Abstract
AIM: To investigate the presence of genetic instability in precancerous lesions of the stomach. METHODS: Fifteen cases of sporadic gastric cancers with a background of intestinal metaplasia were studied by microsatellite assay at nine loci. Altered metaplastic mucosa was microdissected, reconstructed topographically, and examined immunohistochemically with an anti-p53 antibody, comparing its positive area with foci of microsatellite instability in each individual. RESULTS: Alterations at one or more loci were observed in seven of 15 cancers (46.7%) and four of 15 intestinal metaplasias (26.7%). Two cases of replication error positive phenotype had no microsatellite alterations in their metaplastic mucosa. All the microsatellite alterations in the metaplastic mucosa were restricted to incomplete-type intestinal metaplasia around the respective cancers. Moreover, in one case, an identical pattern of microsatellite alteration was detected in the cancer tissue and in the adjacent metaplastic mucosa, suggesting the sequential development of gastric cancer from intestinal metaplasia. Frequent alteration was found at the locus D1S191 (1q), indicating that this locus might be altered early in the development of intestinal-type gastric cancer. No significant association between microsatellite instability and p53 immunoreactivity was observed in the cases examined. CONCLUSION: These results indicate that microsatellite instability may be an early event in stomach carcinogenesis, especially in intestinal-type cancers.
Full text
PDF

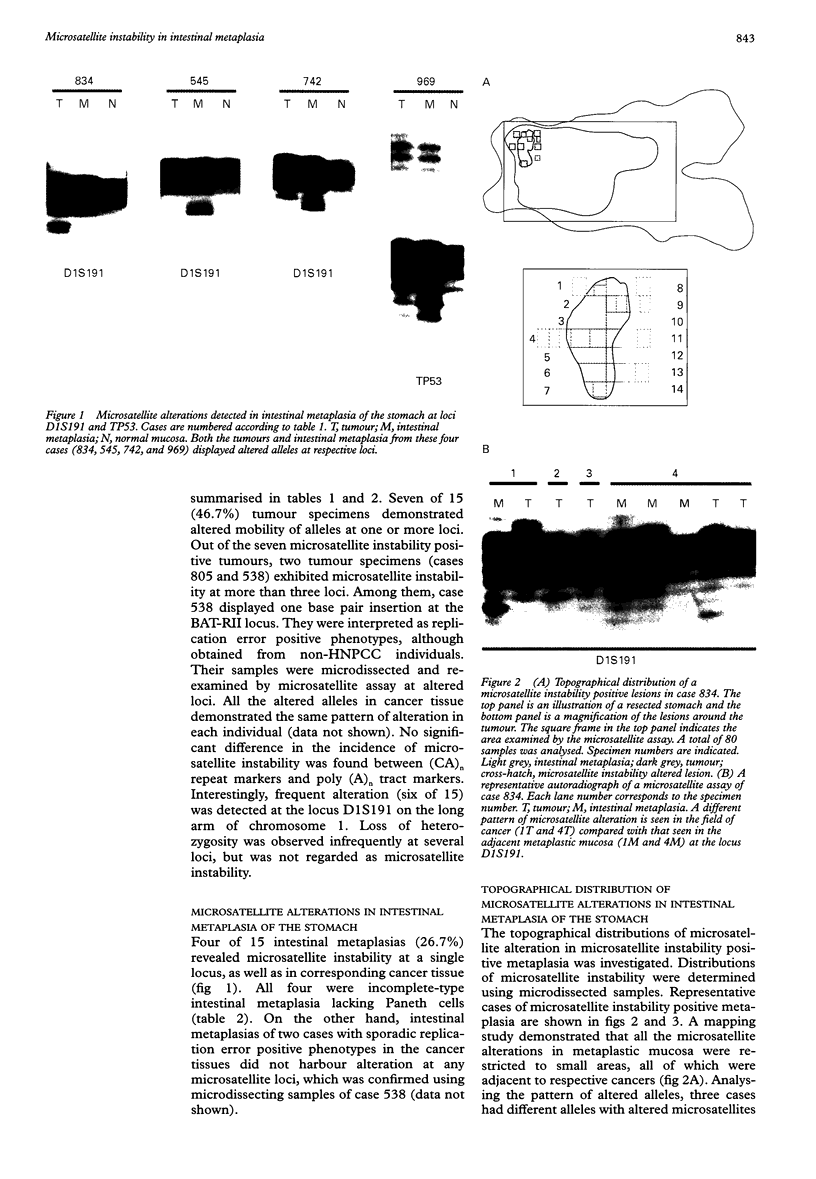
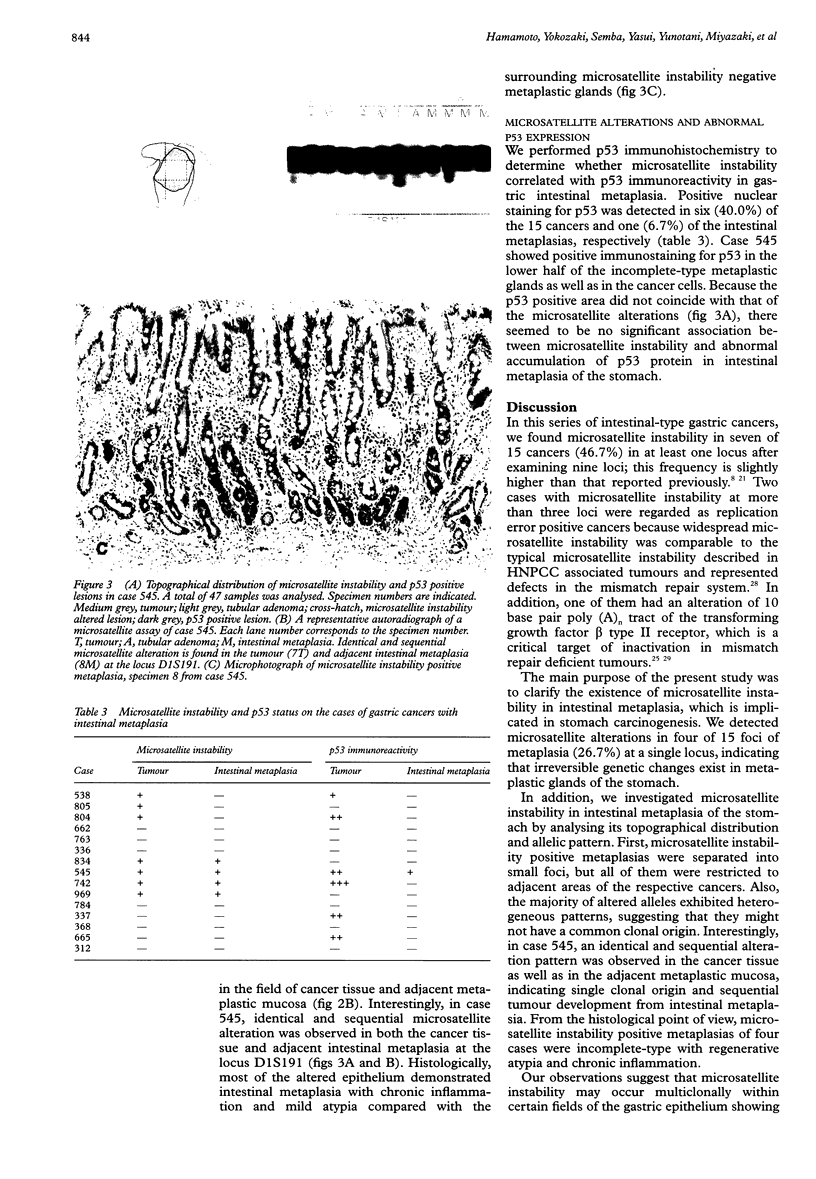


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Bronner C. E., Baker S. M., Morrison P. T., Warren G., Smith L. G., Lescoe M. K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994 Mar 17;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- Burks R. T., Kessis T. D., Cho K. R., Hedrick L. Microsatellite instability in endometrial carcinoma. Oncogene. 1994 Apr;9(4):1163–1166. [PubMed] [Google Scholar]
- Chung Y. J., Song J. M., Lee J. Y., Jung Y. T., Seo E. J., Choi S. W., Rhyu M. G. Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res. 1996 Oct 15;56(20):4662–4665. [PubMed] [Google Scholar]
- Correa P. The new era of cancer epidemiology. Cancer Epidemiol Biomarkers Prev. 1991 Nov-Dec;1(1):5–11. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Gleeson C. M., Sloan J. M., McGuigan J. A., Ritchie A. J., Weber J. L., Russell S. E. Ubiquitous somatic alterations at microsatellite alleles occur infrequently in Barrett's-associated esophageal adenocarcinoma. Cancer Res. 1996 Jan 15;56(2):259–263. [PubMed] [Google Scholar]
- Han H. J., Yanagisawa A., Kato Y., Park J. G., Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993 Nov 1;53(21):5087–5089. [PubMed] [Google Scholar]
- Horii A., Han H. J., Shimada M., Yanagisawa A., Kato Y., Ohta H., Yasui W., Tahara E., Nakamura Y. Frequent replication errors at microsatellite loci in tumors of patients with multiple primary cancers. Cancer Res. 1994 Jul 1;54(13):3373–3375. [PubMed] [Google Scholar]
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Ming S. C., Goldman H., Freiman D. G. Intestinal metaplasia and histogenesis of carcinoma in human stomach. Light and electron microscopic study. Cancer. 1967 Sep;20(9):1418–1429. doi: 10.1002/1097-0142(196709)20:9<1418::aid-cncr2820200908>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Sugano H., Takagi K., Kumakura K. [Histogenesis of cancer of the stomach, with special reference to light and electron microscopic and statistical studies of primary microcarcinoma of the stomach]. Gan No Rinsho. 1969 Jul;15(7):627–647. [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Ochiai A., Yamauchi Y., Hirohashi S. p53 mutations in the non-neoplastic mucosa of the human stomach showing intestinal metaplasia. Int J Cancer. 1996 Feb 20;69(1):28–33. doi: 10.1002/(SICI)1097-0215(19960220)69:1<28::AID-IJC6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Liu B., Parsons R., Lengauer C., Palombo F., D'Arrigo A., Markowitz S., Willson J. K., Kinzler K. W. Mutations of GTBP in genetically unstable cells. Science. 1995 Jun 30;268(5219):1915–1917. doi: 10.1126/science.7604266. [DOI] [PubMed] [Google Scholar]
- Parsons R., Myeroff L. L., Liu B., Willson J. K., Markowitz S. D., Kinzler K. W., Vogelstein B. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res. 1995 Dec 1;55(23):5548–5550. [PubMed] [Google Scholar]
- Peltomäki P., Lothe R. A., Aaltonen L. A., Pylkkänen L., Nyström-Lahti M., Seruca R., David L., Holm R., Ryberg D., Haugen A. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993 Dec 15;53(24):5853–5855. [PubMed] [Google Scholar]
- Rhyu M. G., Park W. S., Meltzer S. J. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene. 1994 Jan;9(1):29–32. [PubMed] [Google Scholar]
- Semba S., Yokozaki H., Yamamoto S., Yasui W., Tahara E. Microsatellite instability in precancerous lesions and adenocarcinomas of the stomach. Cancer. 1996 Apr 15;77(8 Suppl):1620–1627. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1620::AID-CNCR30>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Shiao Y. H., Rugge M., Correa P., Lehmann H. P., Scheer W. D. p53 alteration in gastric precancerous lesions. Am J Pathol. 1994 Mar;144(3):511–517. [PMC free article] [PubMed] [Google Scholar]
- Strickler J. G., Zheng J., Shu Q., Burgart L. J., Alberts S. R., Shibata D. p53 mutations and microsatellite instability in sporadic gastric cancer: when guardians fail. Cancer Res. 1994 Sep 1;54(17):4750–4755. [PubMed] [Google Scholar]
- Tahara E., Kuniyasu H., Yasui W., Yokozaki H. Gene alterations in intestinal metaplasia and gastric cancer. Eur J Gastroenterol Hepatol. 1994 Dec;6 (Suppl 1):S97–102. [PubMed] [Google Scholar]
- Tahara H., Kuniyasu H., Yokozaki H., Yasui W., Shay J. W., Ide T., Tahara E. Telomerase activity in preneoplastic and neoplastic gastric and colorectal lesions. Clin Cancer Res. 1995 Nov;1(11):1245–1251. [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Vasen H. F., Mecklin J. P., Khan P. M., Lynch H. T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991 May;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- Wu C., Akiyama Y., Imai K., Miyake S., Nagasaki H., Oto M., Okabe S., Iwama T., Mitamura K., Masumitsu H. DNA alterations in cells from hereditary non-polyposis colorectal cancer patients. Oncogene. 1994 Mar;9(3):991–994. [PubMed] [Google Scholar]
- Yasui W., Ji Z. Q., Kuniyasu H., Ayhan A., Yokozaki H., Ito H., Tahara E. Expression of transforming growth factor alpha in human tissues: immunohistochemical study and northern blot analysis. Virchows Arch A Pathol Anat Histopathol. 1992;421(6):513–519. doi: 10.1007/BF01606881. [DOI] [PubMed] [Google Scholar]
- Yokozaki H., Kuniyasu H., Kitadai Y., Nishimura K., Todo H., Ayhan A., Yasui W., Ito H., Tahara E. p53 point mutations in primary human gastric carcinomas. J Cancer Res Clin Oncol. 1992;119(2):67–70. doi: 10.1007/BF01209657. [DOI] [PMC free article] [PubMed] [Google Scholar]