Abstract
The many interactions between the nervous and the immune systems, which are active in both physiological and pathological states, have recently become more clearly delineated with the discovery of a meningeal lymphatic system capable of carrying fluid, immune cells and macromolecules from the CNS to the draining deep cervical lymph nodes. The exact localization of the meningeal lymphatic vasculature and the path of drainage from the CSF to the lymphatics remain, however, poorly understood. Here we discuss the potential differences between peripheral and CNS lymphatic vessels and examine the purported mechanisms of CNS lymphatic drainage, along with how these may fit into established patterns of CSF flow.
Function of the meningeal lymphatics
The historical view of the immune privilege of the CNS has been challenged over the past twenty years by a body of work demonstrating that immune surveillance of the CNS is an important aspect of its homeostasis as well as response to injury and neurodegenerative conditions [1–9]. The meninges are an essential immunological site that allows CNS immune surveillance to ensure its proper function [6,7,10–12]. In searching for the pathways of movement of immune cells throughout the meninges, a system of vessels that run along the perisinusal space has recently been demonstrated [1,13,14]. These vessels have immunohistological and structural characteristics of lymphatic vessels, and are capable of carrying fluid and macromolecules [1,13]. They express all traditional markers of tissue lymphatic endothelial cells, including Prox1, CD31, Lyve-1, Podoplanin, VEGFR3 and CCL21 (see glossary)[1,13]. Furthermore, functional experiments demonstrated that these meningeal lymphatic vessels carry numerous immune cells under physiological conditions, suggesting their role in normal immune surveillance of the brain [1].
Apart from its role in immune surveillance, a CNS lymphatic system is likely to play a role in waste clearance from the brain parenchyma. A system of CSF-ISF exchange, called the ‘glymphatic’ system, may be responsible for clearance of hydrophilic and lipophilic compounds as well as of waste products from the brain parenchyma into the CSF [15–19]. Subsequently, macromolecules and other waste products are assumed to be cleared from the CSF via drainage through the nasal mucosa lymphatics into cervical lymph nodes [9,20], purportedly via the cribriform plate [21,22].
Despite accumulating evidence regarding the various pathways and fluid dynamics of the CNS, a number of important details in the anatomic and physiologic pathways of lymphatic drainage remain unclear. We highlight these controversies below.
Dynamics of CNS fluids
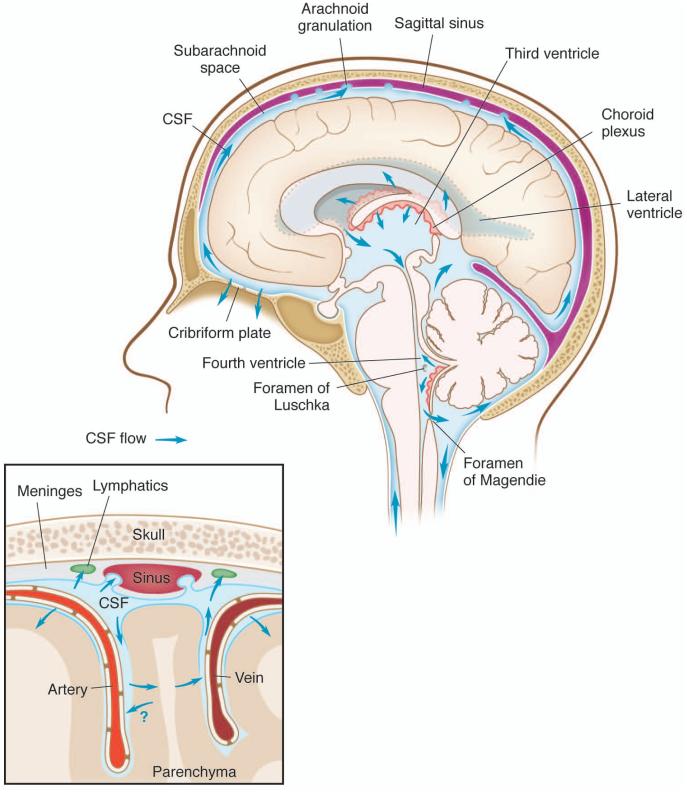
CSF flow is a tightly regulated phenomenon, but with complex fluid dynamics that are as yet incompletely characterized [23–25]. CSF is produced by the choroid plexus, flows through the lateral and third ventricles, and exits through the foramina of Luschke and Magendie, to reach the subarachnoid space over the convexities (Fig. 1). CSF leaves the intracranial circulation by draining into the dural venous sinuses through arachnoid granulations, which contain valves that prevent backflow of blood or CSF back into the CSF compartment [26–29] (Fig. 1). Granulations have been found in the subarachnoid space between the arachnoid and the dura mater, and were assumed to demonstrate a similar pulsatile flow to CSF in larger intracranial compartments [26–29]. CSF is also absorbed in the paraneural sheaths of cranial and spinal nerves, into lymphatic vessels which run close to these structures [21,30], but the contribution of each route for the drainage of CSF needs to be re-assessed in the light of newly discovered paths [1,13].
Figure 1. Production and circulation of CSF within the CNS.
Schematic representation of the pattern of intracranial CSF flow. A. CSF is produced by the choroid plexus of the lateral and fourth ventricles, and flows from the third ventricle to the fourth ventricle through the cerebral aqueduct. After circulating over the hemispheres, CSF absorption into the superior sagittal sinus, transverse sinus, and sigmoid sinuses is via arachnoid granulations, as well as efflux from the CNS along the olfactory nerves through the cribriform plate. B. Schematic representation of CSF-ISF flow from and to the subarachnoid space. CSF can diffuse in and out of the brain parenchyma along the perivascular space.
Intracranial CSF flow is linked to parenchymal ISF circulation, and the relationship is governed by bulk flow forces, arterial and venous pressures, and intracranial pressure [15–18]. First described in the 1980’s and recently re-born, a CSF-ISF exchange system, now termed a ‘glymphatic’ system [15,16,31] has been proposed as a system for the removal of macromolecules from the brain parenchyma into the CSF. This system relies on the influx of CSF into the brain parenchyma through peri-arterial spaces and efflux, along with both hydrophilic and lipophilic compounds, through the para-venous spaces back into the subarachnoid space [15]. The efficiency of the system relies on arteriole pulsatility and CSF pressure, and appears to be dependent (at least partially) on the water channel AQP4 [15,17,18,32]. More interestingly, this system appears to be more efficient during sleep [18]. Whether ISF flows from the brain parenchyma (into CSF) is along periarterial or perivenular spaces remains a matter of debate [33,34] and needs to be better understood. The meningeal lymphatic system has emerged as a new player in CSF flow, and the relationship between the meningeal lymphatic system and the other paths previously described needs to be addressed.
Macromolecules, such as amyloid beta, are supposedly cleared from the CNS parenchyma via different mechanisms, such as phagocytosis and proteolytic degradation by mononuclear phagocytes [35–37] and vascular smooth muscle cells [38], transcytosis across the blood-brain barrier [39–41] and via ISF bulk flow [18,42,43]. Previous studies have demonstrated that around 85% of Aβ is eliminated from the brain by transvascular clearance under normal physiological conditions in mice, while a smaller percentage is cleared by the other routes, notably ISF bulk flow [44,45]. The latter is of particular importance since Aβ has been shown in both mice and humans [46,47] to accumulate along the path of ISF flow, suggesting a potential dysfunction of these routes in the pathology of Alzheimer’s disease. Given that mice lacking meningeal lymphatic vessels show dysfunction in their ability to clear parenchymal macromolecules [13], studies will be required to address the anatomical and functional relationship between the ISF flow and the meningeal lymphatic drainage.
Transvascular clearance of Aβ has been shown to occur within the brain parenchyma [37,44,45,48]. This clearing is mediated by receptor-dependant transcytosis, mainly by the low-density receptor related protein (LRP1) transporter [39,44,45,48]. The ApoE family of molecules has been shown to participate in the transvascular clearing of Aβ [49,50]. Indeed, Ab binding to apoE2 or apoE3 is cleared rapidly through LRP1, but binding to apoE4, a genetic susceptibility genes for AD, Aβ is only slowly cleared via the very-low density lipoprotein receptor (VLDLR)-mediated internalization and transcytosis [49,50]. Similar phenomenon may occur across the lymphatic endothelial cells, which express the scavenger receptors, SRB1 [51], known to bind fibrillar amyloid beta [52]. Unraveling the mechanism of macromolecule clearance from the CNS parenchyma by lymphatic endothelial cells is the next step in understanding this complex interaction.
One can hypothesize that the meningeal lymphatic system is not implicated in the drainage of the water content of the CSF, since no change in intracranial pressure was reported in mice lacking meningeal lymphatic vasculature [13]. Similarly, the multiple paths of drainage of CSF might have different functions; the cribriform plate path along with the arachnoid granulations could serve as valves for the maintenance of CSF pressure, while the meningeal lymphatic vasculature would have a more immunological function, i.e. drainage of macromolecules and immune cells into the cervical lymph nodes to maintain brain immune surveillance. More studies investigating the interaction between the different drainage paths are necessary to demonstrate the selectivity and functional relevance of each of these paths for the maintenance of brain function.
Meningeal lymphatic drainage – anatomical considerations
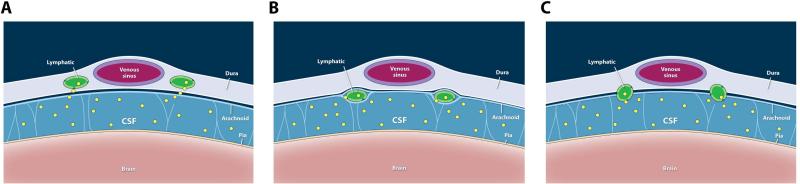
Where do CNS lymphatic vessels actually run? We demonstrated that lymphatic vessels are associated with sinuses [1], and Aspelund et al. also demonstrated lymphatic vessels at the base of the skull and along the dural middle meningeal artery [13]. The middle meningeal arteries in human run on the outer surface of the dura, and are therefore not part of the CNS. Due to the diminutive size of mouse meninges, it has not been possible to definitely separate the meningeal layers and identify the precise location of meningeal lymphatic vessels. Three possibilities exist: lymphatic vessels may run within dural leaflets, on the inside surface of the dura, or in the subarachnoid space with the cortical veins (Fig. 2 a-c).
Figure 2. Possible localizations of CNS lymphatic vessels within the meninges.
A. Lymphatic vessels are located within the dura layers in close apposition to the venous sinuses. B. Lymphatics are located at the interface between the dura and the arachnoid layers. C. Lymphatics are exposed to the subarachnoid space. Macromolecules (yellow) are seen within the subarachnoid space and within the lymphatic vessels into which they are draining.
The precise localization of the meningeal lymphatic vessels is of primary importance to understand how they are able to drain tracers and cells from the CSF [1,13]. If the meningeal lymphatics are localized within the subarachnoid space, they would be “bathed” in the CSF, which could therefore easily diffuse into the meningeal lymphatic vasculature. If the meningeal lymphatic vessels are indeed localized within the dural leaflets, or on the inside surface of the dura, however, then they are physically separated from the subarachnoid space. The question is then how can tracer, injected into the CSF, the lateral ventricle or the brain parenchyma, reach the meningeal lymphatic vessels? Studies have suggested that CSF is transported across the arachnoid membrane to the dura [53–55], which would explain the accumulation of CSF tracers in dural-localized lymphatic vessels. Furthermore, expression of transporters by arachnoid cells [56] could also allow the transfer of tracer from the CSF into the dura. Another intriguing possibility would be if there is an arachnoid granulation-like structure, which may mediate drainage of CSF into dural lymphatic vasculature across the arachnoid and inner layer of the dura. Further anatomical studies characterizing the meningeal lymphatics are required to better understand the drainage of the CSF by the meningeal lymphatic system.
Meningeal lymphatic drainage – environmental considerations
In peripheral tissues, macromolecules are able to diffuse into lymphatic vessels from the interstitial fluid through permeable endothelial cell junctions [57]. Specialized features of lymphatic vessels in the periphery allow cells and molecules to enter, including a lack of pericytes, and a discontinuous basement membrane. There are different types of lymphatic vessels, including initial and collecting vessels, the latter of which contain bileaflet valves to prevent backflow of lymph [58–60]. Lymph is pushed anterograde within lymphatic vessels by the action of the surrounding smooth muscle cells of the collecting vessels and arterial pulsations [57]. Meningeal lymphatics share features of initial vessels (lack of valves and surrounding smooth muscle cells) [1], except at the base of the skull where potential lymphatic valves have been detected [13], suggesting a transition from initial to collecting vessels as these vessels are exiting the CNS.
Contrary to the peripheral tissue, the meningeal lymphatics are likely to be exposed to an environment with different physical properties that might affect the development and behavior of the vessels. The meningeal lymphatic vasculature has less tissue coverage and smaller diameter than peripheral lymphatics [1], suggesting physical environmental pressure or other factors might inhibit the sprouting and enlargement of the meningeal lymphatic vasculature. Furthermore, a single injection of recombinant VEGFc is able to induce lymphatic vasculature sprouting and proliferation in peripheral organs [61,62], whereas in the meninges, only a small increase in diameter is observed [1]. This observation reinforces the hypothesis that the meningeal environment might be limiting the expansion of the meningeal vasculature due to CSF flow dynamics. One could wonder if the small diameter of the meningeal lymphatic vasculature might limit the amount or quality of antigen being drained into the cervical lymph nodes and participate in the immune privilege of the brain. Further studies on the development and plasticity of the meningeal lymphatic vasculature will be necessary to appreciate the function of these vessels in the drainage of macromolecules and cells from the CNS and understand their role in the brain’s immune privilege.
Concluding remarks
The emergence of growing consensus around the role of meningeal immunity in CNS surveillance in physiological states has generated a number of important discoveries that have helped to characterize the specialized CNS immune system. One of these is the presence of meningeal lymphatic vessels capable of carrying fluid, immune cells, and macromolecules from within the CNS and CSF. These findings, although they help explain how the CNS and peripheral immune systems may be linked, raise a number of questions that remain to be fully elucidated (see Outstanding Questions Box). Meningeal lymphatic vessels may provide a key component of the mechanism for the immune response to CNS insult from traumatic injury or stroke. On the other hand, meningeal lymphatic dysfunction may manifest in a variety of different ways, with implications for a number of neurological diseases and neurodegenerative conditions.
Outstanding Questions Box.
How can meningeal lymphatic vessels absorb macromolecules and immune cells from the CSF if they are located within the dura? What are the forces that govern this absorption and how to they relate to physiologic changes in CSF hemodynamic forces?
Where precisely do meningeal lymphatic vessels run? Are there equivalent structures to somatic pre-collecting and collecting lymphatic vessels at different locations within the intracranial space?
Trends Box.
Functional classic lymphatic vessels exist in the dura, and can function to drain fluid and immune cells from the meninges, the parenchyma and the CSF.
The meningeal lymphatic system is necessary for the efficient clearance of brain ISF, and may be a common pathway for removal of wastes initially cleared from brain parenchyma through the glymphatic system of CSF-ISF exchange.
The precise location of meningeal lymphatic vessels within the layers of the meninges needs to be further investigated.
The hemodynamics of the flow and the access of the CSF to meningeal lymphatic vessels are still poorly understood.
Acknowledgments
We would like to thank Anita Impagliazzo for figure artwork. This work was primarily supported by a grant from the National Institute on Aging. NIH (AG034113 award to J.K.).
Glossary
- BBB
Blood-brain barrier. This essential component of the immunological privilege of the CNS is composed of tight junctions of cerebral endothelial cells, along with astrocyte foot processes and pericytes.
- CCL21
Chemokine (C-C motif) ligand 21 is a cytokine of the CC chemokine family, which functions as an immune cell chemoattractant protein.
- CD31
Also known as platelet endothelial cell adhesion molecule (PECAM-1), CD31 is an endothelial marker. It is found on the surface of platelets, monocytes, neutrophils, some T cells, and endothelial cells, among other cell types throughout the body.
- Lyve-1
Extracellular link domain containing 1, XLKD1, or lymphatic vessel endothelial hyaluran receptor-1. This membrane glycoprotein is a cell surface receptor on lymphatic endothelial cells.
- Podoplanin
Type-1 membrane glycoprotein of uncertain function that is found in lung alveolar cells, kidney podocytes, and lymphatic endothelial cells.
- Prox1
Prospero homeobox protein 1, a transcription factor involved in developmental processes in a number of organs, including development of the lymphatic system. VEGFR3: Vascular endothelial growth factor receptor 3 is a tyrosine-protein kinase cell surface receptor for VEGFc and VEGFd, which has been implicated in lymphangiogenesis.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 doi: 10.1038/nature14432. DOI: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cserr HF, et al. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. Zurich Switz. 1992;2:269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 3.Steinman L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 4.Walsh JT, et al. Regulatory T cells in central nervous system injury: a double-edged sword. J. Immunol. Baltim. Md 1950. 2014;193:5013–5022. doi: 10.4049/jimmunol.1302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz M, Raposo C. Protective Autoimmunity: A Unifying Model for the Immune Network Involved in CNS Repair. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2014;20:343–358. doi: 10.1177/1073858413516799. [DOI] [PubMed] [Google Scholar]
- 6.Kipnis J, et al. Pro-cognitive properties of T cells. Nat. Rev. Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 8.Derecki NC, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louveau A, et al. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russi AE, Brown MA. The meninges: new therapeutic targets for multiple sclerosis. Transl. Res. J. Lab. Clin. Med. 2015;165:255–269. doi: 10.1016/j.trsl.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weller RO. Microscopic morphology and histology of the human meninges. Morphol. Bull. Assoc. Anat. 2005;89:22–34. doi: 10.1016/s1286-0115(05)83235-7. [DOI] [PubMed] [Google Scholar]
- 12.Decimo I, et al. Meninges: from protective membrane to stem cell niche. Am. J. Stem Cells. 2012;1:92–105. [PMC free article] [PubMed] [Google Scholar]
- 13.Aspelund A, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andres KH, et al. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat. Embryol. (Berl.) 1987;175:289–301. doi: 10.1007/BF00309843. [DOI] [PubMed] [Google Scholar]
- 15.Iliff JJ, et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012;4:147ra111–147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 2013;11:107. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thrane VR, et al. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci. Rep. 2013;3 doi: 10.1038/srep02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kress BT, et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harling-Berg C, et al. Role of cervical lymph nodes in the systemic humoral immune response to human serum albumin microinfused into rat cerebrospinal fluid. J. Neuroimmunol. 1989;25:185–193. doi: 10.1016/0165-5728(89)90136-7. [DOI] [PubMed] [Google Scholar]
- 21.Kida S, et al. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 22.Harris MG, et al. Immune privilege of the CNS is not the consequence of limited antigen sampling. Sci. Rep. 2014;4:4422. doi: 10.1038/srep04422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spector R, et al. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp. Neurol. 2015;273:57–68. doi: 10.1016/j.expneurol.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinker T, et al. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kido DK, et al. Human spinal arachnoid villi and granulations. Neuroradiology. 1976;11:221–228. doi: 10.1007/BF00328377. [DOI] [PubMed] [Google Scholar]
- 27.Go KG, et al. Fluid secretion in arachnoid cysts as a clue to cerebrospinal fluid absorption at the arachnoid granulation. J. Neurosurg. 1986;65:642–648. doi: 10.3171/jns.1986.65.5.0642. [DOI] [PubMed] [Google Scholar]
- 28.Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J. Neurosurg. 1985;63:867–875. doi: 10.3171/jns.1985.63.6.0867. [DOI] [PubMed] [Google Scholar]
- 29.Tripathi BJ, Tripathi RC. Vacuolar transcellular channels as a drainage pathway for cerebrospinal fluid. J. Physiol. 1974;239:195–206. doi: 10.1113/jphysiol.1974.sp010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston M, et al. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradbury MW, et al. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol. 1981;240:F329–336. doi: 10.1152/ajprenal.1981.240.4.F329. [DOI] [PubMed] [Google Scholar]
- 32.Iliff JJ, et al. Cerebral Arterial Pulsation Drives Paravascular CSF– Interstitial Fluid Exchange in the Murine Brain. J. Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schley D, et al. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 2006;238:962–974. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Carare RO, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 35.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simard AR, et al. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Bakker ENTP, et al. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2016;36:181–194. doi: 10.1007/s10571-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell RD, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat. Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zlokovic BV, et al. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J. Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Z, et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deane R, et al. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarasoff-Conway JM, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mawuenyega KG, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibata M, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deane R, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Morris AWJ, et al. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. (Berl.) 2016;131:725–736. doi: 10.1007/s00401-016-1555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preston SD, et al. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol. Appl. Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 48.Storck SE, et al. Endothelial LRP1 transports amyloid-β(1-42) across the blood-brain barrier. J. Clin. Invest. 2016;126:123–136. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bell RD, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deane R, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J. Clin. Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim HY, et al. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17:671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Kunjathoor VV, et al. beta-Amyloid promotes accumulation of lipid peroxides by inhibiting CD36-mediated clearance of oxidized lipoproteins. J. Neuroinflammation. 2004;1:23. doi: 10.1186/1742-2094-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston M, et al. Possible role of the cavernous sinus veins in cerebrospinal fluid absorption. Cerebrospinal Fluid Res. 2007;4:3. doi: 10.1186/1743-8454-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zenker W, et al. Morphological indications for considerable diffuse reabsorption of cerebrospinal fluid in spinal meninges particularly in the areas of meningeal funnels. An electronmicroscopical study including tracing experiments in rats. Anat. Embryol. (Berl.) 1994;189:243–258. doi: 10.1007/BF00239012. [DOI] [PubMed] [Google Scholar]
- 55.Squier W, et al. Demonstration of fluid channels in human dura and their relationship to age and intradural bleeding. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2009;25:925–931. doi: 10.1007/s00381-009-0888-5. [DOI] [PubMed] [Google Scholar]
- 56.Yasuda K, et al. Drug transporters on arachnoid barrier cells contribute to the blood-cerebrospinal fluid barrier. Drug Metab. Dispos. Biol. Fate Chem. 2013;41:923–931. doi: 10.1124/dmd.112.050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alitalo K. The lymphatic vasculature in disease. Nat. Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 58.Dejana E, et al. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Kerjaschki D. The lymphatic vasculature revisited. J. Clin. Invest. 2014;124:874–877. doi: 10.1172/JCI74854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sweet DT, et al. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J. Clin. Invest. 2015;125:2995–3007. doi: 10.1172/JCI79386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alessio SD’, et al. VEGF-C–dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Invest. 2014;124:3863–3878. doi: 10.1172/JCI72189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aspelund A, et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Invest. 2014;124:3975–3986. doi: 10.1172/JCI75395. [DOI] [PMC free article] [PubMed] [Google Scholar]