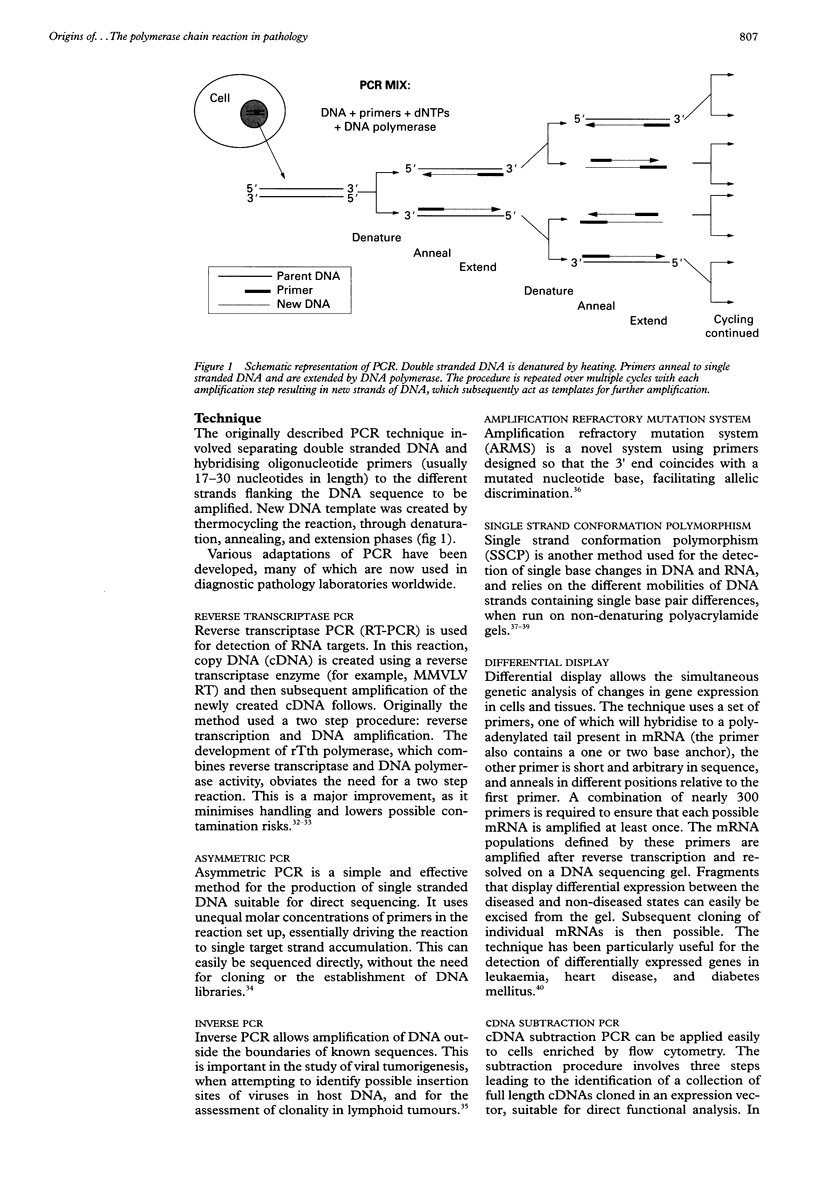
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott A. Roche faces charges over Taq patent claim. Nature. 1996 Aug 22;382(6593):660–660. doi: 10.1038/382660a0. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Brady G., Iscove N. N. Construction of cDNA libraries from single cells. Methods Enzymol. 1993;225:611–623. doi: 10.1016/0076-6879(93)25039-5. [DOI] [PubMed] [Google Scholar]
- Buongiorno-Nardelli M., Amaldi F. Autoradiographic detection of molecular hybrids between RNA and DNA in tissue sections. Nature. 1970 Mar 7;225(5236):946–948. doi: 10.1038/225946a0. [DOI] [PubMed] [Google Scholar]
- Clarke A. M., Mapstone N. P., Quirke P. Molecular biology made easy. The polymerase chain reaction. Histochem J. 1992 Dec;24(12):913–926. doi: 10.1007/BF01046497. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G., Pardue M. L. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci U S A. 1969 Jun;63(2):378–383. doi: 10.1073/pnas.63.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Greenfield C., Sinickas V., Harrison L. C. Detection of cytomegalovirus by the polymerase chain reaction. A simple, rapid and sensitive non-radioactive method. Med J Aust. 1991 Mar 18;154(6):383–385. doi: 10.5694/j.1326-5377.1991.tb121127.x. [DOI] [PubMed] [Google Scholar]
- Grünewald K., Feichtinger H., Weyrer K., Dietze O., Lyons J. DNA isolated from plastic embedded tissue is suitable for PCR. Nucleic Acids Res. 1990 Oct 25;18(20):6151–6151. doi: 10.1093/nar/18.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer R. L., Koshland D. E., Jr The Molecule of the Year. Science. 1989 Dec 22;246(4937):1543–1546. doi: 10.1126/science.2688087. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Holland P. M., Abramson R. D., Watson R., Gelfand D. H. Detection of specific polymerase chain reaction product by utilizing the 5'----3' exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7276–7280. doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Todd K., Birnstiel M. L., Bird A. Molecular hybridization of 3H-labelled ribosomal RNA with DNA in ultrathin sections prepared for electron microscopy. Biochim Biophys Acta. 1971 Feb 11;228(3):761–766. doi: 10.1016/0005-2787(71)90746-5. [DOI] [PubMed] [Google Scholar]
- John H. A., Birnstiel M. L., Jones K. W. RNA-DNA hybrids at the cytological level. Nature. 1969 Aug 9;223(5206):582–587. doi: 10.1038/223582a0. [DOI] [PubMed] [Google Scholar]
- Kallioniemi A., Kallioniemi O. P., Sudar D., Rutovitz D., Gray J. W., Waldman F., Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992 Oct 30;258(5083):818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Chang K. S., Cabanillas F., Freireich E. J., Trujillo J. M., Stass S. A. Detection of minimal residual cells carrying the t(14;18) by DNA sequence amplification. Science. 1987 Jul 10;237(4811):175–178. doi: 10.1126/science.3110950. [DOI] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N. A. Representational difference analysis: finding the differences between genomes. Trends Genet. 1995 Aug;11(8):303–307. doi: 10.1016/s0168-9525(00)89087-3. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Patel P., Wainscoat J. S., Sampietro M., Gillmer M. D., Fleming K. A. Prenatal sex determination by DNA amplification from maternal peripheral blood. Lancet. 1989 Dec 9;2(8676):1363–1365. doi: 10.1016/s0140-6736(89)91969-7. [DOI] [PubMed] [Google Scholar]
- McDougall J. K., Dunn A. R., Jones K. W. In situ hybridization of adenovirus RNA and DNA. Nature. 1972 Apr 14;236(5346):346–348. doi: 10.1038/236346a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B. The unusual origin of the polymerase chain reaction. Sci Am. 1990 Apr;262(4):56-61, 64-5. doi: 10.1038/scientificamerican0490-56. [DOI] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary J. J., Chetty R., Graham A. K., McGee J. O. In situ PCR: pathologist's dream or nightmare? J Pathol. 1996 Jan;178(1):11–20. doi: 10.1002/(SICI)1096-9896(199601)178:1<11::AID-PATH459>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G., Jeanteur P., Croissant O. Evidence for and localization of vegetative viral DNA replication by autoradiographic detection of RNA-DNA hybrids in sections of tumors induced by Shope papilloma virus. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1876–1880. doi: 10.1073/pnas.68.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Khorana H. G. Studies on polynucleotides. The linkage of deoxyribopolynucleotide templates to cellulose and its use in their replication. J Biol Chem. 1974 Aug 25;249(16):5213–5221. [PubMed] [Google Scholar]
- Päbo S. Ancient DNA: extraction, characterization, molecular cloning, and enzymatic amplification. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1939–1943. doi: 10.1073/pnas.86.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Yoon H. S., Sommer S. S. Screening for mutations by RNA single-strand conformation polymorphism (rSSCP): comparison with DNA-SSCP. Nucleic Acids Res. 1992 Feb 25;20(4):871–878. doi: 10.1093/nar/20.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Martin W. J., Arnheim N. Analysis of DNA sequences in forty-year-old paraffin-embedded thin-tissue sections: a bridge between molecular biology and classical histology. Cancer Res. 1988 Aug 15;48(16):4564–4566. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M. Forensic applications of DNA fingerprinting. Mol Biotechnol. 1994 Feb;1(1):13–27. doi: 10.1007/BF02821508. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orita M., Shiraishi M., Hayashi K., Sekiya T. Detection of ras gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990 Jul;5(7):1037–1043. [PubMed] [Google Scholar]
- Thomas M. R., Tutschek B., Frost A., Rodeck C. H., Yazdani N., Craft I., Williamson R. The time of appearance and disappearance of fetal DNA from the maternal circulation. Prenat Diagn. 1995 Jul;15(7):641–646. doi: 10.1002/pd.1970150709. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse W. T., Forget B. G. Reverse transcription and direct amplification of cellular RNA transcripts by Taq polymerase. Gene. 1990 Apr 16;88(2):293–296. doi: 10.1016/0378-1119(90)90047-u. [DOI] [PubMed] [Google Scholar]
- Tutschek B., Sherlock J., Halder A., Delhanty J., Rodeck C., Adinolfi M. Isolation of fetal cells from transcervical samples by micromanipulation: molecular confirmation of their fetal origin and diagnosis of fetal aneuploidy. Prenat Diagn. 1995 Oct;15(10):951–960. doi: 10.1002/pd.1970151010. [DOI] [PubMed] [Google Scholar]
- Tutschek B., Thomas M., Williamson R., Rodeck C. H. Nichtinvasive Pränataldiagnostik an fetalen Zellen im mütterlichen Blut. Gynakologe. 1995 Oct;28(5):289–301. [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]
- Wan J. H., Trainor K. J., Brisco M. J., Morley A. A. Monoclonality in B cell lymphoma detected in paraffin wax embedded sections using the polymerase chain reaction. J Clin Pathol. 1990 Nov;43(11):888–890. doi: 10.1136/jcp.43.11.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warford A. In situ hybridisation: a new tool in pathology. Med Lab Sci. 1988 Oct;45(4):381–394. [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]


