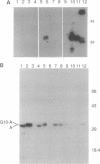
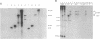Abstract
An antiserum raised against a peptide was used to select a unique RNA species from a degenerate pool of RNAs designed to resemble an autoantibody recognition site in U1 RNA. The peptide and the selected RNA epitope could compete for antibody binding, suggesting that both RNA and peptide epitopes occupy the same or overlapping antigen-combining sites. Thus, the RNA epitope functioned as a specific inhibitor of the antibody-antigen interaction. We demonstrate that the RNA epitope can be used to tag unrelated RNA molecules and also to detect the presence of the antibody. We propose that sequence-specific recognition of RNA by antibodies may involve protein-RNA contacts similar to those occurring in other nucleic acid-binding proteins. In addition, these findings are compatible with the suggestion that nucleic acid-binding autoantibodies may arise through immunological cross-reactivity between proteins and nucleic acids.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley R. C., Keene J. D. Recognition of U1 and U2 small nuclear RNAs can be altered by a 5-amino-acid segment in the U2 small nuclear ribonucleoprotein particle (snRNP) B" protein and through interactions with U2 snRNP-A' protein. Mol Cell Biol. 1991 Apr;11(4):1829–1839. doi: 10.1128/mcb.11.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. C., Bernstein R. M., Mathews M. B. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J Exp Med. 1986 May 1;163(5):1281–1291. doi: 10.1084/jem.163.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. C., Mathews M. B. Autoreactive epitope defined as the anticodon region of alanine transfer RNA. Science. 1987 Nov 20;238(4830):1116–1119. doi: 10.1126/science.2446387. [DOI] [PubMed] [Google Scholar]
- Chambers J. C., Keene J. D. Isolation and analysis of cDNA clones expressing human lupus La antigen. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2115–2119. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa O., Pinatel K., Monier J. C. Antibodies to ribonucleic acids detected by ELISA in systemic lupus erythematosus, systemic sclerosis and rheumatoid arthritis. J Rheumatol. 1985 Oct;12(5):928–933. [PubMed] [Google Scholar]
- Deutscher S. L., Keene J. D. A sequence-specific conformational epitope on U1 RNA is recognized by a unique autoantibody. Proc Natl Acad Sci U S A. 1988 May;85(10):3299–3303. doi: 10.1073/pnas.85.10.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Steinberg A. D., Schechter A. N. The reaction of SLE antibodies with native, single stranded RNA: radioassay and binding specificities. J Immunol. 1978 Feb;120(2):550–557. [PubMed] [Google Scholar]
- Ellington A. D., Szostak J. W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990 Aug 30;346(6287):818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- King G. C., Coleman J. E. Two-dimensional 1H NMR of gene 5 protein indicates that only two aromatic rings interact significantly with oligodeoxynucleotide bases. Biochemistry. 1987 May 19;26(10):2929–2937. doi: 10.1021/bi00384a039. [DOI] [PubMed] [Google Scholar]
- Lutz-Freyermuth C., Query C. C., Keene J. D. Quantitative determination that one of two potential RNA-binding domains of the A protein component of the U1 small nuclear ribonucleoprotein complex binds with high affinity to stem-loop II of U1 RNA. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6393–6397. doi: 10.1073/pnas.87.16.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian I. S., Bradwell A. R., Olson A. J. Structure, function and properties of antibody binding sites. J Mol Biol. 1991 Jan 5;217(1):133–151. doi: 10.1016/0022-2836(91)90617-f. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., Grudier J. P., Gilkeson G. S. A role for immunogenic DNA in the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1990 Feb;33(2):153–159. doi: 10.1002/art.1780330202. [DOI] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Raz E., Brezis M., Rosenmann E., Eilat D. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989 May 1;142(9):3076–3082. [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Origins of anti-DNA autoantibodies. J Clin Invest. 1985 Feb;75(2):321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tsai D. E., Harper D. S., Keene J. D. U1-snRNP-A protein selects a ten nucleotide consensus sequence from a degenerate RNA pool presented in various structural contexts. Nucleic Acids Res. 1991 Sep 25;19(18):4931–4936. doi: 10.1093/nar/19.18.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990 Aug 3;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Keene J. D. Autoantibodies specific for U1 RNA and initiator methionine tRNA. J Biol Chem. 1986 Apr 25;261(12):5467–5472. [PubMed] [Google Scholar]
- van Venrooij W. J., Hoet R., Castrop J., Hageman B., Mattaj I. W., van de Putte L. B. Anti-(U1) small nuclear RNA antibodies in anti-small nuclear ribonucleoprotein sera from patients with connective tissue diseases. J Clin Invest. 1990 Dec;86(6):2154–2160. doi: 10.1172/JCI114954. [DOI] [PMC free article] [PubMed] [Google Scholar]