically-induced permanent cerebral ischemia. Our findings here demonstrate the protective effect of the CB1 antagonist in a permanent model of cerebral ischemia and correlate with work previously done in our laboratory with ischemia/reperfusion injury [7-10]. Moreover, our results here challenge the long standing notion that CB1 activation serves a neuroprotective role by demonstrating that CB1 receptor blockade can also protect against CNS injury in select models. Having determined that the CB1 receptor antagonist was protective in a permanent ischemia model, we also demonstrated that the effect was not 5HT1A dependent. In the last component of the study we went on to demonstrate that the CB1 receptor antagonist was also protective when administered one hour after ischemia.
In our recent publication exploring an ischemia/reperfusion model, the protective effects of SR141716A and O-1966, through the 5HT1A and CB2 receptors respectively, decreased infarct volume at least in part through a mechanism of increased cerebral blood flow via dilation of arteriolar vessels during occlusion. It is interesting to note that the CB1 receptor was not necessary for this protection [7]. In contrast with the ischemia/reperfusion model, there is almost no return of flow to the ischemic core with the permanent photochemical model. Since the factors contributing to infarct size mechanistically differ between transient and permanent ischemia, it was important to determine if the CB1 receptor antagonist could also attenuate injury in a permanent ischemia model.
Due to the fact that activation of the 5TH1A receptor was shown to be a requirement for the protective effect of the CB1 receptor antagonist in the transient ischemia model, we investigated the possibility that antagonism of this receptor might also block the protective effect of SR141716A in the permanent ischemia model. This was found not to be the case, as the addition of WAY-100635 to antagonize the 5HT1A receptor failed to prevent the attenuation of infarct size produced by SR141716A. In the permanent photochemical model, the ischemic core remains almost completely devoid of flow and differs from the mechanism in transient ischemia induced by a filament in many models. It is, however, possible that in the photochemical stroke, interventions that preserve the penumbra could reduce infarct size.
A possible explanation for the protective effect of the CB1 antagonist in the permanent ischemia model may be the prevention of a cerebral steal syndrome, or in other words, the diversion of blood flow from regions of marginal perfusion by the dilation of vessels in healthy regions of the brain. Multiple studies have shown increases in cannabinoids following cerebral ischemia with elevations being recorded as early as 20 minutes after the onset of ischemia but also noted at as much as 20 hours later [5,6,11,20,34,35]. Decreases in enzymes responsible for the degradation of anandamide were also seen and concentrations of cannabinoids have been noted to be proportional to ischemic time [5,11]. Several lines of evidence have demonstrated that the endogenous cannabinoids anandamide (AEA) and 2-arachdonylglycerol (2-AG), which can activate the CB1 receptor, produce vasodilation [36-40]. Both endogenously produced cannabinoids increase after cerebral ischemia and multiple reports have demonstrated a hypotensive and vasodilatory action of AEA and 2-AG that is blocked by SR141716A [6,20,41-43]. Further, inhibition of the CB1 receptor was shown to cause vasoconstriction in response to a Thromboxane A2 mimic [44]. Therefore increases in AEA and/or 2-AG may induce vasodilation in healthy regions of the brain and compromise the penumbra following stroke. CB1 antagonism may block this effect while maintaining perfusion pressure in the penumbra and protecting against secondary injury.
A second goal of this investigation was to determine if SR141716A would have a protective effect when administered after ischemia. While both pre and post-treatment with SR141716A was protective, the effect was larger in pretreatment groups, as might be expected. If SR141716A acts to prohibit a cerebral steal syndrome, then its activity at the onset of ischemia explains the greater benefit when given as a pretreatment. The 5HT1A receptor did not prove to be necessary for the protective effect of SR141716A and we hypothesize that protection likely comes from inhibiting a cerebral steal syndrome. However, the possibility that a non CB1/CB2 cannabinoid receptor contributes to this protection cannot fully be excluded at this time [38]. Interestingly, SR141716A has been shown to prevent the downregulation of NMDA receptors but not affect glutamate release, an effect that would in theory contribute to excitotoxic injury [21]. This does not however appear to be the case since infarct size was reduced by the compound [20,21].
The improved neurological function observed with SR141716A as a pretreatment parallels the preservation of brain tissue. Interestingly, despite almost no change in infarct fraction or volume among the treatment groups, the addition of WAY-100635 tended to produce a less favorable clinical outcome with worse neurological function compared to SR141716A alone, although it was not significantly different than the SR141716 pretreatment group. This discrepancy in neurological outcome may be secondary to anti-serotonergic effects of WAY-100635 or off target receptor involvement that affects behavior. Our laboratory has recently begun utilizing novel objection exploration and operant learning to evaluate neurological function after cerebral ischemia and these tests may yield additional and more precise insight to our present findings [45]. The preservation of body weight can be seen as a surrogate marker of well-being and neurological function as animals that maintained body weight were able to continue to feed, hydrate, and recover. SR141716A pretreatment mice had the greatest conservation of weight with only 5.44% lost. While SR141716A served to reduce infarct volume and infarct fraction in both pre and post-treatment, its neurological preservation was only seen with pretreatment.
In conclusion, the goal of this investigation was to determine if a CB1 antagonist would improve outcome in a model of permanent cerebral ischemia. SR141716A proved to be protective and reduce the size of infarctions in the brain and this effect did not depend on the 5HT1A receptor. Animals treated with SR141716A as a pretreatment achieved more favorable clinical function scores. While animals that received SR141716A after the injury showed a trend of neurological improvement, that data failed to reach statistical significance unlike animals in the pretreatment group. It should not be overlooked that numerous surgical procedures place patients at greater risk for cerebral ischemia and pretreatment in these cases could be of therapeutic benefit. Further studies will be necessary to precisely define the mechanism of action responsible for this protective effect.
Highlights.
The CB1 antagonist, SR141716A is protective in photochemically induced ischemia.
The CB1 antagonist, SR141716A, results in reduced size of infarctions.
The CB1 antagonist, SR141716A, does not rely on 5HT1AR for its protective effect.
The SR141716A improves neurological function after stroke when given prior to injury.
Acknowledgements
This work was supported in part by grants from National Institute of Drugs of Abuse, T32 DA007237 (EMU) and P30DA013429 (EMU).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
7. Ethical Responsibility of Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Subcommittee AHASCaSS: Executive summary: Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 4.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillard CJ. Role of cannabinoids and endocannabinoids in cerebral ischemia. Curr Pharm Des. 2008;14:2347–2361. doi: 10.2174/138161208785740054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muthian S, Rademacher DJ, Roelke CT, Gross GJ, Hillard CJ. Anandamide content is increased and cb1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience. 2004;129:743–750. doi: 10.1016/j.neuroscience.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Mahadevan A, Amere M, Li H, Ganea D, Tuma RF. Unique effects of compounds active at both cannabinoid and serotonin receptors during stroke. Translational Stroke Research. 2012;3:348–356. doi: 10.1007/s12975-012-0197-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Martin BR, Adler MW, Razdan RJ, Kong W, Ganea D, Tuma RF. Modulation of cannabinoid receptor activation as a neuroprotective strategy for eae and stroke. J Neuroimmune Pharmacol. 2009;4:249–259. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. Cb2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, Martin BR, Adler MW, Razdan RK, Ganea D, Tuma RF. Modulation of the balance between cannabinoid cb(1) and cb(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152:753–760. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amantea D, Spagnuolo P, Bari M, Fezza F, Mazzei C, Tassorelli C, Morrone LA, Corasaniti MT, Maccarrone M, Bagetta G. Modulation of the endocannabinoid system by focal brain ischemia in the rat is involved in neuroprotection afforded by 17beta-estradiol. FEBS J. 2007;274:4464–4775. doi: 10.1111/j.1742-4658.2007.05975.x. [DOI] [PubMed] [Google Scholar]
- 12.Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA. Increased severity of stroke in cb1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22:9771–9775. doi: 10.1523/JNEUROSCI.22-22-09771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-ag) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 14.van der Stelt M, Veldhuis WB, Bär PR, Veldink GA, Vliegenthart JF, Nicolay K. Neuroprotection by delta9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. J Neurosci. 2001;21:6475–6479. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin KL, Mao XO, Goldsmith PC, Greenberg DA. Cb1 cannabinoid receptor induction in experimental stroke. Ann Neurol. 2000;48:257–261. [PubMed] [Google Scholar]
- 16.Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin K, Greenberg DA. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA. Molecular mechanisms of cannabinoid protection from neuronal excitotoxicity. Mol Pharmacol. 2006;69:691–696. doi: 10.1124/mol.105.016428. [DOI] [PubMed] [Google Scholar]
- 18.Shen M, Thayer SA. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol Pharmacol. 1998;54:459–462. doi: 10.1124/mol.54.3.459. [DOI] [PubMed] [Google Scholar]
- 19.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. Cb1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 20.Berger C, Schmid PC, Schabitz WR, Wolf M, Schwab S, Schmid HH. Massive accumulation of nacylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J Neurochem. 2004;88:1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- 21.Sommer C, Schomacher M, Berger C, Kuhnert K, Müller HD, Schwab S, Schäbitz WR. Neuroprotective cannabinoid receptor antagonist sr141716a prevents downregulation of excitotoxic nmda receptors in the ischemic penumbra. Acta Neuropathol. 2006;112:277–286. doi: 10.1007/s00401-006-0110-8. [DOI] [PubMed] [Google Scholar]
- 22.Spikes JD. Applications of dye-sensitized photoreactions in neurobiology. Photochem Photobiol. 1991;54:1079–1092. doi: 10.1111/j.1751-1097.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Reempts J, Borgers M. Histopathological characterization of photochemical damage in nervous tissue. Histol Histopathol. 1994;9:185–195. [PubMed] [Google Scholar]
- 24.Dietrich WD, Watson BD, Busto R, Ginsberg MD, Bethea JR. Photochemically induced cerebral infarction. I. Early microvascular alterations. Acta Neuropathol. 1987;72:315–325. doi: 10.1007/BF00687262. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Li Y, Lu H, Tong S. Real-time high resolution laser speckle imaging of cerebral vascular changes in a rodent photothrombosis model. Biomed Opt Express. 2014;5:1483–1493. doi: 10.1364/BOE.5.001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fletcher A. A pharmacological profile of the selective silent 5-ht1a receptor antagonist, way-100635. Eur J Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with way-100635, a potent, selective and silent 5-ht1a receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 28.Martel JC, Leduc N, Ormière AM, Faucillon V, Danty N, Culie C, Cussac D, Newman-Tancredi A. Way-100635 has high selectivity for serotonin 5-ht(1a) versus dopamine d(4) receptors. Eur J Pharmacol. 2007;574:15–19. doi: 10.1016/j.ejphar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Kleinschnitz C, Braeuninger S, Pham M, Austinat M, Nölte I, Renné T, Nieswandt B, Bendszus M, Stoll G. Blocking of platelets or intrinsic coagulation pathway-driven thrombosis does not prevent cerebral infarctions induced by photothrombosis. Stroke. 2008;39:1262–1268. doi: 10.1161/STROKEAHA.107.496448. [DOI] [PubMed] [Google Scholar]
- 30.Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann KA. A reproducible model of middle cerebral artery occlusion in mice: Hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–375. doi: 10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 32.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 33.Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neurosci. 2003;23:7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schäbitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, Piomelli D. Release of fatty acid amides in a patient with hemispheric stroke: A microdialysis study. Stroke. 2002;33:2112–2114. doi: 10.1161/01.str.0000023491.63693.18. [DOI] [PubMed] [Google Scholar]
- 36.Wagner JA, Járai Z, Bátkai S, Kunos G. Hemodynamic effects of cannabinoids: Coronary and cerebral vasodilation mediated by cannabinoid cb(1) receptors. Eur J Pharmacol. 2001;423:203–210. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- 37.Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 38.Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from cb1 or cb2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- 40.Varga K, Lake K, Martin BR, Kunos G. Novel antagonist implicates the cb1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- 41.Fowler CJ, Rojo ML, Rodriguez-Gaztelumendi A. Modulation of the endocannabinoid system: Neuroprotection or neurotoxicity? Exp Neurol. 2010;224:37–47. doi: 10.1016/j.expneurol.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Mukhopadhyay S, Tulis DA. Endocannabinoid regulation of matrix metalloproteinases: Implications in ischemic stroke. Cardiovasc Hematol Agents Med Chem. 2007;5:311–318. doi: 10.2174/187152507782109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degn M, Lambertsen KL, Petersen G, Meldgaard M, Artmann A, Clausen BH, Hansen SH, Finsen B, Hansen HS, Lund TM. Changes in brain levels of n-acylethanolamines and 2-arachidonoylglycerol in focal cerebral ischemia in mice. J Neurochem. 2007;103:1907–1916. doi: 10.1111/j.1471-4159.2007.04892.x. [DOI] [PubMed] [Google Scholar]
- 44.Hillard CJ, Ho WS, Thompson J, Gauthier KM, Wheelock CE, Huang H, Hammock BD. Inhibition of 2-arachidonoylglycerol catabolism modulates vasoconstriction of rat middle cerebral artery by the thromboxane mimetic, u-46619. Br J Pharmacol. 2007;152:691–698. doi: 10.1038/sj.bjp.0707468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ronca RD, Myers AM, Ganea D, Tuma RF, Walker EA, Ward SJ. A selective cannabinoid cb2 agonist attenuates damage and improves memory retention following stroke in mice. Life Sci. 2015;138:72–77. doi: 10.1016/j.lfs.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]