Abstract
DCs, like the sensory neurons, express vanilloid receptor 1 (VR1). Here we demonstrate that the VR1 agonists, capsaicin (CP) and resiniferatoxin (RTX), enhance antiviral CTL responses by increasing MHC class I-restricted viral antigen presentation in dendritic cells (DCs). Bone marrow-derived DCs (BM-DCs) were infected with a recombinant vaccinia virus (VV) expressing OVA (VV-OVA), and then treated with CP or RTX. Both CP and RTX increased MHC class I-restricted presentation of virus-encoded endogenous OVA in BM-DCs. Oral administration of CP or RTX significantly increased MHC class I-restricted OVA presentation by splenic and lymph node DCs in VV-OVA-infected mice, as assessed by directly measuring OVA peptide SIINFEKL-Kb complexes on the cell surface and by performing functional assays using OVA-specific CD8 T cells. Accordingly, oral administration of CP or RTX elicited potent OVA-specific CTL activity in VV-OVA-infected mice. The results from this study demonstrate that VR1 agonists enhance anti-viral CTL responses, as well as a neuro-immune connection in anti-viral immune responses.
Keywords: Antigen presentation, Dendritic cell, MHC class I, VR1 agonist
INTRODUCTION
Vanilloid receptor 1 (VR1), also known as transient receptor potential cation channel subfamily V member 1, is a non-selective cation channel that is predominantly expressed by sensory neurons of dorsal root and trigeminal ganglia (1). VR1 is activated by a diverse chemical ligands such as capsaicin (CP), resiniferatoxin (RTX), allyl isothiocyanate, and anandamide, as well as by temperatures greater than 43℃ (2,3,4). VR1 activation leads to a painful, burning sensation. CP, the pungent component of hot peppers, is the best known VR1 agonist (5). RTX is an irritant component of a cactus-like plant, Euphorbia resinifera (6). CP and RTX activate and then desensitize VR1. RTX is much more potent than CP in inducing analgesia (2,7).
CP can modulate many parameters of immune responses. CP was shown to inhibit Con-A-induced lymphocyte proliferation (8); the production of cytokines such as GM-CSF, IFN-γ, and IL-2 from PMA-activated Jurkat T cells (9); prostaglandin E2 (PGE2) and NO production in LPS-stimulated peritoneal macrophages (10); LPS- and IFN-γ-mediated NO production, iNOS protein and mRNA expression, and LPS- and PMA- induced COX-2 expression and PGE2 production in RAW264.7 macrophages (11); T cell differentiation in neonatal rats (12); and the accumulation of DCs and other inflammatory cells around small pulmonary vessels during the pulmonary response to inhaled antigens in rats (13). Some of the diverse immunomodulatory activities of CP are due to its binding to VR1 on DCs. DCs, like the sensory neurons, express VR1 (14,15). CP was shown to induce the maturation of immature DCs in VR1+/+ mice, but not in VR1–/– mice (14). In addition, intradermal CP injection induced DC migration to the draining lymph nodes in VR1+/+ mice, but not in VR1–/– mice (14). CP also increased the capability of exogenous antigen presentation in association with MHC class I molecules in the DCs of VR1+/+ mice, but not of VR1–/– mice. In contrast to the promotion of MHC class I-restricted exogenous antigen presentation, CP inhibited MHC class II-restricted exogenous antigen presentation by suppressing transcription of class II transactivator genes in murine peritoneal macrophages (16).
In the present study, we showed that CP and RTX increased MHC class I-restricted presentation of virus-encoded endogenous antigens in DCs, both in vitro and in vivo, and enhanced viral antigen-specific CTL activity in virus-infected mice. These results demonstrate a neuro-immune connection in anti-viral immune responses and also imply that the oral consumption of CP, a pungent component of pepper, could be useful in enhancing anti-viral CTL responses.
MATERIALS AND METHODS
Cell lines, reagents, and mice
A T cell hybridoma, B3Z86/90.14 (B3Z), was provided by Dr. Nilabh Shastri (University of California, Berkeley, CA, USA) (17). The T cell hybridomas, CD8 OVA1.3 and DOBW, were provided by Dr. Clifford V. Harding (Case Western Reserve University, Cleveland, OH, USA) (18,19). Recombinant vaccinia virus encoding OVA (VV-OVA) was provided by Dr. Jonathan W. Yewdell (National Institutes of Health, Bethesda, MD, USA) (20). Both capsaicin (CP) and resiniferatoxin (RTX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The compounds were dissolved in absolute ethanol for in vitro experiments or in ethanol/tween-20/PBS (10/10/80) for in vivo experiments. Male, C57BL/6 and BALB/c mice (8~12-week-old) were purchased from Orient Co., Ltd. (Seoul, Korea). Mice were studied according to the protocols approved by the Animal Care Committee of Chungbuk National University.
Generation of bone marrow-derived DCs (BM-DCs)
BM-DCs were generated as described previously (21). Briefly, total BM cells obtained from mouse femurs were cultured in 6-well plates (5×106/well) in culture medium containing 40 ng/ml GM-CSF and 20 ng/ml IL-4 (both from CreaGene, Seongnam, Korea). After 3 and 4 days, non-adherent cells were removed by gentle shaking and replacing the medium. Immature BM-DCs were harvested by gentle pipetting on day 6.
In vitro viral antigen-presentation assay
Immature BM-DCs (1×107/ml) were infected with VV-OVA (multiplicity of infection [MOI]=5) for 20 min at 37℃. Virus-infected DCs were diluted to 1×106/ml with DMEM containing 10% FBS and 1 µM cytosine β-D-arabinofuranoside (Sigma-Aldrich), and distributed to a 96-well microtiter plate (100 µl/well). After adding the indicated quantities of VR1 agonists, the plate was incubated for 6 h at 37℃. After fixing the cells with ice-cold, 1.0% paraformaldehyde for 5 min at room temperature, class I MHC-complexed OVA peptide quantities were assessed using CD8 OVA 1.3 cells (2×105/well), which recognize OVA[257-264]-Kb complexes and secrete IL-2 (22).
VR1 agonist treatment and VV-OVA infection
C57BL/6 (H-2b) mice were orally administrated CP (10 mg/kg) or RTX (0.1 mg/kg) for 3 days, and then infected with VV-OVA (5×104 CFUs/mouse, i.v. administration). After infection, the mice were orally administered the same doses of CP or RTX for 5 consecutive days. On the final day of final VR1 agonist administration, functional assays were performed, such as the in vivo antigen-presentation assay, in vivo CTL assay, and OT-I T cell-adaptive transfer assay were performed.
In vivo viral antigen-presentation assay
DCs were isolated from the lymph nodes (popliteal, inguinal, mesenteric, and axillary) and spleens were collected and pooled (≥10 mice per treatment group), using a negative-selection method, as described previously (23). Class I-restricted T cell activation assays were then performed as described previously (17) using B3Z cells, which express β-galactosidase when activated with OVA[257-264]-Kb complexes. The B3Z cell responses were determined by X-gal (5-bromo-4-chloro-3-indoyl-β- D-galactopyranoside) staining (23).
Phenotype analysis
Cells were stained with mAbs recognizing murine cell surface markers as described previously (22), and flow cytometric analysis was performed on a FACS Canto II (BD Biosciences, San Jose, CA, USA). mAbs (anti-H2-Kb, anti-I-Ab, anti-CD80, anti-CD86, and an isotype-matched control) were purchased from BD Biosciences. The mAbs recognizing OVA[257-264]-Kb complexes, Alexa 488-conjugated 25-D1.16, was kindly provided by Dr. Jonathan W. Yewdell (National Institutes of Health, Bethesda, MD) (20).
In vivo CTL assay
In vivo evaluation of the cytotoxic activities was performed using 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes)-labeled target cells prepared from syngeneic spleen and lymph node cell suspensions, as described previously (23). Specific in vivo cytotoxicity was determined for the lymph node and spleen cells isolated from the recipient mice 18 h after the i.v. injection of target cells. The ratio between the percentage of uncoated vs. OVA[257-264]-coated (CFSElow/CFSEhigh) cells was calculated to obtain a numerical value of the cytotoxicity.
OT-I T cell transfer experiment
CD8+ T cells were isolated from the pooled spleens and lymph nodes of OT-I mice using a CD8+ T Cell Isolation kit (Miltenyi Biotec Inc., Auburn, CA, USA). The cells were labelled with 50 µM CFSE, and injected into the tail vein of mice (1×107 cells/mouse). After 4 days, proliferation of CFSE-labeled OT-I CD8+ T cells was determined for the lymph node and spleen cells isolated from the recipient mice by flow cytometry.
Intracellular IFN-γ staining
Intracellular IFN-γ staining was performed as described previously (24) using a FITC-labeled anti-CD8 mAb and a PE-conjugated anti-IFN-γ mAb (BD Biosciences) (24). Cells were then analyzed using a FACSCanto II flow cytometer (BD Biosciences), and the number of CD8+ cells expressing IFN-γ was enumerated using FlowJo software (TreeStar, Ashland, OR, USA).
Preparation of microspheres containing OVA (OVA-microspheres)
OVA-microspheres were prepared using a homogenization/ solvent evaporation method, as described previously (21), with 400 µl of OVA-containing water (50 mg OVA/ ml) and 2 ml of ethyl acetate containing poly(lactic-coglycolic acid) (100 mg/ml, Sigma-Aldrich). Fluorescent microspheres were prepared by adding FITC (5 µg/ml) to the ethyl acetate phase.
Exogenous antigen-presentation assays
BM-DCs, generated from the BM cells of C57BL/6 or BALB/c mice, were seeded in 96-well microtiter plates (1×105/well) in the presence of different concentrations of CP or RTX, incubated for 4 h at 37℃, and then OVA-microspheres (50 µg/ml as OVA) were added. After 2 h incubation at 37℃, the cells were fixed with 1.0% paraformaldehyde for 5 min at room temperature. Class I MHC-complexed OVA peptide quantities were assessed using CD8 OVA 1.3 cells (2×105/well), which recognize OVA[257-264]-Kb complexes and secrete IL-2 (22). Class II MHC-complexed OVA peptide quantities were assessed using DOBW cells (1×105/well), which recognize OVA[323-339]-I-Ad complexes and secrete IL-2 (19).
Statistical analysis
The statistical significance of the difference between the control and treatment groups was assessed using a one-way ANOVA followed by a Student's t-test.
RESULTS
VR1 agonists increase MHC class I-restricted OVA presentation in BM-DCs infected with VV-OVA
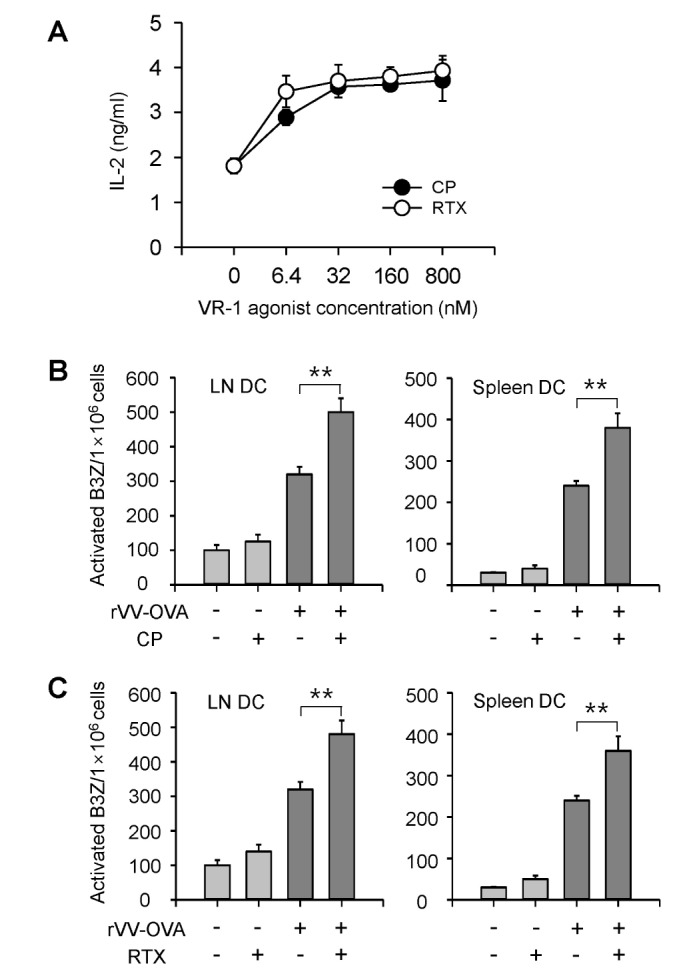
To examine the effects of VR1 agonists on MHC class I-restricted endogenous antigen presentation, BM-DCs were infected with VV-OVA (MOI=5), and then cultured in the presence of different concentrations of CP or RTX. After 6 h, the cells were fixed with paraformaldehyde, washed, and then the amounts of MHC class I (Kb)-OVA peptide complexes on the cell surface were assessed using CD8 OVA1.3 cells. Both CP and RTX increased MHC class I-restricted presentation of virus-encoded OVA (Fig. 1A). The ability of CP and RTX to increase MHC class I-restricted endogenous antigen presentation reached near maximum levels at 32 nM and 6.4 nM, respectively, showing that RTX is much more potent than CP in increasing MHC class I-restricted endogenous antigen presentation in DCs.
Figure 1. VR1 agonists increase MHC class I-restricted OVA presentation in DCs infected with VV-OVA. (A) BM-DCs (1×105/well) generated from C57BL/6 (H-2b) mice were infected with VV-OVA (MOI=5), and cultured for 6 h in the presence of the indicated amounts of CP or RTX. The amounts of H-2Kb-OVA peptide complexes were assessed using CD8 OVA1.3 cells. (B, C) VR1 agonists increased MHC class I-restricted DC presentation of OVA peptides in mice infected with VV-OVA. C57BL/6 mice were orally administered CP (10 mg/kg) or RTX (0.1 mg/kg) was for 3 days, and then then were infected with VV-OVA (5×104 CFUs/mouse, i.v.). After virus infection, the mice were orally administrated the same doses of CP or RTX for consecutive 5 days. On the final day of VR1 agonist administration, DCs were isolated from the lymph nodes and spleens of the mice, and the amounts of Kb-OVA peptide complexes on the cell surface were evaluated using OVA-specific CD8 T hybridoma cells (B3Z cells). **p<0.01.
VR1 agonists increase MHC class I-restricted DC presentation of OVA peptides in mice infected with VV-OVA
To examine whether VR1 agonists also increase MHC class I-restricted endogenous antigen presentation in vivo , CP (10 mg/kg) or RTX (0.1 mg/kg) was orally administered to C57BL/6 mice for 3 days, followed by infection with VV-OVA (5×104 CFUs/mouse, i.v.). After virus infection, the mice were orally administrated with the same doses of CP or RTX for consecutive 5 days. On the final day of VR1 agonist administration, DCs were isolated from the lymph nodes and spleens of the mice, and the amounts of Kb-OVA peptide complexes on the cell surface were evaluated using OVA-specific CD8 T hybridoma cells (B3Z cells). Both CP and RTX significantly increased MHC class I-restricted presentation of virus-encoded OVA in both lymph node DCs and spleen DCs (Fig. 1B, C).
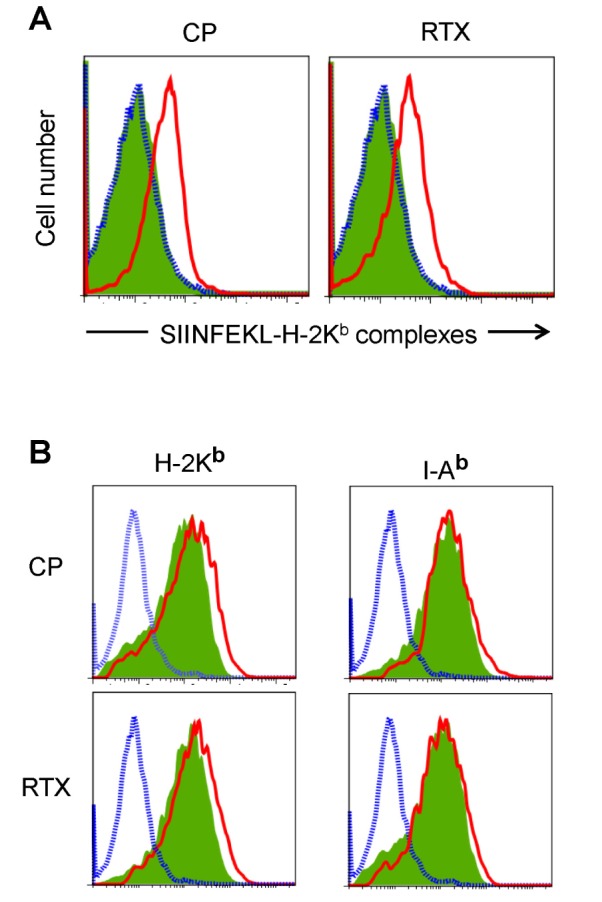
The amounts of Kb-OVA peptide complexes on the cell surface of lymph node and spleen DCs were directly determined using the F(ab')2 fragment of a SIINFEKL-H-2Kb-specific mAb (25-D1.16). Oral administration of CP and RTX (Fig. 2A) profoundly increased the cell surface expression of SIINFEKL-H-2Kb complexes in the spleen DCs of VV-OVA-infected mice. We then examined whether CP and RTX increased the expression of MHC class I molecules on DCs. Both CP and RTX increased the expression levels of H-2Kb molecules, but not the expression levels of I-Ab molecules, in the spleen DCs of VV-OVA-infected mice (Fig. 2B).
Figure 2. VR1 agonists increased the expression of SIINFEKL-H-2Kb complexes in mice infected with VV-OVA. Mice were orally administered CP or RTX for 3 days, infected with VV-OVA, and then treated with the same doses of CP or RTX for consecutive 5 days, as described in Fig. 1. DCs were isolated from the lymph nodes and spleens. (A) The amounts of Kb-OVA peptide complexes on the cell surface were directly determined using the F(ab')2 fragment of a SIINFEKL-H-2Kb-specific mAb (25-D1.16). (B) Expression levels of H-2Kb and I-Ab molecules. Blue dotted line, isotype control; green shaded area, DCs from mice not treated with a VR1 agonist (CP or RTX); red line, DCs from VR1 agonist-treated mouse.
Oral administration of VR1 agonists increased OVA-specific CTL activity in mice infected with VV-OVA
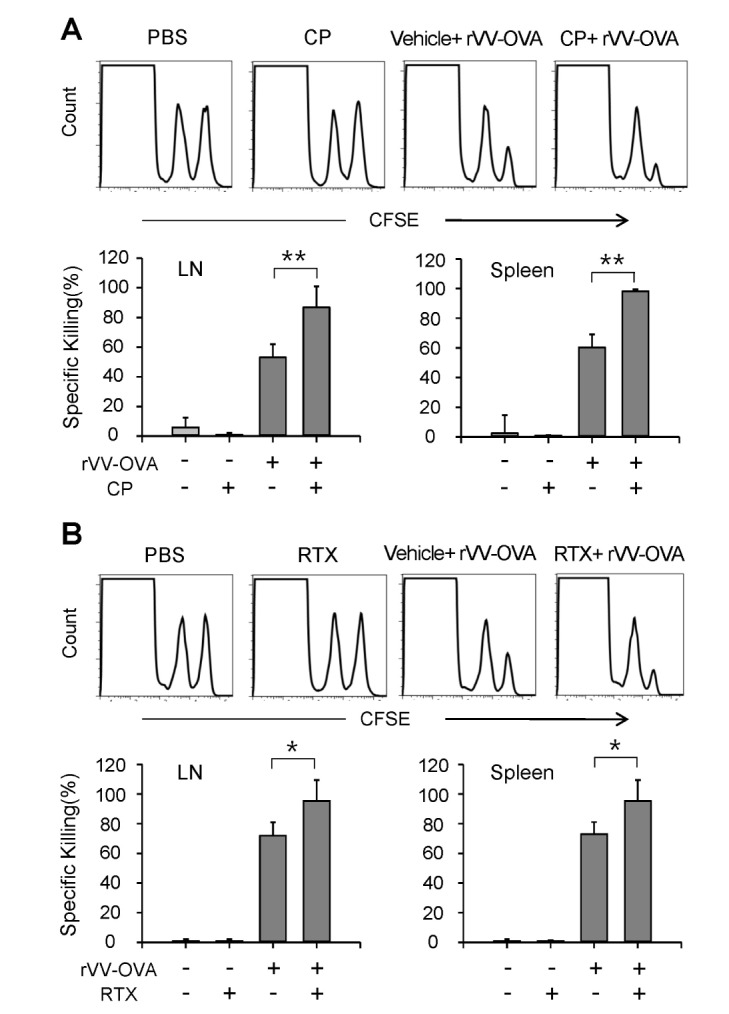
The effects of VR1 agonists on the induction of CTL activity were examined in mice orally administered CP or RTX for 3 days, infected with VV-OVA, and then treated with the same doses of CP or RTX for consecutive 5 days, as described in Fig. 1. On the final day of VR1 agonist administration, in vivo CTL assays were performed using CFSE-labeled syngeneic target cells. Representative histograms are shown in Fig. 3. Oral administration of CP (Fig. 3A) or RTX (Fig. 3B) significantly increased OVA-specific CTL activities in the lymph nodes and spleens.
Figure 3. VR1 agonists increased OVA-specific CTL activity in mice injected with VV-OVA. Mice were orally administered CP or RTX for 3 days, infected with VV-OVA, and then treated with the same doses of CP or RTX for consecutive 5 days, as described in Fig. 1. On the final day of VR1 agonist administration, and in vivo CTL assays were performed using CFSE-labeled syngeneic target cells. (A) Representative histograms of lymph node and spleen cells, and the percentage of specific killing of OVA[257-264] peptide-pulsed target cells in the lymph nodes and spleens are shown (n=5 for each group). Each data point represents the mean±SD of values obtained from 2 individual experiments. *p<0.05; **p<0.01.
Oral CP administration increased the proliferation and activation of adaptively transferred OT-I cells
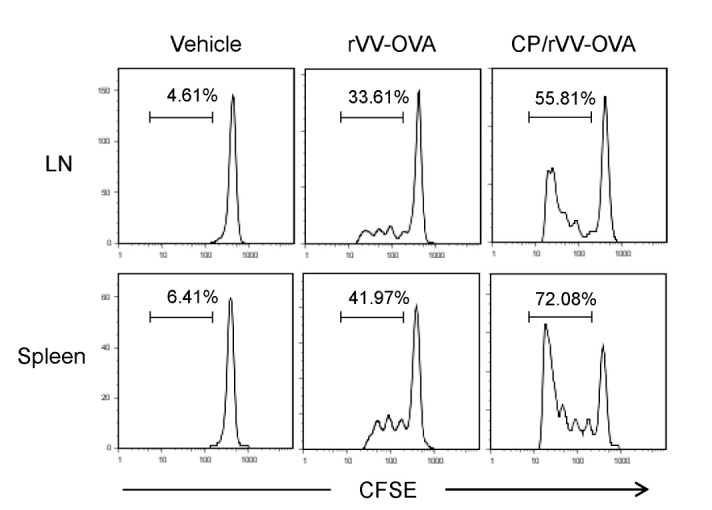
To confirm that CP increases MHC class I-restricted OVA presentation in DCs of VV-OVA infected mice, an adaptive transfer experiment was performed, in which OVA-specific CD8+ T cells (OT-I cells) were transferred to VV-OVA-infected mice. Mice were orally administered CP, infected with VV-OVA, and then treated with the same doses of CP for consecutive 5 days, as described in Fig. 1. On the final day of CP administration, CFSE-labeled OT-I cells were injected through the tail vein. After 4 days, proliferation of OT-I cells in the spleens and lymph nodes was determined by the progressive dilution of CFSE intensity, as determined by flow cytometry. Representative histograms are shown in Fig. 4. OT-1 cells in the lymph node and spleen expanded significantly in CP-treated mice, compared with vehicle-administered control mice.
Figure 4. CP increased the proliferation of adaptively transferred OTI cells. Mice were orally administered CP, infected with VV-OVA, and then treated with the same doses of CP for consecutive 5 days, as described in Fig. 1. On the final day of CP administration, CFSE-labeled OT-I cells were injected through the tail vein. After 4 days, proliferation of OT-I cells in the spleens and lymph nodes was determined by flow cytometry.
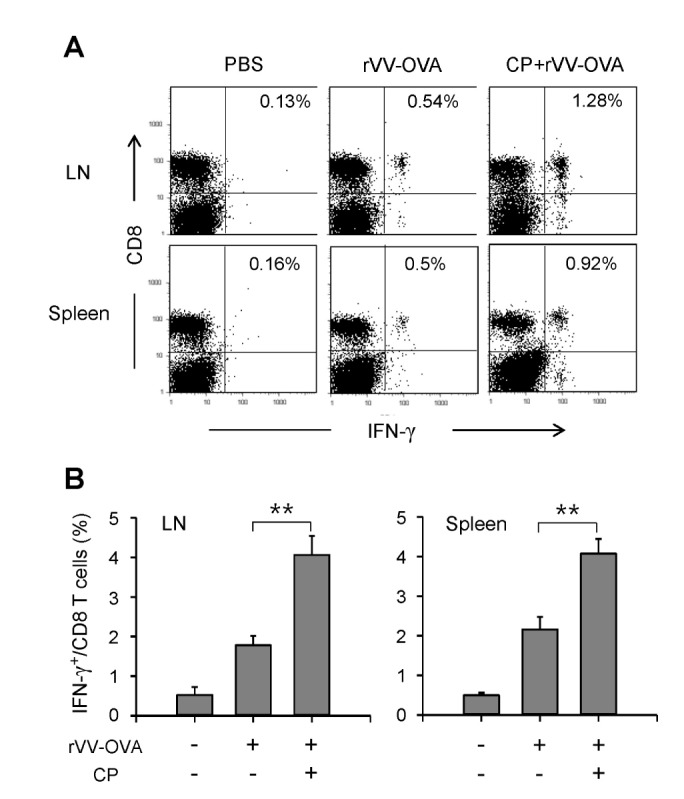
To examine the effects of CP on IFN-γ production in CD8 T cells, total spleen cells and lymph node cells were isolated from mice treated with CP and adaptively transferred with OT-1 cells, and were re-stimulated with OVA[257-264]-SIINFEKL peptide-pulsed normal BM-DCs in the presence of monensin for 6 h. The cells were stained with an anti-CD8 mAb, permeabilized, and stained with an anti-IFN-γ mAb. Representative histograms are shown in Fig. 5A. Oral administration of CP significantly increased the frequency of IFN-γ-producing CD8 T cells among total CD8 T cells (Fig. 5B).
Figure 5. CP increased IFN-γ producing CD8 T cells in mice infected with rVV-OVA. Mice were orally administered CP, infected with VV-OVA, and then treated with the same doses of CP for consecutive 5 days, as described in Fig. 1. On the final day of CP administration, CFSE-labeled OT-I cells were injected through the tail vein. After 4 days, the lymph node and spleen cells were isolated and restimulated for 6 h with OVA[257-264] peptide-pulsed DCs. The cells were stained with an anti-CD8 mAb, then permeabilized and stained with an anti-IFN-γ mAb. (A) Representative histograms are shown. (B) The percentages of IFN-γ+ cells among total CD8+ T cells are shown graphically (n=5 for each group). **p<0.01.
VR1 agonists increase class I, but not class II, MHC-restricted processing of exogenous antigen in BM-DCs
The effects of VR1 agonists on the MHC-restricted exogenous antigen presentation were examined using BM-DCs. For MHC class I-restricted exogenous antigen presentation assays, BM-DCs were generated from BM cells of C57BL/6 mice, treated with the indicated concentrations of CP or RTX for 4 h, and then allowed to phagocytose OVA-microspheres for 2 h. The BM-DCs were washed and fixed with paraformaldehyde. Class I MHC-complexed OVA peptide quantities were assessed using CD8 OVA 1.3 hybriboma cells. The results showed that CP and RTX efficiently enhanced class I MHC-restricted exogenous OVA presentation (Fig. 6A). For MHC class II-restricted exogenous antigen presentation assays, BM-DCs were generated from the BM cells of BALB/c mice, treated with the indicated concentrations of CP or RTX for 4 h, and then allowed to phagocytose OVA-microspheres for 2 h. The BM-DCs were washed and fixed with paraformaldehyde. Class II MHC-complexed OVA peptide quantities were assessed using DOBW cells. The results showed that CP and RTX did not affect class II MHC-restricted exogenous OVA presentation in BM-DCs (Fig. 6B).
Figure 6. VR1 agonists increases class I, but not class II, MHC-restricted presentation of exogenous antigen in BM-DCs. (A) BM-DCs generated from BM cells of C57BL/6 mice were treated with the indicated concentrations of CP or RTX for 4 h, and then allowed to phagocytose OVA-microspheres for 2 h. After fixing, the amounts of H-2Kb-OVA peptide complexes were assessed using CD8 OVA 1.3 cells. (B) BM-DCs generated from BM cells of BALB/c mice were treated with the indicated concentrations of CP or RTX for 4 h, and then allowed to phagocytose OVA-microspheres for 2 h. After fixing, the amounts of I-Ad-OVA peptide complexes were assessed using DOBW cells. (C, D) Effects of CP and RTX on phagocytic activity. BM-DCs generated from BM cells of C57BL/6 mice were incubated with FITC-labeled OVA-microspheres, washed, harvested, and analyzed by flow cytometry. A representative set of histograms from 3 individual experiments is shown. Shaded histograms, phagocytic activity of DCs in the absence of CP or RTX; thick line histograms, phagocytic activity of DCs in the presence of CP or RTX.
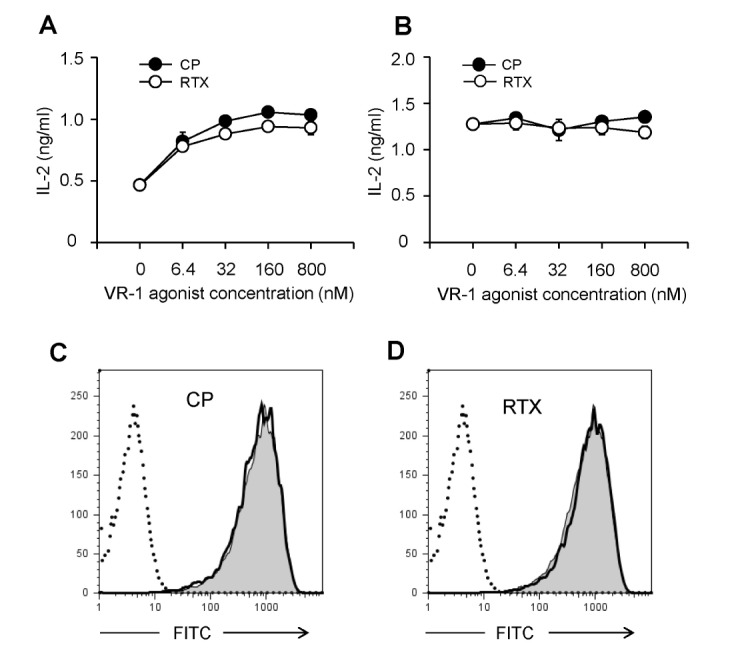
To test whether the class I MHC-restricted antigen presentation-enhancing activity of CP and RTX was due to the enhancement of phagocytic activity, BM-DCs generated from BM cells of C57BL/6 mice were incubated with FITC-labeled OVA-microspheres, washed, and then harvested by gentle pipetting after cooling on ice. Flow cytometric analysis of the harvested cells showed that CP and RTX did not affect the phagocytic activity of BM-DCs (Fig. 6C, D).
DISCUSSION
The major finding of the present study is that VR1 agonists, CP and RTX, enhance MHC class I-restricted viral antigen presentation in DCs infected with a virus. We used VV-OVA as a model virus to infect DCs. Both CP and RTX increased MHC class I (Kb)-restricted OVA peptide presentation in BM-DCs infected with VV-OVA. The in vivo relevance of the enhancement of MHC class I-restricted endogenous antigen presentation was studied in mice orally administered a VR1 agonist and infected with VV-OVA, which showed that that both CP and RTX significantly increased MHC class I-restricted OVA peptide presentation in vivo. Consistent with these results, both CP and RTX increased the production of OVA-specific CTLs in mice infected with VV-OVA.
The results from numerous studies have shown that CP exerts anti-inflammatory properties mainly via inhibiting innate immune responses (9,11,13). CP can inhibit adaptive immune responses, such as lymphocyte proliferation and T cell differentiation (8,12), and reduce IgA and IgG production (25). In contrast, CP exerts adjuvant activity on antigen-specific T cell responses. For instance, when CP and OVA were co-injected into mice that were adaptively transferred with OVA-specific T cells (OT1 cells), CP increased OT1 cell proliferation and the generation of OVA-specific CTLs (14). This study could represent an indirect demonstration of the enhancing effect of CP on the MHC class I-restricted exogenous antigen presentation. In contrast to the effects of CP on MHC class I-restricted exogenous antigen presentation, CP was previously reported to inhibit MHC class II-restricted exogenous antigen presentation (16). CP inhibited IFN-γ-induced MHC class II expression by suppressing CIITA expression in macrophages. To date, the effects of CP on MHC class I-restricted endogenous antigen presentation has not been studied in detail. Here, we demonstrated that CP and RTX increase MHC class I-restricted endogenous antigen presentation in DCs both in vitro and in vivo. The MHC class I-restricted endogenous antigen presentation-enhancing activity of CP and RTX was confirmed by directly measuring OVA peptide SIINFEKL-Kb complexes on the cell surface and by performing functional assays using OVA-specific T cells. It is also noteworthy that CP and RTX were both administered orally in this study, whereas CP was injected intradermally in an experiment showing that CP increased the MHC class I-restricted exogenous antigen presentation (14).
Both CP and RTX increased MHC class I-restricted exogenous antigen presentation (cross-presentation), but did not affect MHC class II-restricted exogenous antigen presentation (Fig. 6). The classical paradigm of antigen presentation by professional APCs is that endogenous antigens are presented via MHC-I molecules to CD8+ T cells, whereas exogenous antigens are presented via MHC-II molecules to CD4+ T cells (26). Subsequently, it was demonstrated that professional APCs also process exogenous antigens for presentation by MHC-I molecules to CD8+ T cells. This process was termed crosspresentation (27,28,29). The current study and the earlier study by Basu and Srivastava (14) demonstrated that CP increased the MHC class I-restricted antigen presentation of both exogenous and endogenous antigens. CP, however, inhibited MHC class II-restricted antigen presentation of exogenous antigens (16). The discrepancy in the effects of CP on MHC class II-restricted exogenous antigen presentation may due to differences in the cell type used and in the stimulation conditions. When examining the effects of CP on MHC class II-restricted exogenous antigen presentation, we used immature DCs, while Mahmoud et al. used IFN-γ-activated macrophages (16).
RTX is a much more potent VR1 agonist than is CP (2). In agreement, RTX was much more potent than CP in increasing MHC class I-restricted exogenous antigen presentation in DCs. When examining the effects of CP and RTX on the MHC class I-restricted endogenous antigen presentation in vivo, mice were fed with 10 mg/kg CP and 0.1 mg/kg RTX, and similar results were obtained in functional assays, such as in vivo CTL assays (Fig. 3), OT1 cell-proliferation assays (Fig. 4), and intracellular staining of IFN-γ-producing CD8 T cells (Fig. 5). Thus, the potency of CP and RTX in increasing MHC class I-restricted endogenous antigen presentation appears to be directly proportional to the potency of VR1 receptor binding.
ACKNOWLEDGEMENTS
This study was supported by the research grant of the Chungbuk National University in 2014.
Abbreviations
- BM-DCs
bone marrow-derived DCs
- CP
capsaicin
- DC
dendritic cell
- MOI
multiplicity of infection
- PGE2
prostaglandin E2
- RTX
resiniferatoxin
- VR1
vanilloid receptor 1
- VV
vaccinia virus
Footnotes
CONFLICTS OF INTEREST: The authors have no financial conflict of interest.
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Szallasi A, Blumberg PM. Vanilloid(Capsaicin)receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 3.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 4.Everaerts W, Gees M, Alpizar YA, Farre R, Leten , C, Apetrei A, Dewachter I, van LF, Vennekens R, De RD, Nilius B, Voets T, Talavera K. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 5.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 6.Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 7.Bley KR. Recent developments in transient receptor potential vanilloid receptor 1 agonist-based therapies. Expert Opin Investig Drugs. 2004;13:1445–1456. doi: 10.1517/13543784.13.11.1445. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson G, Ahlstedt S. Altered lymphocyte proliferation of immunized rats after neurological manipulation with capsaicin. Int J Immunopharmacol. 1988;10:747–751. doi: 10.1016/0192-0561(88)90028-8. [DOI] [PubMed] [Google Scholar]
- 9.Gertsch J, Guttinger M, Sticher O, Heilmann J. Relative quantification of mRNA levels in Jurkat T cells with RT-real time-PCR (RT-rt-PCR): new possibilities for the screening of anti-inflammatory and cytotoxic compounds. Pharm Res. 2002;19:1236–1243. doi: 10.1023/a:1019818814336. [DOI] [PubMed] [Google Scholar]
- 10.Kim CS, Kawada T, Kim BS, Han IS, Choe SY, Kurata T, Yu R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-α degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003;15:299–306. doi: 10.1016/s0898-6568(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen CW, Lee ST, Wu WT, Fu WM, Ho FM, Lin WW. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br J Pharmacol. 2003;140:1077–1087. doi: 10.1038/sj.bjp.0705533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoni G, Perfumi MC, Pompei P, Spreghini E, Lucciarini R, Martarelli D, Staffolani M, Piccoli M. Impairment of rat thymocyte differentiation and functions by neonatal capsaicin treatment is associated with induction of apoptosis. J Neuroimmunol. 2000;104:37–46. doi: 10.1016/s0165-5728(99)00249-0. [DOI] [PubMed] [Google Scholar]
- 13.Kradin R, MacLean J, Duckett S, Schneeberger EE, Waeber C, Pinto C. Pulmonary response to inhaled antigen: neuroimmune interactions promote the recruitment of dendritic cells to the lung and the cellular immune response to inhaled antigen. Am J Pathol. 1997;150:1735–1743. [PMC free article] [PubMed] [Google Scholar]
- 14.Basu S, Srivastava P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci USA. 2005;102:5120–5125. doi: 10.1073/pnas.0407780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nevius E, Srivastava PK, Basu S. Oral ingestion of capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunol. 2012;5:76–86. doi: 10.1038/mi.2011.50. [DOI] [PubMed] [Google Scholar]
- 16.Mahmoud ME, Nikami H, Shiina T, Takewaki T, Shimizu Y. Capsaicin inhibits IFN-gamma-induced MHC class II expression by suppressing transcription of class II transactivator gene in murine peritoneal macrophages. Int Immunopharmacol. 2010;10:86–90. doi: 10.1016/j.intimp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Karttunen, J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci USA. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- 19.Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991;147:2860–2863. [PubMed] [Google Scholar]
- 20.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 21.Lee YR, Yang IH, Lee YH, Im SA, Song S, Li H, Han K, Kim K, Eo SK, Lee CK. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005;105:3951–3955. doi: 10.1182/blood-2004-10-3927. [DOI] [PubMed] [Google Scholar]
- 22.Lee YH, Lee YR, Kim KH, Im SA, Song S, Lee MK, Kim Y, Hong JT, Kim K, Lee CK. Baccatin III, a synthetic precursor of taxol, enhances MHC-restricted antigen presentation in dendritic cells. Int Immunopharmacol. 2011;11:985–991. doi: 10.1016/j.intimp.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Lee YH, Lee YR, Im SA, Park SI, Kim KH, Gerelchuluun T, Song S, Kim K, Lee CK. Calcineurin inhibitors block MHC-restricted antigen presentation in vivo. J Immunol. 2007;179:5711–5716. doi: 10.4049/jimmunol.179.9.5711. [DOI] [PubMed] [Google Scholar]
- 24.Park CS, Im SA, Song S, Kim K, Lee CK. Identification of HLA-A2-restricted immunogenic peptides derived from a xenogenic porcine major histocompatibility complex. Xenotransplantation. 2014;21:465–472. doi: 10.1111/xen.12119. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson G, Alving K, Ahlstedt S. Effects on immune responses in the rats after neuromanipulation with capsaicin. Int Immunopharmacol. 1991;13:21–26. doi: 10.1016/0192-0561(91)90021-x. [DOI] [PubMed] [Google Scholar]
- 26.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 27.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding CV. Phagocytic processing of antigens for presentation by MHC molecules. Trends Cell Biol. 1995;5:105–109. doi: 10.1016/s0962-8924(00)88959-x. [DOI] [PubMed] [Google Scholar]
- 29.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1:126–134. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]


